化工进展 ›› 2023, Vol. 42 ›› Issue (6): 2916-2943.DOI: 10.16085/j.issn.1000-6613.2022-1429
甲基芳烃液相选择性催化氧化催化剂研究进展
- 北京理工大学化学与化工学院,北京 100081
-
收稿日期:2022-08-01修回日期:2022-10-28出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:吴芹,黎汉生 -
作者简介:殷鹏镇(1999—),男,硕士研究生,研究方向为工业催化。E-mail:YPZ990728@163.com。 -
基金资助:中国石油科技创新基金(2020D-5007-0402)
Advances in catalysts for liquid-phase selective oxidation of methyl aromatic hydrocarbons
YIN Pengzhen( ), WU Qin(
), WU Qin( ), LI Hansheng(
), LI Hansheng( )
)
- School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, China
-
Received:2022-08-01Revised:2022-10-28Online:2023-06-25Published:2023-06-29 -
Contact:WU Qin, LI Hansheng
摘要:
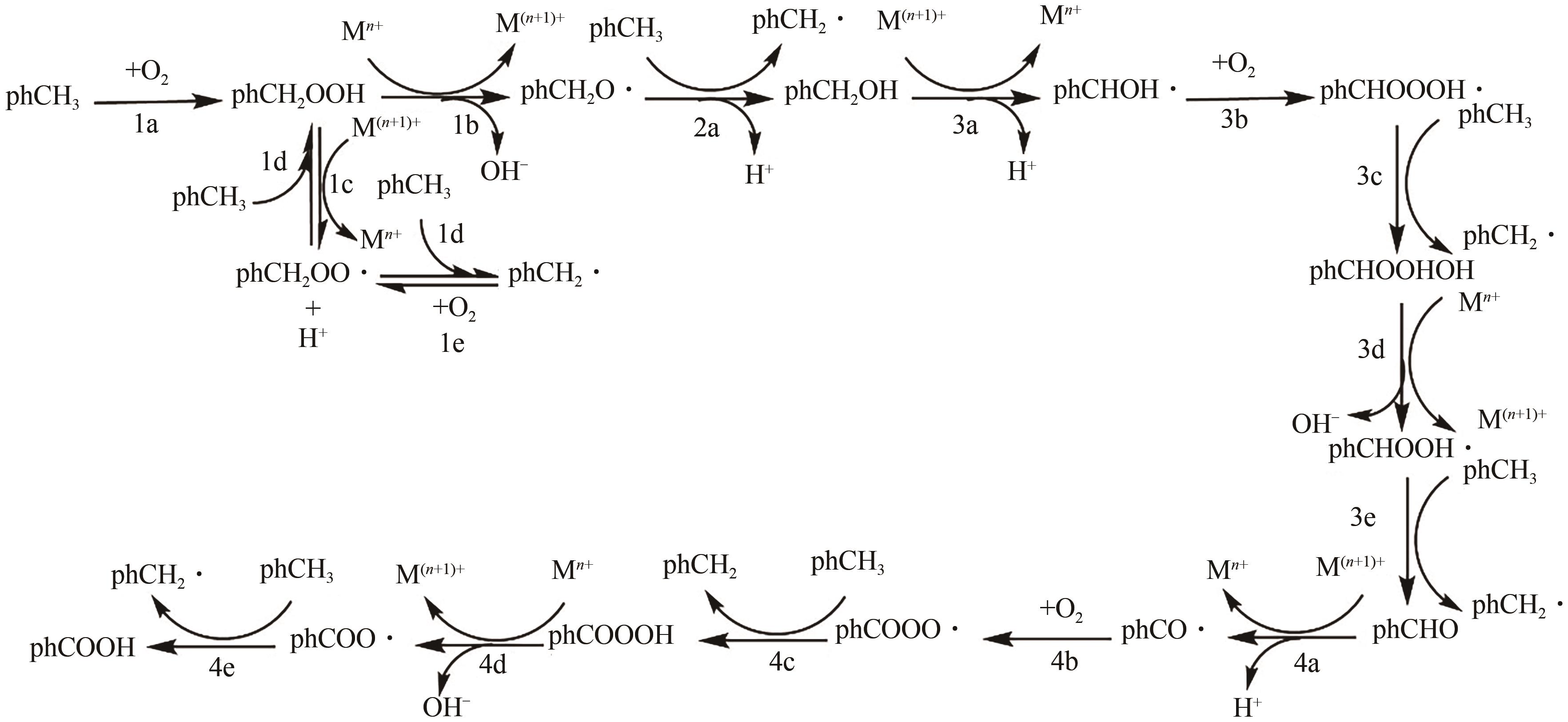
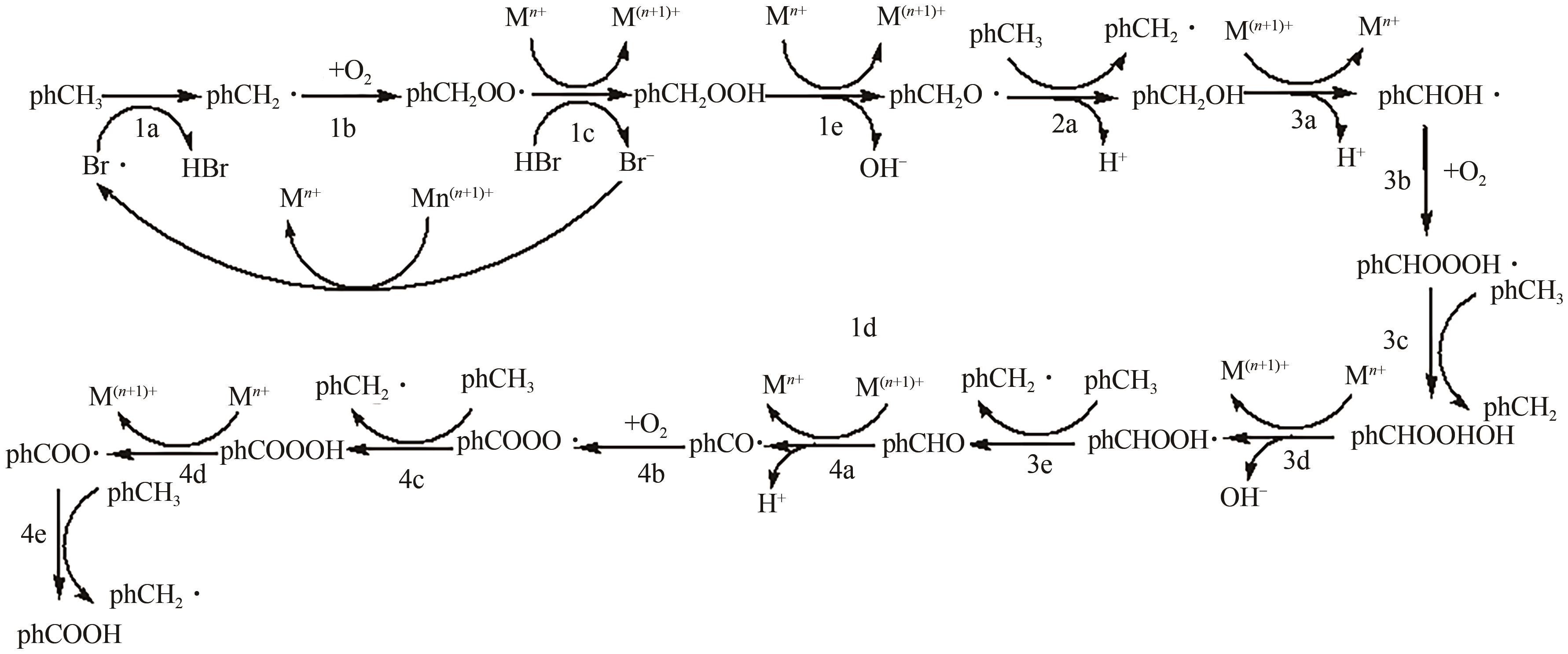
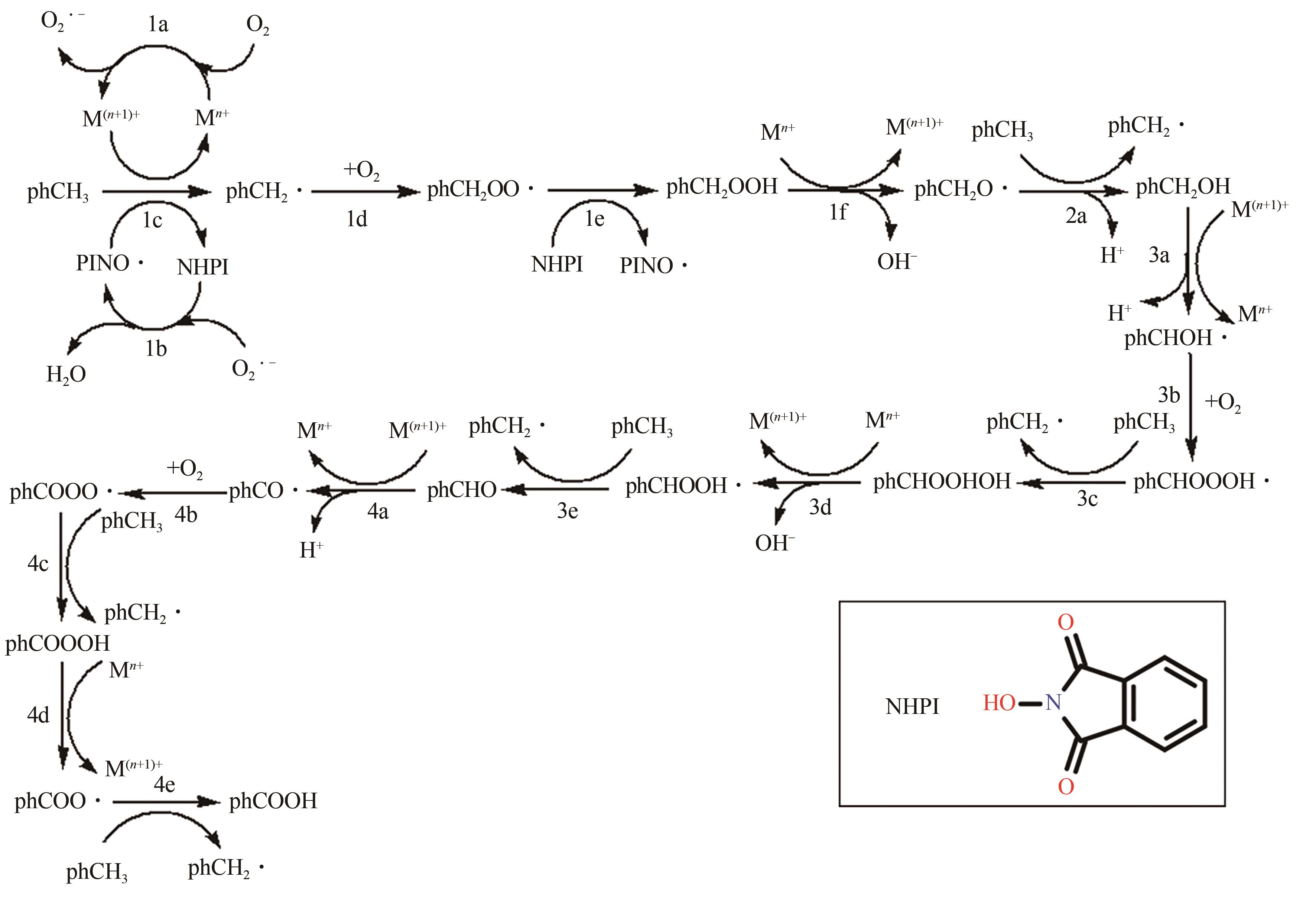
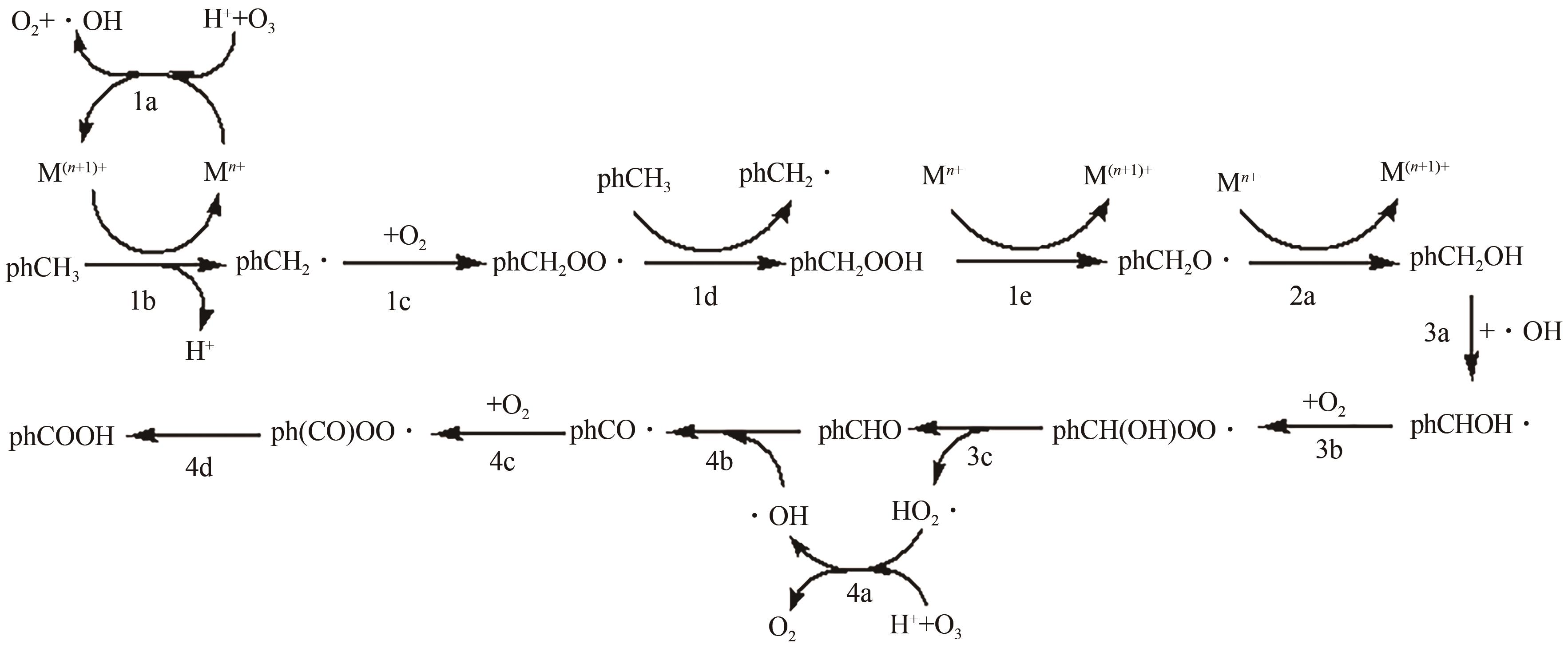
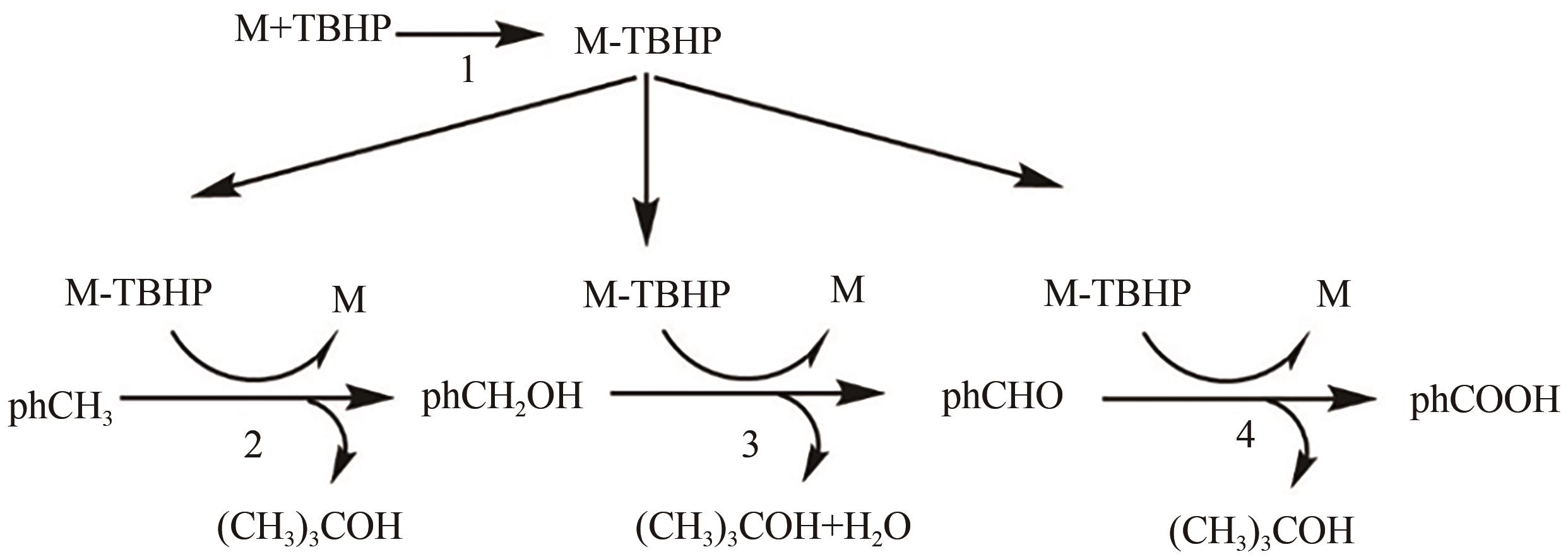
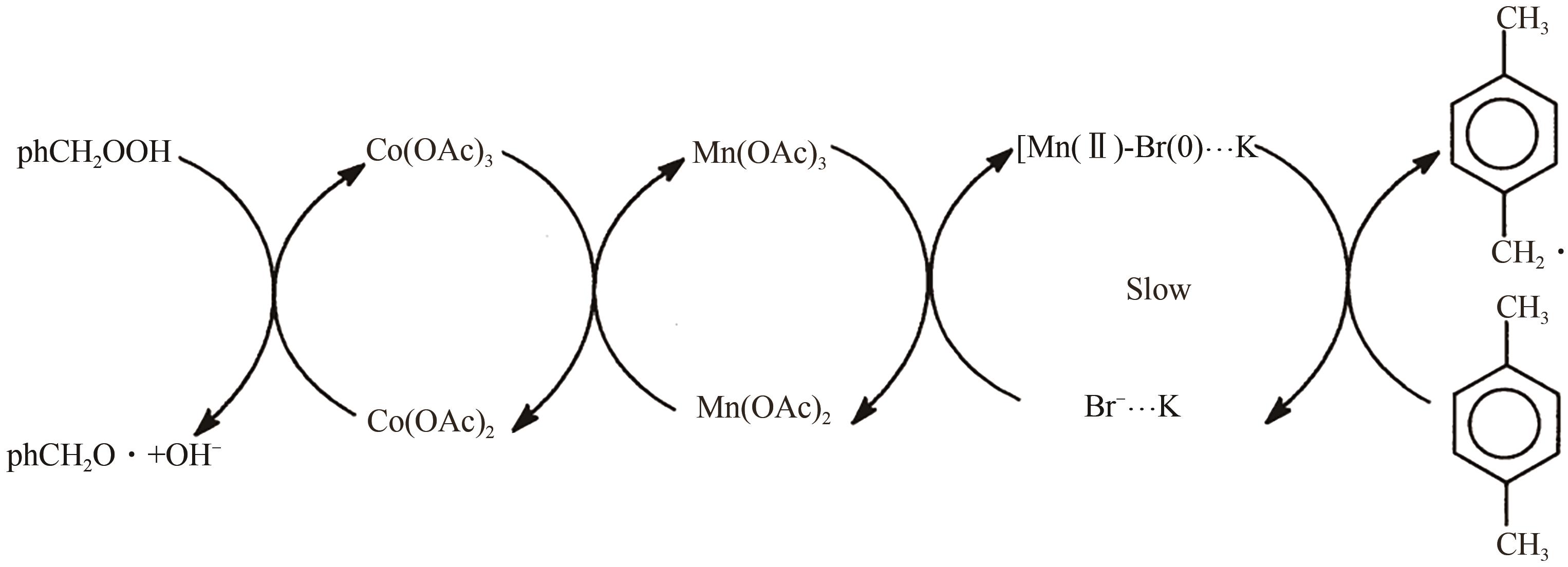
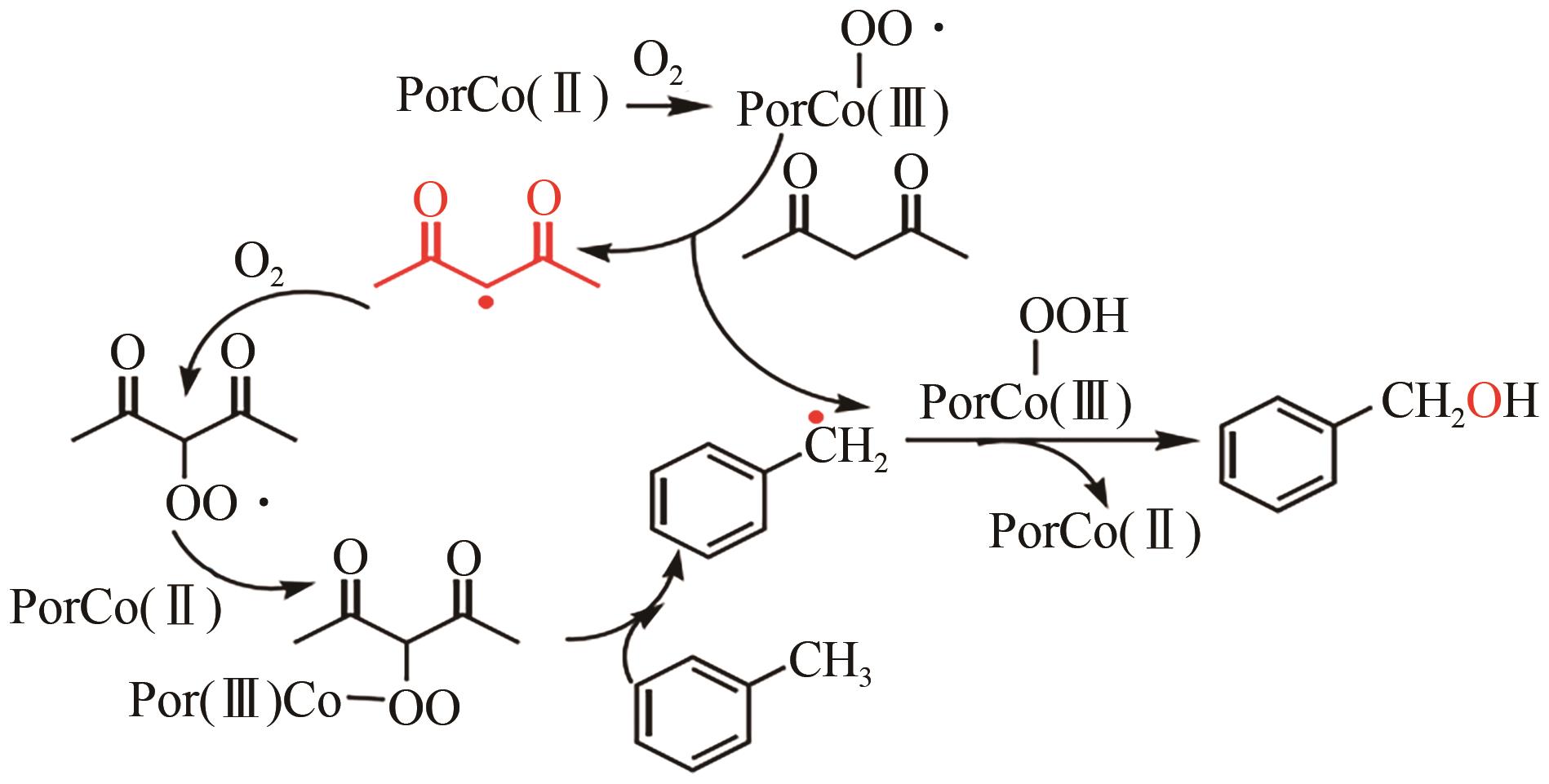
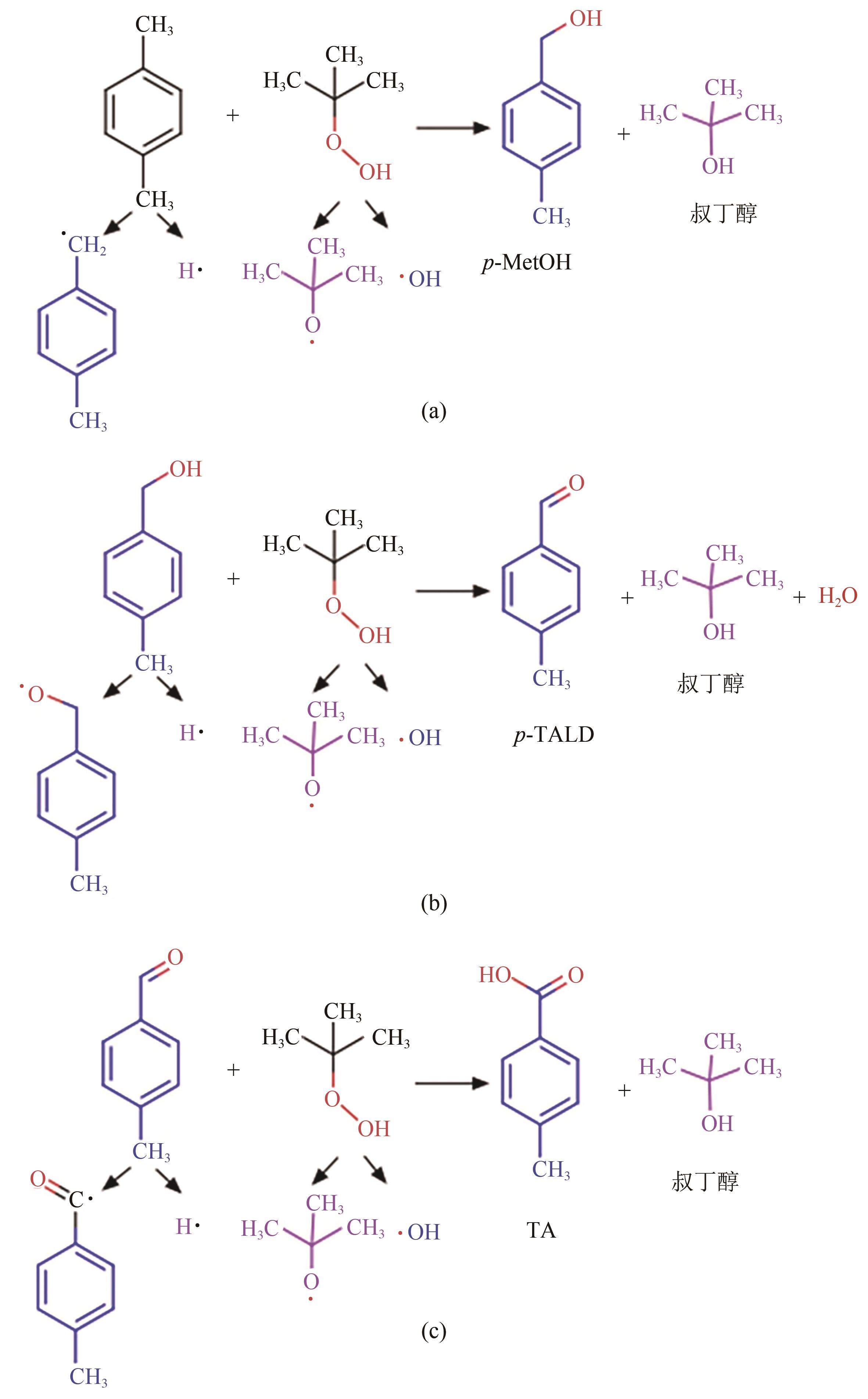
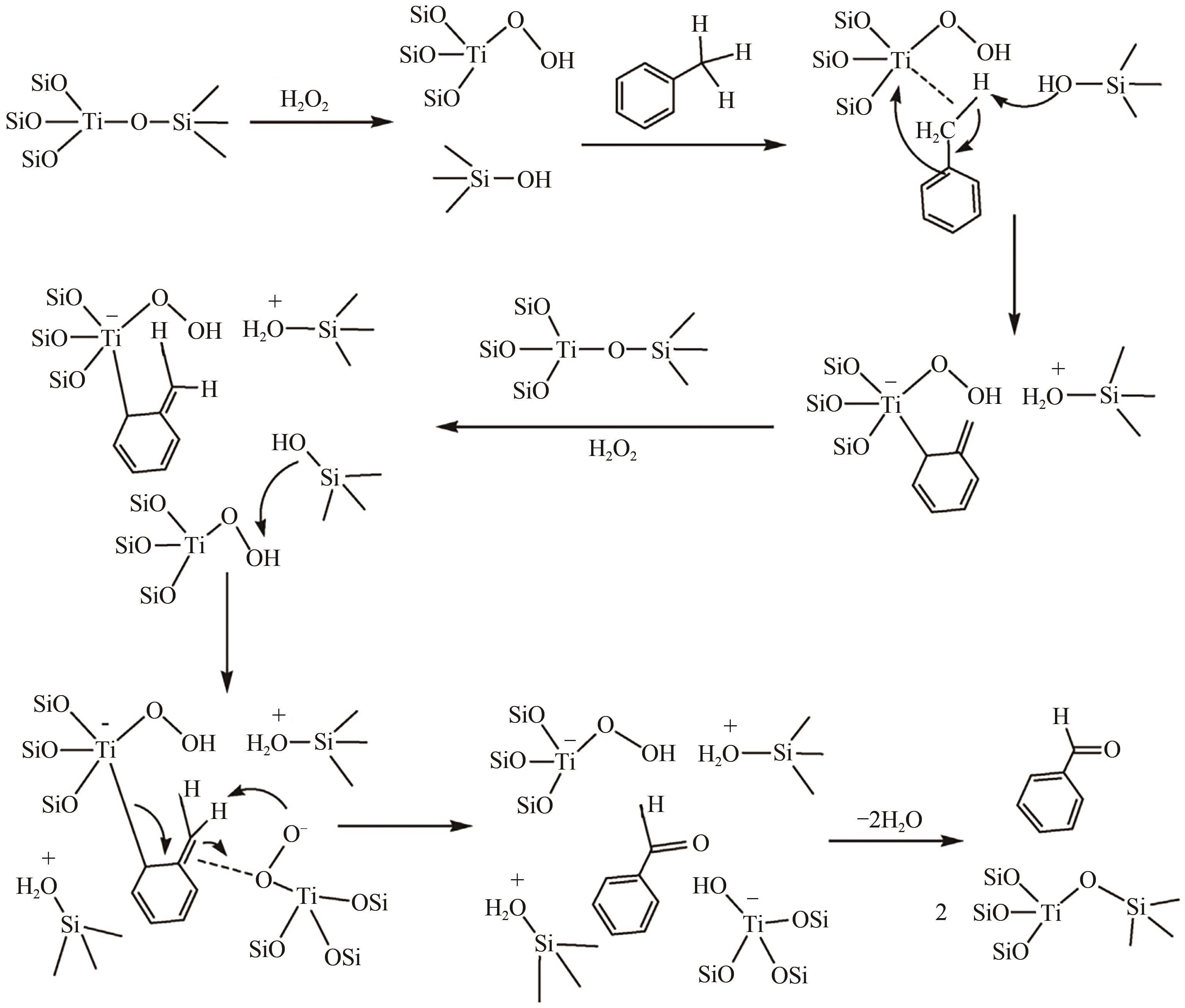
甲基芳烃衍生的芳甲醇、芳甲醛、芳甲酸等高附加值产品作为重要化工中间体,一直在国内外市场处于供不应求状态。其通常以甲基芳烃为原料,通过液相选择性催化氧化技术可控制备。该技术因其转化率高、成本低、安全性好等优点,成为目前工业上该类产品的主要生产方法,而开发高性能催化剂是该技术的关键问题之一。因此,本文在总结甲基芳烃催化氧化机理的基础上,综述了近年来金属盐催化剂、金属络合物催化剂、金属有机框架(MOF)催化剂、金属氧化物催化剂及其他催化剂在甲基芳烃液相选择性催化氧化方面的应用研究进展。研究者从提升催化剂活性、稳定性及降低制备成本出发,对不同种类的催化剂进行改进,发现金属盐及金属络合物催化剂性能的提高在于开发相应载体,而MOFs催化剂在于开发高活性的Co、Mn金属MOFs催化剂,金属氧化物催化剂则需要在现有基础上优化制备工艺。
中图分类号:
引用本文
殷鹏镇, 吴芹, 黎汉生. 甲基芳烃液相选择性催化氧化催化剂研究进展[J]. 化工进展, 2023, 42(6): 2916-2943.
YIN Pengzhen, WU Qin, LI Hansheng. Advances in catalysts for liquid-phase selective oxidation of methyl aromatic hydrocarbons[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2916-2943.
| 催化剂 | 反应条件 |
|---|---|
| 乙酸钴(Ⅱ) | 空气液相氧化,160℃,0.98MPa |
| 硬脂酸钴 | |
| 钴盐,NaBr | 富氧液相氧化,140~170℃,常压 |
| KMnO4,溴化物>0.03%, CH3CN>0.05% | 原子氧液相氧化 |
| 环烷酸钴 | 90℃,1.47MPa,氧或空气液相氧化 |
| 辛酸钴 | 150~175℃,0.34~0.52MPa |
乙酸钴(Ⅱ) 苯甲酸钴(Ⅱ) | 170℃,0.69MPa |
| 钴 | 空气液相氧化,>125℃,>0.2MPa |
| 钴盐0.1%~0.3% | 空气液相氧化,121~177℃,0.2MPa |
| 可溶性钴盐0.1%~0.3% | 空气液相氧化,0.2MPa |
月桂酸钴(Ⅱ) 月桂酸锰(Ⅱ) | 液相氧化,130℃,氧气压力0.49MPa |
乙酰丙酮酸钴0.003~0.02mol/L 苯甲醛0.01~0.1mol/L | 液相氧化 |
表1 甲苯液相催化氧化常用催化剂[3]
| 催化剂 | 反应条件 |
|---|---|
| 乙酸钴(Ⅱ) | 空气液相氧化,160℃,0.98MPa |
| 硬脂酸钴 | |
| 钴盐,NaBr | 富氧液相氧化,140~170℃,常压 |
| KMnO4,溴化物>0.03%, CH3CN>0.05% | 原子氧液相氧化 |
| 环烷酸钴 | 90℃,1.47MPa,氧或空气液相氧化 |
| 辛酸钴 | 150~175℃,0.34~0.52MPa |
乙酸钴(Ⅱ) 苯甲酸钴(Ⅱ) | 170℃,0.69MPa |
| 钴 | 空气液相氧化,>125℃,>0.2MPa |
| 钴盐0.1%~0.3% | 空气液相氧化,121~177℃,0.2MPa |
| 可溶性钴盐0.1%~0.3% | 空气液相氧化,0.2MPa |
月桂酸钴(Ⅱ) 月桂酸锰(Ⅱ) | 液相氧化,130℃,氧气压力0.49MPa |
乙酰丙酮酸钴0.003~0.02mol/L 苯甲醛0.01~0.1mol/L | 液相氧化 |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VOSO4 | O2 | 氢溴酸 | 100℃、0.1MPa | 乙酸 | 苯甲酸 | 20h | 99 | 98 | [ |
| 2 | 萘酸钴 | O2 | 无 | 140℃、2MPa | [Emim]BF4 | 苯甲酸 | 2h | 31 | 60 | [ |
| 3 | Co(OAc)2 | O3 | KBr | 80℃、0.8L/min | 乙酸 | 对苯二甲酸 | 6h | 96 | 84 | [ |
| 4 | Mn(OAc)2 | O3 | 硫酸 | 60℃、6.0 × 10-3L/s | 乙酸 | 苯甲酸 | 75min | 27 | 82.50 | [ |
| 5 | CoBr2/MnBr2 | O2 | 无 | 110℃ | 乙酸 | 苯甲酸 | 10h | 16 | 95 | [ |
| 6 | Co(acac)2/Mn(acac)2 | O2 | 苯甲酸 | 165℃、0.6MPa | 无 | 苯甲醛 | 4h | 45 | 15 | [ |
| 7 | Co(OAc)2/Mn(OAc)2 | O2 | H3PW12O40 | 215℃、3MPa | 无 | 间苯二甲酸 | 3h | 92 | 31.5 | [ |
| 8 | VO(OAc)2/Zr(OAc)2 | H2O2 | 无 | 90℃、H2O2(30%)5mL | 乙酸 | 苯甲醛 | 4h | 20.3 | 70.3 | [ |
| 9 | Co(OAc)2/Mn(OAc)2 | O2 | 1,1,2,2-四溴乙烷 | 110℃ | 乙酸 | 苯甲醛 | 8h | 67 | 67.60 | [ |
| 10 | Co(OAc)2 | O2 | 溴苄/4-二甲氨基吡啶(DMAP) | 160℃ | 无 | 苯甲酸 | 80min | 42 | 76 | [ |
| 11 | Co(OAc)2 | O2 | NHPI | 170~190℃、0.8MPa | 无 | 苯甲酸 | 20h | 84 | 93 | [ |
| 12 | Co(OAc)2 | O2 | NHPI | 50℃、1.0MPa | 二氯甲烷 | 苯甲酸 | 3h | 58.7 | 95 | [ |
| 13 | MnCl2 | O2 | NHPI | 100℃、0.05L/min | 乙酸 | 苯甲酸 | 5h | 40.4 | 71.3 | [ |
| 14 | MnII1CoII10@MCM-41/HNT | O2 | 溴化钾 | 200~250℃、2.0MPa | 乙酸和乙腈 | 对苯二甲酸 | 3h | 99 | 93.80 | [ |
| 15 | Cu-MCM-41 | O2 | 溴化十六烷基三甲铵 | 80℃ | 无 | 2,5-二羟基对苯二甲酸 | 5h | 21.70 | 73.00 | [ |
| 17 | Co(NO3)2/SiO2 | O2 | NHPI | 90℃、2MPa | HFIP | 苯甲醛 | 5h | 42.5 | 52.60 | [ |
| 16 | Co(OAc)2/SiO2 | O2 | NHPI | 90℃、2MPa | HFIP | 苯甲醛 | 5h | 42.50 | 73.10 | [ |
| 18 | CuBr2@g-C3N4 | TBHP | 无 | 120℃ | 无 | 苯甲醛 | 3~6h | 55 | 95 | [ |
| 19 | Cu-CNB | TBHP | 无 | 70℃ | 乙腈 | 苯甲醛 | 16h | 6.30 | 99 | [ |
| 20 | 聚(4-乙烯基吡啶-共二苯基苯)负载Fe(Ⅲ)催化剂 | H2O2 | 无 | 80℃ | 乙腈 | 苯甲酸 | 6h | 90 | 96 | [ |
表2 金属盐催化剂体系及其在甲基芳烃催化氧化反应中应用
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VOSO4 | O2 | 氢溴酸 | 100℃、0.1MPa | 乙酸 | 苯甲酸 | 20h | 99 | 98 | [ |
| 2 | 萘酸钴 | O2 | 无 | 140℃、2MPa | [Emim]BF4 | 苯甲酸 | 2h | 31 | 60 | [ |
| 3 | Co(OAc)2 | O3 | KBr | 80℃、0.8L/min | 乙酸 | 对苯二甲酸 | 6h | 96 | 84 | [ |
| 4 | Mn(OAc)2 | O3 | 硫酸 | 60℃、6.0 × 10-3L/s | 乙酸 | 苯甲酸 | 75min | 27 | 82.50 | [ |
| 5 | CoBr2/MnBr2 | O2 | 无 | 110℃ | 乙酸 | 苯甲酸 | 10h | 16 | 95 | [ |
| 6 | Co(acac)2/Mn(acac)2 | O2 | 苯甲酸 | 165℃、0.6MPa | 无 | 苯甲醛 | 4h | 45 | 15 | [ |
| 7 | Co(OAc)2/Mn(OAc)2 | O2 | H3PW12O40 | 215℃、3MPa | 无 | 间苯二甲酸 | 3h | 92 | 31.5 | [ |
| 8 | VO(OAc)2/Zr(OAc)2 | H2O2 | 无 | 90℃、H2O2(30%)5mL | 乙酸 | 苯甲醛 | 4h | 20.3 | 70.3 | [ |
| 9 | Co(OAc)2/Mn(OAc)2 | O2 | 1,1,2,2-四溴乙烷 | 110℃ | 乙酸 | 苯甲醛 | 8h | 67 | 67.60 | [ |
| 10 | Co(OAc)2 | O2 | 溴苄/4-二甲氨基吡啶(DMAP) | 160℃ | 无 | 苯甲酸 | 80min | 42 | 76 | [ |
| 11 | Co(OAc)2 | O2 | NHPI | 170~190℃、0.8MPa | 无 | 苯甲酸 | 20h | 84 | 93 | [ |
| 12 | Co(OAc)2 | O2 | NHPI | 50℃、1.0MPa | 二氯甲烷 | 苯甲酸 | 3h | 58.7 | 95 | [ |
| 13 | MnCl2 | O2 | NHPI | 100℃、0.05L/min | 乙酸 | 苯甲酸 | 5h | 40.4 | 71.3 | [ |
| 14 | MnII1CoII10@MCM-41/HNT | O2 | 溴化钾 | 200~250℃、2.0MPa | 乙酸和乙腈 | 对苯二甲酸 | 3h | 99 | 93.80 | [ |
| 15 | Cu-MCM-41 | O2 | 溴化十六烷基三甲铵 | 80℃ | 无 | 2,5-二羟基对苯二甲酸 | 5h | 21.70 | 73.00 | [ |
| 17 | Co(NO3)2/SiO2 | O2 | NHPI | 90℃、2MPa | HFIP | 苯甲醛 | 5h | 42.5 | 52.60 | [ |
| 16 | Co(OAc)2/SiO2 | O2 | NHPI | 90℃、2MPa | HFIP | 苯甲醛 | 5h | 42.50 | 73.10 | [ |
| 18 | CuBr2@g-C3N4 | TBHP | 无 | 120℃ | 无 | 苯甲醛 | 3~6h | 55 | 95 | [ |
| 19 | Cu-CNB | TBHP | 无 | 70℃ | 乙腈 | 苯甲醛 | 16h | 6.30 | 99 | [ |
| 20 | 聚(4-乙烯基吡啶-共二苯基苯)负载Fe(Ⅲ)催化剂 | H2O2 | 无 | 80℃ | 乙腈 | 苯甲酸 | 6h | 90 | 96 | [ |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CoTPP | O2 | 乙酰丙酮 | 165℃ | 乙腈 | 苯甲酸 | 4h | 14.6 | 61.40 | [ |
| 2 | VOTPP | O2 | 无 | 145℃、0.8MPa | 无 | 苯甲酸 | 4h | 23.0 | 86.00 | [ |
| 3 | T(p-Cl)PPMnCl | O2 | NHPI、CTAB | 100℃,0.05L/min | 无 | 苯甲酸 | 6h | 40.95 | 65.14 | [ |
| 4 | [T(p-Cl)PPFe]2O | 空气 | 无 | 190℃、1.0MPa | 无 | 苯甲醛和苯甲醇 | 90min | 8.71 | 4.20 | [ |
| 5 | Schiff碱锰(Ⅲ)配合物 | 空气 | 无 | 120℃、0.05L/h | 无 | 对苯二甲酸 | 12h | 55 | 90 | [ |
| 6 | Co(Ⅱ)TPP/CTS | O2 | 无 | 145℃、0.8MPa | 无 | 苯甲醛和苯甲醇 | 4.5h | 8.93 | 66 | [ |
| 7 | MnTPP/CTS | O2 | 无 | 195℃、0.6MPa | 无 | 苯甲醛和苯甲醇 | 2h | 4.8 | 95 | [ |
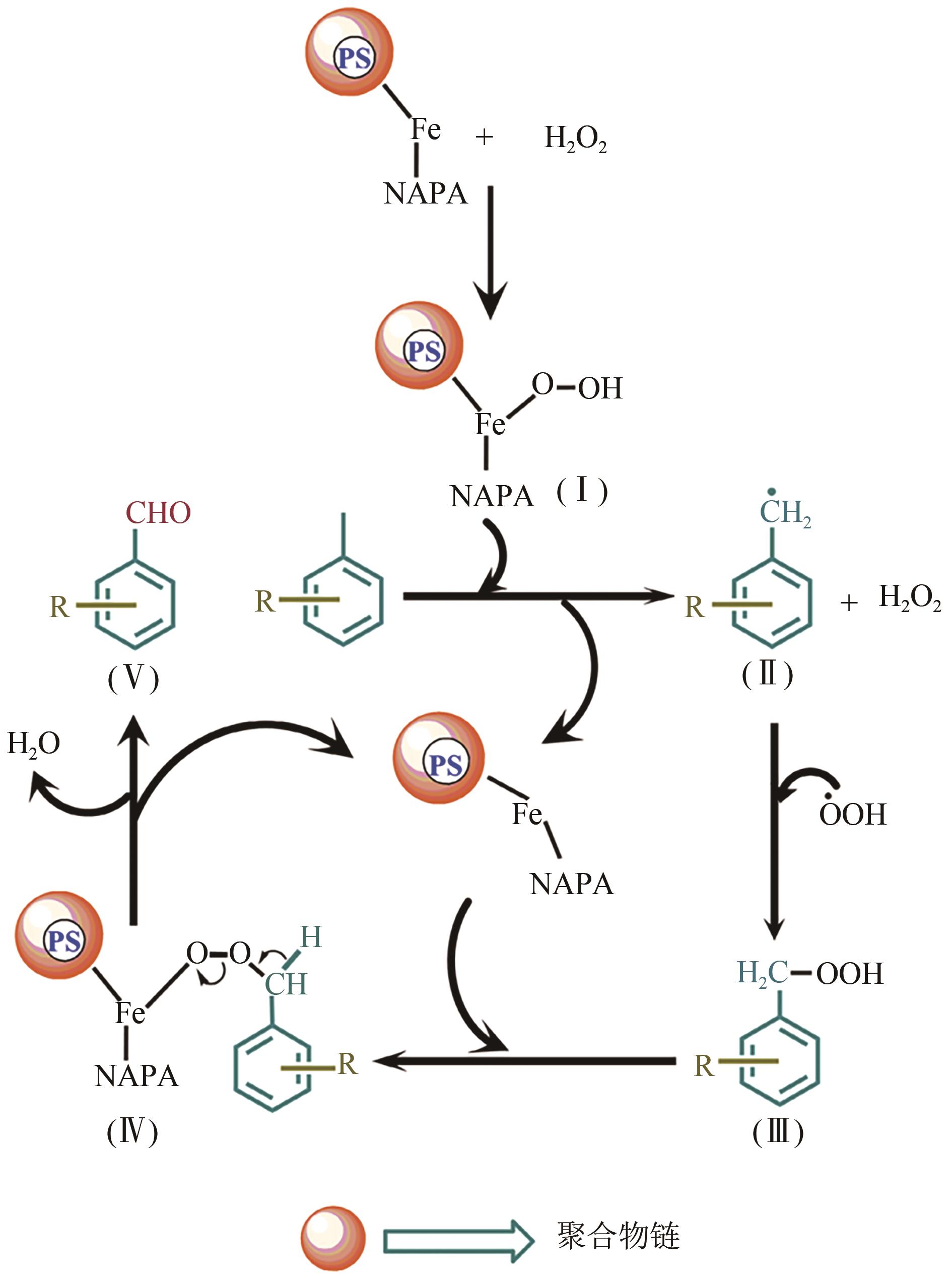
| 8 | PS-Fe-NAPA | H2O2 | 无 | 60℃ | 乙腈 | 苯甲醛 | 7h | 78 | 86 | [ |
| 9 | LDH-[NAPABA-M] | TBHP | 无 | 100℃ | 无 | 苯甲醛 | 7h | 55.30 | 86.10 | [ |
表3 不同金属络合物催化剂在甲基芳烃催化氧化中的性能
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CoTPP | O2 | 乙酰丙酮 | 165℃ | 乙腈 | 苯甲酸 | 4h | 14.6 | 61.40 | [ |
| 2 | VOTPP | O2 | 无 | 145℃、0.8MPa | 无 | 苯甲酸 | 4h | 23.0 | 86.00 | [ |
| 3 | T(p-Cl)PPMnCl | O2 | NHPI、CTAB | 100℃,0.05L/min | 无 | 苯甲酸 | 6h | 40.95 | 65.14 | [ |
| 4 | [T(p-Cl)PPFe]2O | 空气 | 无 | 190℃、1.0MPa | 无 | 苯甲醛和苯甲醇 | 90min | 8.71 | 4.20 | [ |
| 5 | Schiff碱锰(Ⅲ)配合物 | 空气 | 无 | 120℃、0.05L/h | 无 | 对苯二甲酸 | 12h | 55 | 90 | [ |
| 6 | Co(Ⅱ)TPP/CTS | O2 | 无 | 145℃、0.8MPa | 无 | 苯甲醛和苯甲醇 | 4.5h | 8.93 | 66 | [ |
| 7 | MnTPP/CTS | O2 | 无 | 195℃、0.6MPa | 无 | 苯甲醛和苯甲醇 | 2h | 4.8 | 95 | [ |
| 8 | PS-Fe-NAPA | H2O2 | 无 | 60℃ | 乙腈 | 苯甲醛 | 7h | 78 | 86 | [ |
| 9 | LDH-[NAPABA-M] | TBHP | 无 | 100℃ | 无 | 苯甲醛 | 7h | 55.30 | 86.10 | [ |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间/h | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cu-MOF | H2O2 | 无 | 30℃ | 乙腈 | 4-羟甲基苯甲酸 | 5 | 85.50 | 99 | [ |
| 2 | Co-MOF-74 | O2 | 无 | 80℃ | 乙腈 | 苯甲醛 | 6 | 12.10 | 63 | [ |
| 3 | ZIF-67-24 | O2 | NHPI | 40℃ | HFIP | 苯甲醛 | 4 | 87.90 | 66 | [ |
| 4 | Cu-BTC | O2 | NHPI | 110℃ | 无 | 苯甲醛和苯甲醇 | 2 | 7.6 | 50 | [ |
| 5 | Co-BTC | O2 | NDHPI | 150℃ | 乙腈 | 对苯二甲酸 | 12 | 100 | 97 | [ |
| 6 | Ag-Cu-BTC | O2 | 无 | 160℃、1.0MPa | 无 | 苯甲醛 | 4 | 12.70 | 99 | [ |
| 7 | MnCo-MOF-74 | O2 | 无 | 80℃ | 无 | 苯甲醛 | 6 | 17.60 | 98 | [ |
| 8 | Co-MOF-74@Mn-MOF-74 | TBHP | 无 | 80℃ | 乙腈 | 苯甲醛 | 6 | 22.4 | 98.1 | [ |
表4 不同MOFs催化剂在甲基芳烃催化氧化中的性能
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间/h | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cu-MOF | H2O2 | 无 | 30℃ | 乙腈 | 4-羟甲基苯甲酸 | 5 | 85.50 | 99 | [ |
| 2 | Co-MOF-74 | O2 | 无 | 80℃ | 乙腈 | 苯甲醛 | 6 | 12.10 | 63 | [ |
| 3 | ZIF-67-24 | O2 | NHPI | 40℃ | HFIP | 苯甲醛 | 4 | 87.90 | 66 | [ |
| 4 | Cu-BTC | O2 | NHPI | 110℃ | 无 | 苯甲醛和苯甲醇 | 2 | 7.6 | 50 | [ |
| 5 | Co-BTC | O2 | NDHPI | 150℃ | 乙腈 | 对苯二甲酸 | 12 | 100 | 97 | [ |
| 6 | Ag-Cu-BTC | O2 | 无 | 160℃、1.0MPa | 无 | 苯甲醛 | 4 | 12.70 | 99 | [ |
| 7 | MnCo-MOF-74 | O2 | 无 | 80℃ | 无 | 苯甲醛 | 6 | 17.60 | 98 | [ |
| 8 | Co-MOF-74@Mn-MOF-74 | TBHP | 无 | 80℃ | 乙腈 | 苯甲醛 | 6 | 22.4 | 98.1 | [ |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间/h | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mn3O4 | O2 | 无 | 195℃、1MPa | 无 | 苯甲酸 | 3 | 36.4 | 89.3 | [ |
| 2 | γ-MnO2 | O2 | 无 | 110℃、9mL/min | AIBN | 苯甲酸 | 48 | 47.8 | 57 | [ |
| 3 | MnO2 | O2 | NHPI | 110℃、0.3MPa | 无 | 苯甲酸 | 3 | 94.40 | 98.40 | [ |
| 4 | CeO2 | O2 | 无 | 70℃、0.1MPa | 无 | 对苯二甲酸 | 25 | 40 | 52 | [ |
| 5 | Mn3O4/碳纳米管 | O2 | 无 | 90℃、15mL/min | 无 | 苯甲醛 | 12 | 24.63 | 90.49 | [ |
| 6 | CoO x /SiO2 | O2 | NHPI | 25℃、0.1MPa | HFIP | 苯甲醛 | 4 | 91 | 68 | [ |
| 7 | CoO x /SiO2 | O2 | NHPI | 30℃、0.1MPa | HFIP | 苯甲醛 | 4 | 98.50 | 62.50 | [ |
| 8 | In-TUD-1 | TBHP | 无 | 80℃ | 乙酸 | 苯甲醛 | 5 | 48 | 48 | [ |
| 9 | Cu-Fe-O | O2 | 吡啶 | 190℃、1.0MPa | 无 | 苯甲醛 | 2 | 7 | 86 | [ |
| 10 | Cu x Zn y O | O2 | 无 | 250℃ | 无 | 苯甲醛 | 4 | 65 | 99 | [ |
| 11 | Cu x Mn3.66-x Mo3O12 | O2 | 溴化钠 | 170℃、1100mL/min | 乙酸 | 苯甲醛 | 4 | 60 | 57.40 | [ |
| 12 | Mn/Fe/O | TBHP | 无 | 100℃ | 乙腈 | 间苯二甲酸 | 24 | 98 | 93 | [ |
| 13 | Ag-WO3微球 | H2O2 | 无 | 70℃ | 二甲基亚砜 | 间苯二甲酸 | 10 | 99 | 96 | [ |
| 14 | HDPA-Fe2O3/Al2O3 | O2 | 无 | 185℃、2.0MPa | 水 | 苯甲醛 | 99 | [ | ||
| 15 | Cu-Cr双金属氧化物/g-C3N4 | H2O2 | 无 | 75℃ | 乙腈 | 间苯二甲酸 | 5 | 46.50 | 42.90 | [ |
| 16 | MnMoO4纳米催化材料 | H2O2 | 无 | 80℃ | 无 | 苯甲醛 | 78 | [ |
表5 不同金属氧化物基催化剂在甲基芳烃催化氧化中的性能
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间/h | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mn3O4 | O2 | 无 | 195℃、1MPa | 无 | 苯甲酸 | 3 | 36.4 | 89.3 | [ |
| 2 | γ-MnO2 | O2 | 无 | 110℃、9mL/min | AIBN | 苯甲酸 | 48 | 47.8 | 57 | [ |
| 3 | MnO2 | O2 | NHPI | 110℃、0.3MPa | 无 | 苯甲酸 | 3 | 94.40 | 98.40 | [ |
| 4 | CeO2 | O2 | 无 | 70℃、0.1MPa | 无 | 对苯二甲酸 | 25 | 40 | 52 | [ |
| 5 | Mn3O4/碳纳米管 | O2 | 无 | 90℃、15mL/min | 无 | 苯甲醛 | 12 | 24.63 | 90.49 | [ |
| 6 | CoO x /SiO2 | O2 | NHPI | 25℃、0.1MPa | HFIP | 苯甲醛 | 4 | 91 | 68 | [ |
| 7 | CoO x /SiO2 | O2 | NHPI | 30℃、0.1MPa | HFIP | 苯甲醛 | 4 | 98.50 | 62.50 | [ |
| 8 | In-TUD-1 | TBHP | 无 | 80℃ | 乙酸 | 苯甲醛 | 5 | 48 | 48 | [ |
| 9 | Cu-Fe-O | O2 | 吡啶 | 190℃、1.0MPa | 无 | 苯甲醛 | 2 | 7 | 86 | [ |
| 10 | Cu x Zn y O | O2 | 无 | 250℃ | 无 | 苯甲醛 | 4 | 65 | 99 | [ |
| 11 | Cu x Mn3.66-x Mo3O12 | O2 | 溴化钠 | 170℃、1100mL/min | 乙酸 | 苯甲醛 | 4 | 60 | 57.40 | [ |
| 12 | Mn/Fe/O | TBHP | 无 | 100℃ | 乙腈 | 间苯二甲酸 | 24 | 98 | 93 | [ |
| 13 | Ag-WO3微球 | H2O2 | 无 | 70℃ | 二甲基亚砜 | 间苯二甲酸 | 10 | 99 | 96 | [ |
| 14 | HDPA-Fe2O3/Al2O3 | O2 | 无 | 185℃、2.0MPa | 水 | 苯甲醛 | 99 | [ | ||
| 15 | Cu-Cr双金属氧化物/g-C3N4 | H2O2 | 无 | 75℃ | 乙腈 | 间苯二甲酸 | 5 | 46.50 | 42.90 | [ |
| 16 | MnMoO4纳米催化材料 | H2O2 | 无 | 80℃ | 无 | 苯甲醛 | 78 | [ |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 选择性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HPW@C(H2O2改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 乙酸 | 间苯二甲酸 | >99% | [ |
| 2 | HPW@C(硝酸改性) | H2O2 | 无 | 30℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
| 3 | HPW@C(ZnCl2改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
| 4 | HPW@C(乙酸改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
表6 不同物质改性后的磷钨酸催化剂在甲基芳烃催化氧化中的性能
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 选择性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | HPW@C(H2O2改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 乙酸 | 间苯二甲酸 | >99% | [ |
| 2 | HPW@C(硝酸改性) | H2O2 | 无 | 30℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
| 3 | HPW@C(ZnCl2改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
| 4 | HPW@C(乙酸改性) | H2O2 | 无 | 40℃、3.0MPa、300r/min | 无 | 间苯二甲酸 | >99% | [ |
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni6(C8H9S)12 | H2O2 | 无 | 80℃ | 无 | 苯甲醛 | 4h | 100 | 91 | [ |
| 2 | CuNPs | O2 | 无 | 65℃、2.0MPa | 无 | 苯甲醛 | 8h | 11.50 | 66.5 | [ |
| 3 | W/HAp | H2O2 | 无 | 55℃、900r/min | 无 | 对苯二甲醛 | 4h | 24 | >63 | [ |
| 4 | TS-1 | H2O2 | 无 | 80℃ | 乙腈 | 苯甲醛 | 4h | 97 | 100 | [ |
| 5 | NHSI | O2 | 无 | 100℃ | 无 | 对苯二甲酸 | 24h | 100 | 98.3 | [ |
表7 其他催化剂在甲基芳烃催化氧化中的性能
| 序号 | 催化剂 | 氧化剂 | 助剂 | 操作条件 | 溶剂 | 产物 | 时间 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni6(C8H9S)12 | H2O2 | 无 | 80℃ | 无 | 苯甲醛 | 4h | 100 | 91 | [ |
| 2 | CuNPs | O2 | 无 | 65℃、2.0MPa | 无 | 苯甲醛 | 8h | 11.50 | 66.5 | [ |
| 3 | W/HAp | H2O2 | 无 | 55℃、900r/min | 无 | 对苯二甲醛 | 4h | 24 | >63 | [ |
| 4 | TS-1 | H2O2 | 无 | 80℃ | 乙腈 | 苯甲醛 | 4h | 97 | 100 | [ |
| 5 | NHSI | O2 | 无 | 100℃ | 无 | 对苯二甲酸 | 24h | 100 | 98.3 | [ |
| 1 | CAO Xing, HAN Tong, PENG Qing, et al. Modifications of heterogeneous photocatalysts for hydrocarbon C—H bond activation and selective conversion[J]. Chemical Communications, 2020, 56(90): 13918-13932. |
| 2 | Laura TORRENTE-MURCIANO, SOLSONA Benjamín, AGOURAM Saïd, et al. Low temperature total oxidation of toluene by bimetallic Au-Ir catalysts[J]. Catalysis Science & Technology, 2017, 7(13): 2886-2896. |
| 3 | 赵劲松, 雷庆英. 甲苯经苯甲酸制苯酚[J]. 四川化工, 1989(3): 39-43. |
| ZHAO Jingsong, LEI Qingying. Toluene is prepared from benzoic acid to phenol[J]. Sichuan Chemical Industry, 1989(3): 39-43. | |
| 4 | OTTENBACHER Roman V, TALSI Evgenii P, BRYLIAKOV Konstantin P. Recent progress in catalytic oxygenation of aromatic C—H groups with the environmentally benign oxidants H2O2 and O2 [J]. Applied Organometallic Chemistry, 2020, 34(11): 5900. |
| 5 | 宋梦迪, 刘莹, 李歆, 等. 典型芳香烃大气氧化机理研究进展[J]. 化学学报, 2021, 79(10): 1214-1231. |
| SONG Mengdi, LIU Ying, LI Xin, et al. Advances on atmospheric oxidation mechanism of typical aromatic hydrocarbons[J]. Acta Chimica Sinica, 2021, 79(10): 1214-1231. | |
| 6 | LISICKI Dawid, MACIEJ Artur, ORLINSKA Beata. Selective aerobic oxidation of toluene in the presence of Co2+ and task-specific organic salts, including ionic liquids[J]. Industrial & Engineering Chemistry Research, 2021, 60(30): 11579-11589. |
| 7 | Quanming LYU, CAI Yifu, WANG Shilin, et al. Experiments and kinetic modeling on the Co/Mn/Br catalyzed oxidation of prehnitene to mellophanic acid in the liquid phase[J]. Industrial & Engineering Chemistry Research, 2020, 59(43): 19226-19234. |
| 8 | YANG Yufei, MA Jieyi, WU Junyan, et al. Experimental and theoretical study on N-hydroxyphthalimide and its derivatives catalyzed aerobic oxidation of cyclohexylbenzene[J]. Chinese Journal of Chemical Engineering, 2022, 44(4): 124-130. |
| 9 | WANNA Wondemagegn H, DAMODAR Janmanchi, NATARAJAN Thiyagarajan, et al. Selective oxidation of simple aromatics catalyzed by nano-biomimetic metal oxide catalysts: A mini review[J]. Frontiers in Chemistry, 2020, 8: 589178. |
| 10 | CHEN Guanyi, WANG Zhi, LIN Fawei, et al. Comparative investigation on catalytic ozonation of VOCs in different types over supported MnO x catalysts[J]. Journal of Hazardous Materials, 2020, 391: 122218. |
| 11 | Diptangshu Datta MAL, KHILARI Santimoy, PRADHAN Debabrata. Efficient and selective oxidation of toluene to benzaldehyde on Manganese tungstate nanobars: A noble metal-free approach[J]. Green Chemistry, 2018, 20(10): 2279-2289. |
| 12 | BAI Jiatong, HUANG Jiangnan, JIANG Qi, et al. Radical propagation facilitating aerobic oxidation of substituted aromatics promoted by tert-butyl hydroperoxide[J]. ChemistrySelect, 2021, 6(27): 6895-6903. |
| 13 | LI Xingxing, GUO Lulu, HE Pengcheng, et al. Co-SBA-15-immobilized NDHPI as a new composite catalyst for toluene aerobic oxidation[J]. Catalysis Letters, 2017, 147(4): 856-864. |
| 14 | WANG Qinbo, LI Xi, WANG Lijun, et al. Kinetics of p-xylene liquid-phase catalytic oxidation to terephthalic acid[J]. Industrial & Engineering Chemistry Research, 2005, 44(2): 261-266. |
| 15 | NAKAI Takeo, IWAI Toshiyuki, MIHARA Masatoshi, et al. Novel oxidation of toluenes catalyzed by reusable vanadyl(Ⅳ) sulfate under mild conditions with molecular oxygen[J]. Tetrahedron Letters, 2010, 51(17): 2225-2227. |
| 16 | MENG Yan, LIANG Bin, TANG Shengwei. A study on the liquid-phase oxidation of toluene in ionic liquids[J]. Applied Catalysis A: General, 2012, 439/440: 1-7. |
| 17 | PAN Helin, LI Shuting, SHU Mingjie, et al. p-Xylene catalytic oxidation to terephthalic acid by ozone[J]. ScienceAsia, 2018, 44(3): 212-217. |
| 18 | POTAPENKO E V, ANDREEV P Y. Catalytic oxidation of toluene by ozone in the acetic acid-sulfuric acid system[J]. Russian Journal of Applied Chemistry, 2011, 84(6): 984-987. |
| 19 | YUAN Haoran, FANG Xiang, MA Qiyi, et al. New mechanistic insight into the aerobic oxidation of methylaromatic compounds catalyzed by Co-Mn-Br and its applications[J]. Journal of Catalysis, 2016, 339: 284-291. |
| 20 | BUKHARKINA TATIANA V, DIGUROV NIKOLAI G. Kinetics of aerobic liquid-phase oxidation of organic compounds[J]. Organic Process Research & Development, 2004, 8(3): 320-329. |
| 21 | 张祥富, 时效天. 甲苯常压催化氧化制苯甲酸及其它芳烃的氧化反应的研究[J]. 合成化学, 1998, 6(4): 433-437. |
| ZHANG Xiangfu, SHI Xiaotian. Study on catalytic oxidation of toluene to benzene carbonic acid and other aromatic hydrocarbon[J]. Chinese Journal of Synthetic Chemistry, 1998, 6(4): 433-437. | |
| 22 | BLACKBURN Dale W. Catalysis of organic reactions[M]. New York: M. Dekker, 1990. |
| 23 | 金少瑾, 陈纪忠. 乙酰丙酮盐催化甲苯液相氧化制苯甲醛的研究[J]. 高校化学工程学报, 2014, 28(2)311-316. |
| JIN Shaojin, CHEN Jizhong. Preparation of benzaldehyde by aerobic liquid-phase oxidation of toluene with Co(acac)2 [J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(2)311-316. | |
| 24 | Haifeng LYU, WU Sanqiang, LIU Nian, et al. A study on the m-xylene oxidation to isophthalic acid under the catalysis of bromine-free homogeneous catalytic system[J]. Chemical Engineering Journal, 2011, 172(2/3): 1045-1053. |
| 25 | WANG Xueqin, CAO Xiuli, HU Xiaoke, et al. Effect of zirconium addition on vanadium-catalyzed toluene oxidation by H2O2 in CH3COOH[J]. Journal of Molecular Catalysis A: Chemical, 2012, 357: 1-10. |
| 26 | LI Meng, NIU Fenghui, BUSCH Daryle H, et al. Kinetic investigations of p-xylene oxidation to terephthalic acid with a Co/Mn/Br catalyst in a homogeneous liquid phase[J]. Industrial & Engineering Chemistry Research, 2014, 53(22): 9017-9026. |
| 27 | Yue LYU, LI Caiting, DU Xueyu, et al. Catalytic removal of toluene over manganese oxide-based catalysts: A review[J]. Environmental Science and Pollution Research, 2020, 27(3): 2482-2501. |
| 28 | WU Xiankun, DENG Zilei, YAN Jiujuan, et al. Effect of Acetic anhydride on the oxidation of toluene to benzaldehyde with metal/bromide catalysts[J]. Industrial & Engineering Chemistry Research, 2014, 53(38): 14601-14606. |
| 29 | ZHANG Zhan, GAO Jin, MA Hong, et al. 4-N, N-dimethylamino pyridine promoted oxidation of toluene catalyzed by cobalt acetate and benzyl bromide[J]. Chinese Journal of Catalysis, 2013, 33(7): 1198-1202. |
| 30 | JHUNG Sung Hwa, LEE Ki Hwa, PARK Youn-Seok. Effects of alkali metals on the liquid phase oxidation of p-xylene[J]. Applied Catalysis A: General, 2002, 230(1/2): 31-40. |
| 31 | LIANG Yufeng, WANG Xiaoyang, TANG Conghui, et al. NHPI and palladium cocatalyzed aerobic oxidative acylation of arenes through a radical process[J]. Chemical Communications, 2016, 52(7): 1416-1419. |
| 32 | YASUTAKA Ishii, SATOSHI Sakaguchi, TAKAHIRO Iwahama. Innovation of hydrocarbon oxidation with molecular oxygen and related reactions[J]. Advanced Synthesis & Catalysis, 2001, 343(5): 393-427. |
| 33 | 卓广澜, 赵卫娟, 姜玄珍. 甲苯氧化制苯甲酸的新催化体系[J]. 有机化学, 2004, 24(8): 962-965. |
| ZHUO Guanglan, ZHAO Weijuan, JIANG Xuanzhen. A novel catalyst system for the oxidation of toluene to benzoic acid[J]. Chinese Journal of Organic Chemistry, 2004, 24(8): 962-965. | |
| 34 | 方亚辉, 户安军, 李斌栋, 等. NHPI/过渡金属催化甲苯氧化制苯甲酸[J]. 化工进展, 2006, 25(11): 1358-1361. |
| FANG Yahui, HU Anjun, LI Bindong, et al. Oxidation of toluene catalyzed by NHPI/transition metals[J]. Chemical Industry and Engineering Progress, 2006, 25(11): 1358-1361. | |
| 35 | WEI Gaoling, LIU Peng, CHEN Dong, et al. Activity of manganese oxides supported on halloysite towards the thermal catalytic oxidation of formaldehyde: Constraint from the manganese precursor[J]. Applied Clay Science, 2019, 182: 105280. |
| 36 | EDUARD Karakhanov, ANTON Maximov, ANNA Zolotukhina, et al. Manganese and cobalt doped hierarchical mesoporous halloysite-based catalysts for selective oxidation of p-xylene to terephthalic acid[J]. Catalysts, 2019, 10(1): 7. |
| 37 | LI Ying, DUAN Deliang, WU Mingzhu, et al. One-step synthesis of 2, 5-dihydroxyterephthalic acid by the oxidation of p-xylene over M-MCM-41 (M=Fe, Fe/Cu, Cu) catalysts[J]. Chemical Engineering Journal, 2016, 306: 777-783. |
| 38 | XU Jinyang, SHI Guojun, LIANG Yuxin, et al. Selective aerobic oxidation of toluene to benzaldehyde catalyzed by covalently anchored N-hydroxyphthalimide and cobaltous ions[J]. Molecular Catalysis, 2021, 503: 111440. |
| 39 | SHI Guojun, LU Qiuting, XU Jinyang, et al. Co-immobilization of N-hydroxyphthalimide and cobaltous ions as a recyclable catalyst for selective aerobic oxidation of toluene to benzaldehyde[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106234. |
| 40 | CHAI Bo, LIU Chun, YAN Juntao, et al. In-situ synthesis of WO3 nanoplates anchored on g-C3N4 Z-scheme photocatalysts for significantly enhanced photocatalytic activity[J]. Applied Surface Science, 2018, 448: 1-8. |
| 41 | PADMA Rani Verma, SOUMEN Payra, FAHMIDA Khan, et al. CuBr2@g-C3N4-catalyzed highly selective aerobic oxidation of alcohol and toluene derivatives[J]. ChemistrySelect, 2020, 5(6): 1950-1955. |
| 42 | 马帅帅, 顾建东, 高媛, 等. 多孔超薄g-C3N4纳米片负载Pt复合材料的制备及其光催化性能[J]. 无机化学学报, 2021, 37(8): 1439-1448. |
| MA Shuaishuai, GU Jiandong, GAO Yuan, et al. Preparation and photocatalytic activity of holey ultrathin g-C3N4 nanosheets-supported Pt composite[J]. Chinese Journal of Inorganic Chemistry, 2021, 37(8)1439-1448. | |
| 43 | WANG Yong, LI Haoran, YAO Jia, et al. Synthesis of boron doped polymeric carbon nitride solids and their use as metal-free catalysts for aliphatic C—H bond oxidation[J]. Chemical Science, 2011, 2(3): 446-450. |
| 44 | HAN Hongling, DING Guodong, WU Tianbin, et al. Cu and boron doped carbon nitride for highly selective oxidation of toluene to benzaldehyde[J]. Molecules, 2015, 20(7): 12686-12697. |
| 45 | GAULI Kamlesh, RAM R N, SONI Hemant P. Oxidation of toluene using polymer anchored Ni(Ⅱ) complex as catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2005, 242(1/2): 161-167. |
| 46 | KARUEHANON Weeranuch, SIRATHANYAROTE Chinnapat, PATTARAWARAPAN Mookda. Poly(4-vinylpyridine-co-divinylbenzene) supported iron(Ⅲ) catalyst for selective oxidation of toluene to benzoic acid with H2O2 [J]. Tetrahedron, 2012, 68(46): 9423-9428. |
| 47 | ANBARASU G, MALATHY M, KARTHIKEYAN P, et al. Silica functionalized Cu(Ⅱ) acetylacetonate Schiff base complex: An efficient catalyst for the oxidative condensation reaction of benzyl alcohol with amines[J]. Journal of Solid State Chemistry, 2017, 253: 305-312. |
| 48 | SHEN Haimin, QI Bei, HU Mengyun, et al. Selective solvent-free and additive-free oxidation of primary benzylic C—H bonds with O2 catalyzed by the combination of metalloporphyrin with N-hydroxyphthalimide[J]. Catalysis Letters, 2020, 150(11): 3096-3111. |
| 49 | ZHOU Xiantai, CHEN Hongyu, HAN Qi, et al. Acetylacetone as an oxygen activator to improve efficiency for aerobic oxidation of toluene and its derivatives by using cobalt meso-tetraphenylporphyrin[J]. New Journal of Chemistry, 2020, 44(25): 10286-10291. |
| 50 | JIA Jiaojiao, CHEN Xi, ZHAI Lijun, et al. Oxidation of toluene to benzoic acid via VOTPP catalyst synthesized with an improved method[J]. Monatshefte Für Chemie-Chemical Monthly, 2020, 151(10): 1549-1555. |
| 51 | LászlóJ CSÁNYI, Károly JÁKY. Liquid-phase oxidation of hydrocarbons in the presence of different types of phase-transfer reagents[J]. Journal of Molecular Catalysis A: Chemical, 1997, 120(1/2/3): 125-138. |
| 52 | COLONNA S, MOLINARI H, BANFI S, et al. Synthetic enzymes—4: Highly enantioselective epoxidation by means of polyaminoacids in a triphase system: influence of structural variations within the catalysts[J]. Tetrahedron, 1983, 39(9): 1635-1641. |
| 53 | DENG Wei, WAN Yanping, JIANG Hui, et al. Solvent-free aerobic oxidation of toluene over metalloporphyrin/NHPI/CTAB: Synergy and mechanism[J]. Catalysis Letters, 2014, 144(2): 333-339. |
| 54 | LUO Weiping, SUN Jun, YE Jun, et al. Continuous gas-liquid aerobic oxidation of toluene catalyzed by[T(p-Cl)PPFe]2O in a series of three stirred tank reactors[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3061-3067. |
| 55 | CHAUHAN Dheeraj Singh, M A Jafar MAZUMDER, QURAISHI M A, et al. Chitosan-cinnamaldehyde Schiff base: A bioinspired macromolecule as corrosion inhibitor for oil and gas industry[J]. International Journal of Biological Macromolecules, 2020, 158: 127-138. |
| 56 | LI Jianzhang, YANG Zhuzhu, HE Xiyang, et al. An efficient aerobic oxidation for p-xylene to p-toluic acid by unsymmetrical schiff base Manganese(Ⅲ) complexes with pendant benzo-10-aza-crown ether or morpholino-groups[J]. Journal of Chemical Research, 2010, 34(10): 581-584. |
| 57 | 张昕, 张贵泉, 林涛, 等. 甲苯液相选择氧化反应催化剂研究进展[J]. 化工进展, 2010, 29(10): 1890-1897. |
| ZHANG Xin, ZHANG Guiquan, LIN Tao, et al. Advances in catalysts for liquid-phase selective oxidation of toluene[J]. Chemical Industry and Engineering Progress, 2010, 29(10): 1890-1897. | |
| 58 | CRESTINI Claudia, PASTORINI Alessandra, TAGLIATESTA Pietro. Metalloporphyrins immobilized on motmorillonite as biomimetic catalysts in the oxidation of lignin model compounds[J]. Journal of Molecular Catalysis A: Chemical, 2004, 208(1/2): 195-202. |
| 59 | HUANG G, WANG A P, LIU S Y, et al. An efficient oxidation of toluene over Co(Ⅱ)TPP supported on chitosan using air[J]. Catalysis Letters, 2007, 114(3): 174-177. |
| 60 | HUANG Guan, LUO Jin, DENG Caocheng, et al. Catalytic oxidation of toluene with molecular oxygen over manganese tetraphenylporphyrin supported on chitosan[J]. Applied Catalysis A: General, 2008, 338(1/2): 83-86. |
| 61 | Manirul Islam SK, SUMANTRA Paul, ANUPAM Singha Roy, et al. Selective oxidation of organic substrates in presence of H2O2 using a polymer-anchored iron(Ⅲ)-ferrocene complex[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2013, 23(3): 560-570. |
| 62 | PRIYANKA Basu, TUSAR Kanto Dey, ANIRUDDHA Ghosh, et al. Designing of a new heterogeneous polymer supported naphthyl-azo iron catalyst for the selective oxidation of substituted methyl benzenes[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2018, 28(3): 1158-1170. |
| 63 | JAGAT Singh Kirar, SAVITA Khare, NEHA Tiwari. Transition metal Schiff base complexes supported on layered double hydroxide: Synthesis, characterization and catalytic activity for the oxidation of toluene[J]. Reaction Kinetics, Mechanisms and Catalysis, 2021, 132(2): 1025-1046. |
| 64 | PEEDIKAKKAL Abdul Malik P, ABIOLA Azeez Jimoh, SHAIKH M N, et al. Mixed-metal metal-organic frameworks as catalysts for liquid-phase oxidation of toluene and cycloalkanes[J]. Arabian Journal for Science and Engineering, 2017, 42(10): 4383-4390. |
| 65 | SUN Hua, YU Xiaolin, MA Xiuyun, et al. MnO x -CeO2 catalyst derived from metal-organic frameworks for toluene oxidation[J]. Catalysis Today, 2020, 355: 580-586. |
| 66 | LI Ying, WU Mingzhu, CHEN Daomei, et al. One-step highly selective oxidation of p-xylene to 4-hydroxymethylbenzoic acid over Cu-MOF catalysts under mild conditions[J]. Molecular Catalysis, 2019, 477: 110542. |
| 67 | LIAO Yijun, ZHANG Lin, WESTON Mitchell H, et al. Tuning ethylene gas adsorption via metal node modulation: Cu-MOF-74 for a high ethylene deliverable capacity[J]. Chemical Communications, 2017, 53(67): 9376-9379. |
| 68 | HUANG Cheng, GU Xiangyu, SU Xiaoyan, et al. Controllable synthesis of Co-MOF-74 catalysts and their application in catalytic oxidation of toluene[J]. Journal of Solid State Chemistry, 2020, 289: 121497. |
| 69 | DUAN Jingui, LI Yanshuo, PAN Yichang, et al. Metal-organic framework nanosheets: An emerging family of multifunctional 2D materials[J]. Coordination Chemistry Reviews, 2019, 395(Sep.): 25-45. |
| 70 | XIAO Yepeng, SONG Bingcheng, CHEN Yaju, et al. ZIF-67 with precursor concentration-dependence morphology for aerobic oxidation of toluene[J]. Journal of Organometallic Chemistry, 2020, 930: 121597. |
| 71 | LOMIG Hamon, ELSA Jolimaitre, PIRNGRUBER Gerhard D. CO2 and CH4 separation by adsorption using Cu-BTC metal-organic framework[J]. Industrial & Engineering Chemistry Research, 2010, 49(16): 7497-7503. |
| 72 | BAO Li, LI Xingxing, WU Zhaowei, et al. N-hydroxyphthalimide incorporated onto Cu-BTC metal organic frameworks: An novel catalyst for aerobic oxidation of toluene[J].Research on Chemical Intermediates, 2016, 42(6): 5527-5539. |
| 73 | KOSHINO Nobuyoshi, CAI Yang, ESPENSON James H. Kinetic study of the phthalimide N-oxyl (PINO) radical in acetic acid. hydrogen abstraction from C—H bonds and evaluation of O—H bond dissociation energy of N-hydroxyphthalimide[J]. The Journal of Physical Chemistry A, 2003, 107(21): 4262-4267. |
| 74 | XU Luo, CHEN Dawei, JIANG Haoran, et al. Efficient oxidation of p-xylene to terephthalic acid by using N, N-dihydroxypyromellitimide in conjunction with Co-benzenetricarboxylate[J]. Applied Catalysis A: General, 2020, 599: 117569. |
| 75 | KATO Chika Nozaki, HASEGAWA Mari, SATO Tomohiko, et al. Microporous dinuclear copper(Ⅱ) trans-1, 4-cyclohexanedicarboxylate: Heterogeneous oxidation catalysis with hydrogen peroxide and X-ray powder structure of peroxo copper(Ⅱ) intermediate[J]. Journal of Catalysis, 2005, 230(1): 226-236. |
| 76 | SUN Zhiguo, LI Gang, ZHANG Yue, et al. Ag-Cu-BTC prepared by postsynthetic exchange as effective catalyst for selective oxidation of toluene to benzaldehyde[J]. Catalysis Communications, 2015, 59: 92-96. |
| 77 | HUANG Cheng, LIU Rui, YANG Wenyu, et al. Enhanced catalytic activity of MnCo-MOF-74 for highly selective aerobic oxidation of substituted toluene[J]. Inorganic Chemistry Frontiers, 2018, 5(8): 1923-1932. |
| 78 | LI Hui, YUE Fan, XIE Hongtao, et al. Hollow shell-in-shell Ni3S4@Co9S8 tubes derived from core-shell Ni-MOF-74@Co-MOF-74 as efficient faradaic electrodes[J]. CrystEngComm, 2018, 20(7): 889-895. |
| 79 | GU Xiangyu, HUANG Cheng, XU Zengchuang, et al. Core-shell[email protected]catalysts with controllable shell thickness and their enhanced catalytic activity for toluene oxidation[J]. Journal of Solid State Chemistry, 2021, 294: 121803. |
| 80 | REZAEI Ebrahim, SOLTAN Jafar, CHEN Ning. Catalytic oxidation of toluene by ozone over alumina supported manganese oxides: Effect of catalyst loading[J]. Applied Catalysis B: Environmental, 2013, 136/137: 239-247. |
| 81 | XIA Yunsheng, XIA Lu, LIU Yuxi, et al. Concurrent catalytic removal of typical volatile organic compound mixtures over Au-Pd/α-MnO2 nanotubes[J]. Journal of Environmental Sciences, 2018, 64: 276-288. |
| 82 | LI Xiaoqiang, XU Jie, WANG Feng, et al. Direct oxidation of toluene to benzoic acid with molecular oxygen over Manganese oxides[J]. Catalysis Letters, 2006, 108(3): 137-140. |
| 83 | JIN Lei, CHEN Chunhu, CRISOSTOMO Vincent Mark B, et al. γ-MnO2 octahedral molecular sieve: Preparation, characterization, and catalytic activity in the atmospheric oxidation of toluene[J]. Applied Catalysis A: General, 2009, 355(1/2): 169-175. |
| 84 | YANG Guanyu, ZHENG Liwen, WU Guanghui, et al. Manganese dioxide and N-hydroxyphthalimide. An effective catalytic system for oxidation of nitrotoluenes with molecular oxygen[J]. Advanced Synthesis & Catalysis, 2007, 349(16): 2445-2448. |
| 85 | JIANG Jun, JING Yuanyuan, ZHANG Yaofa, et al. Highly efficient oxidation of toluene to benzoic acid catalyzed by Manganese dioxide and N-hydroxyphthalimide[J]. Catalysis Letters, 2011, 141(4): 544-548. |
| 86 | DEORI Kalyanjyoti, GUPTA Dinesh, SAHA Basudeb, et al. Introducing nanocrystalline CeO2 as heterogeneous environmental friendly catalyst for the aerobic oxidation of para-xylene to terephthalic acid in water[J]. Journal of Materials Chemistry A, 2013, 1(24): 7091-7099. |
| 87 | WANG Zehua, WU Yuchao, WU Chongchong, et al. Electrophilic oxygen on defect-rich carbon nanotubes for selective oxidation of cyclohexane[J]. Catalysis Science & Technology, 2020, 10(2): 332-336. |
| 88 | FENG Yuwei, ZENG Aiwu. Selective liquid-phase oxidation of toluene with molecular oxygen catalyzed by Mn3O4 nanoparticles immobilized on CNTs under solvent-free conditions[J]. Catalysts, 2020, 10(6): 623. |
| 89 | SHI Guojun, XU Sihao, BAO Yan, et al. Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoO x on SiO2 catalyst in the presence of N-hydroxyphthalimide and hexafluoropropan-2-ol[J]. Catalysis Communications, 2019, 123: 73-78. |
| 90 | EDEN Gaster, SEBASTIAN Kozuch, DORON Pappo. Selective aerobic oxidation of methylarenes to benzaldehydes catalyzed by N-hydroxyphthalimide and cobalt(Ⅱ) acetate in hexafluoropropan-2-ol[J]. Angewandte Chemie International Edition, 2017, 56(21): 5912-5915. |
| 91 | XU Sihao, SHI Guojun, FENG Ya, et al. Synthesis and characterization of highly dispersed cobaltous silicate as a catalyst for selective oxidation of toluene to benzaldehyde[J]. Materials Chemistry and Physics, 2021, 262: 124309. |
| 92 | WANG Zhen, QIN Yi, PAN Feng, et al. Mesoporous silica-supported manganese oxides for complete oxidation of volatile organic compounds: influence of mesostructure, redox properties, and hydrocarbon dimension[J]. Industrial & Engineering Chemistry Research, 2018, 57(22): 7374-7382. |
| 93 | PRANGYA Paramita Das, BISWAJIT Chowdhury. Indium oxide nanoparticles embedded in TUD-1 as a highly selective catalyst for toluene to benzaldehyde oxidation using TBHP as oxidant[J]. Chemical Papers, 2020, 74(7): 2091-2100. |
| 94 | LIU Lizhong, JIA Jinping, SUN Tonghua, et al. A facile method for scalable preparation of mesoporous structured SmMnO3 perovskites sheets for efficient catalytic oxidation of toluene[J]. Materials Letters, 2018, 212: 107-110. |
| 95 | WORAYINGYONG Attera, KANGVANSURA Praewpilin, AUSADASUK Siritha, et al. The effect of preparation: Pechini and Schiff base methods, on adsorbed oxygen of LaCoO3 perovskite oxidation catalysts[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 315(1/2/3): 217-225. |
| 96 | WANG Feng, XU Jie, LI Xiaoqiang, et al. Liquid phase oxidation of toluene to benzaldehyde with molecular oxygen over copper-based heterogeneous catalysts[J]. Advanced Synthesis & Catalysis, 2005, 347(15): 1987-1992. |
| 97 | ALHARBI K H, ALSALME A, ALOUMI A B A, et al. Selective catalytic oxidation of toluene to benzaldehyde: Effect of aging time and calcination temperature using Cu x Zn y O mixed metal oxide nanoparticles[J]. Catalysts, 2021, 11(3): 354. |
| 98 | LI Wang, ZHANG Qingjun, ZENG Aiwu. Controlled synthesis of Cu x Mn3.66- x Mo3O12 with the citrate sol-gel method for the selective liquid-phase toluene oxidation to benzaldehyde by air[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 125(2): 707-731. |
| 99 | NICOLAE Sabina, Florentina NEAŢU, FLOREA Mihaela. Selective catalytic oxidation reaction of p-xylene on manganese-iron mixed oxide materials[J]. Comptes Rendus Chimie, 2018, 21(3/4): 354-361. |
| 100 | ACHARYYA Shankha S, SHILPI Ghosh, RAJARAM Bal. Fabrication of three dimensional (3D) hierarchical Ag/WO3 flower-like catalyst materials for the selective oxidation of m-xylene to isophthalic acid[J]. Chemical Communications, 2015, 51(27): 5998-6001. |
| 101 | 邓长顺, 许孟霞, 董珍, 等. 十六烷基膦酸配合的复合氧化物纳米催化剂稳定的O/W乳液中甲苯单一氧化为苯甲醛[J]. 催化学报, 2020, 41(2): 341-349. |
| DENG Changshun, XU Mengxia, DONG Zhen, et al. Exclusively catalytic oxidation of toluene to benzaldehyde in an O/W emulsion stabilized by hexadecylphosphate acid terminated mixed-oxide nanoparticles[J]. Chinese Journal of Catalysis, 2020, 41(2): 341-349. | |
| 102 | XU Cai, WANG Xiaozhong, CHEN Yingqi, et al. Synergistic effect between Cu-Cr bimetallic oxides supported on g-C3N4 for the selective oxidation of toluene to benzaldehyde[J]. Catalysis Science & Technology, 2019, 9(16): 4441-4450. |
| 103 | HAMZA Shoukat, ALTAF Ataf ALI, MUHAMMAD Hamayun, et al. Catalytic oxidation of toluene into benzaldehyde and benzyl alcohol using molybdenum-incorporated manganese oxide nanomaterials[J]. ACS Omega, 2021, 6(30): 19606-19615. |
| 104 | HOU Zhongyan, ZHOU Xiaoying, LIN Tao, et al. The promotion effect of tungsten on monolith Pt/Ce0.65Zr0.35O2 catalysts for the catalytic oxidation of toluene[J]. New Journal of Chemistry, 2019, 43(15): 5719-5726. |
| 105 | ZHOU Xinzhi, WANG Zhihao, FANG Zhouwen, et al. Production of isophthalic acid from m-xylene catalyzed by Co(Ⅱ) and HPW@C modified with acetic acid[J]. Industrial & Engineering Chemistry Research, 2018, 57(35): 11893-11902. |
| 106 | FANG Zhouwen, WEN Di, WANG Zhihao, et al. Effect of H2O2 modification of H3PW12O40@carbon for m-xylene oxidation to isophthalic acid[J]. Korean Journal of Chemical Engineering, 2018, 35(11): 2172-2184. |
| 107 | WANG Zhihao, YANG Zhilin, WU Shiming, et al. A study on the production of isophthalic acid from m-xylene under the catalysis of cobalt and H3PW12O40/carbon modified by HNO3 solution[J]. International Journal of Chemical Reactor Engineering, 2015, 13(3): 413-425. |
| 108 | WANG Zhihao, LIU Huajie, FANG Zhouwen, et al. Production of isophthalic acid from m-xylene catalyzed by Co(Ⅱ) and HPW@C modified with ZnCl2 solution[J]. The Canadian Journal of Chemical Engineering, 2019, 97(7): 2086-2096. |
| 109 | FAN Liwu, ZHU Ziqin, LIU Minjie, et al. Heat transfer during constrained melting of nano-enhanced phase change materials in a spherical capsule: An experimental study[J]. Journal of Heat Transfer, 2016, 138(12): 122402. |
| 110 | PEMBERE Anthony M S, CUI Chaonan, RAJINI Anumula, et al. A hexagonal Ni6 cluster protected by 2-phenylethanethiol for catalytic conversion of toluene to benzaldehyde[J]. Physical Chemistry Chemical Physics, 2019, 21(32): 17933-17938. |
| 111 | SONG Guangliang, FENG Liang, XU Jie, et al. Liquid-phase oxidation of toluene to benzaldehyde with molecular oxygen catalyzed by copper nanoparticles supported on graphene[J]. Research on Chemical Intermediates, 2018, 44(9): 4989-4998. |
| 112 | GELBARD Georges, GAUDUCHEAU Thierry, VIDAL Elisabeth, et al. Epoxidation with peroxotungstic acid immobilised onto silica-grafted phosphoramides[J]. Journal of Molecular Catalysis A: Chemical, 2002, 182/183: 257-266. |
| 113 | DOMÍNGUEZ María Isabel, COJOCARU Bogdan, TUDORACHE Madalina, et al. Liquid-phase oxidation with hydrogen peroxide of benzyl alcohol and xylenes on Ca10(PO4)6(OH)2-CaWO4 [J]. Comptes Rendus Chimie, 2016, 19(10): 1156-1165. |
| 114 | PETA Sreenivasulu, ZHANG Tao, DUBOVOY Viktor, et al. Facile synthesis of efficient and selective Ti-containing mesoporous silica catalysts for toluene oxidation[J]. Molecular Catalysis, 2018, 444: 34-41. |
| 115 | FALCON H, CAMPOS-MARTIN J M, AL-ZAHRANI S M, et al. Liquid-phase oxidation of p-xylene using N-hydroxyimides[J]. Catalysis Communications, 2010, 12(1): 5-8. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [10] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [11] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [14] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [15] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||