化工进展 ›› 2022, Vol. 41 ›› Issue (9): 5122-5131.DOI: 10.16085/j.issn.1000-6613.2021-2435
废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰
余正伟1,2,3( ), 张晓霞2, 雷杰2, 李澳2, 王光应4, 丁祥1, 龙红明1,2(
), 张晓霞2, 雷杰2, 李澳2, 王光应4, 丁祥1, 龙红明1,2( )
)
- 1.冶金减排与资源综合利用教育部重点实验室(安徽工业大学),安徽 马鞍山 243002
2.安徽工业大学冶金工程学院,安徽 马鞍山 243002
3.冶金过程节能与污染控制安徽省教育厅工程技术研究中心,安徽 马鞍山 243002
4.安徽元琛环保科技股份有限公司,安徽 合肥 230012
-
收稿日期:2021-11-26修回日期:2022-04-12出版日期:2022-09-25发布日期:2022-09-27 -
通讯作者:龙红明 -
作者简介:余正伟(1984—),男,博士,讲师,研究方向为固废资源化利用。E-mail:yuzhengwei@ahut.edu.cn。 -
基金资助:国家自然科学基金(51704009);冶金减排与资源综合利用教育部重点实验室开放基金(JKF21-07);冶金过程节能与污染控制安徽省教育厅工程技术研究中心开放基金(GKF20-2)
Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching
YU Zhengwei1,2,3( ), ZHANG Xiaoxia2, LEI Jie2, LI Ao2, WANG Guangying4, DING Xiang1, LONG Hongming1,2(
), ZHANG Xiaoxia2, LEI Jie2, LI Ao2, WANG Guangying4, DING Xiang1, LONG Hongming1,2( )
)
- 1.Key Laboratory of Metallurgical Emission Reduction & Resources Recycling (Anhui University of Technology), Ministry of Education, Maanshan 243002, Anhui, China
2.School of Metallurgical Engineering, Anhui University of Technology, Maanshan 243002, Anhui, China
3.Energy Saving and Pollution Control in Metallurgical Process Engineering Technology Research Center of Education Department of Anhui Province, Maanshan 243002, Anhui, China
4.Anhui Yuanchen Environmental Protection Technology Company Limited, Hefei 230012, Anhui, China
-
Received:2021-11-26Revised:2022-04-12Online:2022-09-25Published:2022-09-27 -
Contact:LONG Hongming
摘要:
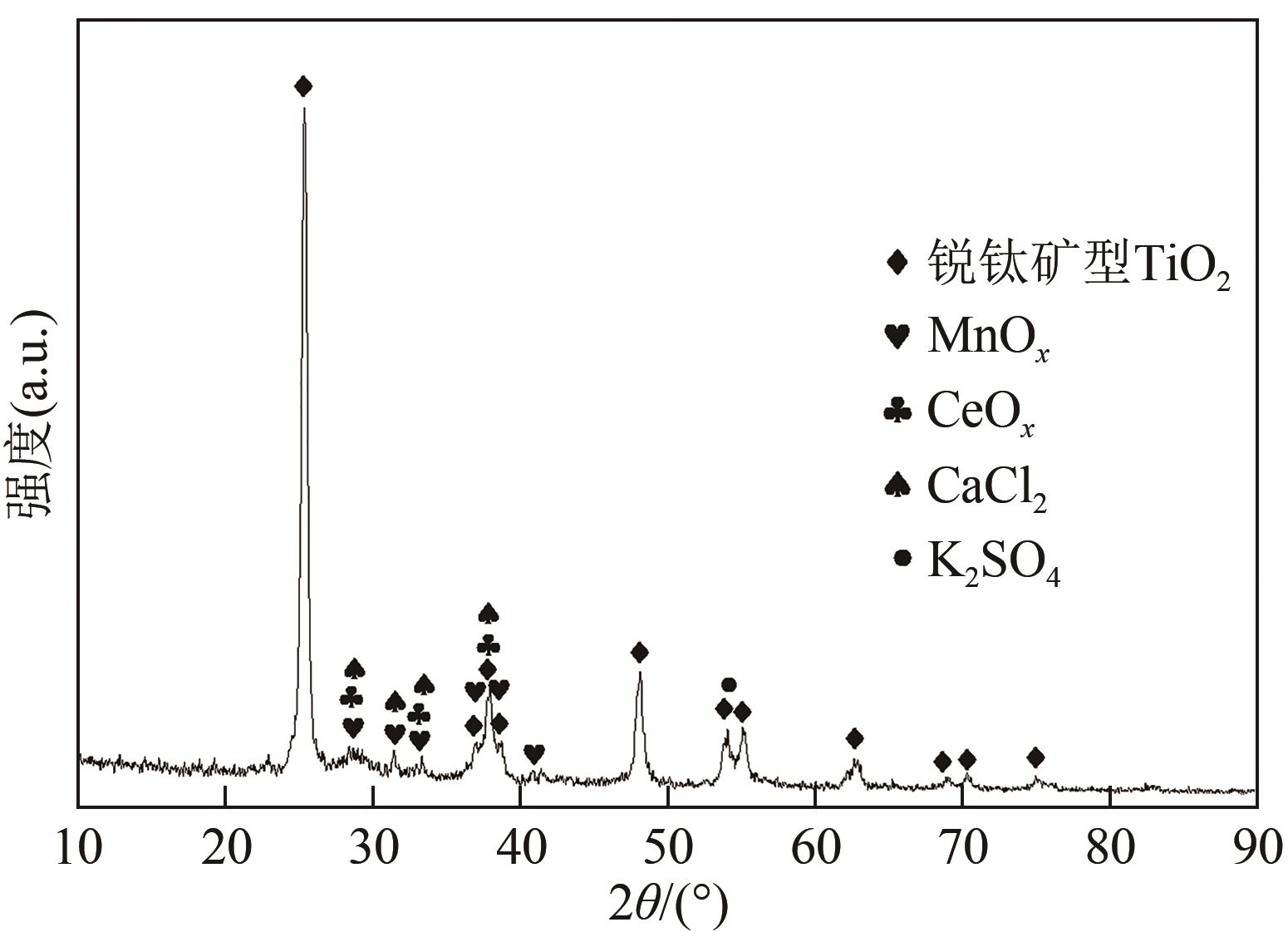
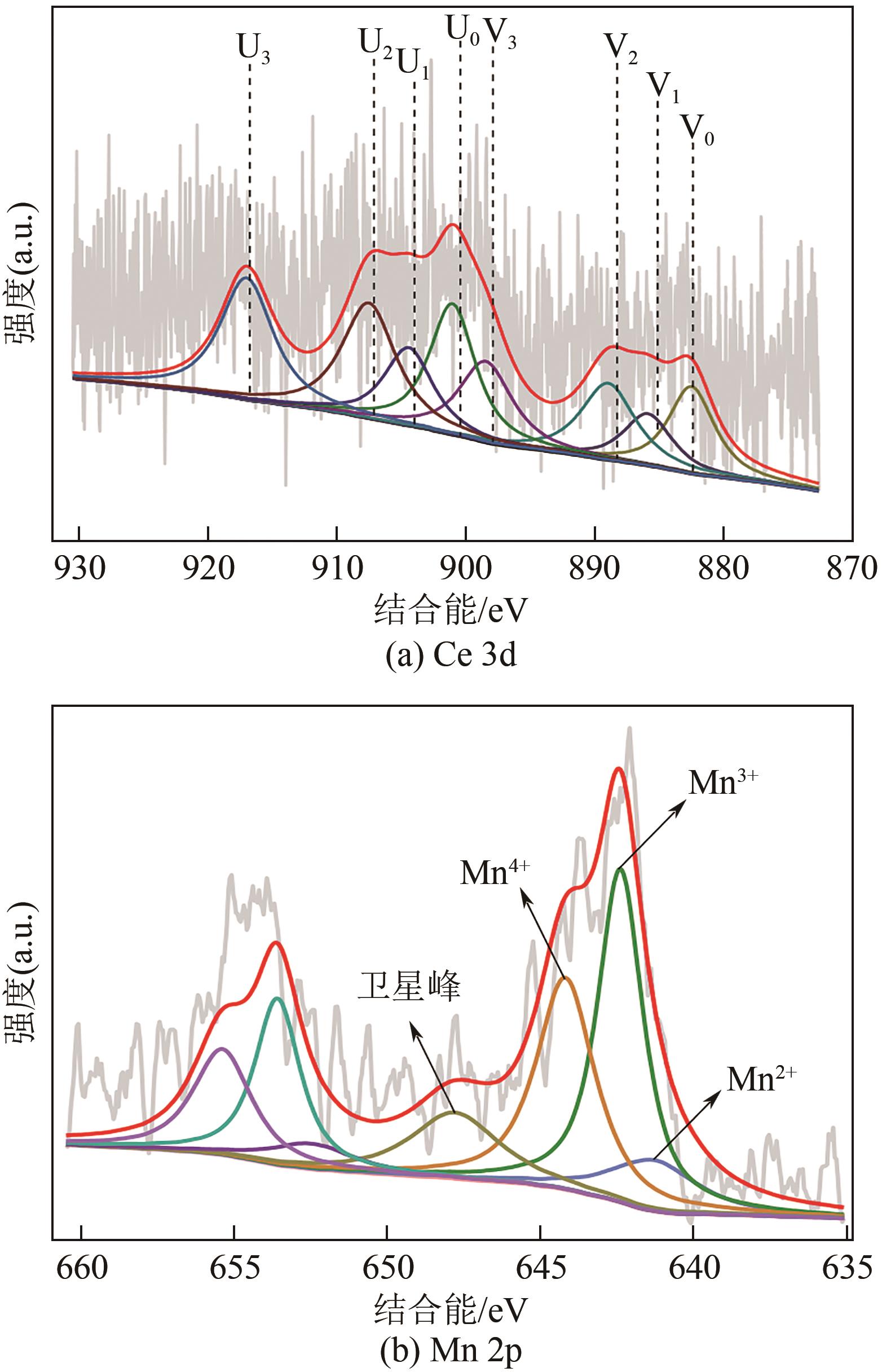
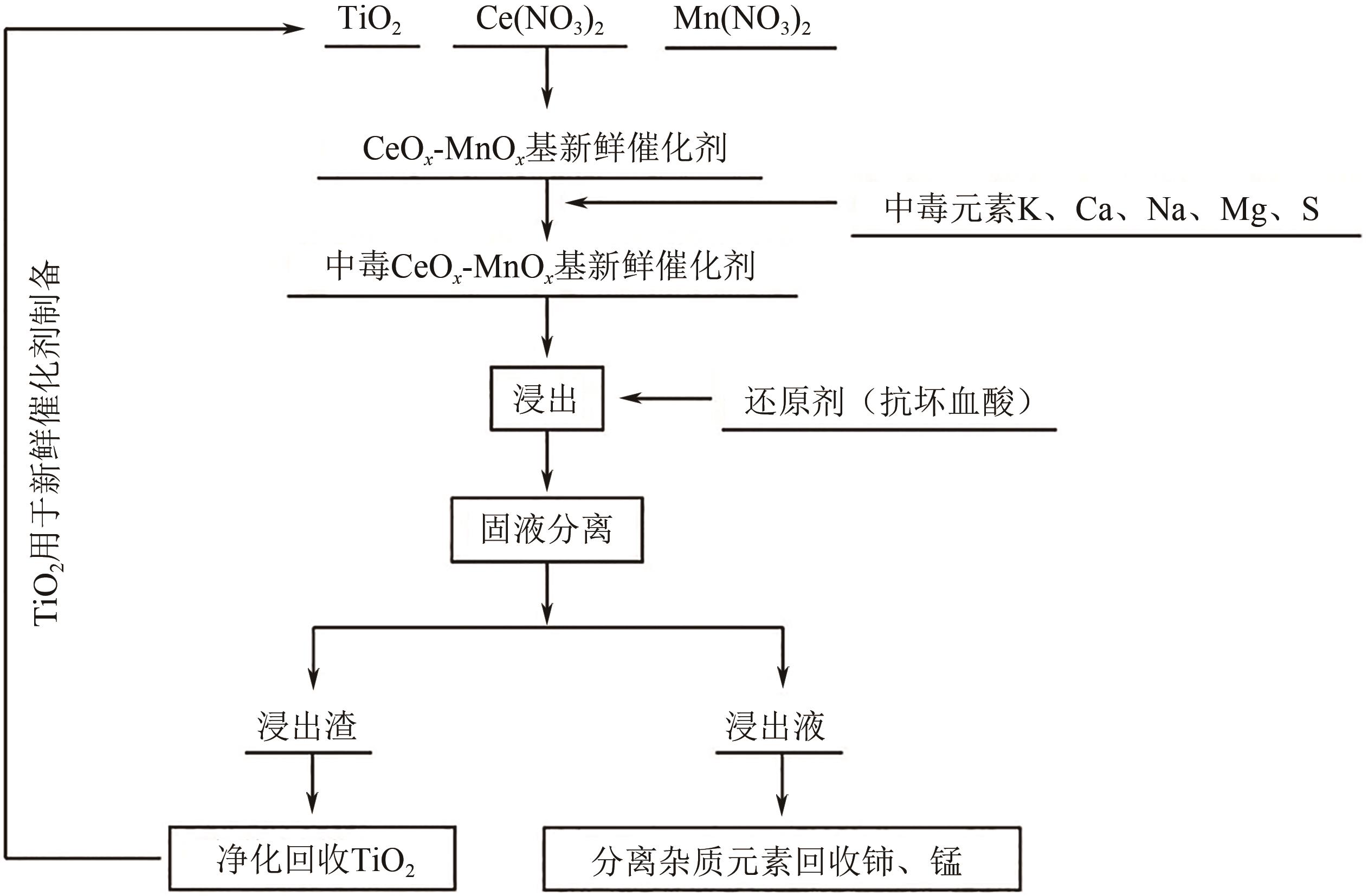
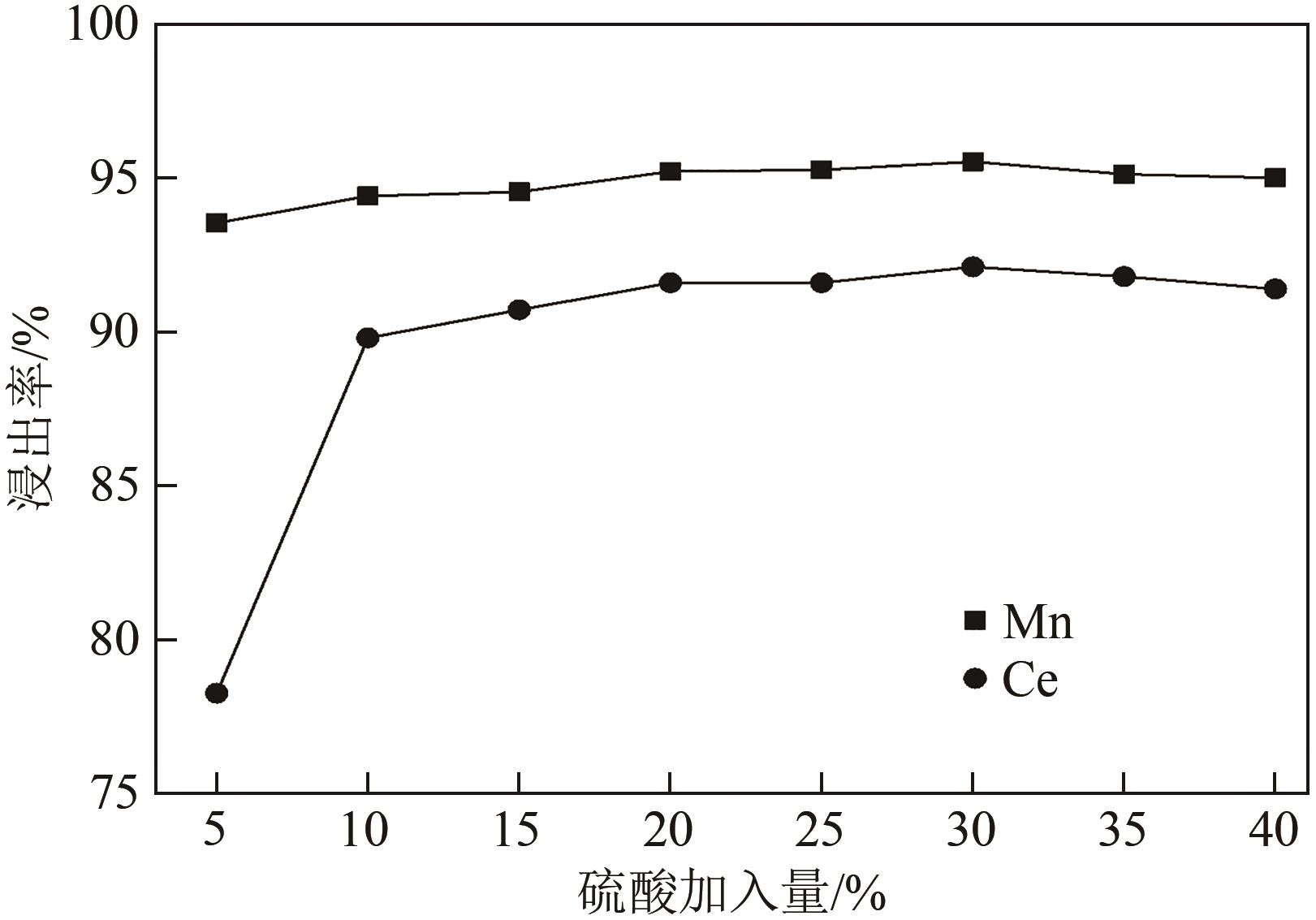
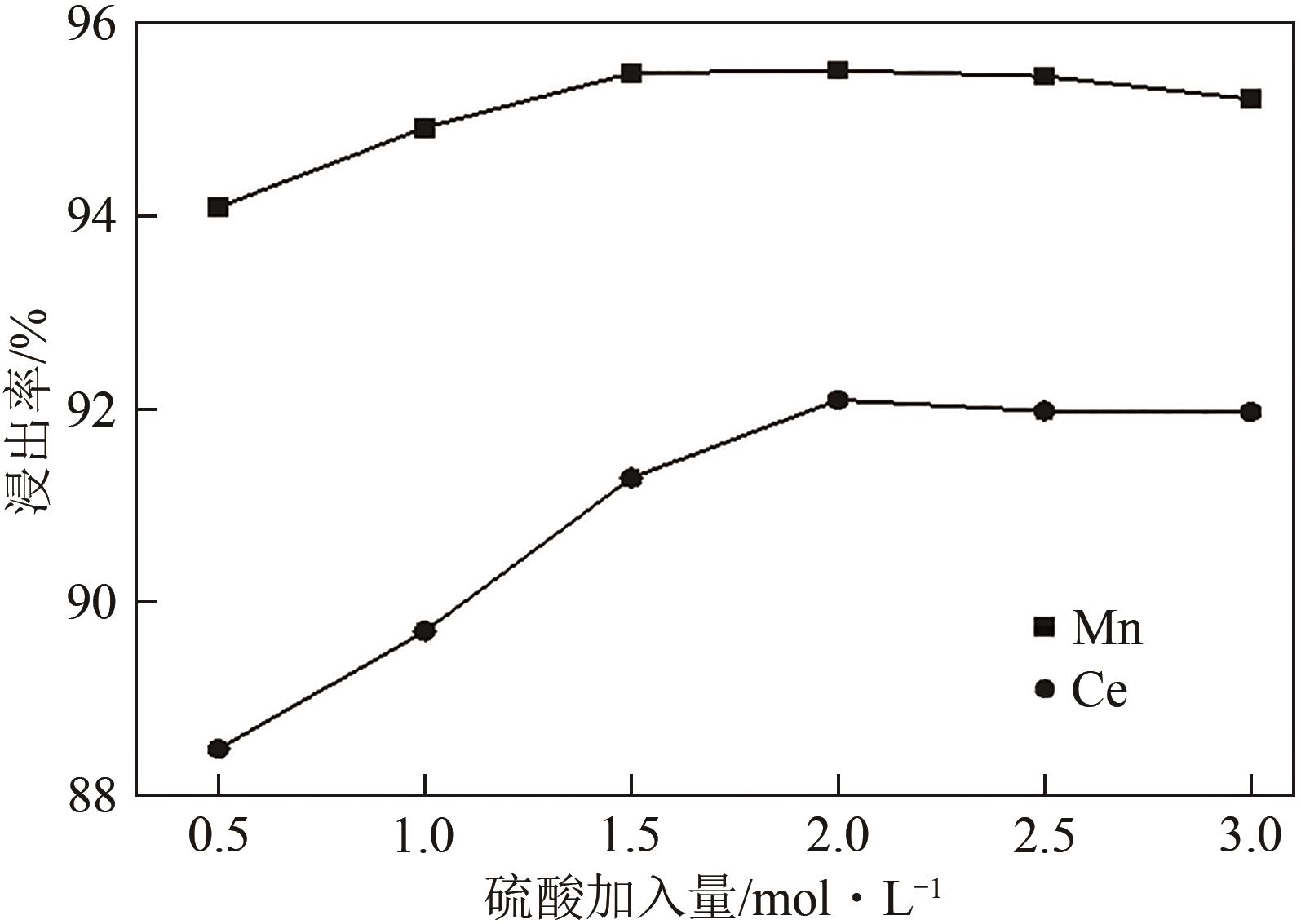
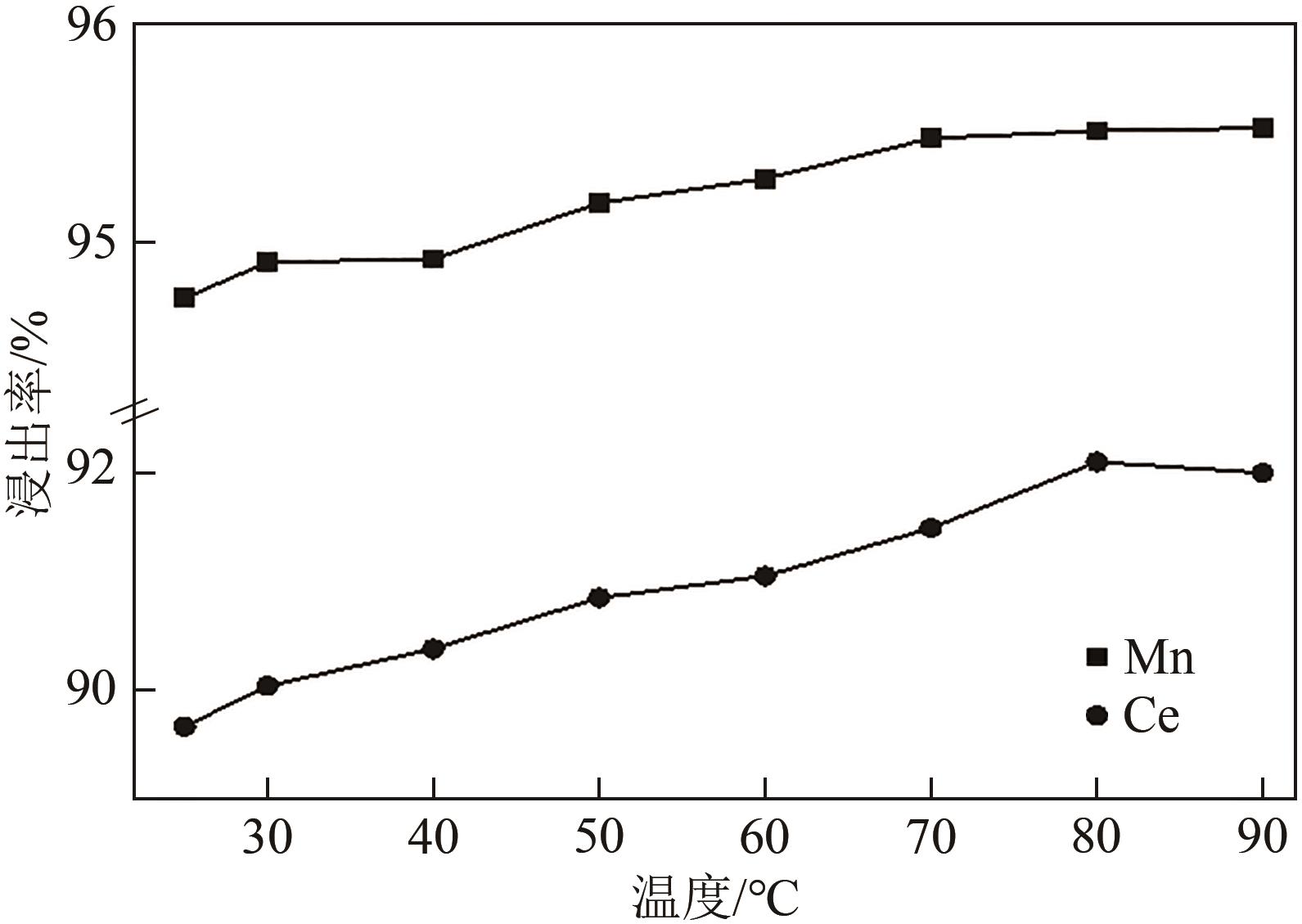
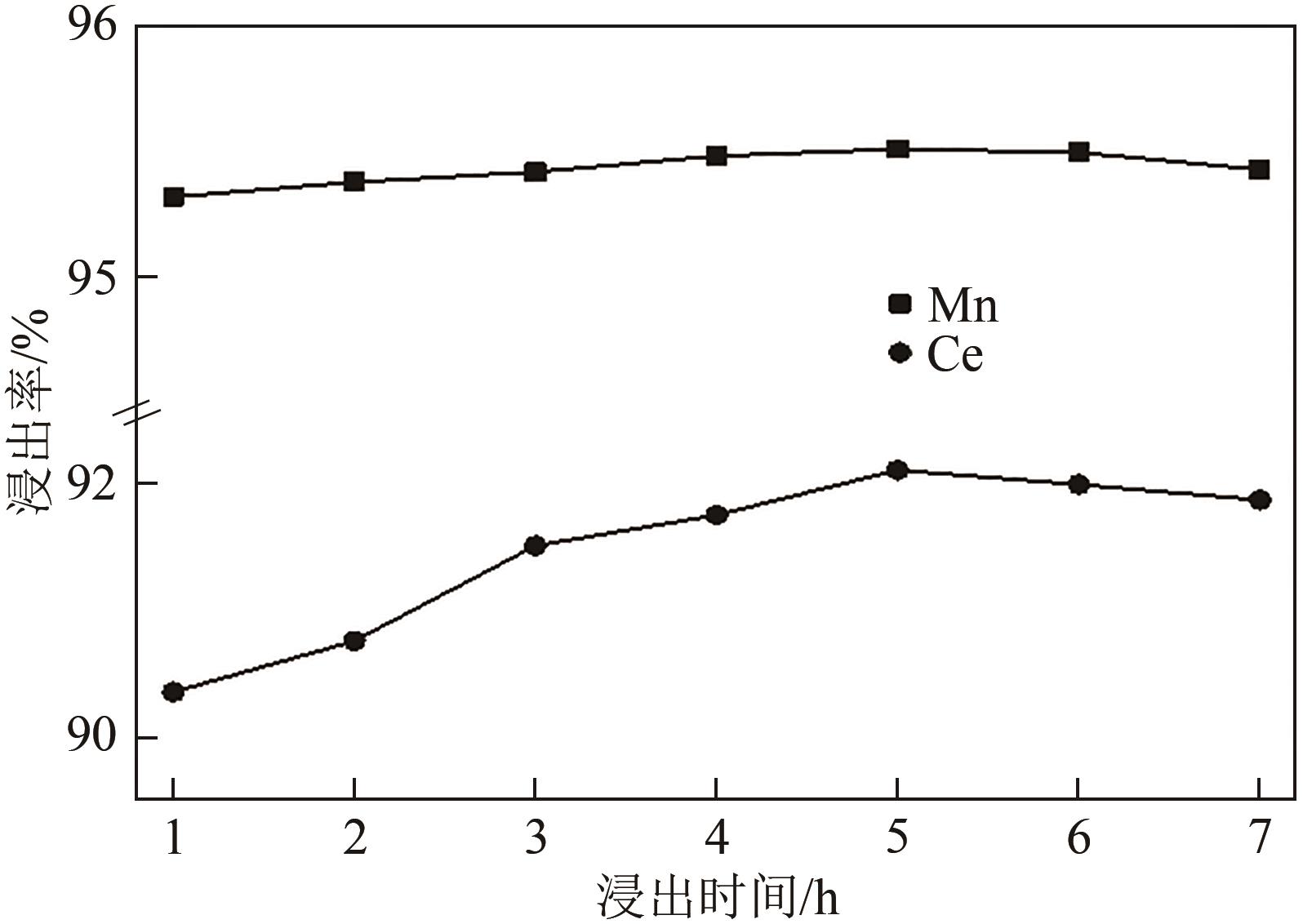
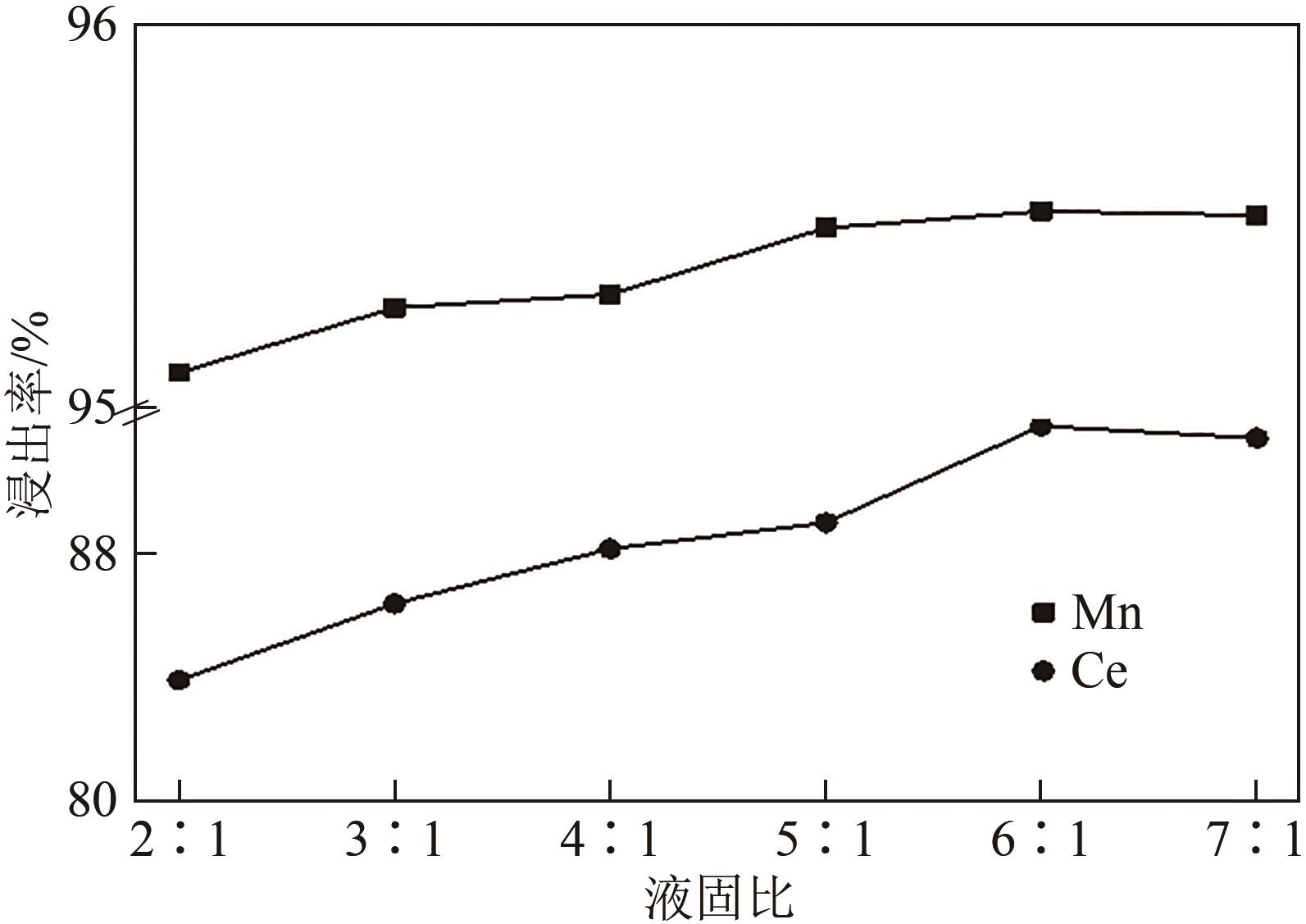
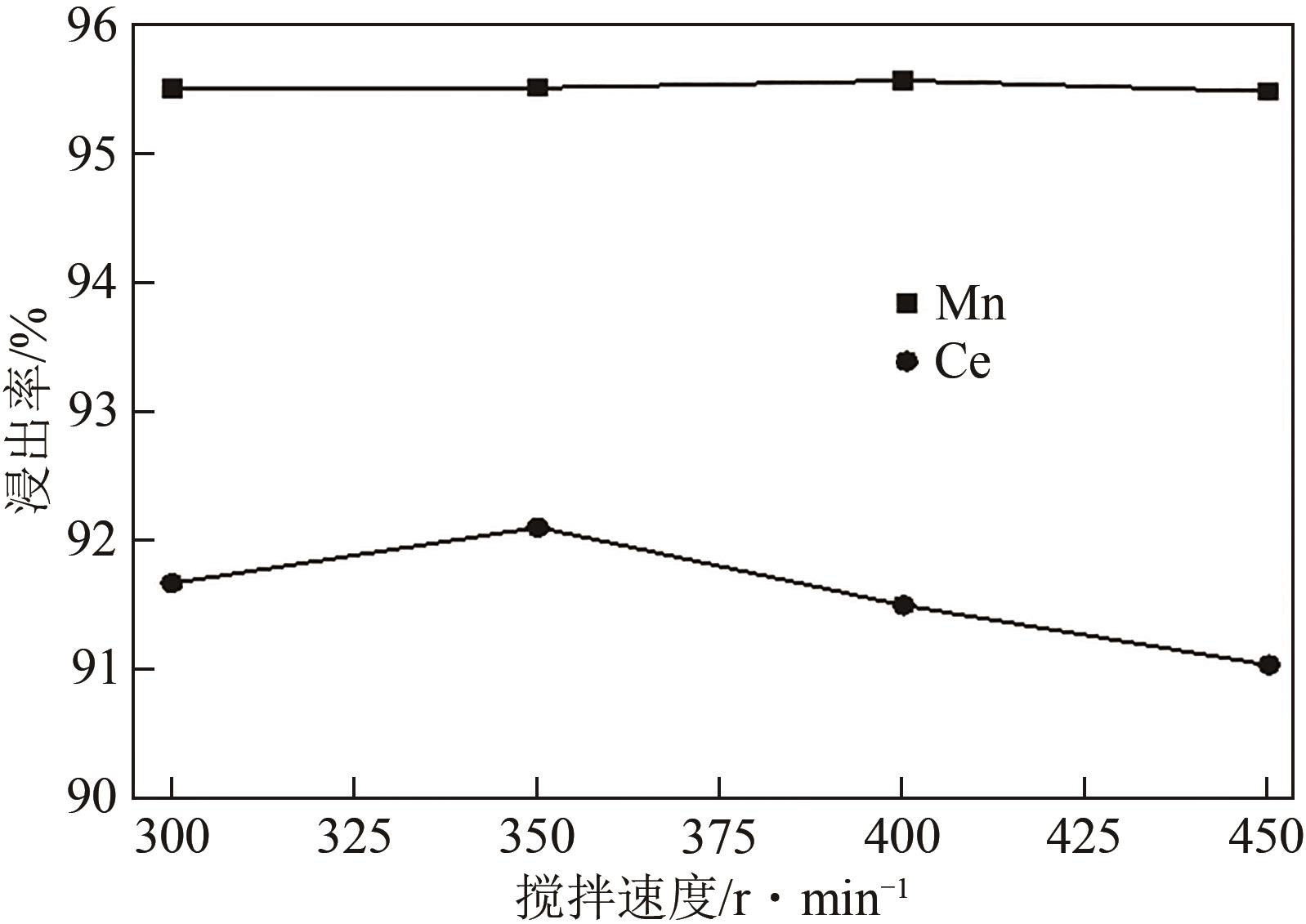
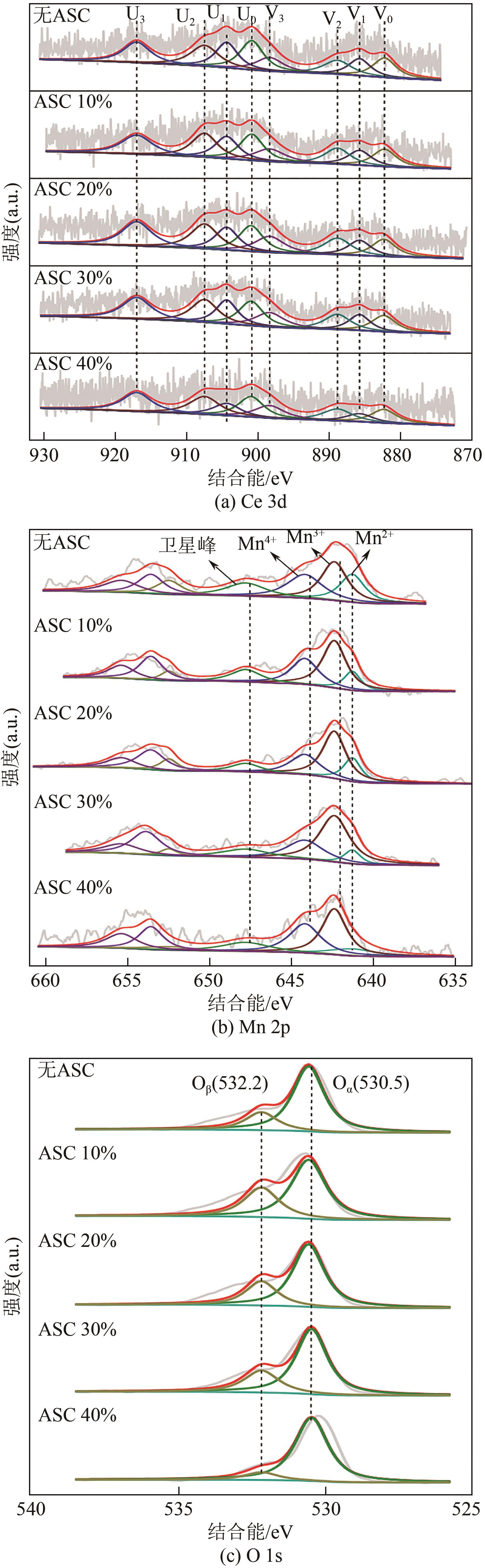
废选择性催化还原(SCR)脱硝催化剂中含有大量的有价金属,直接废弃易造成资源浪费及环境污染。以废CeO x -MnO x 基SCR脱硝催化剂为原料,采用热力学分析结合湿法冶金实验方法,研究了浸出条件对Ce、Mn元素浸出率的影响。结果表明,废催化剂直接酸浸Ce、Mn元素浸出率低,还原-酸浸Ce、Mn元素热力学条件上可行,抗坏血酸对Ce、Mn高价氧化物有明显的还原作用。当抗坏血酸质量分数为30%、硫酸浓度2mol/L、液固比6∶1、搅拌速度350r/min、80℃恒温反应5h时,Ce、Mn的浸出率分别达到92.09%、95.51%。加入抗坏血酸后,部分Ce4+和Mn4+还原为Ce3+和Mn2+,Ce4+/Ce的比值由75.82%降低到71.62%,Mn4+/Mn的比值由29.39%降低到27.17%,同时削弱了高价Ce辅助低价Mn向高价Mn转化的作用,使得Ce、Mn高效浸出,为CeO x -MnO x 基废催化剂中Ce、Mn资源化利用奠定了基础。
中图分类号:
引用本文
余正伟, 张晓霞, 雷杰, 李澳, 王光应, 丁祥, 龙红明. 废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰[J]. 化工进展, 2022, 41(9): 5122-5131.
YU Zhengwei, ZHANG Xiaoxia, LEI Jie, LI Ao, WANG Guangying, DING Xiang, LONG Hongming. Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5122-5131.
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|
| 72.53 | 11.20 | 2.57 | 3.80 | 2.57 | 1.14 | 0.48 | 0.60 | 5.11 |
表1 废CeO x -MnO x 基SCR脱硝催化剂化学成分(质量分数,%)
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|
| 72.53 | 11.20 | 2.57 | 3.80 | 2.57 | 1.14 | 0.48 | 0.60 | 5.11 |
| 样品 | 化合价比例/% |
|---|---|
| Ce3+/Ce | 22.67 |
| Ce4+/Ce | 77.33 |
| Mn2+/Mn | 23.22 |
| Mn3+/Mn | 41.88 |
| Mn4+/Mn | 34.49 |
表2 以XPS能谱峰面积计算化合价比例
| 样品 | 化合价比例/% |
|---|---|
| Ce3+/Ce | 22.67 |
| Ce4+/Ce | 77.33 |
| Mn2+/Mn | 23.22 |
| Mn3+/Mn | 41.88 |
| Mn4+/Mn | 34.49 |
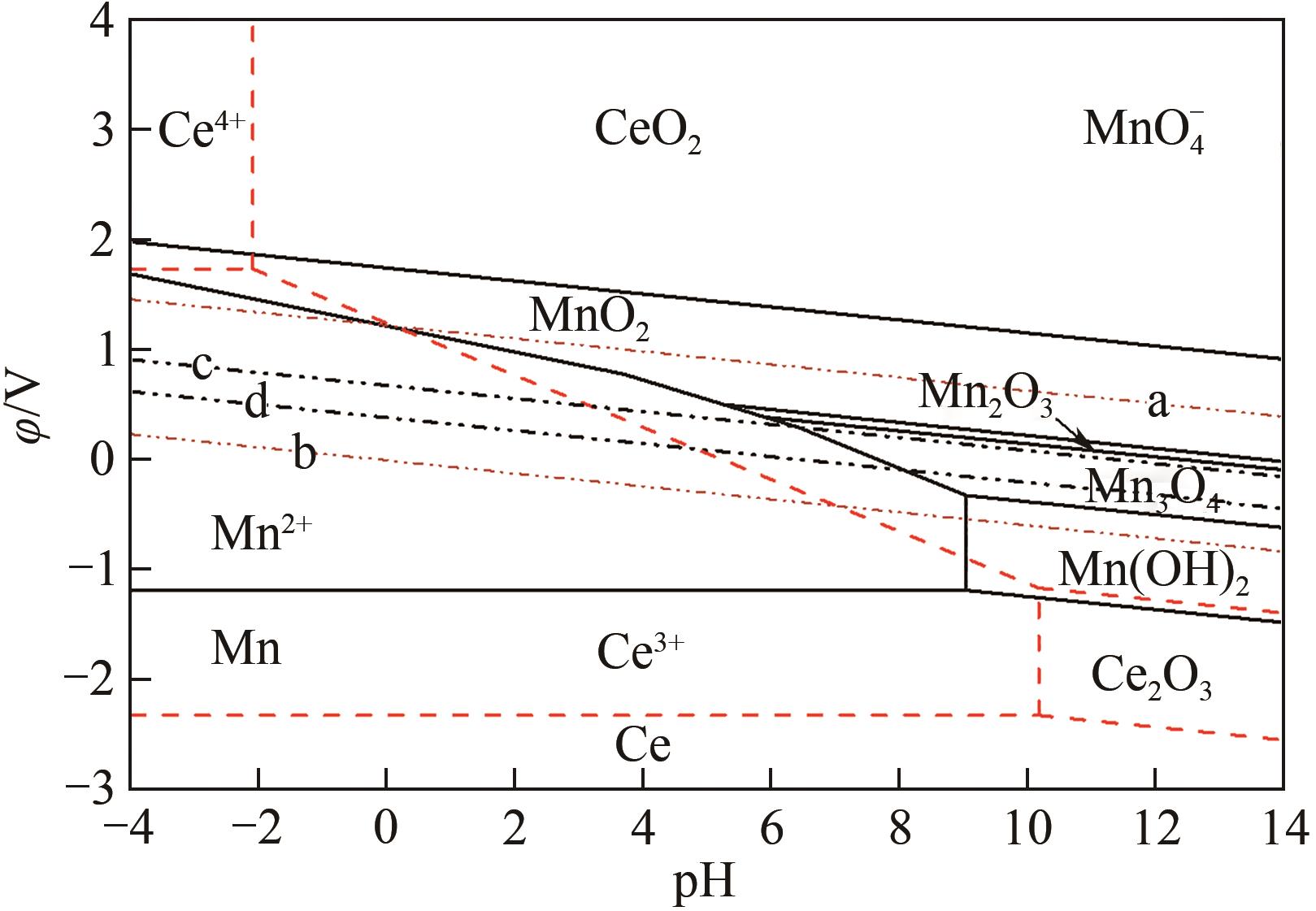
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (1) | Ce3++3e- | φ=-2.3239+0.0197lg |
| (2) | Ce4++e- | φ=1.7432+0.0592lg( |
| (3) | Ce2O3+6H+ | pH=10.1936-3lg |
| (4) | CeO2+4H+ | pH=-2.096-1/4lg |
| (5) | 2CeO2+2H++2e- | φ=-0.5606-0.0592pH |
| (6) | CeO2+4H++e- | φ=1.2485-0.2366pH-0.0592lg |
| (7) | Ce2O3+6H++6e- | φ=-1.7209-0.0592pH |
表3 Ce-H2O系各反应在25℃下对应的φ-pH函数关系
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (1) | Ce3++3e- | φ=-2.3239+0.0197lg |
| (2) | Ce4++e- | φ=1.7432+0.0592lg( |
| (3) | Ce2O3+6H+ | pH=10.1936-3lg |
| (4) | CeO2+4H+ | pH=-2.096-1/4lg |
| (5) | 2CeO2+2H++2e- | φ=-0.5606-0.0592pH |
| (6) | CeO2+4H++e- | φ=1.2485-0.2366pH-0.0592lg |
| (7) | Ce2O3+6H++6e- | φ=-1.7209-0.0592pH |
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (8) | Mn2++2e- | φ=-1.185+0.0296lg |
| (9) | Mn(OH)2+2H+ | pH=9.06-0.0592lg |
| (10) | Mn(OH)2+2H++2e- | φ=-0.649-0.0592pH |
| (11) | Mn3O4+2H++2e- | φ=0.216-0.0592pH |
| (12) | Mn3O4+8H++2e- | φ=1.824-0.237pH-0.0887lg |
| (13) | 3Mn2O3+2H++2e- | φ=0.743-0.0592pH |
| (14) | Mn2O3+6H++2e- | φ=1.443-0.177pH-0.0592lg |
| (15) | 2MnO2+2H++2e- | φ=0.819-0.0592pH |
| (16) | φ=1.754-0.1789pH+0.01972lg | |
| (17) | MnO2+4H++2e- | φ=1.224-0.1183pH-0.02956lg |
表4 Mn-H2O系各反应在25℃下对应的φ-pH函数关系
| 序号 | 平衡反应 | φ-pH关系式 |
|---|---|---|
| a | O2+4H++4e- | φ=1.2290-0.0592pH |
| b | H++2e- | φ=-0.0592pH |
| c | O2+2H++2e- | φ=0.682-0.0592pH |
| d | C6H6O6+2H++2e- | φ=0.39-0.0592pH |
| (8) | Mn2++2e- | φ=-1.185+0.0296lg |
| (9) | Mn(OH)2+2H+ | pH=9.06-0.0592lg |
| (10) | Mn(OH)2+2H++2e- | φ=-0.649-0.0592pH |
| (11) | Mn3O4+2H++2e- | φ=0.216-0.0592pH |
| (12) | Mn3O4+8H++2e- | φ=1.824-0.237pH-0.0887lg |
| (13) | 3Mn2O3+2H++2e- | φ=0.743-0.0592pH |
| (14) | Mn2O3+6H++2e- | φ=1.443-0.177pH-0.0592lg |
| (15) | 2MnO2+2H++2e- | φ=0.819-0.0592pH |
| (16) | φ=1.754-0.1789pH+0.01972lg | |
| (17) | MnO2+4H++2e- | φ=1.224-0.1183pH-0.02956lg |
| 还原剂 | 温度/℃ | 浸出率/% | 残留率/% | |||
|---|---|---|---|---|---|---|
| Ce | Mn | Ti | 浸出渣 | |||
| 无 | 25 | 29.34 | 32.02 | 87.74 | 86.80 | |
| 无 | 80 | 59.72 | 72.55 | 87.83 | 81.70 | |
| 抗坏血酸 | 80 | 92.08 | 95.51 | 91.20 | 77.90 | |
| Na2SO3 | 80 | 57.63 | 92.48 | 89.50 | 78.10 | |
| H2O2 | 80 | 88.49 | 94.10 | 84.73 | 71.10 | |
表5 还原剂种类对废CeO x -MnO x 基SCR脱硝催化剂还原酸浸Ce、Mn的影响
| 还原剂 | 温度/℃ | 浸出率/% | 残留率/% | |||
|---|---|---|---|---|---|---|
| Ce | Mn | Ti | 浸出渣 | |||
| 无 | 25 | 29.34 | 32.02 | 87.74 | 86.80 | |
| 无 | 80 | 59.72 | 72.55 | 87.83 | 81.70 | |
| 抗坏血酸 | 80 | 92.08 | 95.51 | 91.20 | 77.90 | |
| Na2SO3 | 80 | 57.63 | 92.48 | 89.50 | 78.10 | |
| H2O2 | 80 | 88.49 | 94.10 | 84.73 | 71.10 | |
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | 其他 |
|---|---|---|---|---|---|---|
| 93.58 | 0.78 | 0.33 | 0.78 | 0.04 | 0.12 | 4.37 |
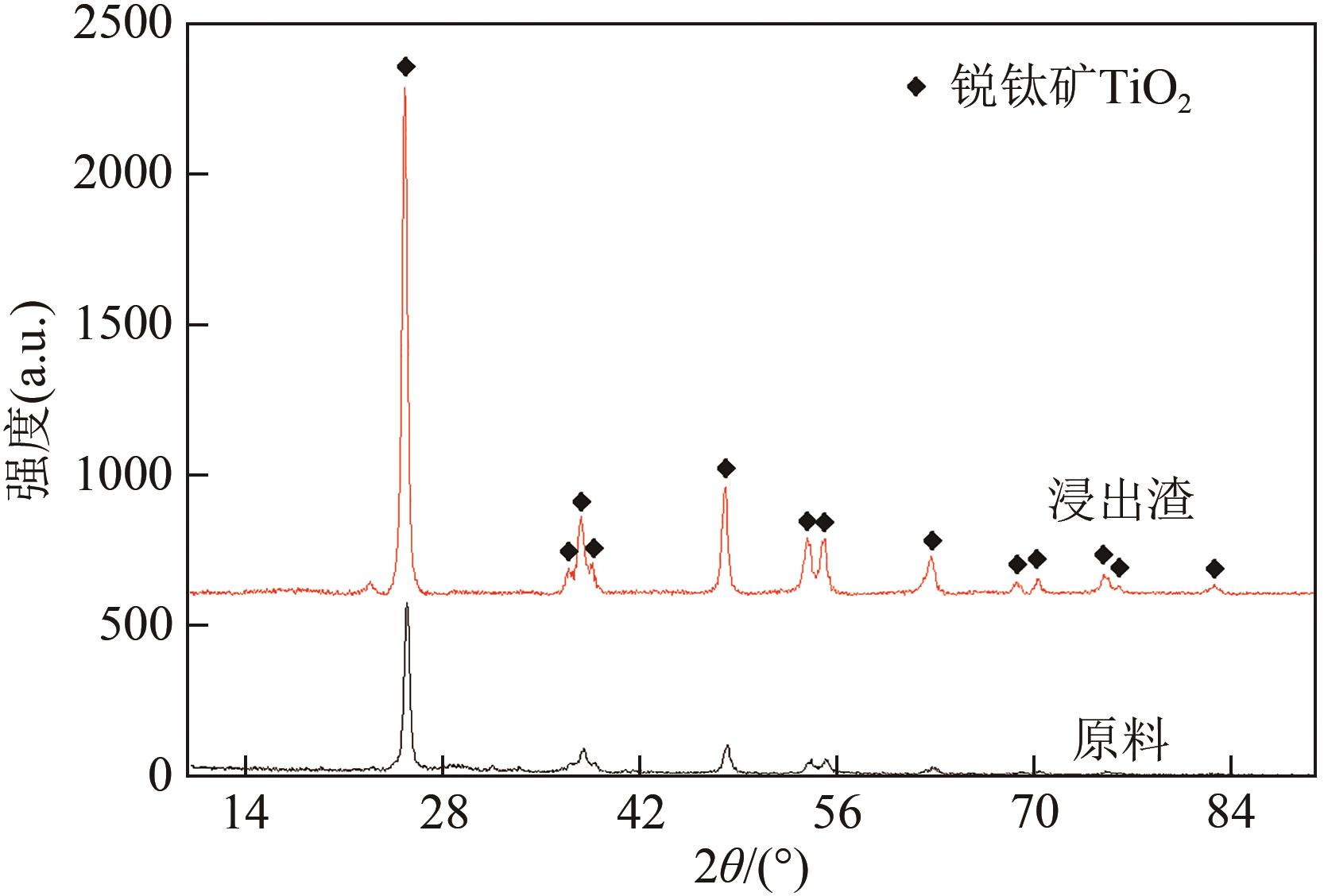
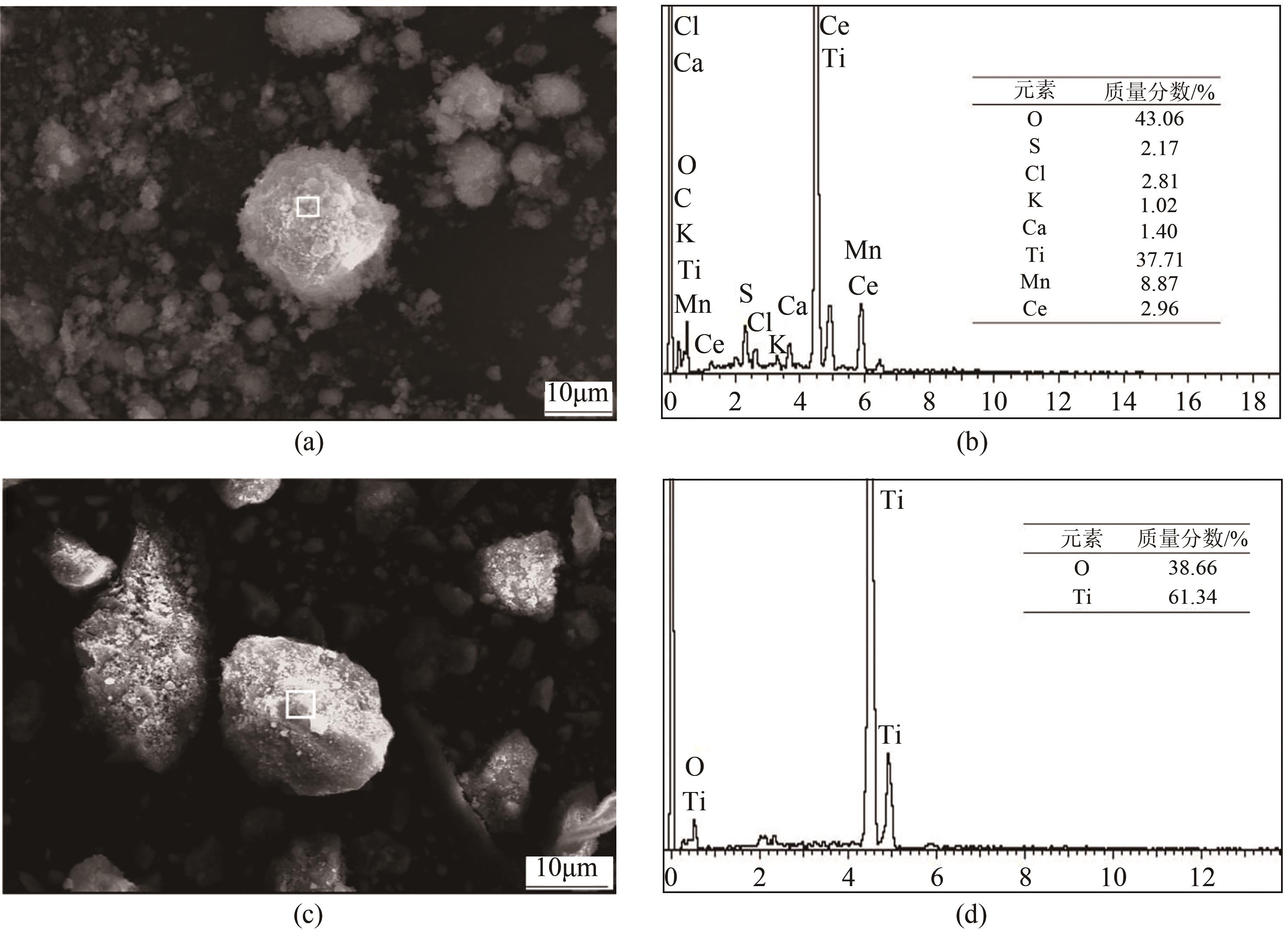
表6 废CeOx-MnOx基SCR脱硝催化剂还原-酸浸渣主要化学成分(质量分数,%)
| TiO2 | MnO | CeO2 | SO3 | CaO | MgO | 其他 |
|---|---|---|---|---|---|---|
| 93.58 | 0.78 | 0.33 | 0.78 | 0.04 | 0.12 | 4.37 |
| 样品 | Ce3+/Ce | Ce4+/Ce | Mn2+/Mn | Mn4+/Mn | Oβ/(Oα+Oβ) | Oα/(Oα+Oβ) |
|---|---|---|---|---|---|---|
| 原料 | 22.67 | 77.33 | 23.22 | 34.49 | 10.88 | 89.12 |
| 抗坏血酸 加入量10% | 24.18 | 75.82 | 23.82 | 29.39 | 27.16 | 72.84 |
| 抗坏血酸 加入量20% | 25.27 | 74.73 | 25.89 | 28.93 | 27.97 | 72.03 |
| 抗坏血酸 加入量30% | 28.38 | 71.62 | 36.35 | 27.17 | 33.47 | 66.53 |
| 抗坏血酸 加入量40% | 28.14 | 71.86 | 36.03 | 26.89 | 21.70 | 78.30 |
表7 以XPS能谱峰面积计算化合价比例 (%)
| 样品 | Ce3+/Ce | Ce4+/Ce | Mn2+/Mn | Mn4+/Mn | Oβ/(Oα+Oβ) | Oα/(Oα+Oβ) |
|---|---|---|---|---|---|---|
| 原料 | 22.67 | 77.33 | 23.22 | 34.49 | 10.88 | 89.12 |
| 抗坏血酸 加入量10% | 24.18 | 75.82 | 23.82 | 29.39 | 27.16 | 72.84 |
| 抗坏血酸 加入量20% | 25.27 | 74.73 | 25.89 | 28.93 | 27.97 | 72.03 |
| 抗坏血酸 加入量30% | 28.38 | 71.62 | 36.35 | 27.17 | 33.47 | 66.53 |
| 抗坏血酸 加入量40% | 28.14 | 71.86 | 36.03 | 26.89 | 21.70 | 78.30 |
| 1 | MOHAN S, DINESHA P, KUMAR S. NO x reduction behaviour in copper zeolite catalysts for ammonia SCR systems: a review[J]. Chemical Engineering Journal, 2020, 384: 123253. |
| 2 | TANG Changjin, ZHANG Hongliang, DONG Lin. Ceria-based catalysts for low-temperature selective catalytic reduction of NO with NH3 [J]. Catalysis Science & Technology, 2016, 6(5): 1248-1264. |
| 3 | 邢奕, 张文伯, 苏伟, 等. 中国钢铁行业超低排放之路[J]. 工程科学学报, 2021, 43(1): 1-9. |
| XING Yi, ZHANG Wenbo, SU Wei, et al. Research of ultra-low emission technologies of the iron and steel industry in China[J]. Chinese Journal of Engineering, 2021, 43(1): 1-9. | |
| 4 | 闫晓淼, 李玉然, 朱廷钰, 等. 钢铁烧结烟气多污染物排放及协同控制概述[J]. 环境工程技术学报, 2015, 5(2): 85-90. |
| YAN Xiaomiao, LI Yuran, ZHU Tingyu, et al. Review of emission and simultaneous control of multiple pollutants from iron-steel sintering flue gas[J]. Journal of Environmental Engineering Technology, 2015, 5(2): 85-90. | |
| 5 | 张洪亮, 施琦, 龙红明, 等. 烧结烟气中氮氧化物脱除工艺分析[J]. 钢铁, 2017, 52(5): 100-106. |
| ZHANG Hongliang, SHI Qi, LONG Hongming, et al. Analysis of NO x removal process in sintering flue gas[J]. Iron & Steel, 2017, 52(5): 100-106. | |
| 6 | 苏玉栋, 李咸伟, 范晓慧. 烧结过程中NO x 减排技术研究进展[J]. 烧结球团, 2013, 38(6): 41-44, 54. |
| SU Yudong, LI Xianwei, FAN Xiaohui. Research progress of NO x reduction technology in sintering process[J]. Sintering and Pelletizing, 2013, 38(6): 41-44, 54. | |
| 7 | LING Shaohua, JING Changyong, ZHANG Lijuan. Analysis denitration technology for iron-steel sintering flue gas[C]//Proceedings of the 2015 International Symposium on Material, Energy and Environment Engineering. November 28-29, 2015. Changsha, China. Paris, France: Atlantis Press, 2015. |
| 8 | 王淑勤, 刘丽凤, 程伟良. 低温SCR脱硝催化技术的应用进展[J]. 能源与环境, 2021(2): 65-69. |
| WANG Shuqin, LIU Lifeng, CHENG Weiliang. Application progress of low-temperature SCR denitrification catalytic technology[J]. Energy and Environment, 2021(2): 65-69. | |
| 9 | 刘福东, 单文坡, 石晓燕, 等. 用于NH3选择性催化还原NO x 的钒基催化剂[J]. 化学进展, 2012, 24(4): 445-455. |
| LIU Fudong, SHAN Wenpo, SHI Xiaoyan, et al. Vanadium-based catalysts for the selective catalytic reduction of NO x with NH3 [J]. Progress in Chemistry, 2012, 24(4): 445-455. | |
| 10 |
丁龙, 钱立新, 杨涛, 等. 烧结烟气中Zn对V2O5-WO3/TiO2催化剂脱除NO x 和二 英性能的影响[J]. 工程科学学报, 2021, 43(8): 1125-1135. 英性能的影响[J]. 工程科学学报, 2021, 43(8): 1125-1135.
|
| DING Long, QIAN Lixin, YANG Tao, et al. Influence of Zn in the iron ore sintering flue gas on the removal of NO x and dioxins by V2O5-WO3/TiO2 catalyst[J]. Chinese Journal of Engineering, 2021, 43(8): 1125-1135. | |
| 11 | ZHANG Qijun, WU Yufeng, ZUO Tieyong. Titanium extraction from spent selective catalytic reduction catalysts in a NaOH molten-salt system: thermodynamic, experimental, and kinetic studies[J]. Metallurgical and Materials Transactions B, 2019, 50(1): 471-479. |
| 12 | 侯学军, 章小明, 程文博, 等. 废钒钛基SCR催化剂的处置方法研究进展[J]. 化工进展, 2021, 40(10): 5313-5324. |
| HOU Xuejun, ZHANG Xiaoming, CHENG Wenbo, et al. Research on disposal methods of spent vanadium-titanium-based catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5313-5324. | |
| 13 | CHOI In Hyeok, MOON Gyeonghye, LEE Jin Young, et al. Extraction of tungsten and vanadium from spent selective catalytic reduction catalyst for stationary application by pressure leaching process[J]. Journal of Cleaner Production, 2018, 197: 163-169. |
| 14 | MOON Gyeonghye, KIM Jin Hyeong, LEE Jin Young, et al. Leaching of spent selective catalytic reduction catalyst using alkaline melting for recovery of titanium, tungsten, and vanadium[J]. Hydrometallurgy, 2019, 189: 105132. |
| 15 | LI Qichao, LIU Zhenyu, LIU Qingya. Kinetics of vanadium leaching from a spent industrial V2O5/TiO2 catalyst by sulfuric acid[J]. Industrial & Engineering Chemistry Research, 2014, 53(8): 2956-2962. |
| 16 | ZHANG Qijun, WU Yufeng, LI Lili, et al. Sustainable approach for spent V2O5-WO3/TiO2 catalysts management: selective recovery of heavy metal vanadium and production of value-added WO3-TiO2 photocatalysts[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12502-12510. |
| 17 | TANG Xingfu, LI Yonggang, HUANG Xiumin, et al. MnO x -CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: effect of preparation method and calcination temperature[J]. Applied Catalysis B: Environmental, 2006, 62(3/4): 265-273. |
| 18 | KLIMCZAK M, KERN P, HEINZELMANN T, et al. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts—Part Ⅰ: V2O5-WO3/TiO2 catalysts[J]. Applied Catalysis B: Environmental, 2010, 95(1/2): 39-47. |
| 19 | 吴维昌. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991. |
| WU Weichang. Manual of standard electrode potentials data[M]. Beijing: Science Press, 1991. | |
| 20 | 梁英教. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社, 1993. |
| LIANG Yingjiao. Manual of inorganic thermodynamics data[M]. Shenyang: Northeast University Press, 1993. | |
| 21 | 叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 2版. 北京: 冶金工业出版社, 2002. |
| YE Dalun, HU Jianhua. Manual of practical inorganic thermodynamics data[M]. 2nd ed. Beijing: Metallurgical Industry Press, 2002. | |
| 22 | 李照刚, 陈为亮, 张建军, 等. 响应曲面法优化软锰矿还原浸出的工艺[J]. 化学工程, 2018, 46(2): 72-78. |
| LI Zhaogang, CHEN Weiliang, ZHANG Jianjun, et al. Reductive leaching technology of pyrolusite optimized by response surface methodology[J]. Chemical Engineering (China), 2018, 46(2): 72-78. | |
| 23 | LI Qian, RAO Xuefei, XU Bin, et al. Extraction of manganese and zinc from their compound ore by reductive acid leaching[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(5): 1172-1179. |
| 24 | 张兆雪. 稀土荧光粉废料碱熔—水浸—还原酸浸回收稀土工艺研究[D]. 赣州: 江西理工大学, 2016. |
| ZHANG Zhaoxue. Process research on recovery of rare earth of waste rare earth fluorescent powders by alkali fusion-washing-reduction acid leaching[D]. Ganzhou: Jiangxi University of Science and Technology, 2016. | |
| 25 | LUO Jian, ZHANG Qiuhua, Javier GARCIA-MARTINEZ, et al. Adsorptive and acidic properties, reversible lattice oxygen evolution, and catalytic mechanism of cryptomelane-type manganese oxides as oxidation catalysts[J]. Journal of the American Chemical Society, 2008, 130(10): 3198-3207. |
| 26 | YE Bora, LEE Minwoo, JEONG Bora, et al. Partially reduced graphene oxide as a support of Mn-Ce/TiO2 catalyst for selective catalytic reduction of NO x with NH3 [J]. Catalysis Today, 2019, 328: 300-306. |
| 27 | LI Lulu, WU Yaohui, HOU Xueyan, et al. Investigation of two-phase intergrowth and coexistence in Mn-Ce-Ti-O catalysts for the selective catalytic reduction of NO with NH3: structure-activity relationship and reaction mechanism[J]. Industrial & Engineering Chemistry Research, 2019, 58(2): 849-862. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [3] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [4] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [8] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [9] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [10] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [11] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [12] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [13] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||