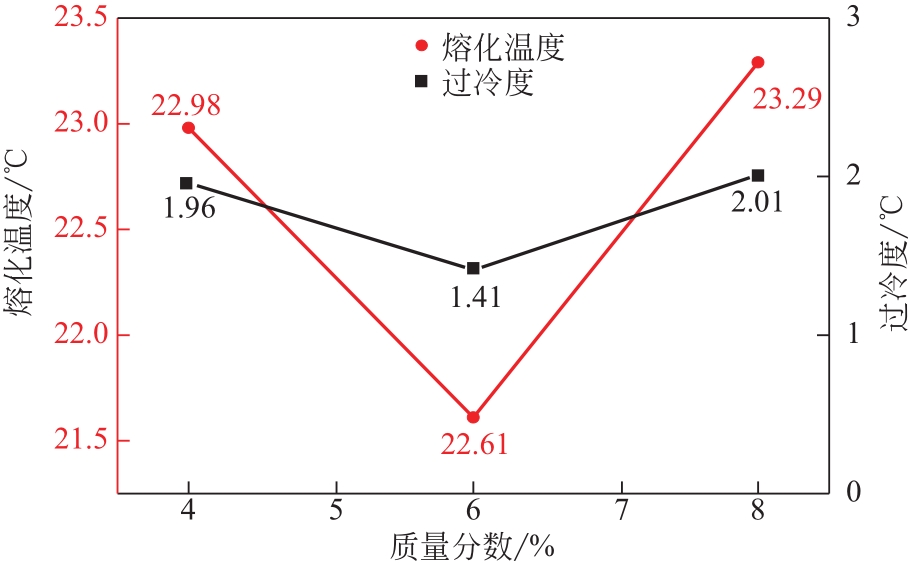
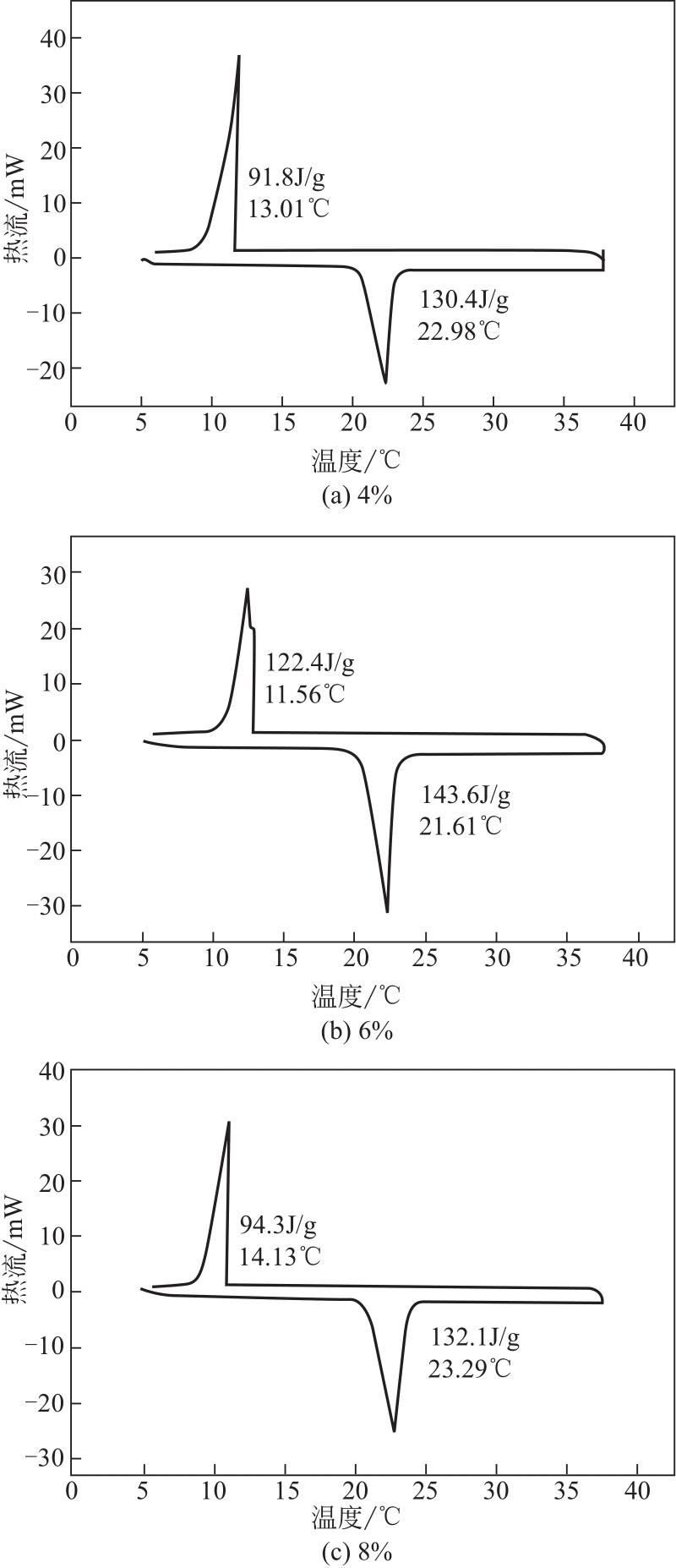
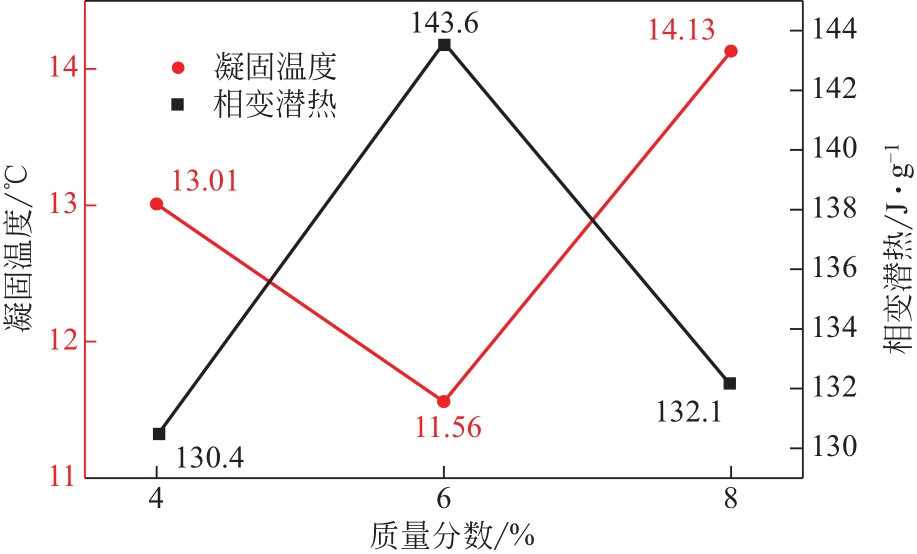
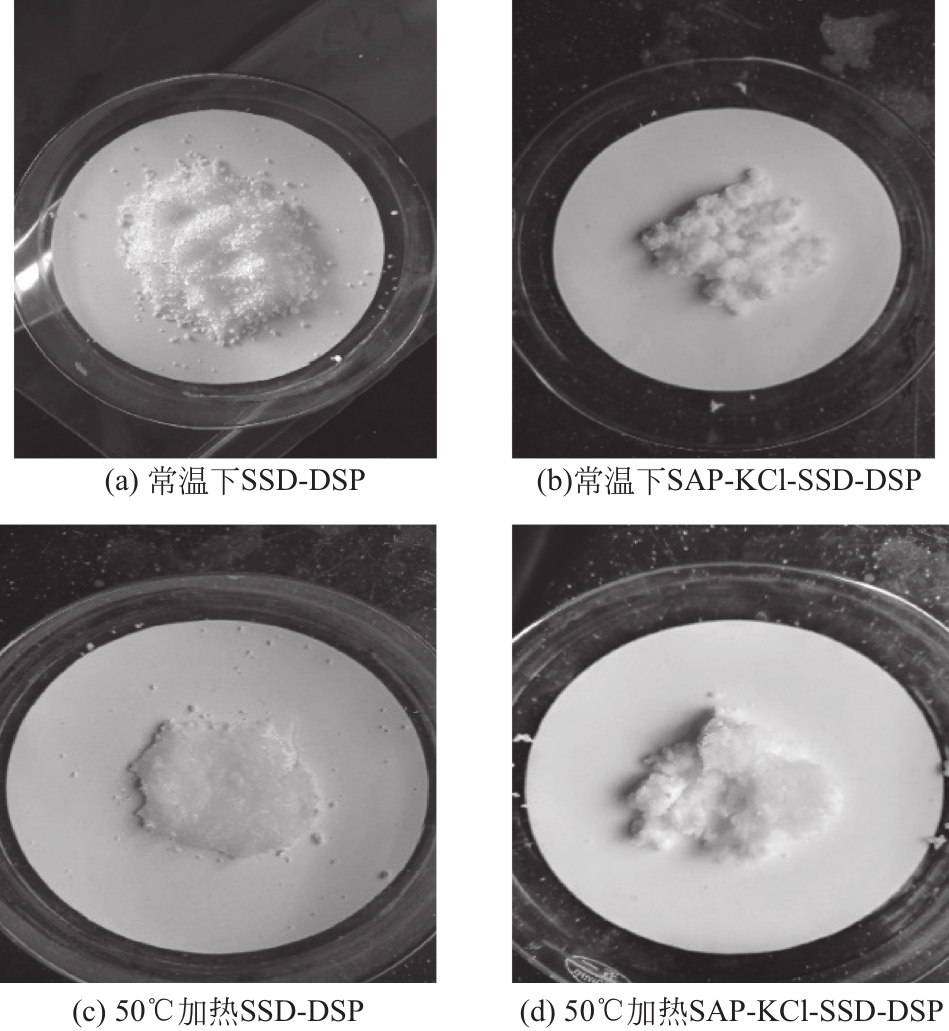
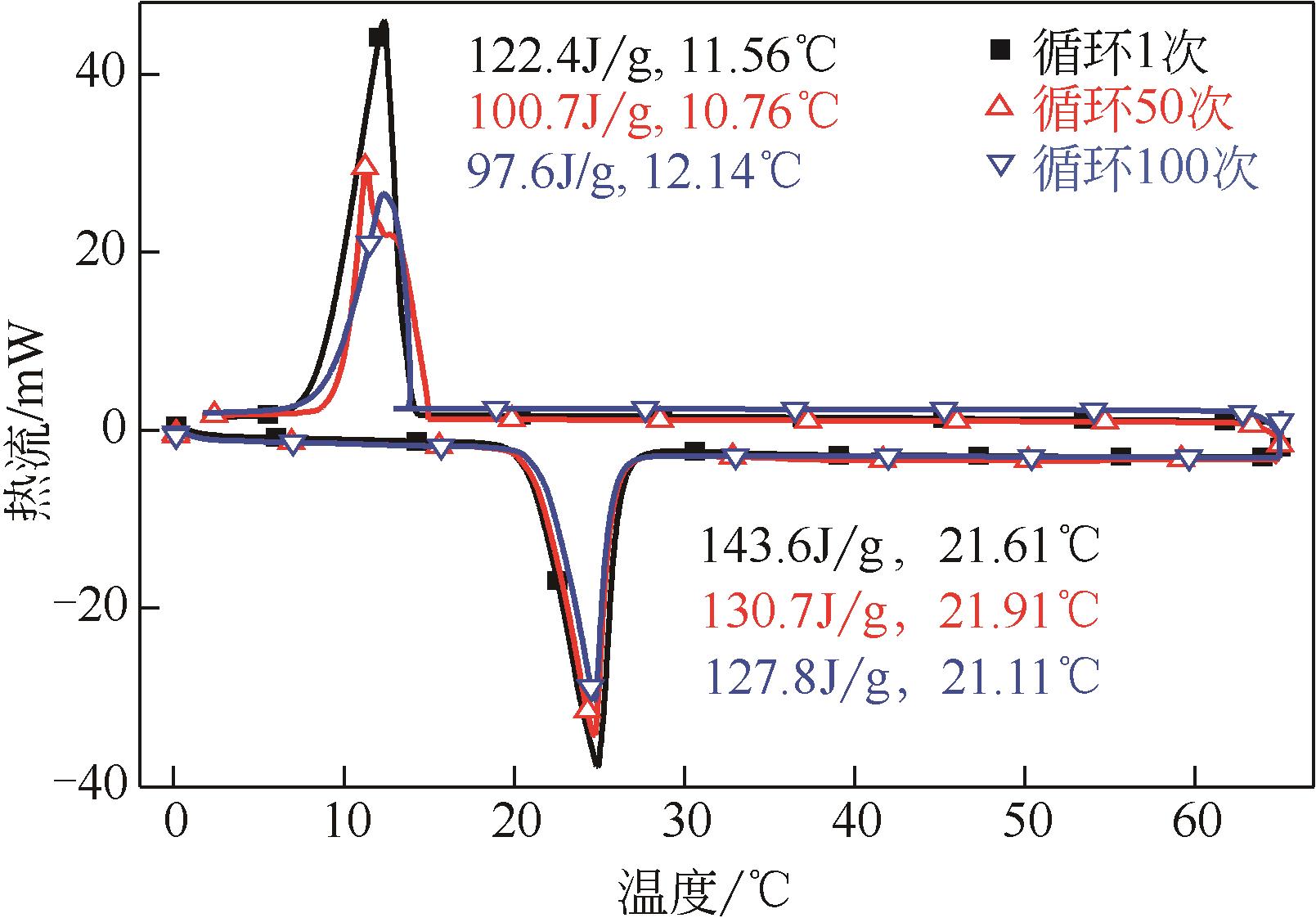
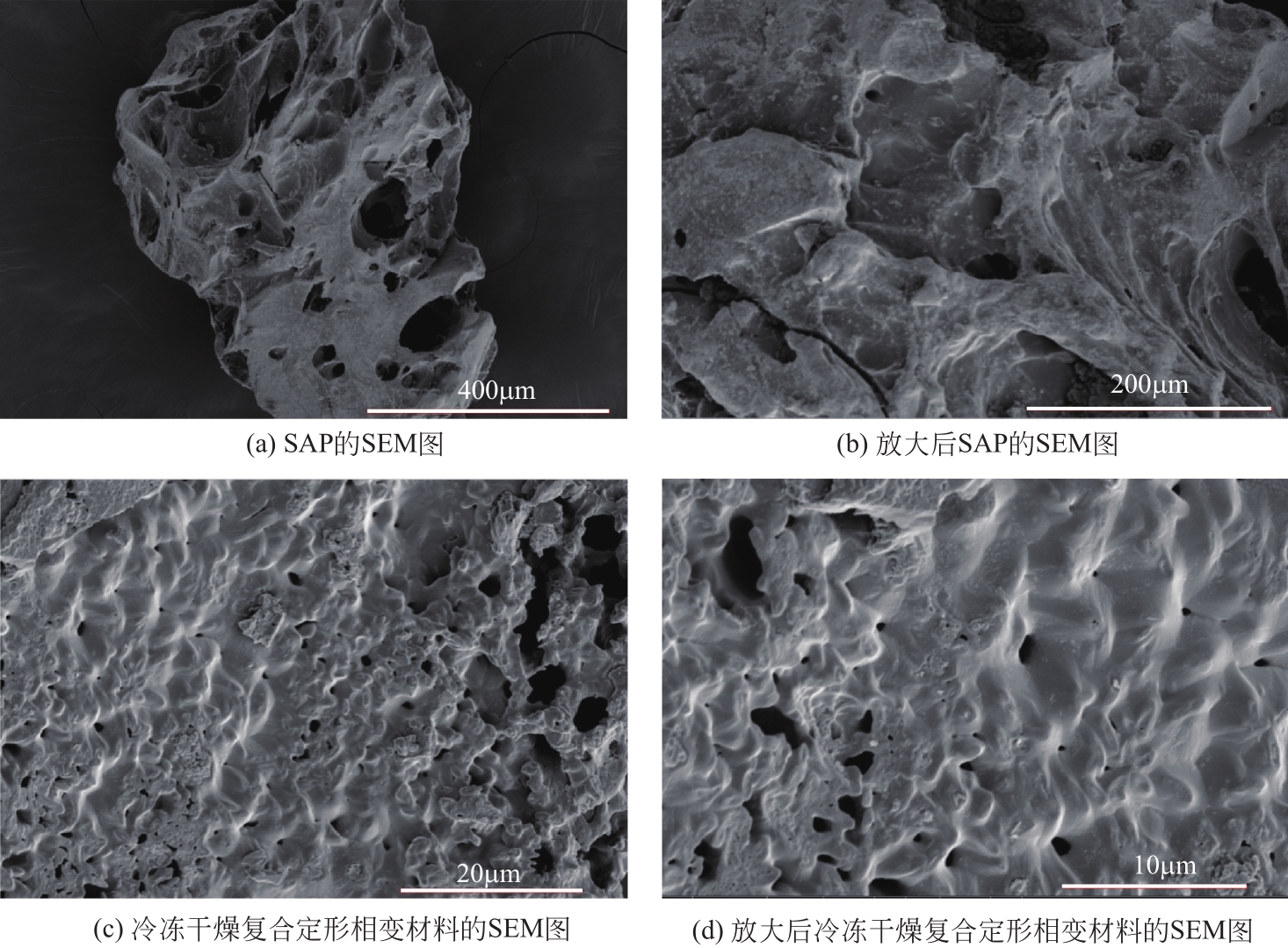
| 1 |
陈灿文, 程军, 胡芬飞. 蓄热材料概述及其应用[J]. 广州化工, 2011, 39(14): 15-17.
|
|
CHEN Canwen, CHENG Jun, HU Fenfei. Research and application of the thermal energy storage material[J]. Guangzhou Chemical Industry, 2011, 39(14): 15-17.
|
| 2 |
李芃, 周沛, 仇中柱. 太阳能蓄热技术研究进展[J]. 制冷空调与电力机械, 2011, 32(1): 73-77.
|
|
LI Peng, ZHOU Pei, QIU Zhongzhu. Research and development of solar energy thermal storage technology[J]. Refrigeration Air Condition & Electric Power Machinery, 2011, 32(1): 73-77.
|
| 3 |
武国峰, 徐跃定, 常志州, 等. 秸秆块墙体日光温室保温蓄热性能分析[J]. 农业环境科学学报, 2015, 34(12): 2402-2409.
|
|
WU Guofeng, XU Yueding, CHANG Zhizhou, et al. Heat-insulating performance of straw-bale wall solar greenhouse[J]. Journal of AgroEnvironment Science, 2015, 34(12): 2402-2409.
|
| 4 |
沈澄, 徐玲玲, 李文浩. 相变储能材料在建筑节能领域的研究进展[J]. 材料导报, 2015, 29(5): 100-104.
|
|
SHEN Cheng, XU Lingling, LI Wenhao. Research progress of phase change energy storage materials in building energy efficiency[J]. Materials Review, 2015, 29(5): 100-104.
|
| 5 |
张森景, 李青达, 张文杰, 等. 温室墙体用蓄热新材料的发展[J]. 陶瓷学报, 2018, 39 (5): 529-538.
|
|
ZHANG Senjing, LI Qingda, ZHANG Wenjie, et al. The development of new heat storage materials for greenhouse walls[J]. Journal of Ceramics, 2018, 39(5): 529-538.
|
| 6 |
铁生年, 蒋自鹏. 相变储能材料在温室大棚中应用研究进展[J]. 硅酸盐通报, 2015, 34(7): 1933-1940.
|
|
Shengnian TIE, JIANG Zipeng. Progress of phase change heat storage material and it’s application in greenhouses[J]. Bulletin of the Chinese Ceramic Society, 2015, 34(7): 1933-1940.
|
| 7 |
徐燕, 刘伟, 于观成, 等. 十水硫酸钠储热材料在农业大棚中的应用[J]. 郑州轻工业学院学报(自然科学版), 2011, 26(2): 98-100.
|
|
XU Yan, LIU Wei, YU Guancheng, et al. Application of Na2SO4·10H2O thermal energy storage materials in the vegetable greenhouse[J]. Journal of Zhengzhou Institute of Light Industry (Natural Science), 2011, 26(2): 98-100.
|
| 8 |
汪翔, 章学来, 华维三, 等. Na2HPO4·12H2O相变储能复合材料制备及热物性[J]. 化工进展, 2019, 38(12): 5457-5464.
|
|
WANG Xiang, ZHANG Xuelai, HUA Weisan, et al. Preparation and thermal properties of Na2HPO4·12H2O composite phase change material for thermal energy storage[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5457-5464.
|
| 9 |
杨小龙, 王宏丽, 许红军, 等. 磷酸氢二钠相变墙板在温室中的应用效果[J]. 上海交通大学学报(农业科学版), 2014, 32(4): 88-94.
|
|
YANG Xiaolong, WANG Hongli, XU Hongjun, et al. Performance of phase change thermal storage wallboard of disodium hydrogen phosphate dodecahydrate in solar greenhouses[J]. Journal of Shanghai Jiaotong University(Agricultural Science), 2014, 32(4): 88-94.
|
| 10 |
苑坤杰, 张正国, 方晓明, 等. 水合无机盐及其复合相变储热材料的研究进展[J]. 化工进展, 2016, 35(6): 1820-1826.
|
|
YUAN Kunjie, ZHANG Zhengguo, FANG Xiaoming, et al. Research progress of inorganic hydrated salts and their phase change heat storage composites[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1820-1826.
|
| 11 |
蒋自鹏, 铁生年. 芒硝基相变材料性能及其在简易温室中升温效果试验[J]. 农业工程学报, 2016, 32(20): 209-216.
|
|
JIANG Zipeng, Shengnian TIE. Property and heat storage performances of Glauber's salt-based phase change materials for solar gree house in Qinghai-Tibet plateau[J]. Transactions of the Chinese Society of Agricultural Engineering, 2016, 32(20): 209-216.
|
| 12 |
鄢瑛, 林小花, 张会平. 十二水磷酸氢二钠相变材料的改性研究[J]. 高校化学工程学报, 2011, 25(6): 1068-1072.
|
|
YAN Ying, LIN Xiaohua, ZHANG Huiping. Modification of disodium hydrogen phosphate dodecahydrate phase change material[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(6): 1068-1072.
|
| 13 |
陈跃, 纪珺, 徐笑锋, 等. 十二水磷酸氢二钠纳米复合相变材料的过冷特性[J]. 化工进展, 2018, 37(7): 2734-2739.
|
|
CHEN Yue, JI Jun, XU Xiaofeng, et al. Supercooling characteristics of disodium hydrogen phosphate nano-composites phase change materials[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2734-2739.
|
| 14 |
LAN X Z, TAN Z C, SHI Q, et al. A novel gelling method for stabilization of phase change material Na2HPO4·12H2O with sodium alginate grafted sodium acrylate[J]. Thermochimica Acta, 2007, 463(1): 18-20.
|
| 15 |
李凤艳, 王鹏, 袁亚东, 等. 相变温度为室温的Na2SO4·10H2O相变储热材料的制备研究[J]. 合成材料老化与应用, 2015, 44(1): 39-41.
|
|
LI Fengyan, WANG Peng, YUAN Yadong, et al. The preparation of phase change material Na2SO4·10H2O with the phase transition temperature at room temperature[J]. Synthetic Materials Aging and Application, 2015, 44(1): 39-41.
|
| 16 |
许红军, 王宏丽, 马江伟, 等. 温室中磷酸氢二钠蓄热体系的制备与性能分析[J]. 农机化研究, 2013, 35(6): 151-154.
|
|
XU Hongjun, WANG Hongli, MA Jiangwei, et al. Preparation of disodium hydrogen phosphate heat storage system in greenhouse[J]. Journal of Agricultural Mechanization Research, 2013, 35(6): 151-154.
|
| 17 |
张森景. Al2O3/Na2SO4·10H2O复合定形相变蓄热材料的制备[D]. 杨凌: 西北农林科技大学, 2020.
|
|
ZHANG Senjing. Preparation of Al2O3/Na2SO4·10H2O composite phase change heat storage materia[D]. Yangling: Northwest A&F University, 2020.
|
| 18 |
张桂芝, 雨田. Na2HPO4·12H2O与聚丙烯酸钠复合相变材料的制备及性能[J]. 科技创新与应用, 2019(16): 50-52.
|
|
ZHANG Guizhi, YU Tian. Preparation and properties of Na2HPO4·12H2O and sodium polyacrylate composite phase change material[J]. Technology Innovation and Application, 2019(16): 50-52.
|
| 19 |
WU Y, WANG T. Preparation and characterization of hydrated salts/silica composite as shape-stabilized phase change material via sol-gel process[J]. Thermochimica Acta, 2014, 591: 10-15.
|
 ), WU Wei, LI Songze, ZHANG Zijian, YI Xuemei(
), WU Wei, LI Songze, ZHANG Zijian, YI Xuemei( )
)