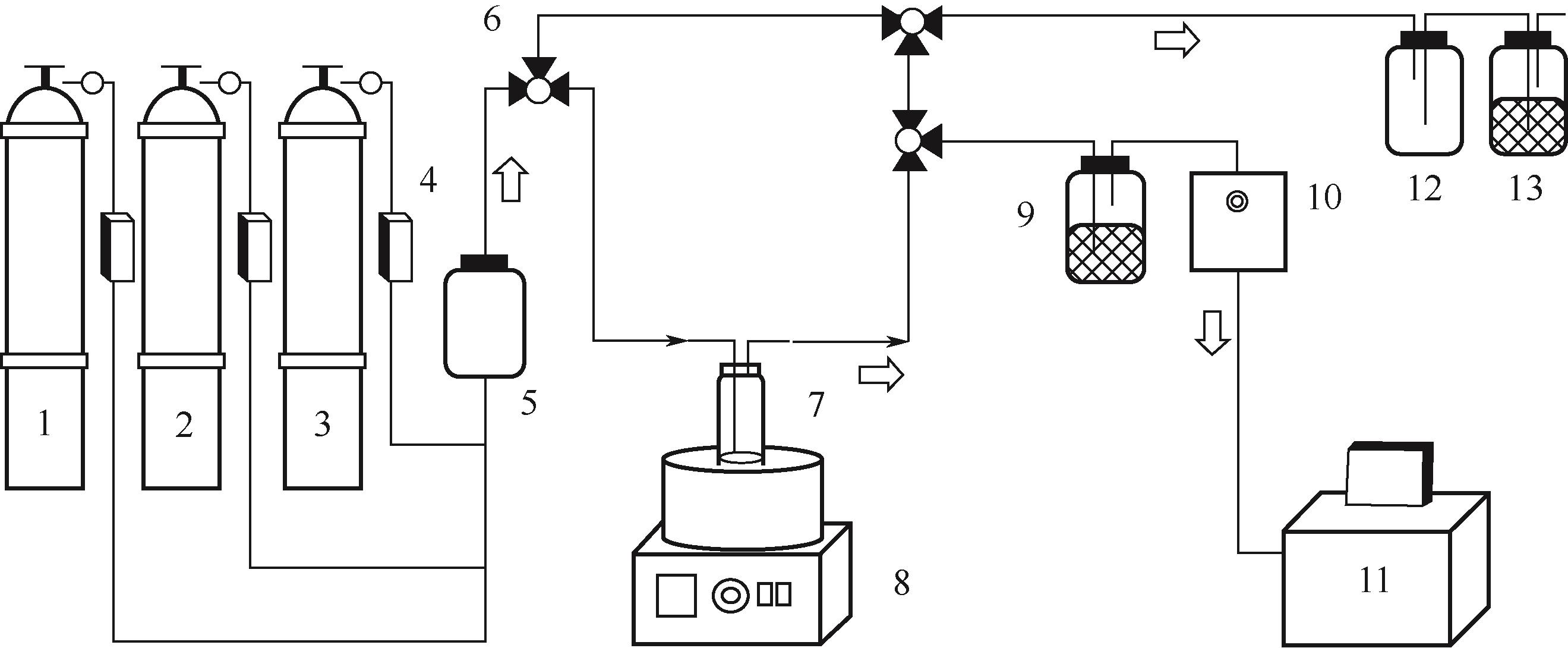
化工进展 ›› 2022, Vol. 41 ›› Issue (2): 1063-1072.DOI: 10.16085/j.issn.1000-6613.2021-0412
电解锰渣浆液烟气脱硫性能及机制
聂紫萌( ), 杨点, 熊玉路, 李英杰(
), 杨点, 熊玉路, 李英杰( ), 田森林, 宁平
), 田森林, 宁平
- 昆明理工大学环境科学与工程学院,云南 昆明 650500
-
收稿日期:2021-03-01修回日期:2021-07-02出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:李英杰 -
作者简介:聂紫萌(1997—),女,硕士研究生,主要研究方向为工业含硫烟气净化技术。E-mail:niezimeng@stu.kust.edu.cn 。 -
基金资助:国家重点研发计划(2018YFC0213405)
Performance and mechanism of electrolytic manganese slag slurry for flue gas desulfurization
NIE Zimeng( ), YANG Dian, XIONG Yulu, LI Yingjie(
), YANG Dian, XIONG Yulu, LI Yingjie( ), TIAN Senlin, NING Ping
), TIAN Senlin, NING Ping
- College of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2021-03-01Revised:2021-07-02Online:2022-02-05Published:2022-02-23 -
Contact:LI Yingjie
摘要:
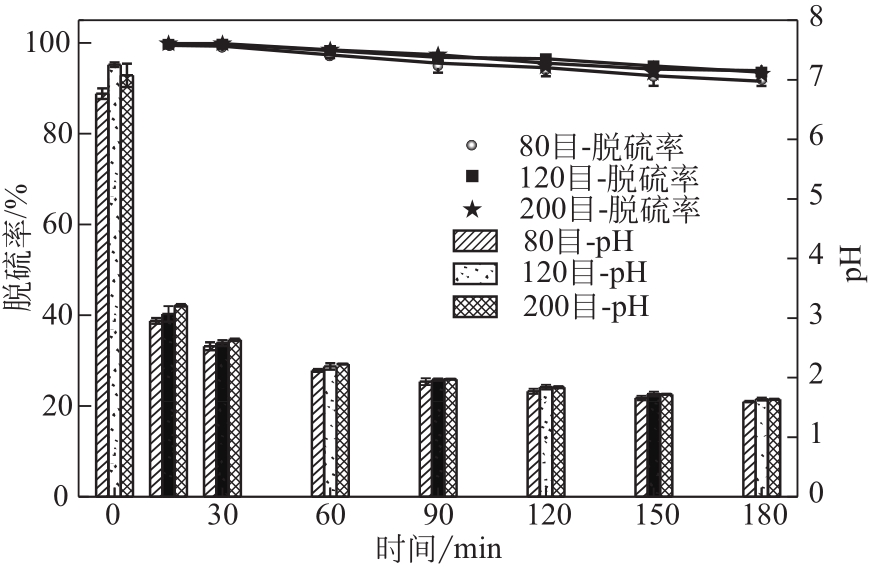
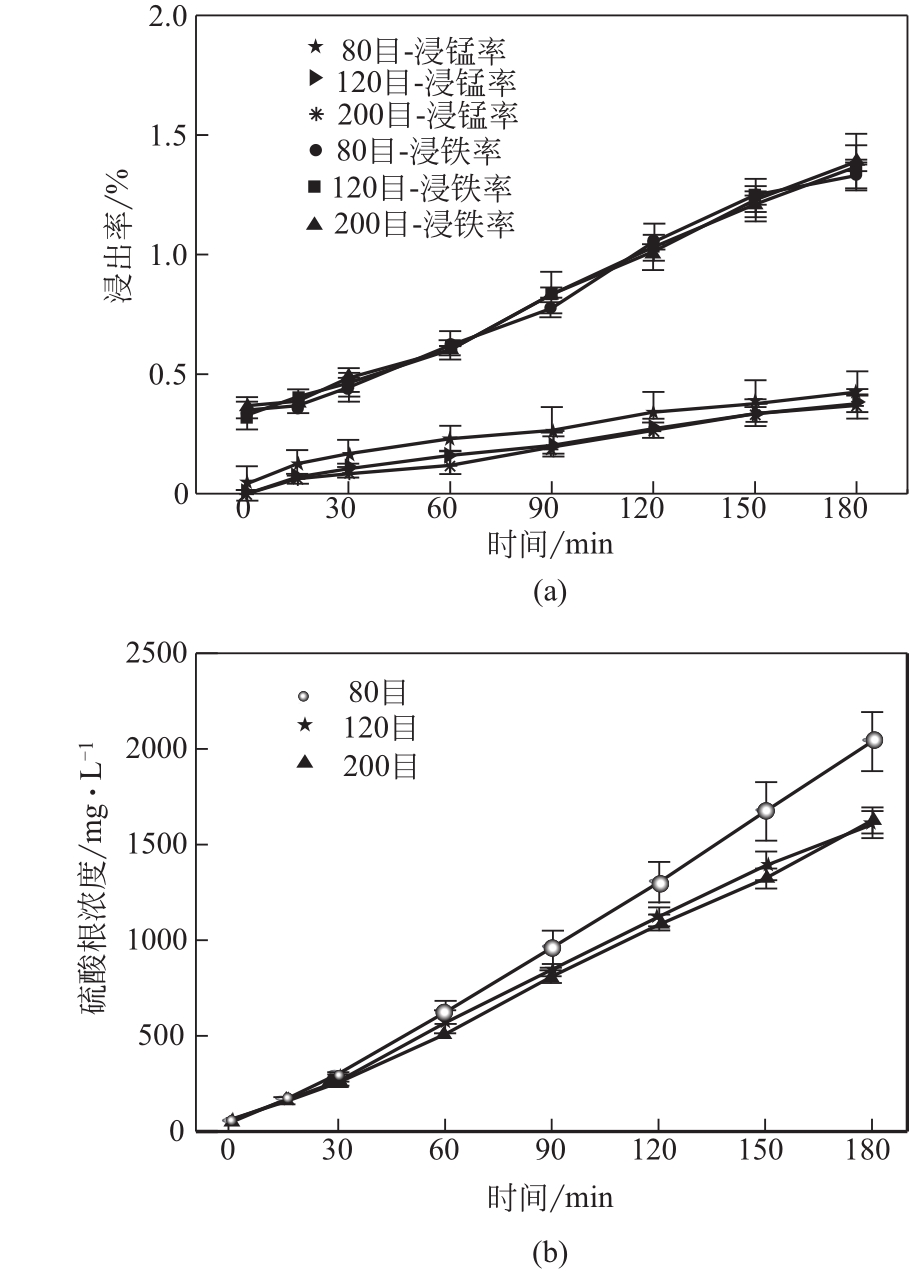
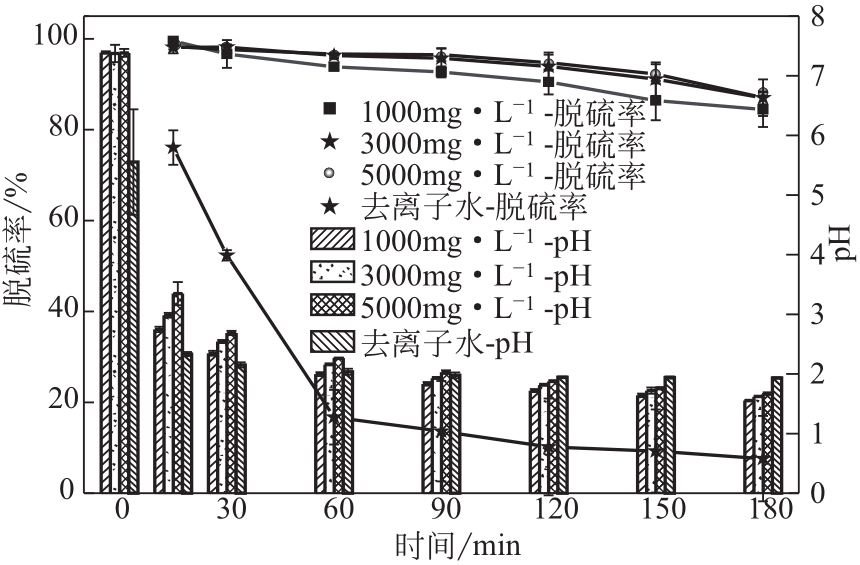
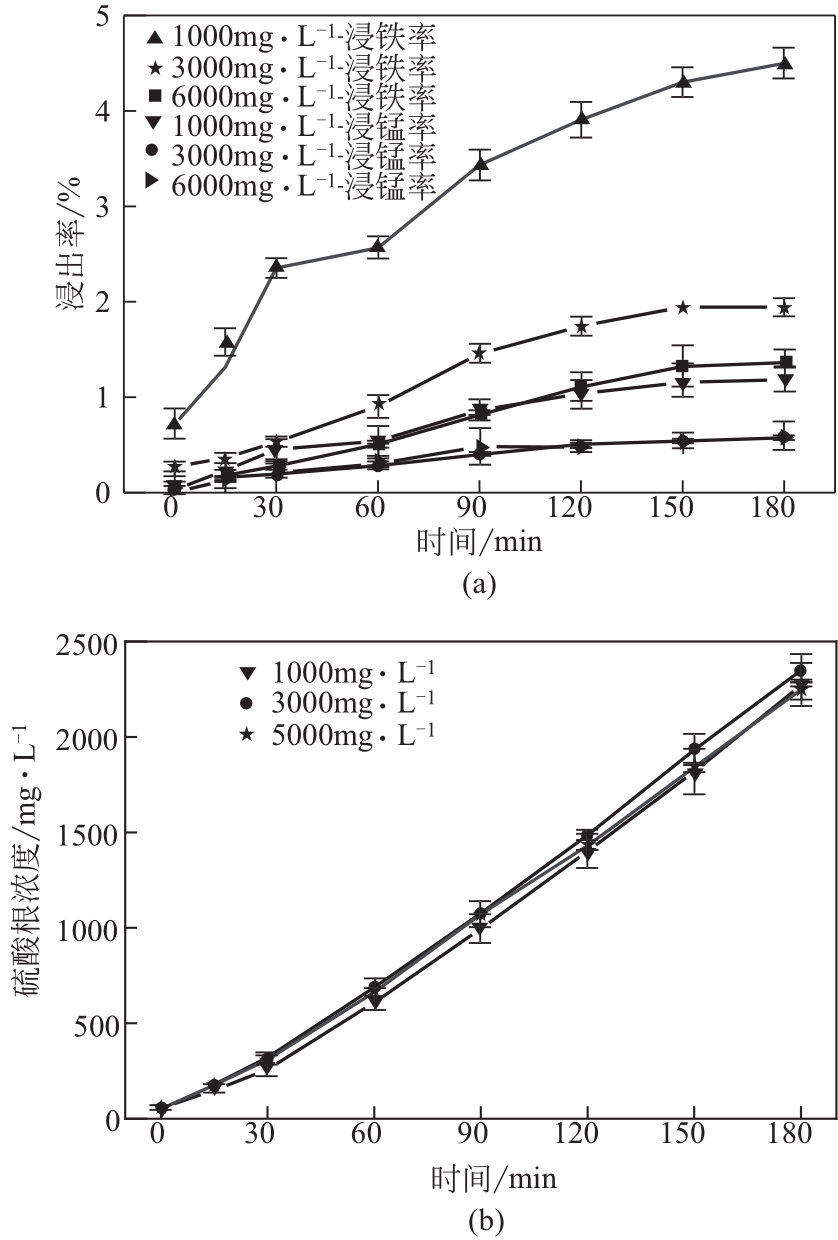
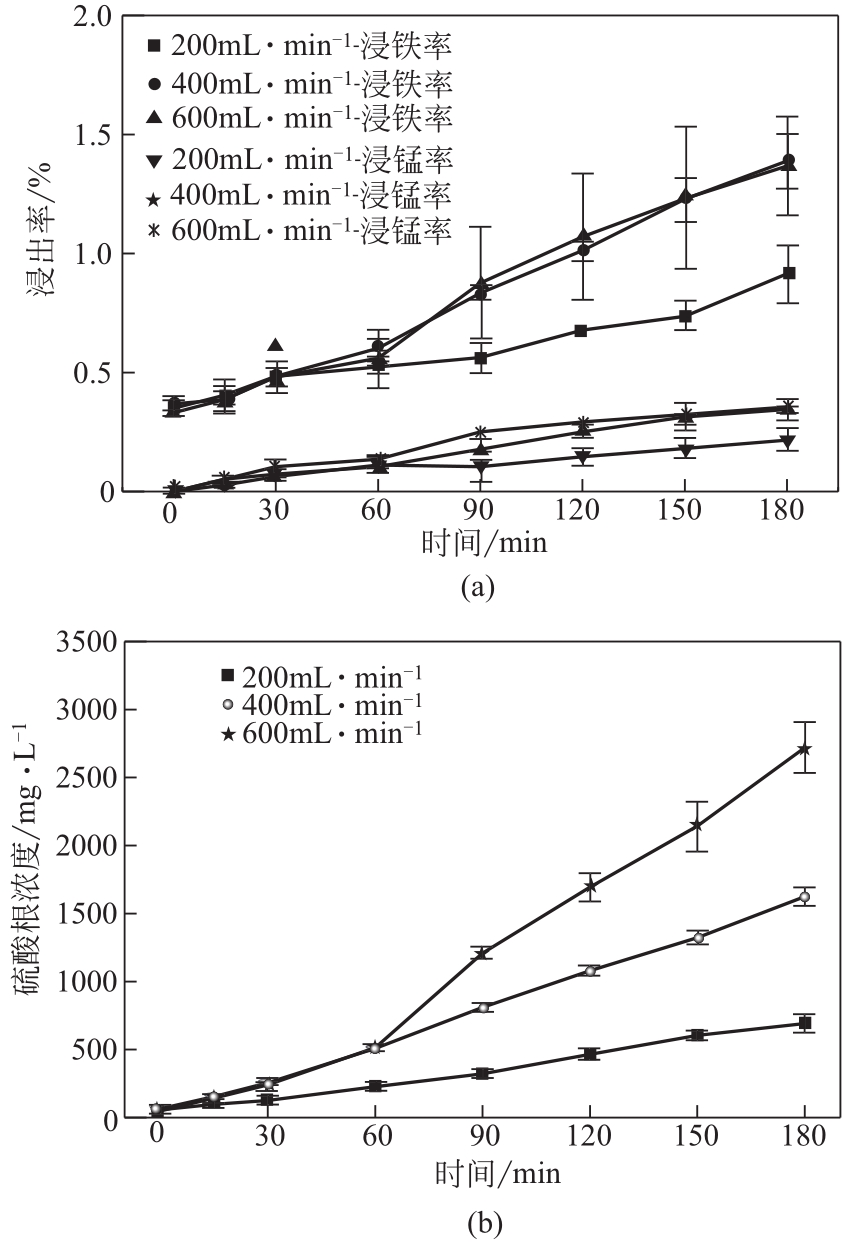
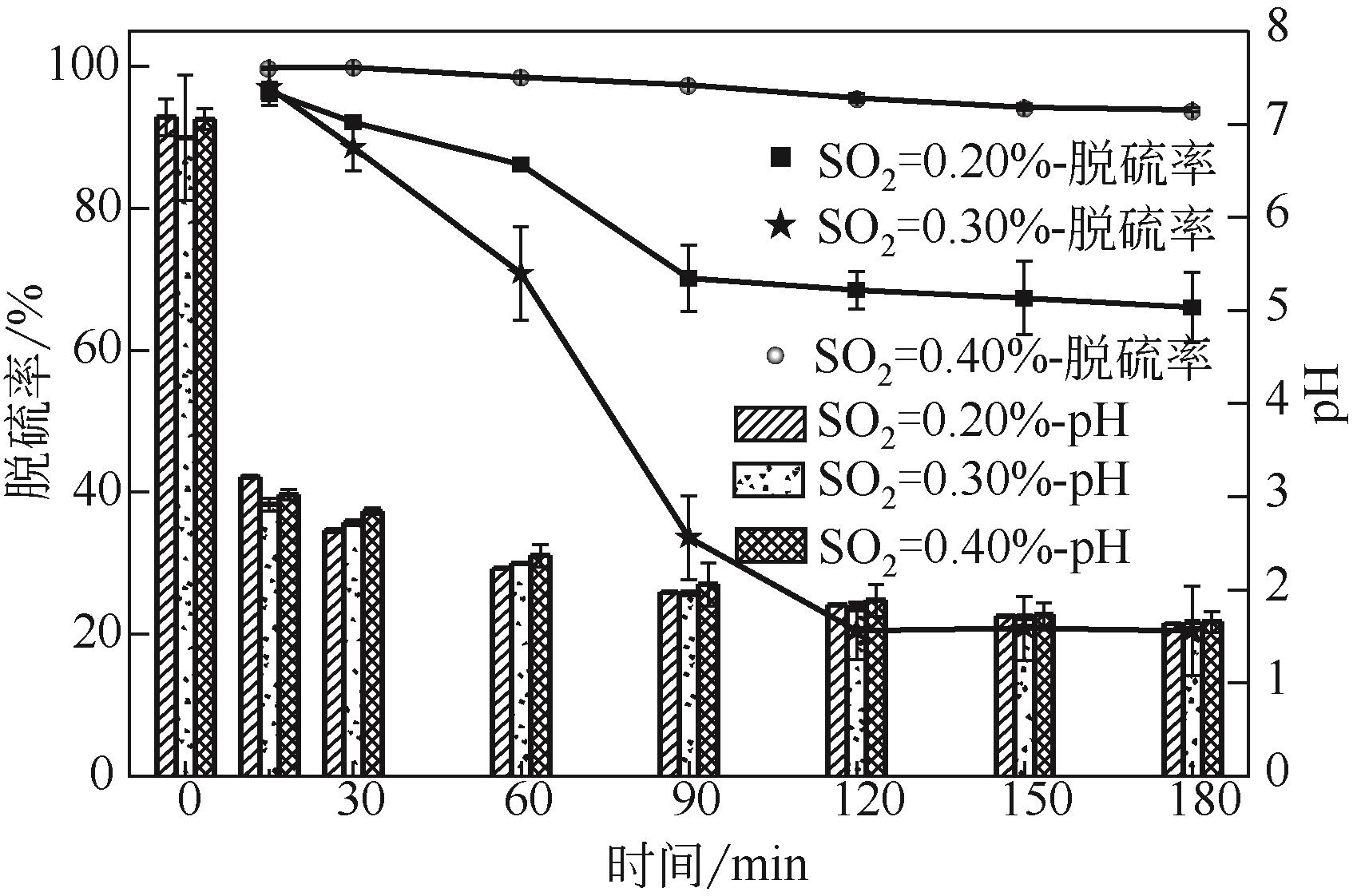
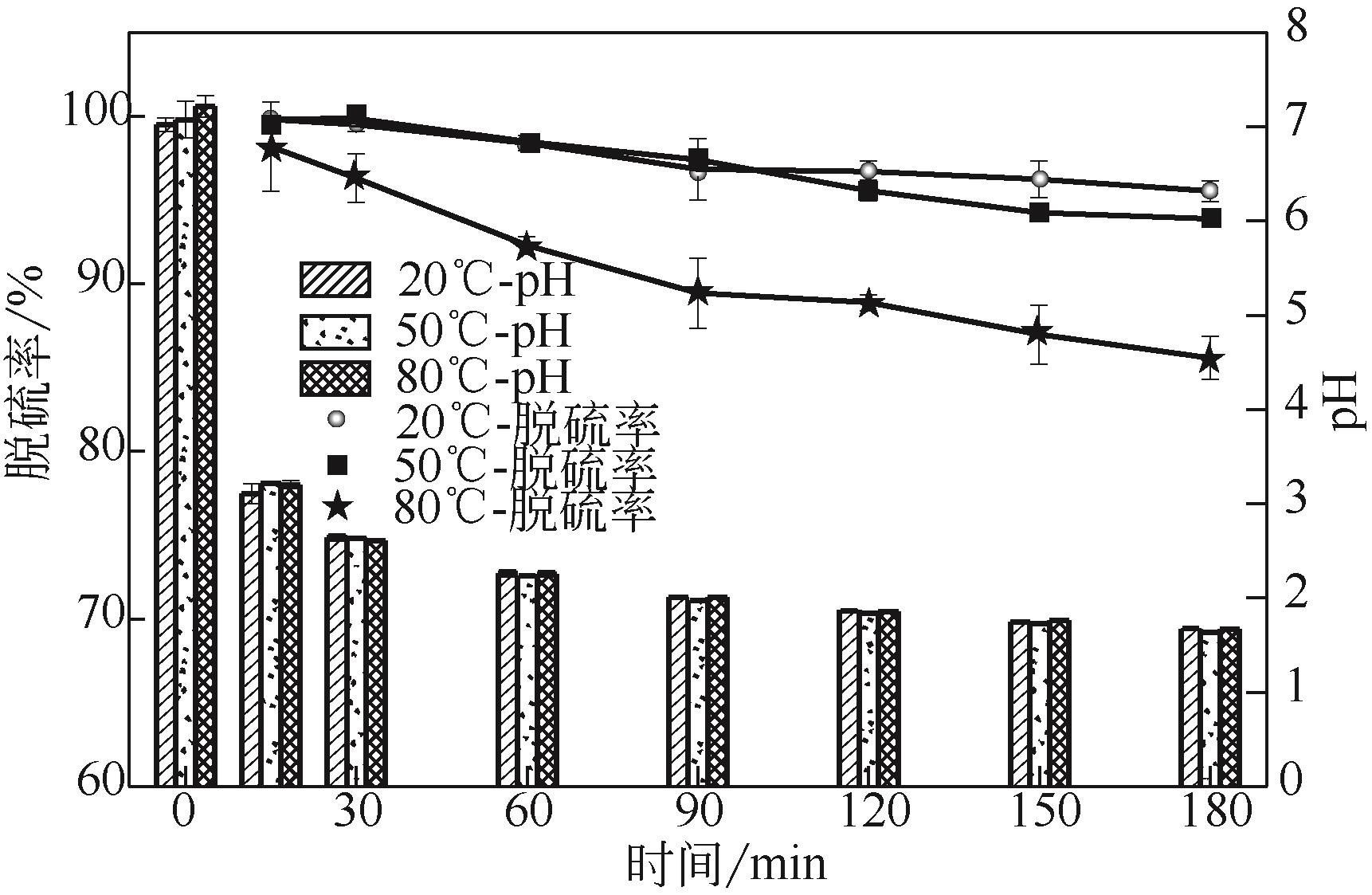
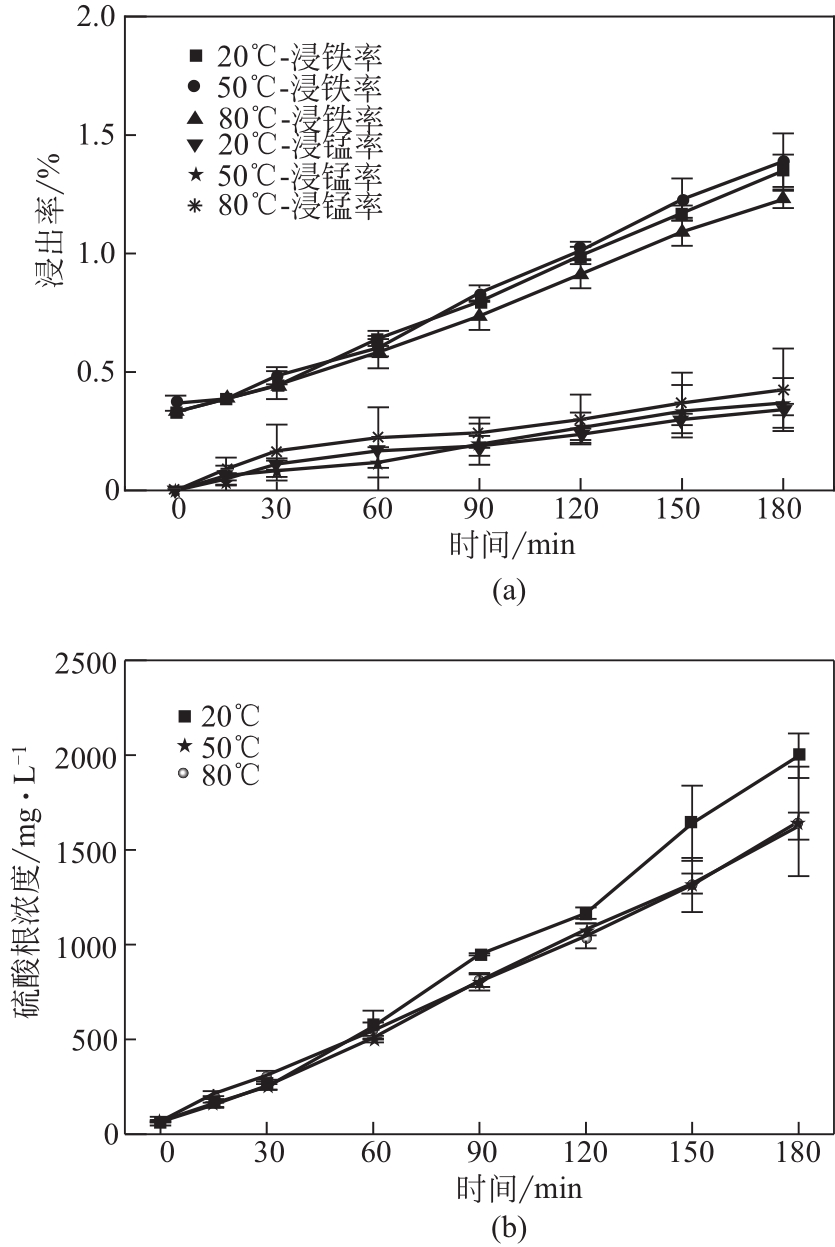
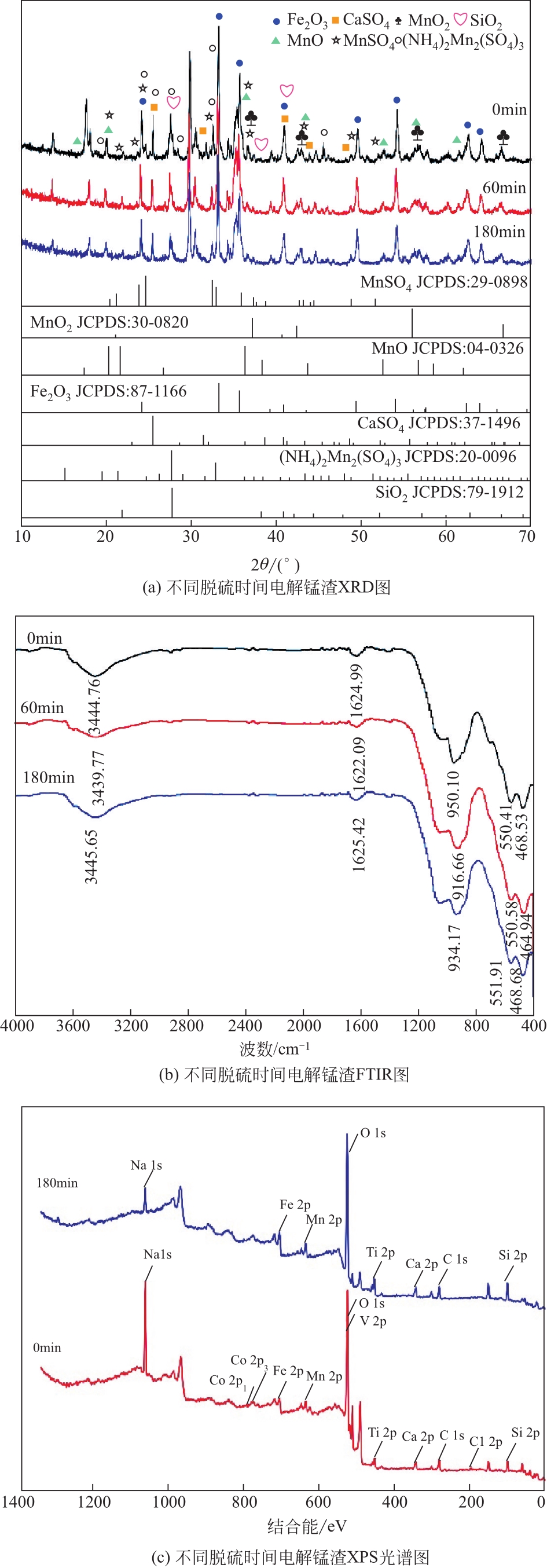
电解锰渣是电解锰生产过程中产生的锰矿石酸浸渣,富含锰、铁等活性组分,理论上可催化氧化SO2实现烟气脱硫,同时脱硫后的电解锰渣可资源化利用,然而目前尚未见电解锰渣矿浆脱硫的研究报道。本文研究了工艺参数对电解锰渣浆液脱除SO2性能的影响,探究了电解锰渣浆液烟气脱硫的过程机制。结果表明:锰渣粒径为200目(<75μm)、锰渣浆液初始浓度5000mg/L、气体流量400mL/min、进口SO2体积分数0.20%、反应温度50℃、反应时间180min的条件下,电解锰渣浆液脱硫率最高可达93.87%。脱硫前后电解锰渣XRD、SEM、XPS表征结果表明,MnO2、MnO、Fe2O3等活性组分参与SO2反应,且浆液中的Mn2+、Fe3+等过渡金属离子液相催化氧化SO2生成H2SO4,实现烟气脱硫。
中图分类号:
引用本文
聂紫萌, 杨点, 熊玉路, 李英杰, 田森林, 宁平. 电解锰渣浆液烟气脱硫性能及机制[J]. 化工进展, 2022, 41(2): 1063-1072.
NIE Zimeng, YANG Dian, XIONG Yulu, LI Yingjie, TIAN Senlin, NING Ping. Performance and mechanism of electrolytic manganese slag slurry for flue gas desulfurization[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1063-1072.
| Fe2O3 | SiO2 | TiO2 | Na2O | MnO | Cr2O3 | CaO | Al2O3 | MgO | P2O5 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 33.64 | 18.97 | 12.82 | 12.29 | 7.57 | 4.83 | 4.42 | 1.97 | 1.41 | 0.29 | 0.10 |
表1 电解锰渣的化学成分(质量分数) (%)
| Fe2O3 | SiO2 | TiO2 | Na2O | MnO | Cr2O3 | CaO | Al2O3 | MgO | P2O5 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 33.64 | 18.97 | 12.82 | 12.29 | 7.57 | 4.83 | 4.42 | 1.97 | 1.41 | 0.29 | 0.10 |
| 时间 | Mn | Fe | Ca | Al | Co | Cu | Ni | Zn | SO |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.14 | 0.13 | 1.52 | 1.19×10-2 | 1.89×10-2 | 1.14×10-3 | 1.15×10-3 | 0.20×10-2 | 63.43 |
| 60min | 2.76 | 0.71 | 6.80 | 3.41 | 2.05×10-2 | 9.43×10-3 | 1.94×10-3 | 3.08×10-2 | 514.86 |
| 180min | 7.35 | 4.19 | 8.65 | 5.54 | 2.13×10-2 | 12.40×10-3 | 4.25×10-3 | 2.48×10-2 | 1623.43 |
| 增长百分比/% | 5150 | 3123.08 | 469.08 | 46454.62 | 12.70 | 987.72 | 269.57 | 1140 | 2459.40 |
表2 不同时间下脱硫浆液中离子浓度变化 (mg/L)
| 时间 | Mn | Fe | Ca | Al | Co | Cu | Ni | Zn | SO |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.14 | 0.13 | 1.52 | 1.19×10-2 | 1.89×10-2 | 1.14×10-3 | 1.15×10-3 | 0.20×10-2 | 63.43 |
| 60min | 2.76 | 0.71 | 6.80 | 3.41 | 2.05×10-2 | 9.43×10-3 | 1.94×10-3 | 3.08×10-2 | 514.86 |
| 180min | 7.35 | 4.19 | 8.65 | 5.54 | 2.13×10-2 | 12.40×10-3 | 4.25×10-3 | 2.48×10-2 | 1623.43 |
| 增长百分比/% | 5150 | 3123.08 | 469.08 | 46454.62 | 12.70 | 987.72 | 269.57 | 1140 | 2459.40 |
| 1 | XU Fuyuan, JIANG Linhua, DAN Zhigang, et al. Water balance analysis and wastewater recycling investigation in electrolytic manganese industry of China—A case study[J]. Hydrometallurgy, 2014, 149: 12-22. |
| 2 | 姚露, 辛广智, 杨林, 等. 铁强化电解锰阳极液体系中氧化锰矿烟气脱硫和锰浸出工艺[J]. 化工进展, 2021, 40(5): 2859-2866. |
| YAO Lu, XIN Guangzhi, YANG Lin, et al. Using iron promoted manganese oxide ore for simmultaneous flue gas desulfurization and Mn leaching: a process study[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2859-2866. | |
| 3 | YANG Chao, Xiaoxin LYU, TIAN Xike, et al. An investigation on the use of electrolytic manganese residue as filler in sulfur concrete[J]. Construction and Building Materials, 2014, 73: 305-310. |
| 4 | NING Duan, WANG Fan, ZHOU Changbo, et al. Analysis of pollution materials generated from electrolytic manganese industries in China[J]. Resources, Conservation and Recycling, 2010, 54(8): 506-511. |
| 5 | ZHANG Ruirui, MA Xiaotian, SHEN Xiaoxu, et al. Life cycle assessment of electrolytic manganese metal production[J]. Journal of Cleaner Production, 2020, 253: 119951. |
| 6 | 何德军, 舒建成, 陈梦君, 等. 电解锰渣建材资源化研究现状与展望[J]. 化工进展, 2020, 39(10): 4227-4237. |
| HE Dejun, SHU Jiancheng, CHEN Mengjun, et al. Current status and future prospects of electrolytic manganese residue reused as building materials[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4227-4237. | |
| 7 | HE Shichao, WILSON B P, LUNDSTRÖM M, et al. Hazard-free treatment of electrolytic manganese residue and recovery of manganese using low temperature roasting-water washing process[J]. Journal of Hazardous Materials, 2021, 402: 123561. |
| 8 | SHU Jiancheng, LIN Fan, CHEN Mengjun, et al. An innovative method to enhance manganese and ammonia nitrogen leaching from electrolytic manganese residue by surfactant and anode iron plate[J]. Hydrometallurgy, 2020, 193:105311. |
| 9 | LI Qingzhu, LIU Qin, PENG Bing, et al. Self-cleaning performance of TiO2-coating cement materials prepared based on solidification/stabilization of electrolytic manganese residue[J]. Construction and Building Materials, 2016, 106: 236-242. |
| 10 | SHU Jiancheng, LIU Renlong, WU Haiping, et al. Adsorption of methylene blue on modified electrolytic manganese residue: kinetics, isotherm, thermodynamics and mechanism analysis[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 82: 351-359. |
| 11 | ZHANG Yuliang, LIU Xiaoming, XU Yingtang, et al. Synergic effects of electrolytic manganese residue-red mud-carbide slag on the road base strength and durability properties[J]. Construction and Building Materials, 2019, 220: 364-374. |
| 12 | TANG Binwen, GAO Shuai, WANG Yaguang, et al. Pore structure analysis of electrolytic manganese residue based permeable brick by using industrial CT[J]. Construction and Building Materials, 2019, 208: 697-709. |
| 13 | 孙丽娜, 李凯, 汤立红, 等. 常见金属氧化物烟气脱硫研究进展[J]. 化工进展, 2017, 36(1): 181-188. |
| SUN Lina, LI Kai, TANG Lihong, et al. Research progress of common metal oxides for flue gas desulfurization[J]. Chemical Industry and Engineering Progress, 2017, 36(1): 181-188. | |
| 14 | 孙峻, 苏仕军, 丁桑岚, 等. 软锰矿浆烟气脱硫资源化利用新工艺[J]. 环境工程, 2007, 25(4): 49-52. |
| SUN Jun, SU Shijun, DING Sanglan, et al. The new process of utilizing the resource from flue gas desulfurization with pyrolusite pulp[J]. Environmental Engineering, 2007, 25(4): 49-52. | |
| 15 | 朱晓帆, 蒋文举, 苏仕军, 等. 软锰矿浆烟气脱硫反应机理研究[J]. 环境污染治理技术与设备, 2002, 3(3): 44-46. |
| ZHU Xiaofan, JIANG Wennju, SU Shijun, et al. The study of reaction mechanism of desulfurization in flue gas with pyrolusite pulp[J]. Techniques and Equipment for Environmental Pollution Control, 2002, 3(3): 44-46. | |
| 16 | 汤争光, 蒋文举. 软锰矿催化氧化二氧化硫的过程与机理研究[J]. 环境科学与技术, 2008, 31(2): 13-15, 37. |
| TANG Zhengguang, JIANG Wenju. Process and mechanism of catalyzed oxidation of SO2 with pyrolusite slurry[J]. Environmental Science & Technology, 2008, 31(2): 13-15, 37. | |
| 17 | 张冬冬, 魏爱斌, 张晋鸣, 等. 液相催化氧化SO2研究进展[J]. 昆明理工大学学报(自然科学版), 2015, 40(5): 97-107. |
| ZHANG Dongdong, WEI Aibin, ZHANG Jinming, et al. Research progress on liquid-phase catalytic oxidization of SO2[J]. Journal of Kunming University of Science and Technology (Natural Science Edition), 2015, 40(5): 97-107. | |
| 18 | COICHEV N, REDDY K B, ELDIK R VAN. The synergistic effect of manganese (Ⅱ) in the sulfite-induced autoxidation of metal ions and complexes in aqueous solution[J]. Atmospheric Environment A: General Topics, 1992, 26(13): 2295-2300. |
| 19 | ZHOU Danna, CHEN Long, LI Jinjun, et al. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: a state-of-the-art minireview[J]. Chemical Engineering Journal, 2018, 346: 726-738. |
| 20 |
HUSS A, LIM P K, ECKERT C A. Oxidation of aqueous sulfur dioxide. ( ): Homogeneous manganese (Ⅱ) and iron (Ⅱ) catalysis at low pH[J]. The Journal of Physical Chemistry, 1982, 86(21): 4224-4228. ): Homogeneous manganese (Ⅱ) and iron (Ⅱ) catalysis at low pH[J]. The Journal of Physical Chemistry, 1982, 86(21): 4224-4228.
|
| 21 | PASIUK-BRONIKOWSKA W, BRONIKOWSKI T. The rate equation for SO2 autoxidation in aqueous MnSO4 solutions containing H2SO4[J]. Chemical Engineering Science, 1981, 36(1):215-219. |
| 22 | HUSS A, LIM P K, ECKERT C A. Oxidation of aqueous sulfur dioxide. (Ⅱ): High-pressure studies and proposed reaction mechanisms[J]. The Journal of Physical Chemistry, 1982, 86(21): 4229-4233. |
| 23 | BERGLUND J, FRONAEUS S, ELDING L I. Kinetics and mechanism for manganese-catalyzed oxidation of sulfur (Ⅳ) by oxygen in aqueous solution[J]. Inorganic Chemistry, 1993, 32(21): 4527-4538. |
| 24 | 陶雷, 王学谦, 宁平, 等. 矿浆烟气脱硫及资源化研究进展[J]. 化工进展, 2017, 36(5): 1868-1879. |
| TAO Lei, WANG Xueqian, NING Ping, et al. Research progress on flue gas desulfurization and utilization with slurry[J]. Chemical Industry and Engineering Progress, 2017, 36(5): 1868-1879. | |
| 25 | 中华人民共和国地质矿产部. 地下水质检验方法. 比浊法测定硫酸根: [S]. 1993. |
| Ministry of Geology and Mineral Resources of the People’s Republic of China. Groundwater quality inspection method. Determination of sulfate by turbidimetric method: [S]. 1993. | |
| 26 | 国家环境保护局. 水质 铁、锰的测定 火焰原子吸收分光光度法: [S]. 北京: 中国标准出版社, 1989. |
| State Bareau of Environmental Protection of the People’s Republic of China. Water quality—Determination of iron and manganese—Flame atomic absorption spectrometric method: [S]. Beijing: Standards Press of China, 1989. | |
| 27 | 袁进, 江霞, 刘一天, 等. 氧化锰共生矿烟气脱硫的机理及影响因素[J]. 环境工程学报, 2018, 12(4): 1104-1111. |
| YUAN Jin, JIANG Xia, LIU Yitian, et al. Mechanism and influence factors of flue gas desulfurization by symbiotic manganese oxides ore[J]. Chinese Journal of Environmental Engineering, 2018, 12(4): 1104-1111. | |
| 28 | 陈红亮, 刘仁龙, 李文生, 等. 电解锰渣的理化特性分析研究[J]. 金属材料与冶金工程, 2014, 42(1): 3-5, 17. |
| CHEN Hongliang, LIU Renlong, LI Wensheng, et al. Physicochemical analysis of electrolytic manganese residue[J]. Metal Materials and Metallurgy Engineering, 2014, 42(1): 3-5, 17. | |
| 29 | 彭铁锋. 电解锰渣制四氧化三锰及其结构性能表征[D]. 重庆: 重庆大学, 2010. |
| PENG Tiefeng. Preparation and characterization of Mn3O4 from electrolytic manganese residue[D]. Chongqing: Chongqing University, 2010. | |
| 30 | 李圭白, 杨艳玲, 李星. 锰化合物净水技术[M]. 北京: 中国建筑工业出版社, 2006: 33. |
| LI Guibai, YANG Yanling, LI Xing. Manganese compound water purification technology[M]. Beijing: China Architecture & Building Press, 2006: 33. | |
| 31 | 陈和生, 孙振亚, 邵景昌. 八种不同来源二氧化硅的红外光谱特征研究[J].硅酸盐通报, 2011, 30(4): 934-937. |
| CHEN Hesheng, SUN Zhenya, SHAO Jingchang. Investigation on FT-IR spectroscopy for eight different sources of SiO2[J]. Bulletin of the Chinese Ceramic Society, 2011, 30(4): 934-937. | |
| 32 | 李昌新, 喻源, 张庆武, 等.合成条件对电解锰渣制备沸石过程中沸石种类和性能的影响[J]. 中南大学学报(自然科学版), 2019, 50(12): 2932-2937. |
| LI Changxin, YU Yuan, ZHANG Qingwu, et al. Effects of synthesis conditions on formation process and property of zeolite prepared from electrolytic manganese residue[J]. Journal of Central South University (Science and Technology), 2019, 50(12): 2932-2937. | |
| 33 | 刘建英, 蒋文举, 谭显东, 等. 低浓度软锰矿浆烟气脱硫过程中的催化作用[J]. 环境工程学报, 2007, 1(6): 72-76. |
| LIU Jianying, JIANG Wenju, TAN Xiandong, et al. Catalysis in the process of flue gas desulphurization by low concentration pyrolusite slurry[J]. Chinese Journal of Environmental Engineering, 2007, 1(6): 72-76. | |
| 34 | 陈昭琼, 童志权. 软锰矿浆烟气脱硫过程机理探讨及过程强化[J]. 湘潭大学自然科学学报, 1998(3): 112-116. |
| CHEN Zhaoqiong, TONG Zhiquan. Approach a mechanism of flue gas desulfurization with pyrolusite slurry and strengthen the process[J]. Natural Science Journal of Xiangtan University, 1998(3):112-116. | |
| 35 | 赵栋, 李光强, 王恒辉, 等. 高磷鲕状赤铁矿酸浸脱磷动力学[J]. 钢铁研究学报, 2017, 29(11): 883-891. |
| ZHAO Dong, LI Guangqiang, WANG Henghui, et al. Kinetics of acid leaching for high phosphorus oolitic hematite dephosphorization[J]. Journal of Iron and Steel Research, 2017, 29(11): 883-891. |
| [1] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [2] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [3] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [4] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [5] | 王保文, 刘同庆, 张港, 李炜光, 林德顺, 王梦家, 马晶晶. CuFe2O4改性脱硫渣氧载体与褐煤的反应特性[J]. 化工进展, 2023, 42(6): 2884-2894. |
| [6] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [7] | 李文秀, 杨宇航, 黄艳, 王涛, 王镭, 方梦祥. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057. |
| [8] | 张晨光, 封硕, 邢玉烨, 沈伯雄, 苏立超. 柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展[J]. 化工进展, 2023, 42(3): 1321-1331. |
| [9] | 陈姝晖, 伍岳, 张文祥, 王闪闪, 马和平. 离子型有机多孔聚合物的制备及其烟气脱硫耦合脱碳性质[J]. 化工进展, 2023, 42(2): 1028-1038. |
| [10] | 郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231. |
| [11] | 高宁博, 胡雅迪, 全翠. 餐厨垃圾的热转化和生物处理研究进展[J]. 化工进展, 2022, 41(S1): 507-515. |
| [12] | 张铭, 高永康, 纪德龙, 刘福杰, 朱文帅, 李华明. 多酸材料在燃油脱硫中的研究进展[J]. 化工进展, 2022, 41(9): 4782-4789. |
| [13] | 李兴, 黄宏宇, 大坂侑吾, 呼和涛力, 肖林发, 李军. 碳材料吸附脱除二氧化硫性能的影响因素[J]. 化工进展, 2022, 41(9): 4963-4972. |
| [14] | 曾一凡, 舒建成, 杨慧敏, 赵志胜, 陈梦君, 杨勇, 刘仁龙. 铵盐体系电解锰渣中石膏的转变规律[J]. 化工进展, 2022, 41(9): 5115-5121. |
| [15] | 余正伟, 张晓霞, 雷杰, 李澳, 王光应, 丁祥, 龙红明. 废CeO x -MnO x 基SCR脱硝催化剂还原酸浸综合回收铈锰[J]. 化工进展, 2022, 41(9): 5122-5131. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||