化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4213-4223.DOI: 10.16085/j.issn.1000-6613.2021-1977
CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响
张嘉琪1,2( ), 林丽娜1,2, 高文桂1,2,3(
), 林丽娜1,2, 高文桂1,2,3( ), 祝星2,3
), 祝星2,3
- 1.昆明理工大学冶金与能源工程学院,云南 昆明 650093
2.昆明理工大学省部共建复杂有色金属资源清洁利用国家重点实验室,云南 昆明 650093
3.冶金节能减排教育部工程研究中心,云南 昆明 650093
-
收稿日期:2021-09-06修回日期:2021-11-18出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:高文桂 -
作者简介:张嘉琪(1997—),女,硕士研究生,研究方向为能源催化。E-mail:382732828@qq.com 。 -
基金资助:省部共建复杂有色金属资源清洁利用国家重点实验室自主课题(CNMRCUTS2009);国家重点研发计划(2018YFB0605402-02)
Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol
ZHANG Jiaqi1,2( ), LIN Lina1,2, GAO Wengui1,2,3(
), LIN Lina1,2, GAO Wengui1,2,3( ), ZHU Xing2,3
), ZHU Xing2,3
- 1.Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2.State Key Laboratory for Clean Utilization of Complex Nonferrous Metal Resources, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
3.Engineering Research Center of Ministry of Education for Energy Conservation and Emission Reduction in Metallurgy, Kunming 650093, Yunnan, China
-
Received:2021-09-06Revised:2021-11-18Online:2022-08-25Published:2022-08-22 -
Contact:GAO Wengui
摘要:
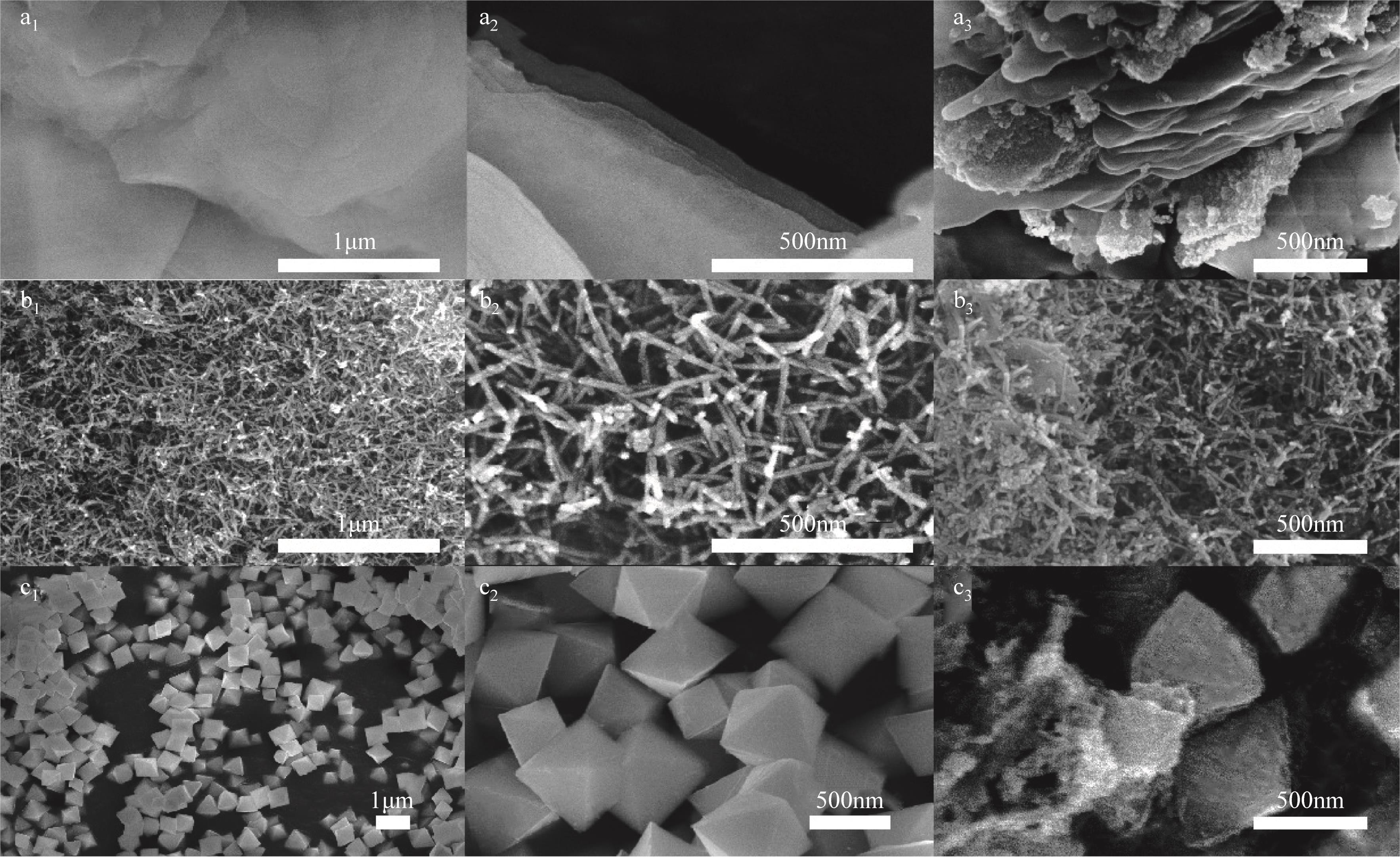
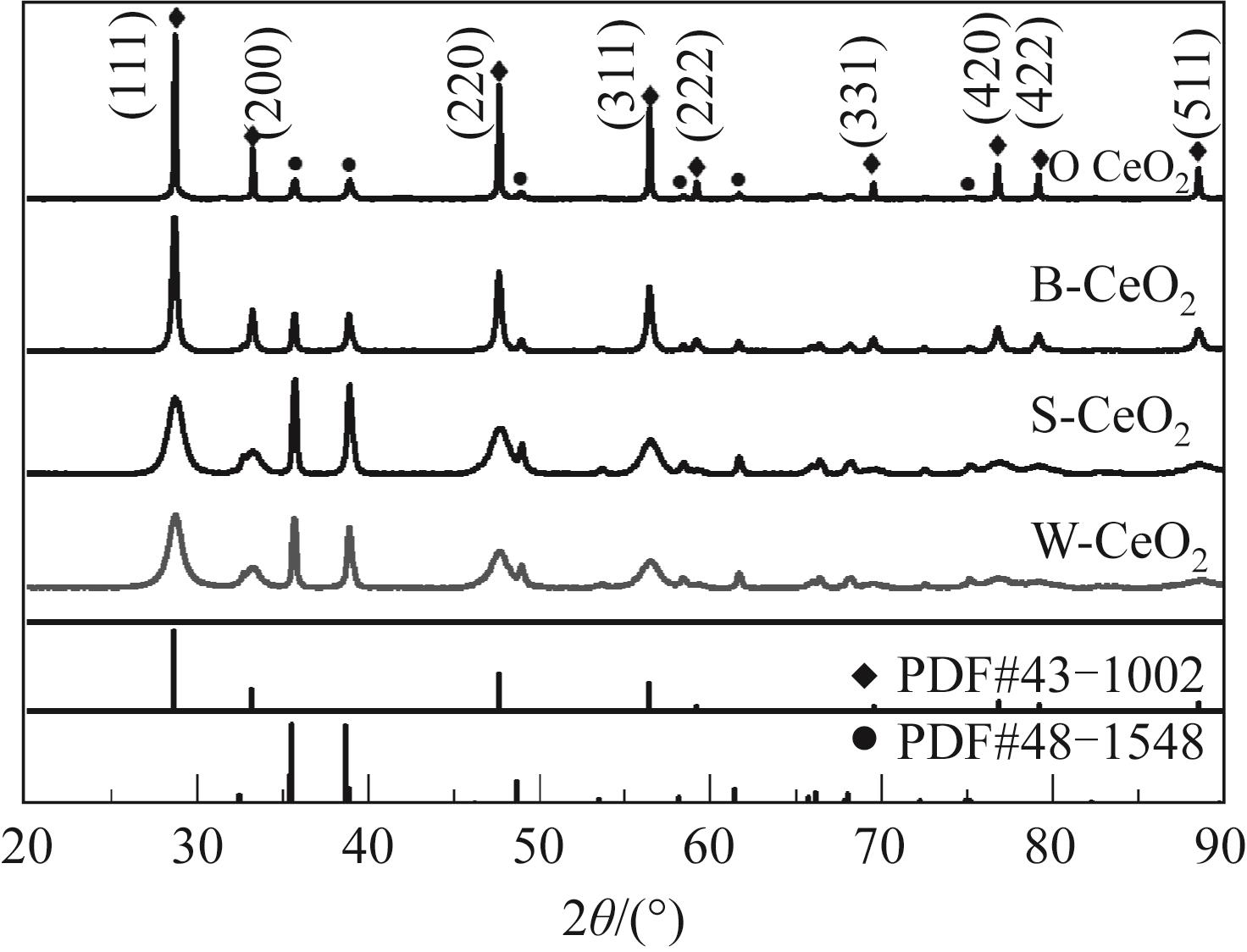
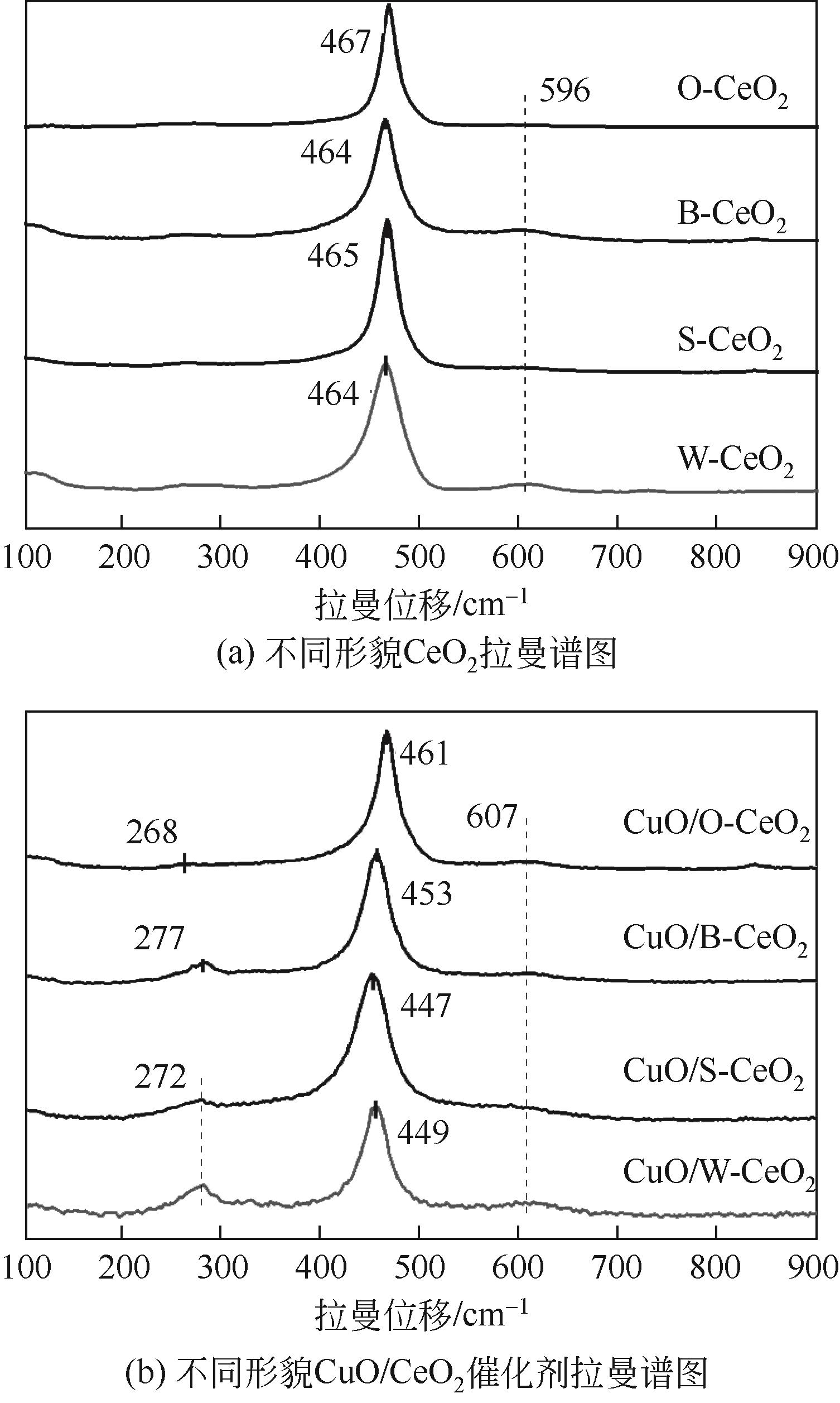
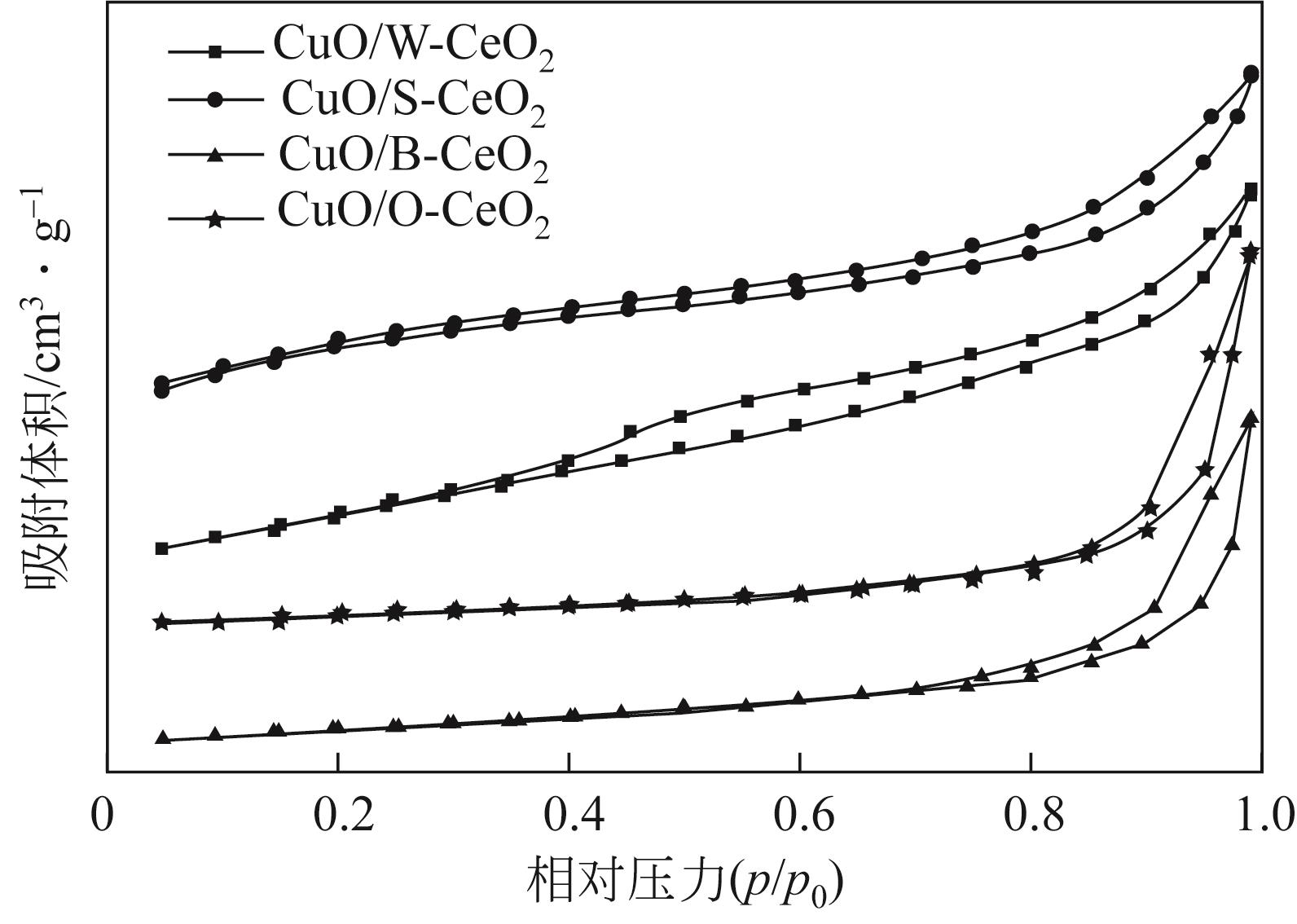
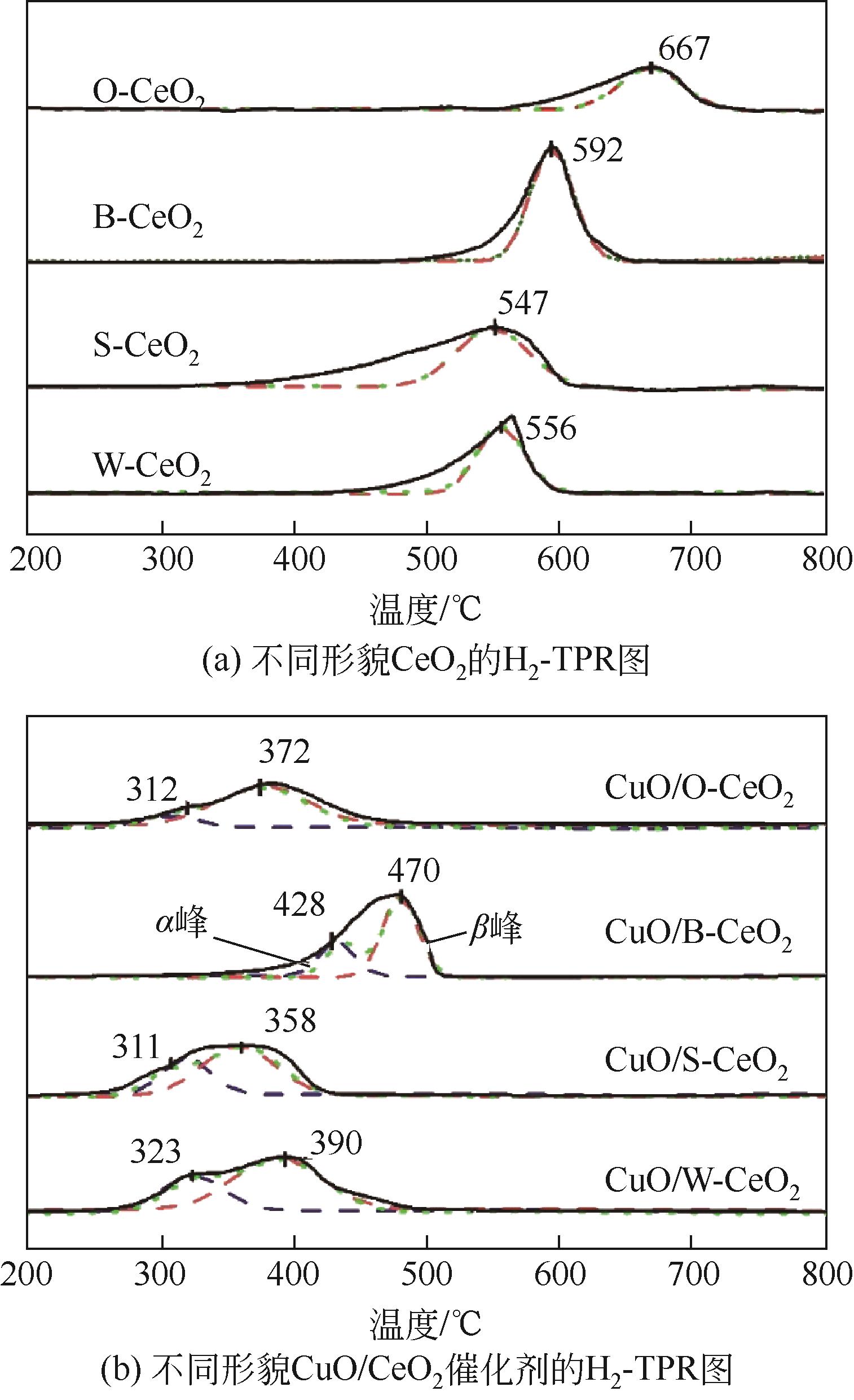
采用水热法制备CeO2纳米颗粒(W-CeO2)、CeO2纳米片(S-CeO2)、CeO2纳米棒(B-CeO2)及CeO2纳米八面体(O-CeO2),用浸渍法负载相同质量分数的铜形成CuO/CeO2催化剂。通过扫描电镜(SEM)、高分辨透射电子显微镜(TEM)、X射线衍射(XRD)、拉曼光谱(Raman)、自动吸附分析仪(BET)、H2程序升温还原(H2-TPR)、N2O滴定等表征技术对催化剂进行表征,并在可控温控压的固定床石英管反应器中对催化剂的催化性能进行评价。研究了不同形貌CuO/CeO2催化剂对CO2加氢制备甲醇的影响;结果表明,CuO/CeO2催化剂的催化活性存在明显的形貌依赖性,催化剂的暴露晶面、比表面积、表面碱性位点、表面氧缺陷的差异均会对CO2转化率、甲醇选择性和产率产生影响。其中,不同形貌CeO2优先暴露晶面的活性顺序为S-CeO2({100}+{110})>W-CeO2{100}>B-CeO2{111}≈O-CeO2{111},暴露晶面活性越高,催化剂表面氧缺陷越多,CuO-CeO2间相互作用越强,则催化活性越好。当为CuO/S-CeO2时,催化剂表面中碱性位点最多,催化剂比表面积为88.8m2/g,铜分散度为19.2%,CO2转化率为6.56%,甲醇选择性和收率为96.3%和0.063g/(gcat·h),催化活性最好,由活性评价试验得转化率由高到低依次为S-CeO2>B-CeO2>W-CeO2>O-CeO2,可知CeO2形貌差异会决定CuO/CeO2催化剂的物化性能和催化活性,从而提升对不同形貌CuO/CeO2催化剂催化CO2加氢制甲醇的基础认识。
中图分类号:
引用本文
张嘉琪, 林丽娜, 高文桂, 祝星. CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响[J]. 化工进展, 2022, 41(8): 4213-4223.
ZHANG Jiaqi, LIN Lina, GAO Wengui, ZHU Xing. Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4213-4223.
| CeO2载体 | SBET/m2·g-1 | ID/IF2g | CuO/CeO2催化剂 | SBET/m2·g-1 | ID/IF2g |
|---|---|---|---|---|---|
| W-CeO2 | 66.9 | 0.035 | CuO/W-CeO2 | 76.4 | 0.126 |
| S-CeO2 | 79.2 | 0.056 | CuO/S-CeO2 | 88.8 | 0.284 |
| B-CeO2 | 114.1 | 0.048 | CuO/B-CeO2 | 122.2 | 0.154 |
| O-CeO2 | 35.8 | 0.026 | CuO/O-CeO2 | 43.5 | 0.067 |
表1 载体及催化剂的比表面积、氧空位浓度比
| CeO2载体 | SBET/m2·g-1 | ID/IF2g | CuO/CeO2催化剂 | SBET/m2·g-1 | ID/IF2g |
|---|---|---|---|---|---|
| W-CeO2 | 66.9 | 0.035 | CuO/W-CeO2 | 76.4 | 0.126 |
| S-CeO2 | 79.2 | 0.056 | CuO/S-CeO2 | 88.8 | 0.284 |
| B-CeO2 | 114.1 | 0.048 | CuO/B-CeO2 | 122.2 | 0.154 |
| O-CeO2 | 35.8 | 0.026 | CuO/O-CeO2 | 43.5 | 0.067 |
| 催化剂 | α | β |  | |||
|---|---|---|---|---|---|---|
| 面积/mV·℃ | Tmax/℃ | 面积/mV·℃ | Tmax/℃ | |||
| CuO/W-CeO2 | 5200 | 323 | 16820 | 390 | 0.236 | |
| CuO/S-CeO2 | 3200 | 311 | 10256 | 358 | 0.238 | |
| CuO/B-CeO2 | 5395 | 428 | 18202 | 470 | 0.229 | |
| CuO/O-CeO2 | 3143 | 312 | 11824 | 372 | 0.210 | |
表2 不同温度下催化剂H2-TPR还原峰数据
| 催化剂 | α | β |  | |||
|---|---|---|---|---|---|---|
| 面积/mV·℃ | Tmax/℃ | 面积/mV·℃ | Tmax/℃ | |||
| CuO/W-CeO2 | 5200 | 323 | 16820 | 390 | 0.236 | |
| CuO/S-CeO2 | 3200 | 311 | 10256 | 358 | 0.238 | |
| CuO/B-CeO2 | 5395 | 428 | 18202 | 470 | 0.229 | |
| CuO/O-CeO2 | 3143 | 312 | 11824 | 372 | 0.210 | |
| 催化剂 | 弱碱性位点 | 中碱性位点 | 强碱性位点 | 总位点 |
|---|---|---|---|---|
| CuO/W-CeO2 | 1220 | 902 | 30 | 2152 |
| CuO/S-CeO2 | 1126 | 1835 | 35 | 2996 |
| CuO/B-CeO2 | 250 | 986 | 11 | 1247 |
| CuO/O-CeO2 | 55 | 579 | 24 | 658 |
表3 不同形貌CuO/CeO2催化剂碱性位点分布表
| 催化剂 | 弱碱性位点 | 中碱性位点 | 强碱性位点 | 总位点 |
|---|---|---|---|---|
| CuO/W-CeO2 | 1220 | 902 | 30 | 2152 |
| CuO/S-CeO2 | 1126 | 1835 | 35 | 2996 |
| CuO/B-CeO2 | 250 | 986 | 11 | 1247 |
| CuO/O-CeO2 | 55 | 579 | 24 | 658 |
| 催化剂 | SCO/% | DCu/% | |||
|---|---|---|---|---|---|
| CuO/W-CeO2 | 5.47 | 99.04 | 0.96 | 5.42 | 15.8 |
| CuO/S-CeO2 | 8.56 | 96.30 | 3.70 | 8.24 | 19.2 |
| CuO/B-CeO2 | 6.01 | 98.09 | 1.91 | 5.90 | 17.4 |
| CuO/O-CeO2 | 3.97 | 99.99 | 0.01 | 3.97 | 11.9 |
表4 不同形貌CuO/CeO2催化剂的CO2催化评价表
| 催化剂 | SCO/% | DCu/% | |||
|---|---|---|---|---|---|
| CuO/W-CeO2 | 5.47 | 99.04 | 0.96 | 5.42 | 15.8 |
| CuO/S-CeO2 | 8.56 | 96.30 | 3.70 | 8.24 | 19.2 |
| CuO/B-CeO2 | 6.01 | 98.09 | 1.91 | 5.90 | 17.4 |
| CuO/O-CeO2 | 3.97 | 99.99 | 0.01 | 3.97 | 11.9 |
| 1 | KOPP R E, KEMP A C, BITTERMANN K, et al. Temperature-driven global sea-level variability in the Common Era[J]. PNAS, 2016, 113(11): E1434-E1441. |
| 2 | KEMP A C, HORTON B P, DONNELLY J P, et al. Climate related sea-level variations over the past two millennia[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(27): 11017-11022. |
| 3 | 郝静, 宛霞. 过去五年史上最热 《2019年全球气候状况声明》发布[J]. 科学大观园, 2020(8): 18-19. |
| HAO Jing, WAN Xia. Hottest on record for the past five years “State of the Global Climate 2019 Statement” released[J]. Grand Garden of Science, 2020(8): 18-19. | |
| 4 | 惠婕. 应对气候变化,拯救我们的星球[J]. 世界环境, 2021(1): 14-15. |
| HUI Jie. Address climate change and save our planet[J]. World Environment, 2021(1): 14-15. | |
| 5 | 赵信国, 刘广绪. 海洋酸化对海洋无脊椎动物的影响研究进展[J]. 生态学报, 2015, 35(7): 2388-2398. |
| ZHAO Xinguo, LIU Guangxu. Advances in the effects of ocean acidification on marine invertebrates[J]. Acta Ecologica Sinica, 2015, 35(7): 2388-2398. | |
| 6 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488 (7411): 294-303. |
| 7 | 王克, 刘芳名, 尹明健, 等. 1.5℃温升目标下中国碳排放路径研究[J]. 气候变化研究进展, 2021, 17(1): 7-17. |
| WANG Ke, LIU Fangming, YIN Mingjian, et al. Research on China’s carbon emissions pathway under the 1.5℃ target[J]. Climate Change Research, 2021, 17(1): 7-17. | |
| 8 | RAMACHANDRIYA K D, KUNDIYANA D K, WILKINS M R, et al. Carbon dioxide conversion to fuels and chemicals using a hybrid green process[J]. Applied Energy, 2013, 112: 289-299. |
| 9 | 徐敏杰, 朱明辉, 陈天元, 等. CO2高值化利用:CO2加氢制甲醇催化剂研究进展[J]. 化工进展, 2021, 40(2): 565-576. |
| XU Minjie, ZHU Minghui, CHEN Tianyuan, et al. High value utilization of CO2: research progress of catalyst for hydrogenation of CO2 to methanol[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 565-576. | |
| 10 | ACRES Gary. Beyond oil and gas: the methanol econnmy[J].Chemistry and Industry, 2006(14): 26-27. |
| 11 | AWAD O I, MAMAT R, IBRAHIM T K, et al. Overview of the oxygenated fuels in spark ignition engine: environmental and performance[J]. Renewable and Sustainable Energy Reviews, 2018, 91: 394-408. |
| 12 | ALI K A, ABDULLAH A Z, MOHAMED A R. Recent development in catalytic technologies for methanol synthesis from renewable sources: a critical review[J]. Renewable and Sustainable Energy Reviews, 2015, 44: 508-518. |
| 13 | KOTHANDARAMAN J, KAR S, GOEPPERT A, et al. Advances in homogeneous catalysis for low temperature methanol reforming in the context of the methanol economy[J]. Topics in Catalysis, 2018, 61(7/8): 542-559. |
| 14 | 贾晨喜, 邵敬爱, 白小薇, 等. 二氧化碳加氢制甲醇铜基催化剂性能的研究进展[J]. 化工进展, 2020, 39(9): 3658-3668. |
| JIA Chenxi, SHAO Jingai, BAI Xiaowei, et al. Review on Cu-based catalysts for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3658-3668. | |
| 15 | HAYWARD J S, SMITH P J, KONDRAT S A, et al. The effects of secondary oxides on copper-based catalysts for green methanol synthesis[J]. ChemCatChem, 2017, 9(9): 1655-1662. |
| 16 | TURSUNOV O, KUSTOV L, TILYABAEV Z. Methanol synthesis from the catalytic hydrogenation of CO2 over CuO-ZnO supported on aluminum and silicon oxides[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 78: 416-422. |
| 17 | HU Nian, LI Xiaoyun, LIU Siming, et al. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation[J]. Chinese Journal of Catalysis, 2020, 41(7): 1081-1090. |
| 18 | YANG Bin, DENG Wei, GUO Limin, et al. Copper-ceria solid solution with improved catalytic activity for hydrogenation of CO2 to CH3OH[J]. Chinese Journal of Catalysis, 2020, 41(9): 1348-1359. |
| 19 | RAUDASKOSKI R, NIEMELÄ M V, KEISKI R L. The effect of ageing time on co-precipitated Cu/ZnO/ZrO2 catalysts used in methanol synthesis from CO2 and H2 [J]. Topics in Catalysis, 2007, 45(1/2/3/4): 57-60. |
| 20 | GRACIANI J, MUDIYANSELAGE K, XU Fang, et al. Catalysis. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO₂[J]. Science, 2014, 345(6196): 546-550. |
| 21 | OUYANG Bi, TAN Weiling, LIU Bing. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation[J]. Catalysis Communications, 2017, 95: 36-39. |
| 22 | KASPAR J. Catalysis by ceria and related materials[M]. Italy: IMPERIAL, 2015: 221-252. |
| 23 | BADWAL S P S, FINI D, CIACCHI F T, et al. Structural and microstructural stability of ceria-gadolinia electrolyte exposed to reducing environments of high temperature fuel cells[J]. Journal of Materials Chemistry A, 2013, 1(36): 10768. |
| 24 | ZHANG Y W, SI R, LIAO C S, et al. Facile alcohothermal synthesis, size-dependent ultraviolet absorption, and enhanced CO conversion activity of ceria nanocrystals[J]. The Journal of Physical Chemistry B, 2003, 107(37): 10159-10167. |
| 25 | ZHANG Jingcai, YANG Hongxiao, WANG Shuping, et al. Mesoporous CeO2 nanoparticles assembled by hollow nanostructures: formation mechanism and enhanced catalytic properties[J]. CrystEngComm, 2014, 16(37): 8777-8785. |
| 26 | CHEN Shilong, XIONG Feng, HUANG Weixin. Surface chemistry and catalysis of oxide model catalysts from single crystals to nanocrystals[J]. Surface Science Reports, 2019, 74(4): 100471. |
| 27 | POLO-GARZON F, BAO Zhenghong, ZHANG Xuanyu, et al. Surface reconstructions of metal oxides and the consequences on catalytic chemistry[J]. ACS Catalysis, 2019, 9(6): 5692-5707. |
| 28 | HUANG Weixin, GAO Yuxian. ChemInform abstract: morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals[J]. Catalysis Science & Technology, 2014, 4(11): 3772-3784. |
| 29 | On the question of speed of growth and dissolution of crystal surfaces[J]. Zeitschrift Für Kristallographie-Crystalline Materials, 1901, 34(1/2/3/4/5/6): 449-530. |
| 30 | ZHANG Dengsong, DU Xianjun, SHI Liyi, et al. Shape-controlled synthesis and catalytic application of ceria nanomaterials[J]. Dalton Transactions, 2012,41(48): 14455-14475. |
| 31 | 位忠斌, 崔育倩, 郭培志, 等. 二氧化铈八面体的水热合成与表征[J]. 无机化学学报, 2011, 27(7): 1399-1404. |
| WEI Zhongbin, CUI Yuqian, GUO Peizhi, et al. Hydrothermal synthesis and characterization of ceria octahedrons[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(7): 1399-1404. | |
| 32 | TANA, ZHANG Milin, LI Juan, et al. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles[J]. Catalysis Today, 2009, 148(1/2): 179-183. |
| 33 | WANG Xue, JIANG Zhiyuan, ZHENG Binjie, et al. Synthesis and shape-dependent catalytic properties of CeO2 nanocubes and truncated octahedra[J]. CrystEngComm, 2012, 14(22): 7579. |
| 34 | SENANAYAKE S D, RAMÍREZ P J, WALUYO I, et al. Hydrogenation of CO2 to methanol on CeO x /Cu(111) and ZnO/Cu(111) catalysts: role of the metal-oxide interface and importance of Ce3+ sites[J]. The Journal of Physical Chemistry C, 2016, 120(3): 1778-1784. |
| 35 | GAO Yuxian, ZHANG Zhenhua, LI Zhaorui, et al. Understanding morphology-dependent CuO x -CeO2 interactions from the very beginning[J]. Chinese Journal of Catalysis, 2020, 41(6): 1006-1016. |
| 36 | HUANG Weixin. Oxide nanocrystal model catalysts[J]. Accounts of Chemical Research, 2016, 49(3): 520-527. |
| 37 | LUO Mengfei, SONG Yupeng, LU Jiqing, et al. Identification of CuO species in high surface area CuO-CeO2 catalysts and their catalytic activities for CO oxidation[J]. The Journal of Physical Chemistry C, 2007, 111(34): 12686-12692. |
| 38 | NOLAN M, GRIGOLEIT S, SAYLE D C, et al. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria[J]. Surface Science, 2005, 576(1/2/3): 217-229. |
| 39 | GAO Yuxian, WANG Wendong, CHANG Sujie, et al. Morphology effect of CeO2 support in the preparation, metal-support interaction, and catalytic performance of Pt/CeO2 catalysts[J]. ChemCatChem, 2013, 5(12): 3610-3620. |
| 40 | GRACIANI J, VIDAL A B, RODRIGUEZ J A, et al. Unraveling the nature of the oxide-metal interaction in ceria-based noble metal inverse catalysts[J]. The Journal of Physical Chemistry C, 2014, 118(46): 26931-26938. |
| 41 | CÁMARA A L, BERA P, CONESA J C, et al. Characterization of active sites/entities and redox/catalytic correlations in copper-ceria-based catalysts for preferential oxidation of CO in H2-rich streams[J]. Catalysts, 2013, 3(2): 378-400. |
| 42 | LI L, ZHAN Y Y, CHEN C Q, et al. Effect of CeO2 support prepared with different methods on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction[J]. Journal of Physical Chemistry, 2009, 25(7): 1397-1404. |
| 43 | KOSTIĆ R, AŠKRABIĆ S, DOHČEVIĆ-MITROVIĆ Z, et al. Low-frequency Raman scattering from CeO2 nanoparticles[J]. Applied Physics A, 2008, 90(4): 679-683. |
| 44 | HE Chi, YU Yanke, YUE Lin, et al. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeO x catalysts synthesized via a simple self-precipitation protocol[J]. Applied Catalysis B: Environmental, 2014, 147: 156-166. |
| 45 | WU Xinping, GONG Xueqing. Clustering of oxygen vacancies at CeO2(111): critical role of hydroxyls[J]. Physical Review Letters, 2016, 116(8): 086102. |
| 46 | MCBRIDE J R, HASS K C, POINDEXTER B D, et al. Raman and X-ray studies of Ce1- x RE x O2- y, where RE=La, Pr, Nd, Eu, Gd, and Tb[J]. Journal of Applied Physics, 1994, 76(4): 2435-2441. |
| 47 | WEBER W H, HASS K C, MCBRIDE J R. Raman study of CeO2: second-order scattering, lattice dynamics, and particle-size effects[J]. Physical Review B, Condensed Matter, 1993, 48(1): 178-185. |
| 48 | 王禹皓. Cu-ZnO-ZrO2界面相互作用及其催化CO2加氢选择性合成甲醇的研究[D]. 昆明: 昆明理工大学, 2018. |
| WANG Yuhao. Cu-ZnO-ZrO2 interfacial interaction and its catalytic hydrogenation of CO2 for selective synthesis of methanol[D]. Kunming: Kunming University of Science and Technology, 2018. | |
| 49 | WANG Xianqin, RODRIGUEZ J A, HANSON J C, et al. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: complex interaction between metallic copper and oxygen vacancies of ceria[J]. The Journal of Physical Chemistry B, 2006, 110(1): 428-434. |
| 50 | YANG Zhijie, HAN Dongqing, MA Donglin, et al. Fabrication of monodisperse CeO2 hollow spheres assembled by nano-octahedra[J]. Crystal Growth & Design, 2010, 10(1): 291-295. |
| 51 | PALOMINO R M, RAMÍREZ P J, LIU Zongyuan, et al. Hydrogenation of CO2 on ZnO/Cu(100) and ZnO/Cu(111) catalysts: role of copper structure and metal-oxide interface in methanol synthesis[J]. The Journal of Physical Chemistry B, 2018, 122(2): 794-800. |
| 52 | SKORODUMOVA N V, BAUDIN M, HERMANSSON K. Surface properties of CeO2 from first principles[J]. Physical Review B, 2004, 69(7): 075401. |
| 53 | SI Rui, RAITANO J, YI Nan, et al. Structure sensitivity of the low-temperature water-gas shift reaction on Cu-CeO2 catalysts[J]. Catalysis Today, 2012, 180(1): 68-80. |
| 54 | KAMMERT J, MOON J, WU Zili. A review of the interactions between ceria and H2 and the applications to selective hydrogenation of alkynes[J]. Chinese Journal of Catalysis, 2020, 41(6): 901-914. |
| 55 | MARTÍNEZ-ARIAS A, GAMARRA D, FERNÁNDEZ-GARCÍA M, et al. Comparative study on redox properties of nanosized CeO2 and CuO/CeO2 under CO/O2 [J]. Journal of Catalysis, 2006, 240(1): 1-7. |
| 56 | ZABILSKIY M, DJINOVIĆ P, TCHERNYCHOVA E, et al. Nanoshaped CuO/CeO2 materials: effect of the exposed ceria surfaces on catalytic activity in N2O decomposition reaction[J]. ACS Catalysis, 2015, 5(9): 5357-5365. |
| 57 | MENON U, POELMAN H, BLIZNUK V, et al. Nature of the active sites for the total oxidation of toluene by CuOCeO2/Al2O3 [J]. Journal of Catalysis, 2012, 295: 91-103. |
| 58 | WANG Shuxian, GUO Ruitang, PAN Weiguo, et al. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals: TPD and DRIFT studies[J]. Catalysis Communications, 2017, 89: 143-147. |
| 59 | GIORDANO F, TROVARELLI A, DE LEITENBURG C, et al. Some insight into the effects of oxygen diffusion in the reduction kinetics of ceria[J]. Industrial & Engineering Chemistry Research, 2001, 40(22): 4828-4835. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [11] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [14] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [15] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||