化工进展 ›› 2023, Vol. 42 ›› Issue (1): 488-496.DOI: 10.16085/j.issn.1000-6613.2022-0542
(NH4)2SO4和Na2SO4混合溶液中(NH4)2SO4结晶动力学及铁/铝/锰/铬等离子对(NH4)2SO4结晶的影响规律
范嘉昊1,2( ), 张洋2(
), 张洋2( ), 范兵强2, 张贺东2, 郑诗礼2, 邹兴1
), 范兵强2, 张贺东2, 郑诗礼2, 邹兴1
- 1.北京科技大学冶金与生态工程学院,北京 100084
2.中国科学院过程工程研究所战略金属资源绿色循环利用国家工程研究中心,北京 100190
-
收稿日期:2022-04-01修回日期:2022-06-06出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:张洋 -
作者简介:范嘉昊(1997—),男,硕士研究生,研究方向工业结晶。E-mail:g20198253@xs.ustb.edu.cn。 -
基金资助:国家重点研发计划(2018YFC1900505)
Crystallization kinetics of (NH4)2SO4 in mixed solution of (NH4)2SO4 and Na2SO4 and the influence of Fe/Al/Mn/Cr ions on crystallization
FAN Jiahao1,2( ), ZHANG Yang2(
), ZHANG Yang2( ), FAN Binqiang2, ZHANG Hedong2, ZHENG Shili2, ZOU Xing1
), FAN Binqiang2, ZHANG Hedong2, ZHENG Shili2, ZOU Xing1
- 1.School of Metallurgical and Ecological Engineering,University of Science & Technology Beijing, Beijing 100084, China
2.National Engineering Research Center for Green Recycling of Strategic Metal Resources, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
-
Received:2022-04-01Revised:2022-06-06Online:2023-01-25Published:2023-02-20 -
Contact:ZHANG Yang
摘要:
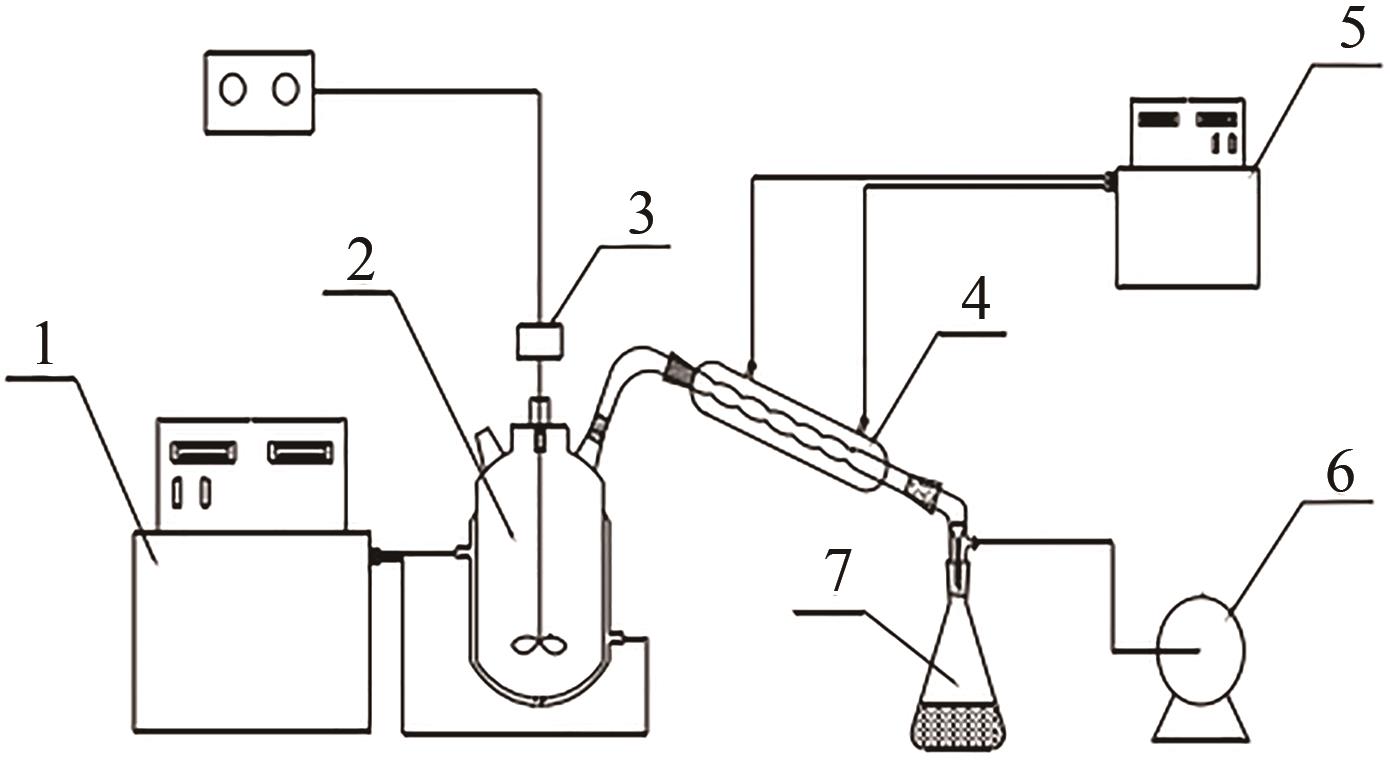
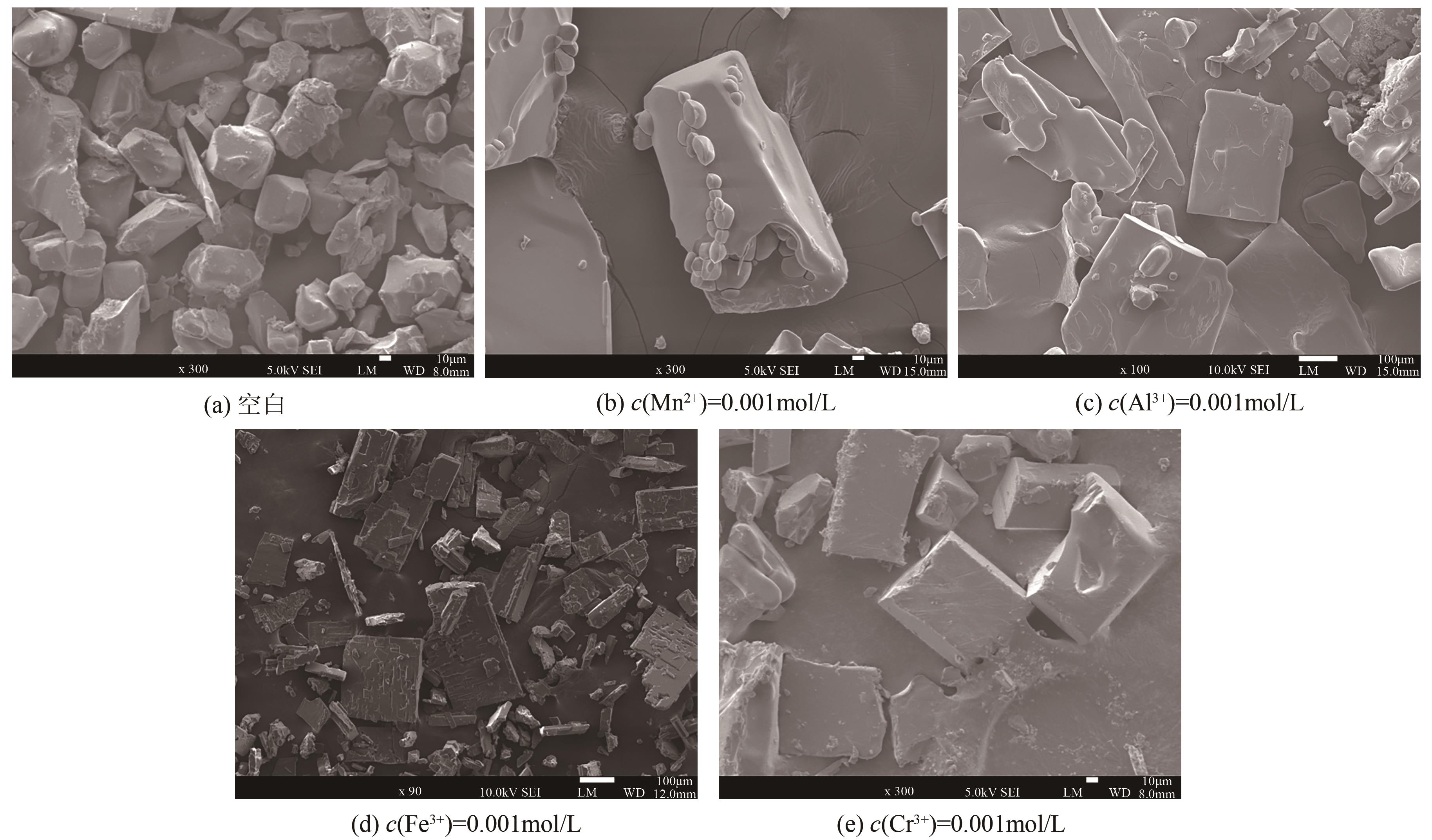
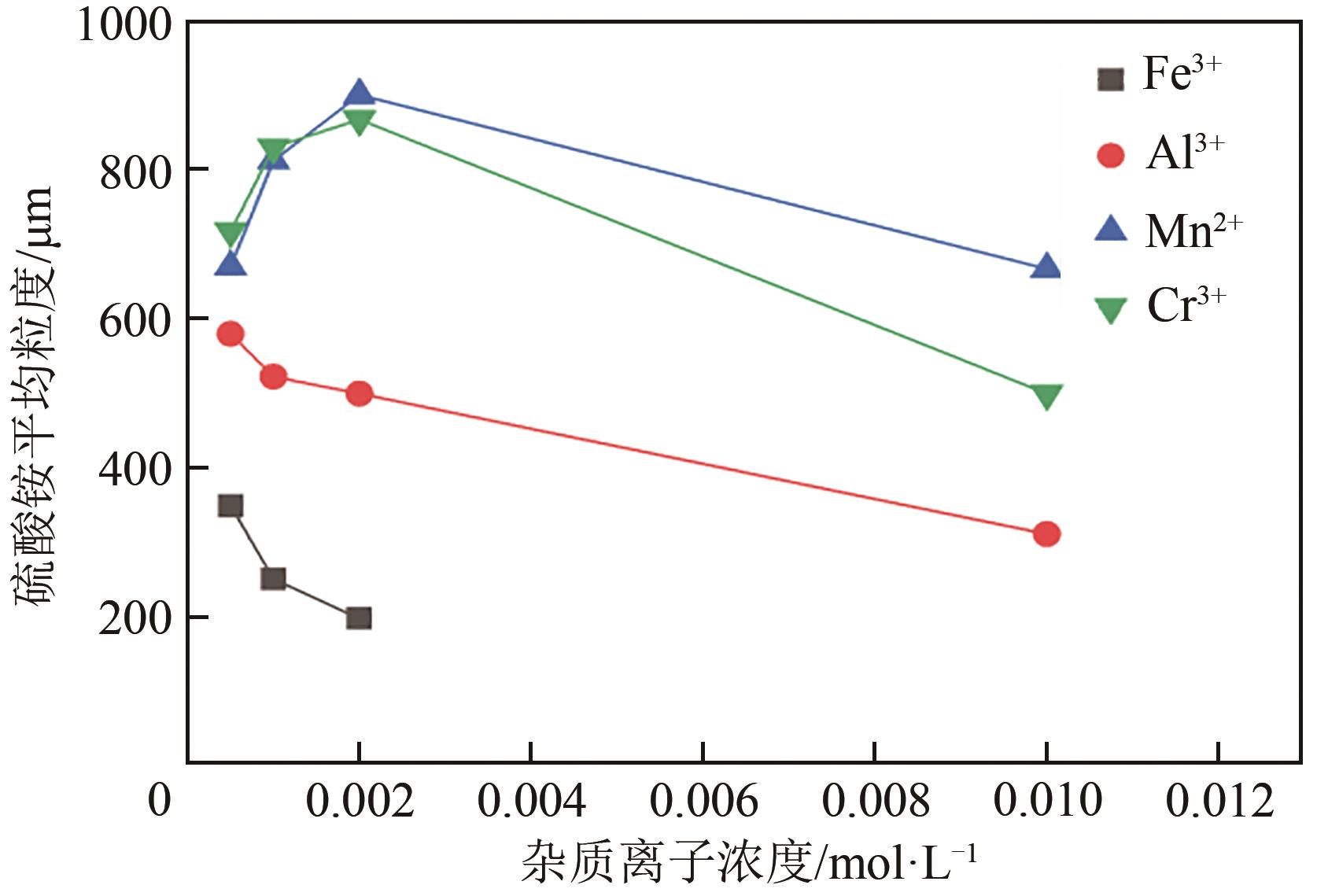
湿法冶金、三元前体制备等过程均会产生含硫酸钠与硫酸铵的高盐废水。研究高盐废水中硫酸铵的结晶动力学,并考察常见金属离子的影响规律对高盐废水处理具有重要意义。本文以含硫酸钠与硫酸铵混合溶液中硫酸铵的结晶过程为例,系统考察了硫酸铵的结晶动力学及铁、铝、锰、铬对硫酸铵结晶的影响规律。含硫酸钠与硫酸铵混合溶液硫酸铵结晶动力学研究结果表明,硫酸铵生长速率和成核速率方程分别为为
中图分类号:
引用本文
范嘉昊, 张洋, 范兵强, 张贺东, 郑诗礼, 邹兴. (NH4)2SO4和Na2SO4混合溶液中(NH4)2SO4结晶动力学及铁/铝/锰/铬等离子对(NH4)2SO4结晶的影响规律[J]. 化工进展, 2023, 42(1): 488-496.
FAN Jiahao, ZHANG Yang, FAN Binqiang, ZHANG Hedong, ZHENG Shili, ZOU Xing. Crystallization kinetics of (NH4)2SO4 in mixed solution of (NH4)2SO4 and Na2SO4 and the influence of Fe/Al/Mn/Cr ions on crystallization[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 488-496.
| 取样编号 | m/kg | σ | |
|---|---|---|---|
| 1 | 4.19×10-5 | 4.19 | 0.014 |
| 2 | 7.99×10-5 | 7.99 | 0.042 |
| 3 | 12.01×10-4 | 12.01 | 0.035 |
| 4 | 19.13×10-4 | 19.13 | 0.05 |
| 5 | 14.24×10-4 | 14.24 | 0.04 |
| 6 | 19.77×10-4 | 19.77 | 0.075 |
| 7 | 23.55×10-4 | 23.55 | 0.05 |
| 8 | 25.52×10-4 | 25.52 | 0.014 |
| 9 | 26.08×10-4 | 26.08 | 0.042 |
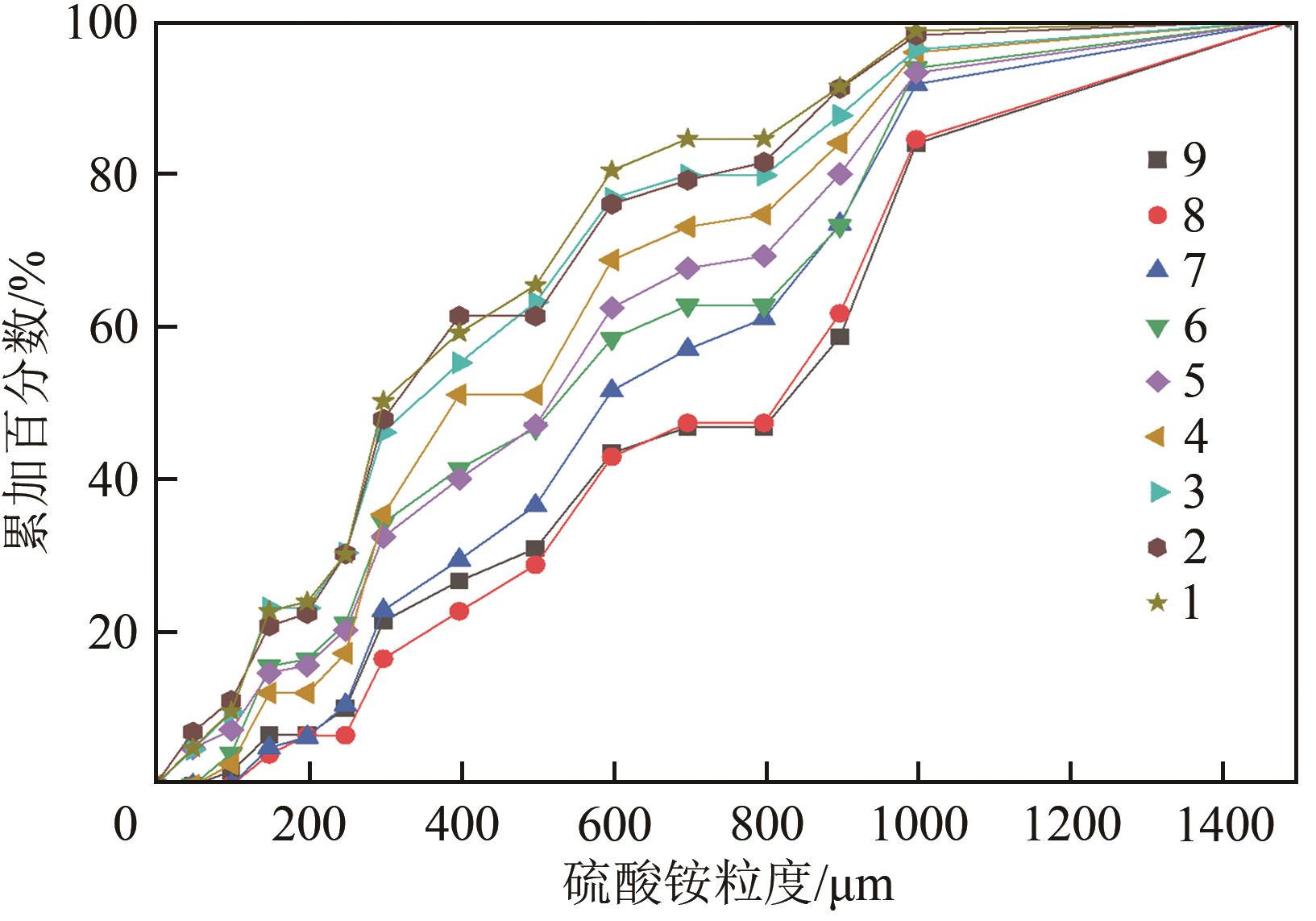
表1 硫酸铵悬浮密度及相对过饱和度
| 取样编号 | m/kg | σ | |
|---|---|---|---|
| 1 | 4.19×10-5 | 4.19 | 0.014 |
| 2 | 7.99×10-5 | 7.99 | 0.042 |
| 3 | 12.01×10-4 | 12.01 | 0.035 |
| 4 | 19.13×10-4 | 19.13 | 0.05 |
| 5 | 14.24×10-4 | 14.24 | 0.04 |
| 6 | 19.77×10-4 | 19.77 | 0.075 |
| 7 | 23.55×10-4 | 23.55 | 0.05 |
| 8 | 25.52×10-4 | 25.52 | 0.014 |
| 9 | 26.08×10-4 | 26.08 | 0.042 |
| 粒度/μm | 累加百分数/% | ΔL/μm | nj /μm-4 | μ0j /μm-3 | μ1j /μm-2 | |
|---|---|---|---|---|---|---|
| 0 | 0 | 50 | 25 | 0 | 0 | 0 |
| 50 | 0 | 50 | 75 | 0 | 0 | 0 |
| 100 | 1.8113208 | 50 | 125 | 4.39072×10-15 | 2.19536×10-13 | 2.7442×10-11 |
| 150 | 6.5660377 | 50 | 175 | 4.20031×10-15 | 2.10015×10-3 | 3.67527×10-11 |
| 200 | 6.5660377 | 50 | 225 | 0 | 0 | 0 |
| 250 | 10.0377358 | 50 | 275 | 7.90341×10-16 | 3.95171×10-14 | 1.08672×10-11 |
| 300 | 21.4339623 | 100 | 350 | 6.29213×10-16 | 6.29213×10-14 | 2.20225×10-11 |
| 400 | 26.7169811 | 100 | 450 | 1.37241×10-16 | 1.37241×10-14 | 6.17587×10-12 |
| 500 | 30.9433962 | 100 | 550 | 6.01347×10-17 | 6.01347×10-15 | 3.30741×10-12 |
| 600 | 43.3962264 | 100 | 650 | 1.07342×10-16 | 1.07342×10-14 | 6.97722×10-12 |
| 700 | 46.7924528 | 100 | 750 | 1.9057×10-17 | 1.9057×10-15 | 1.42927×10-12 |
| 800 | 46.7924528 | 100 | 850 | 0 | 0 | 0 |
| 900 | 58.5660377 | 100 | 950 | 3.25071×10-17 | 3.25071×10-15 | 3.08818×10-12 |
| 1000 | 83.8490566 | 500 | 1250 | 6.12872×10-18 | 3.06436×10-15 | 3.83045×10-12 |
| 1500 | 100 | 500 | 1750 | 1.42677×10-18 | 7.13386×10-16 | 1.24843×10-12 |
| 2000 | μ0=5.71396×10-13 | μ1=1.23141×10-10 |
表2 以1号结晶样品为例计算μ0和μ1
| 粒度/μm | 累加百分数/% | ΔL/μm | nj /μm-4 | μ0j /μm-3 | μ1j /μm-2 | |
|---|---|---|---|---|---|---|
| 0 | 0 | 50 | 25 | 0 | 0 | 0 |
| 50 | 0 | 50 | 75 | 0 | 0 | 0 |
| 100 | 1.8113208 | 50 | 125 | 4.39072×10-15 | 2.19536×10-13 | 2.7442×10-11 |
| 150 | 6.5660377 | 50 | 175 | 4.20031×10-15 | 2.10015×10-3 | 3.67527×10-11 |
| 200 | 6.5660377 | 50 | 225 | 0 | 0 | 0 |
| 250 | 10.0377358 | 50 | 275 | 7.90341×10-16 | 3.95171×10-14 | 1.08672×10-11 |
| 300 | 21.4339623 | 100 | 350 | 6.29213×10-16 | 6.29213×10-14 | 2.20225×10-11 |
| 400 | 26.7169811 | 100 | 450 | 1.37241×10-16 | 1.37241×10-14 | 6.17587×10-12 |
| 500 | 30.9433962 | 100 | 550 | 6.01347×10-17 | 6.01347×10-15 | 3.30741×10-12 |
| 600 | 43.3962264 | 100 | 650 | 1.07342×10-16 | 1.07342×10-14 | 6.97722×10-12 |
| 700 | 46.7924528 | 100 | 750 | 1.9057×10-17 | 1.9057×10-15 | 1.42927×10-12 |
| 800 | 46.7924528 | 100 | 850 | 0 | 0 | 0 |
| 900 | 58.5660377 | 100 | 950 | 3.25071×10-17 | 3.25071×10-15 | 3.08818×10-12 |
| 1000 | 83.8490566 | 500 | 1250 | 6.12872×10-18 | 3.06436×10-15 | 3.83045×10-12 |
| 1500 | 100 | 500 | 1750 | 1.42677×10-18 | 7.13386×10-16 | 1.24843×10-12 |
| 2000 | μ0=5.71396×10-13 | μ1=1.23141×10-10 |
| 取样编号 | μ0/μm-3 | μ1/μm-2 | ||
|---|---|---|---|---|
| 1 | 5.71396×10-13 | 1.23141×10-10 | 1.18142×10-15 | 0.04116 |
| 2 | 1.28025×10-12 | 3.51759×10-10 | 1.04789×10-15 | 0.18793 |
| 3 | 1.90898×10-12 | 5.31568×10-10 | 4.01696×10-15 | 0.14552 |
| 4 | 4.31916×10-12 | 8.03464×10-10 | 1.57388×10-14 | 0.23945 |
| 5 | 1.37625×10-11 | 1.55993×10-9 | 4.67263×10-15 | 0.20499 |
| 6 | 1.656×10-11 | 1.74644×10-9 | 1.09063×10-14 | 0.41233 |
| 7 | 1.00223×10-11 | 2.07534×10-9 | 1.71882×10-14 | 0.25547 |
| 8 | 2.03352×10-11 | 2.30801×10-9 | 1.91881×10-14 | 0.62046 |
| 9 | 3.18481×10-11 | 3.27934×10-9 |
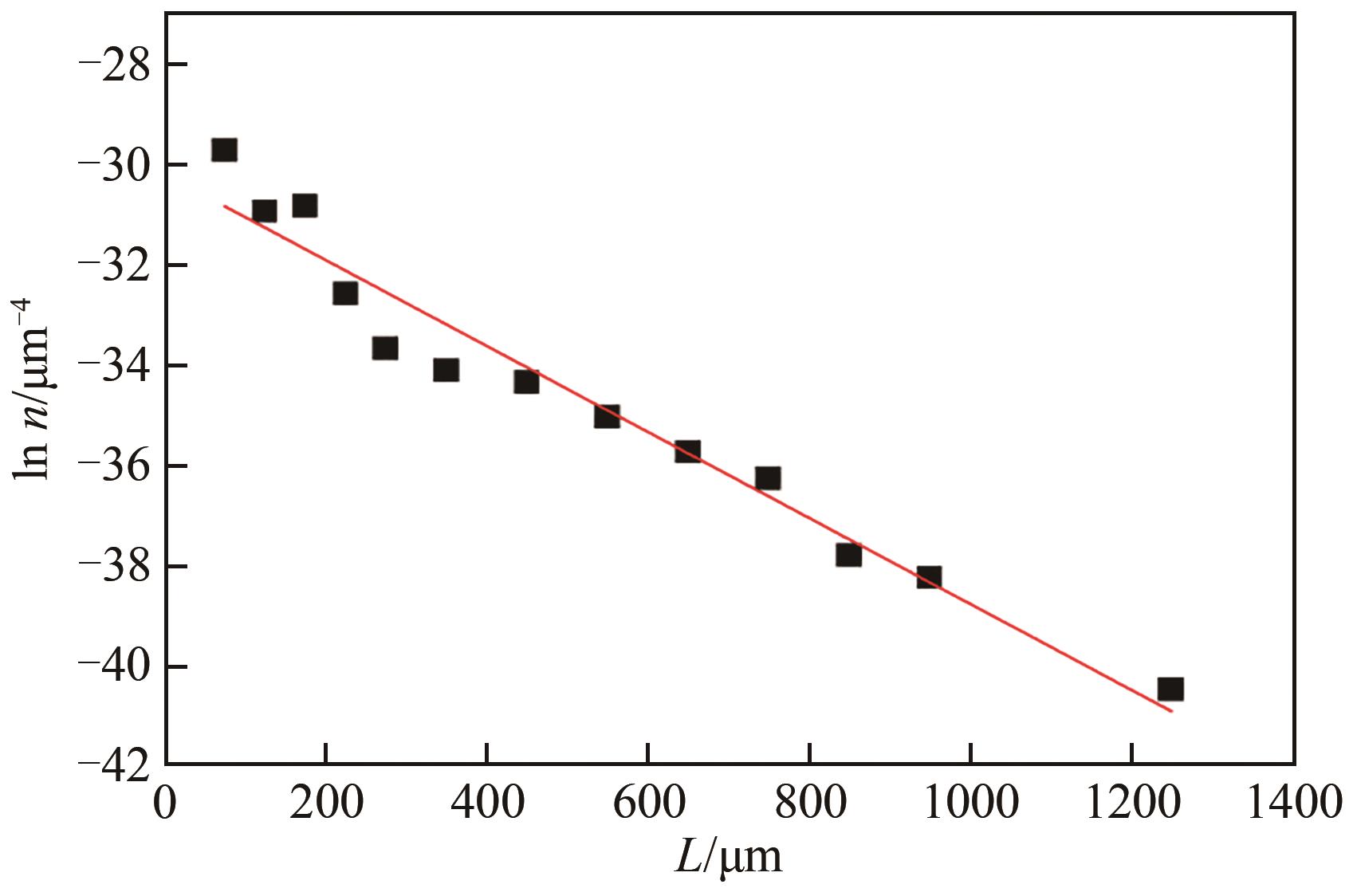
表3 平均成核速率和平均生长速率
| 取样编号 | μ0/μm-3 | μ1/μm-2 | ||
|---|---|---|---|---|
| 1 | 5.71396×10-13 | 1.23141×10-10 | 1.18142×10-15 | 0.04116 |
| 2 | 1.28025×10-12 | 3.51759×10-10 | 1.04789×10-15 | 0.18793 |
| 3 | 1.90898×10-12 | 5.31568×10-10 | 4.01696×10-15 | 0.14552 |
| 4 | 4.31916×10-12 | 8.03464×10-10 | 1.57388×10-14 | 0.23945 |
| 5 | 1.37625×10-11 | 1.55993×10-9 | 4.67263×10-15 | 0.20499 |
| 6 | 1.656×10-11 | 1.74644×10-9 | 1.09063×10-14 | 0.41233 |
| 7 | 1.00223×10-11 | 2.07534×10-9 | 1.71882×10-14 | 0.25547 |
| 8 | 2.03352×10-11 | 2.30801×10-9 | 1.91881×10-14 | 0.62046 |
| 9 | 3.18481×10-11 | 3.27934×10-9 |
| 杂质种类 | 浓度/mol·L-1 | 成核动力学方程 | 生长动力学方程 |
|---|---|---|---|
| Mn2+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Al3+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Fe3+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Cr3+ | 0.001 | ||
| 0.002 | |||
| 0.003 |
表4 硫酸铵结晶动力学方程
| 杂质种类 | 浓度/mol·L-1 | 成核动力学方程 | 生长动力学方程 |
|---|---|---|---|
| Mn2+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Al3+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Fe3+ | 0.001 | ||
| 0.002 | |||
| 0.003 | |||
| Cr3+ | 0.001 | ||
| 0.002 | |||
| 0.003 |
| 杂质种类 | 浓度/mol·L-1 | ||
|---|---|---|---|
| Mn2+ | 0.001 | 1.76419×10-14 | 0.66714 |
| 0.002 | 1.13867×10-14 | 0.82653 | |
| 0.003 | 9.17748×10-15 | 0.33817 | |
| Al3+ | 0.001 | 7.42176×10-15 | 0.61249 |
| 0.002 | 6.34097×10-15 | 0.37135 | |
| 0.003 | 1.76419×10-15 | 0.24965 | |
| Fe3+ | 0.001 | 5.25004×10-15 | 0.44396 |
| 0.002 | 3.54196×10-15 | 0.34471 | |
| 0.003 | 1.19991×10-15 | 0.14457 | |
| Cr3+ | 0.001 | 1.05465×10-14 | 0.74411 |
| 0.002 | 8.94551×10-15 | 0.37585 | |
| 0.003 | 5.11125×10-15 | 0.25918 |
表5 不同浓度杂质条件下硫酸铵结晶平均成核速率和平均生长速率
| 杂质种类 | 浓度/mol·L-1 | ||
|---|---|---|---|
| Mn2+ | 0.001 | 1.76419×10-14 | 0.66714 |
| 0.002 | 1.13867×10-14 | 0.82653 | |
| 0.003 | 9.17748×10-15 | 0.33817 | |
| Al3+ | 0.001 | 7.42176×10-15 | 0.61249 |
| 0.002 | 6.34097×10-15 | 0.37135 | |
| 0.003 | 1.76419×10-15 | 0.24965 | |
| Fe3+ | 0.001 | 5.25004×10-15 | 0.44396 |
| 0.002 | 3.54196×10-15 | 0.34471 | |
| 0.003 | 1.19991×10-15 | 0.14457 | |
| Cr3+ | 0.001 | 1.05465×10-14 | 0.74411 |
| 0.002 | 8.94551×10-15 | 0.37585 | |
| 0.003 | 5.11125×10-15 | 0.25918 |
| 1 | 蒋道利, 王丹, 王海元. 分步结晶技术在酸性铵盐沉钒废水处理中的应用[J]. 中国井矿盐, 2016, 47(1): 9-12. |
| JIANG Daoli, WANG Dan, WANG Haiyuan. The application of fractional crystallization technology in treatment of precipitating vanadium wastewater with acidic ammonium salts[J]. China Well and Rock Salt, 2016, 47(1): 9-12. | |
| 2 | 彭思瑶, 施云海. 硫酸铵介稳区宽度的研究及成核动力学计算[J]. 无机盐工业, 2018, 50(1): 41-45. |
| PENG Siyao, SHI Yunhai. Study on metastable zone width and nucleation kinetics of ammonium sulfate crystallization process[J]. Inorganic Chemicals Industry, 2018, 50(1): 41-45. | |
| 3 | 万雅曼. 硫铵蒸发结晶的工艺研究与优化[D]. 上海: 华东理工大学, 2014. |
| WAN Yaman. Process optimization and research of ammonium sulphate evaporation crystallization[D]. Shanghai: East China University of Science and Technology, 2014. | |
| 4 | 张建华, 叶世超, 段兰娟, 等. 流化床中硫酸铵结晶生长特性实验研究[J]. 现代化工, 2018, 38(2): 170-172, 174. |
| ZHANG Jianhua, YE Shichao, DUAN Lanjuan, et al. Study on crystal growth characteristics of ammonium sulfate in fluidized bed[J]. Modern Chemical Industry, 2018, 38(2): 170-172, 174. | |
| 5 | KITAMURA Mitsutaka, ENDO Hiromi. Growth process of ammonium sulfate crystals produced by secondary nucleation in stirred-tank crystallizer[J]. Journal of Chemical Engineering of Japan, 1991, 24(5): 593-599. |
| 6 | WESTHOFF G M, BUTLER B K, KRAMER H J M, et al. Growth behaviour of crystals formed by primary nucleation on different crystalliser scales[J]. Journal of Crystal Growth, 2002, 237/238/239: 2136-2141. |
| 7 | 靳学利, 李明东, 尤晓宇, 等. 硫酸铵、镁、锰复盐结晶动力学研究[J]. 有色金属科学与工程, 2022, 13(1): 1-7. |
| JIN Xueli, LI Mingdong, YOU Xiaoyu, et al. Study on crystallization kinetics of ammonium sulfate, magnesium and manganese double salt[J]. Nonferrous Metals Science and Engineering, 2022, 13(1): 1-7. | |
| 8 | 张伊恒, 徐兆立. 氯化钠在乙二醇溶液中的结晶动力学分析[J]. 天然气技术与经济, 2017, 11(2): 58-60, 84. |
| ZHANG Yiheng, XU Zhaoli. Crystallization kinetics of sodium chloride in ethylene glycol solution[J]. Natural Gas Technology and Economy, 2017, 11(2): 58-60, 84. | |
| 9 | 杨洪友. 镁、铵在MnSO4溶液中的溶解度及复盐结晶动力学研究[D]. 贵阳: 贵州大学, 2020. |
| YANG Hongyou. Research on the solubility of magnesium and ammonium in MnSO4 solution and the crystallization kinetics of the double salt[D]. Guiyang: Guizhou University, 2020. | |
| 10 | RAULS M, BARTOSCH K, KIND M, et al. The influence of impurities on crystallization kinetics—A case study on ammonium sulfate[J]. Journal of Crystal Growth, 2000, 213(1/2): 116-128. |
| 11 | 殷萍. 硫酸铵蒸发结晶过程研究[D]. 天津: 天津大学, 2007. |
| YIN Ping. Study on evaporation crystallization process of ammonium sulfate[D]. Tianjin: Tianjin University, 2007. | |
| 12 | ÓMEADHRA R, VAN ROSMALEN G M. Modelling of the growth of ammonium sulphate crystals in a DTB crystallizer[J]. Chemical Engineering Science, 1996, 51(16): 3919-3929. |
| 13 | 高毅颖, 张懿, 王静康, 等. 不同类型媒晶剂对硫酸铵晶体形态学指标的影响[J]. 化工学报, 2011, 62(12): 3575-3579. |
| GAO Yiying, ZHANG Yi, WANG Jingkang, et al. Effect of crystallization additives on crystal morphology of ammonium sulfate[J]. CIESC Journal, 2011, 62(12): 3575-3579. | |
| 14 | 代蒙, 黄帮福, 李露, 等. 氨法脱硫中影响硫酸铵结晶的主要因素[J]. 硅酸盐通报, 2021, 40(2): 505-512. |
| DAI Meng, HUANG Bangfu, LI Lu, et al. Main influencing factors of ammonium sulfate crystallization in ammonia desulfurization process[J]. Bulletin of the Chinese Ceramic Society, 2021, 40(2): 505-512. | |
| 15 | CHOI Cheong Song, KIM Ik Soo. Growth kinetics of ammonium sulfate in the ternary system ammonium sulfate-ammonium nitrate-water[J]. Industrial & Engineering Chemistry Research, 1990, 29(7): 1558-1562. |
| 16 | 许小静, 张圆圆, 郑鹏艳, 等. 氨法脱硫中杂质对硫酸铵结晶的影响[J]. 煤炭转化, 2020, 43(6): 75-83. |
| XU Xiaojing, ZHANG Yuanyuan, ZHENG Pengyan, et al. Effects of impurities on ammonium sulfate crystallization in ammonia desulfurization[J]. Coal Conversion, 2020, 43(6): 75-83. | |
| 17 | LARSON Maurice A, MULLIN John W. Crystallization kinetics of ammonium sulphate[J]. Journal of Crystal Growth, 1973, 20(3): 183-191. |
| 18 | 刘宝树, 种悦晖, 孙华, 等. 均一化大颗粒硫酸铵结晶高效制备工艺研究[J]. 无机盐工业, 2017, 49(6): 77-80. |
| LIU Baoshu, CHONG Yuehui, SUN Hua, et al. High efficiency preparation of homogeneous large granular ammonium sulfate crystals[J]. Inorganic Chemicals Industry, 2017, 49(6): 77-80. | |
| 19 | LIU X, ZHONG Q, WANG J, et al. Study on ammonium sulfate crystallization in the ammonium desulphurization process in a coal-based power plant in the petrochemical industry[J]. Energy Sources A: Recovery, Utilization, and Environmental Effects, 2011, 33(22): 2027-2035. |
| 20 | 贾海燕. 影响氨法脱硫溶液结晶成硫酸铵产品的因素及对策[J]. 煤炭加工与综合利用, 2017(12): 30-33, 71, 96. |
| JIA Haiyan. The factors influencing crystallization of ammonia desulfurization solution to ammonium sulfate products and the countermeasures[J]. Coal Processing & Comprehensive Utilization, 2017(12): 30-33, 71, 96. | |
| 21 | 章永洁, 曹吉林, 李琳, 等. 25℃下K2SO4-(NH4)2SO4-CO(NH2)2-H2O四元体系的相平衡[J]. 过程工程学报, 2003, 3(3): 212-217. |
| ZHANG Yongjie, CAO Jilin, LI Lin, et al. Phase equilibrium of the quaternary K2SO4-(NH4)2SO4-CO(NH2)2-H2O system at 25℃[J]. The Chinese Journal of Process Engineering, 2003, 3(3): 212-217. | |
| 22 | FARRELL Robert J, TSAI Yen Cheng. Modeling, simulation and kinetic parameter estimation in batch crystallization processes[J]. AIChE Journal, 1994, 40(4): 586-593. |
| 23 | 陈亮, 肖剑, 谢在库, 等. 高质量分数对二甲苯结晶动力学研究[J]. 化学工程, 2009, 37(10): 22-24, 31. |
| CHEN Liang, XIAO Jian, XIE Zaiku, et al. Crystallization kinetics of p-xylene with high mass fraction[J]. Chemical Engineering (China), 2009, 37(10): 22-24, 31. | |
| 24 | 王道广. MgCl2-CO2-NH3-H2O体系结晶热力学和动力学及其在二氧化碳固定和氧化镁生产中的应用[D]. 北京: 中国科学院大学, 2012. |
| WANG Daoguang. Crystallization thermodynamics and kinetics of the MgCl2-CO2-NH3-H2O system: its application in carbon dioxide sequestration and magnesia production[D]. Beijing: University of Chinese Academy of Sciences, 2012. | |
| 25 | 周堃, 向东妮, 刘海川. MSMPR结晶器中肌肉肌醇的冷却结晶动力学[J]. 化工设计通讯, 2018, 44(11): 158-160. |
| ZHOU Kun, XIANG Dongni, LIU Haichuan. Cooling crystallization kinetics of myo-inositol in MSMPR crysallizer[J]. Chemical Engineering Design Communications, 2018, 44(11): 158-160. | |
| 26 | MERSMANN A, KIND M, STICHLMAIR J. Thermal separation technology[M]. New York: Springer, 2011. |
| 27 | 王禄. 添加剂条件下的甘氨酸结晶过程研究及分子模拟[D]. 石家庄: 河北科技大学, 2017. |
| WANG Lu. Study on the crystallization process of glycine with additives and molecular simulation[D]. Shijiazhuang: Hebei University of Science and Technology, 2017. | |
| 28 | DAVEY R J. The effect of impurity adsorption on the kinetics of crystal growth from solution[J]. Journal of Crystal Growth, 1976, 34(1): 109-119. |
| 29 | CANO H, GABAS N, CANSELIER J P. Experimental study on the ibuprofen crystal growth morphology in solution[J]. Journal of Crystal Growth, 2001, 224(3/4): 335-341. |
| 30 | PUEL F, MARCHAL P, KLEIN J. Habit transient analysis in industrial crystallization using two dimensional crystal sizing technique[J]. Chemical Engineering Research and Design, 1997, 75(2): 193-205. |
| [1] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [2] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [3] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [4] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [5] | 刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103. |
| [6] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [7] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [8] | 王达锐, 孙洪敏, 薛明伟, 王一棪, 刘威, 杨为民. 微波法高效合成全结晶ZSM-5分子筛催化剂及其催化性能[J]. 化工进展, 2023, 42(7): 3582-3588. |
| [9] | 孙征楠, 李洪晶, 荆国林, 张福宁, 颜飚, 刘晓燕. EVA及其改性聚合物在原油降凝剂领域的应用[J]. 化工进展, 2023, 42(6): 2987-2998. |
| [10] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [11] | 曾天续, 张永显, 严渊, 刘宏, 马娇, 党鸿钟, 吴新波, 李维维, 陈永志. 羟胺对硝化菌活性及其动力学参数的影响[J]. 化工进展, 2023, 42(6): 3272-3280. |
| [12] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [13] | 张成松, 张静, 龚斌, 李明洋, 袁佳新, 李宏业. 自吸射流柔性搅拌桨振动特性[J]. 化工进展, 2023, 42(4): 1728-1738. |
| [14] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [15] | 吴霞, 蒋勋涛, 张余晓, 吕慧园, 黄方, 于筱溪. 基于液滴微流控技术的蛋白质结晶[J]. 化工进展, 2023, 42(4): 2024-2030. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||