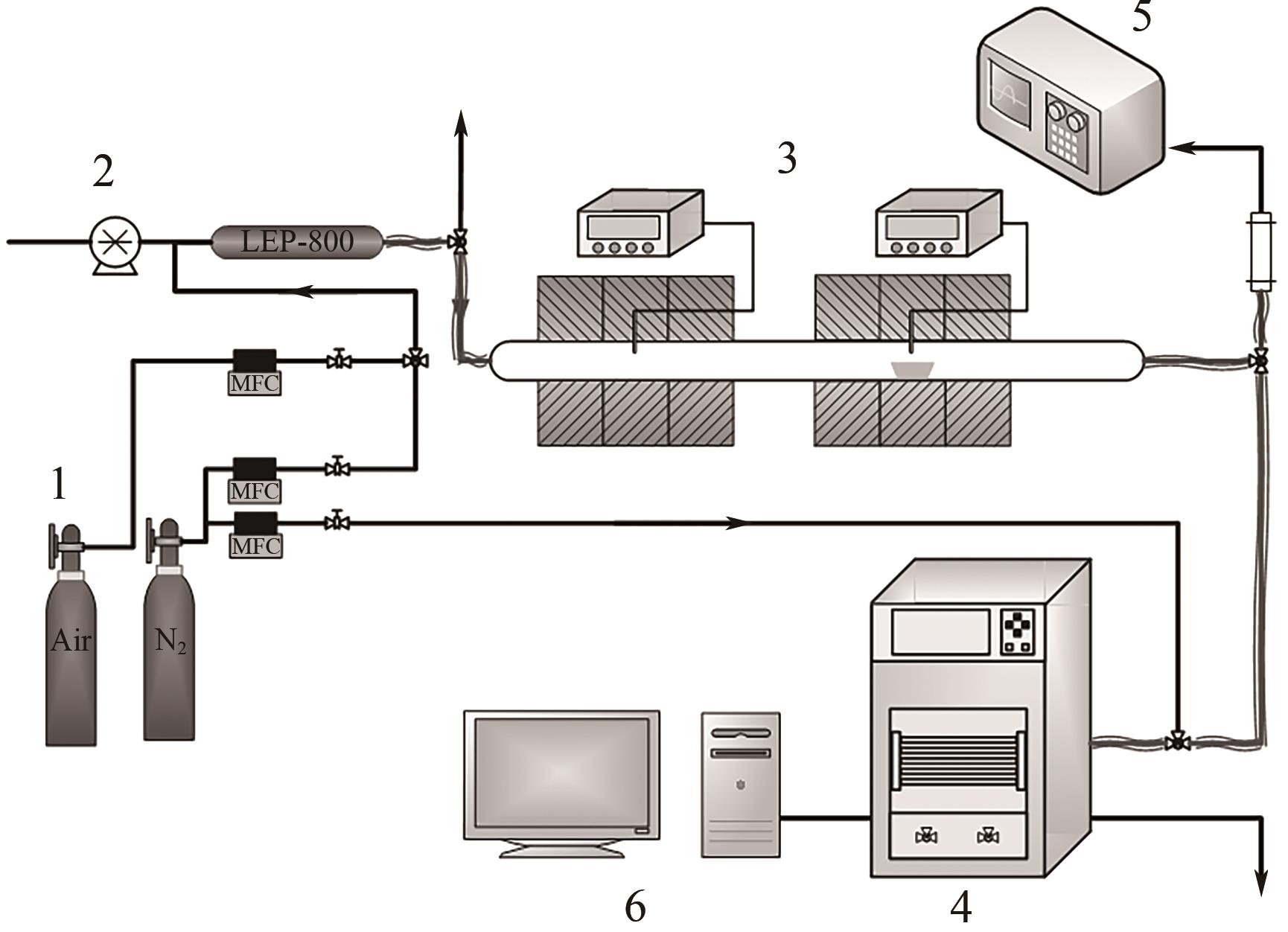
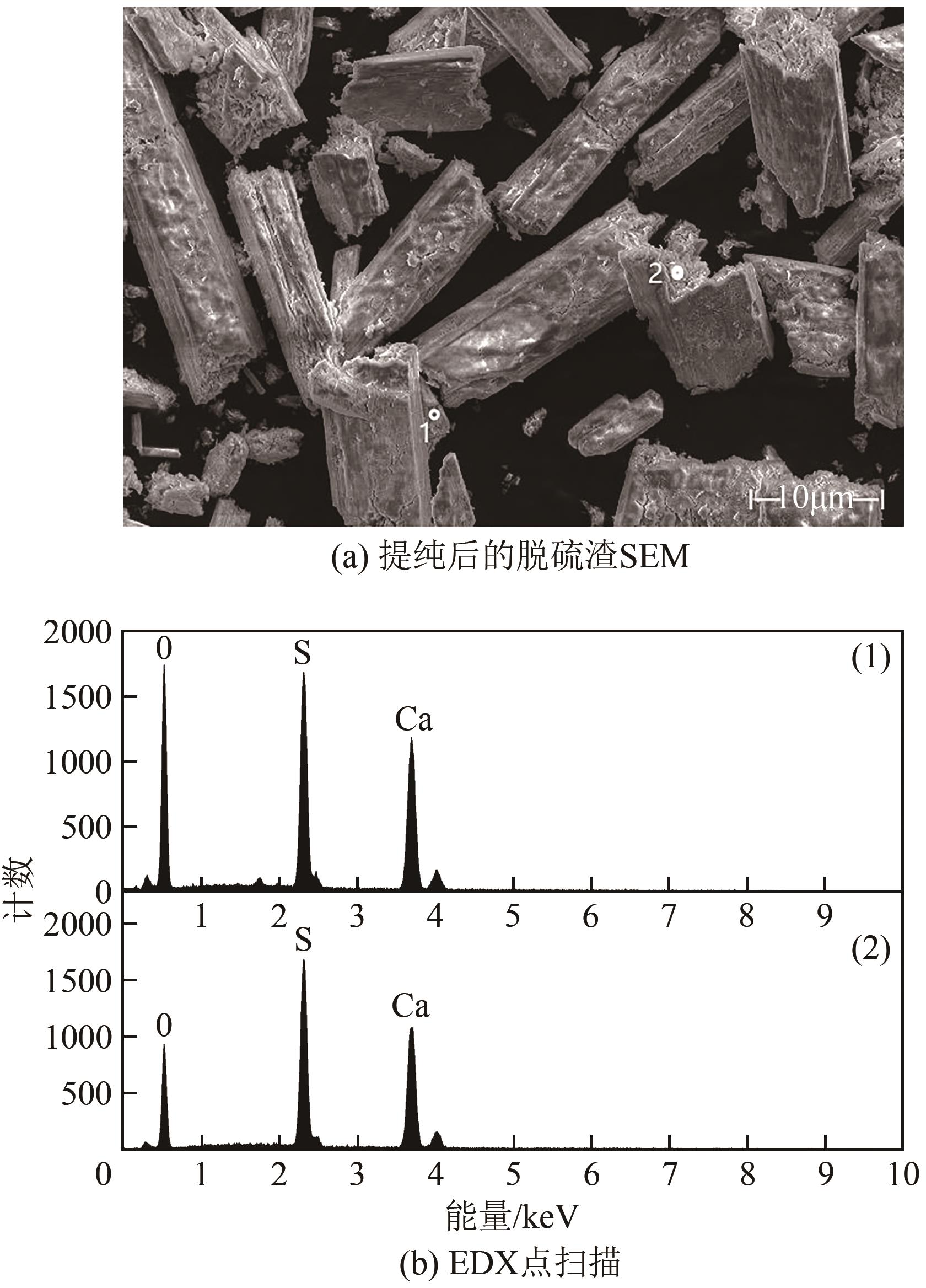
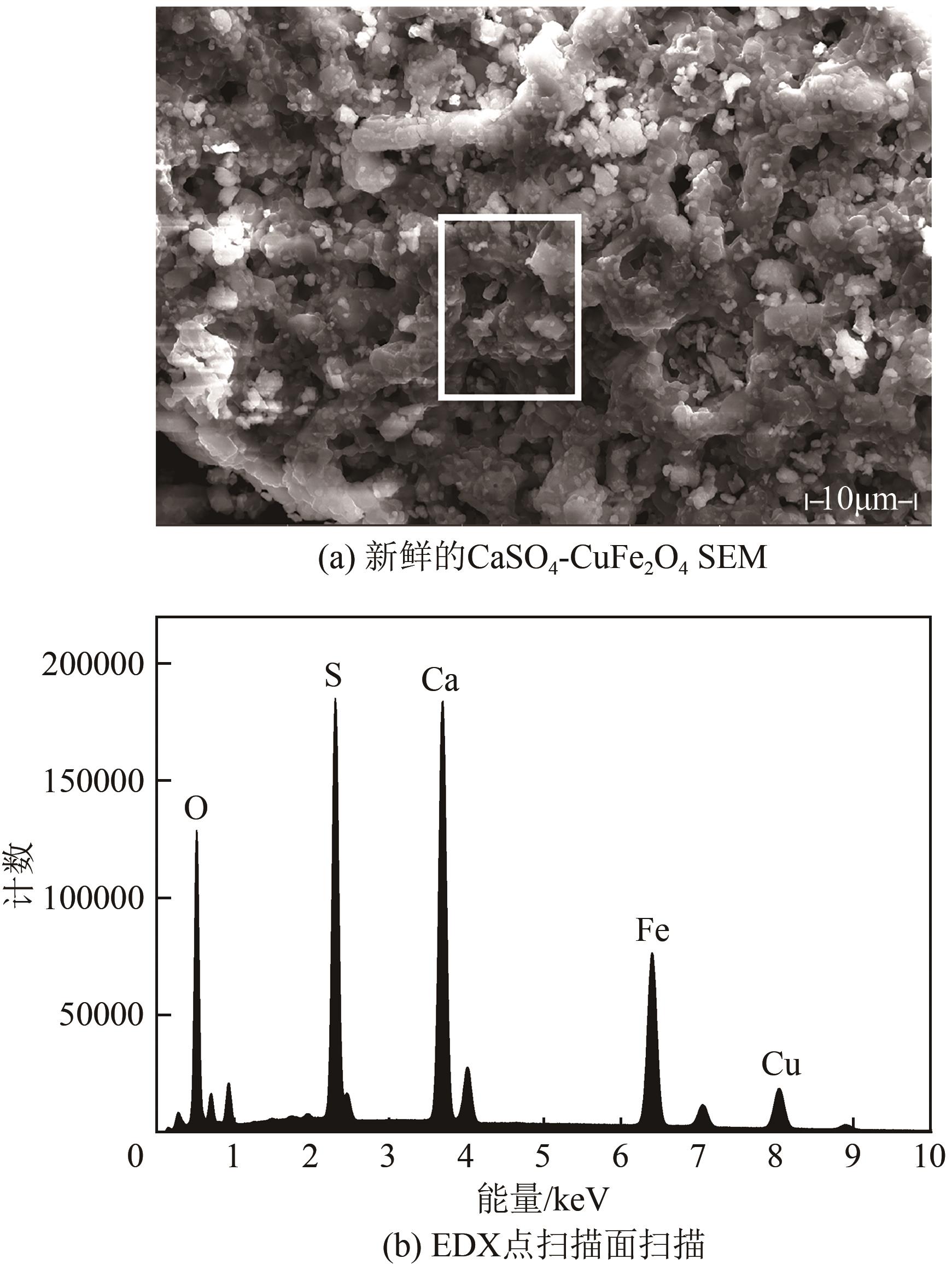
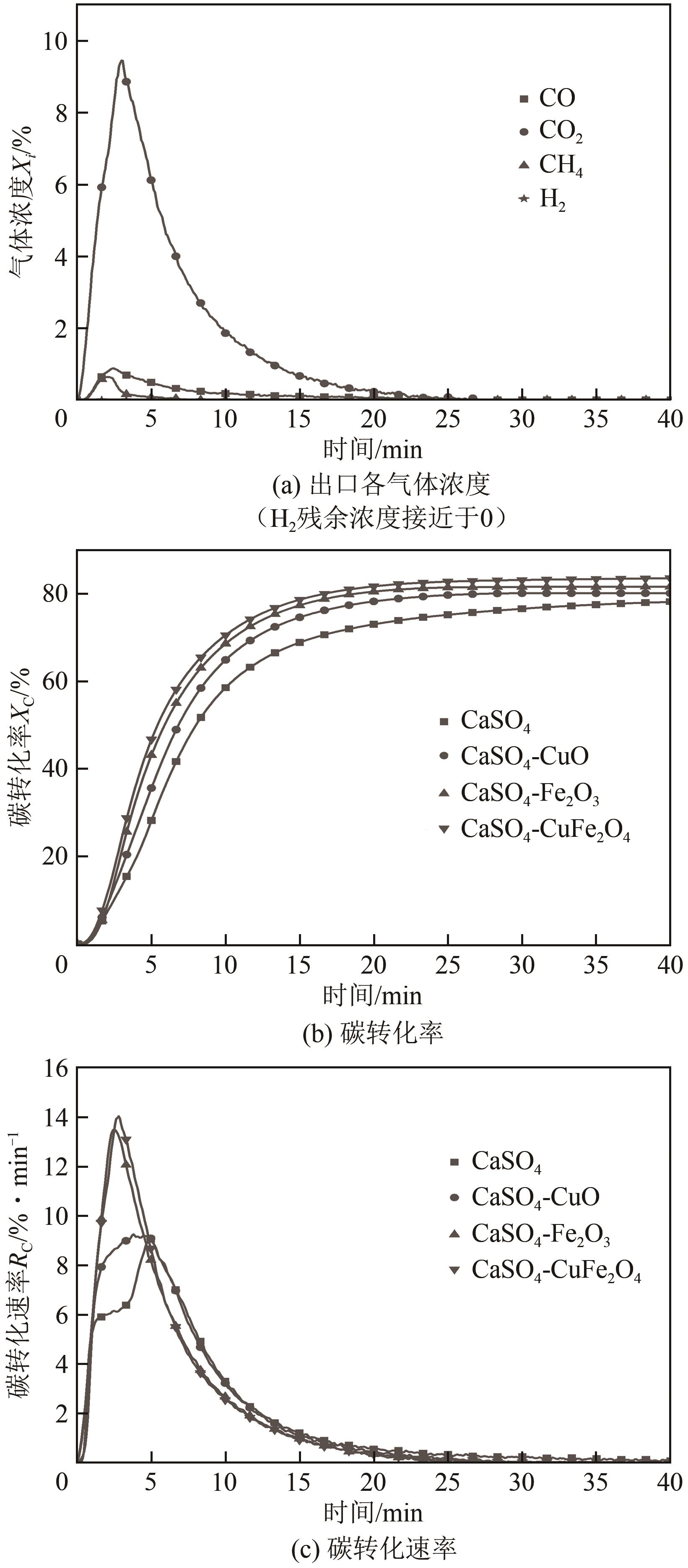
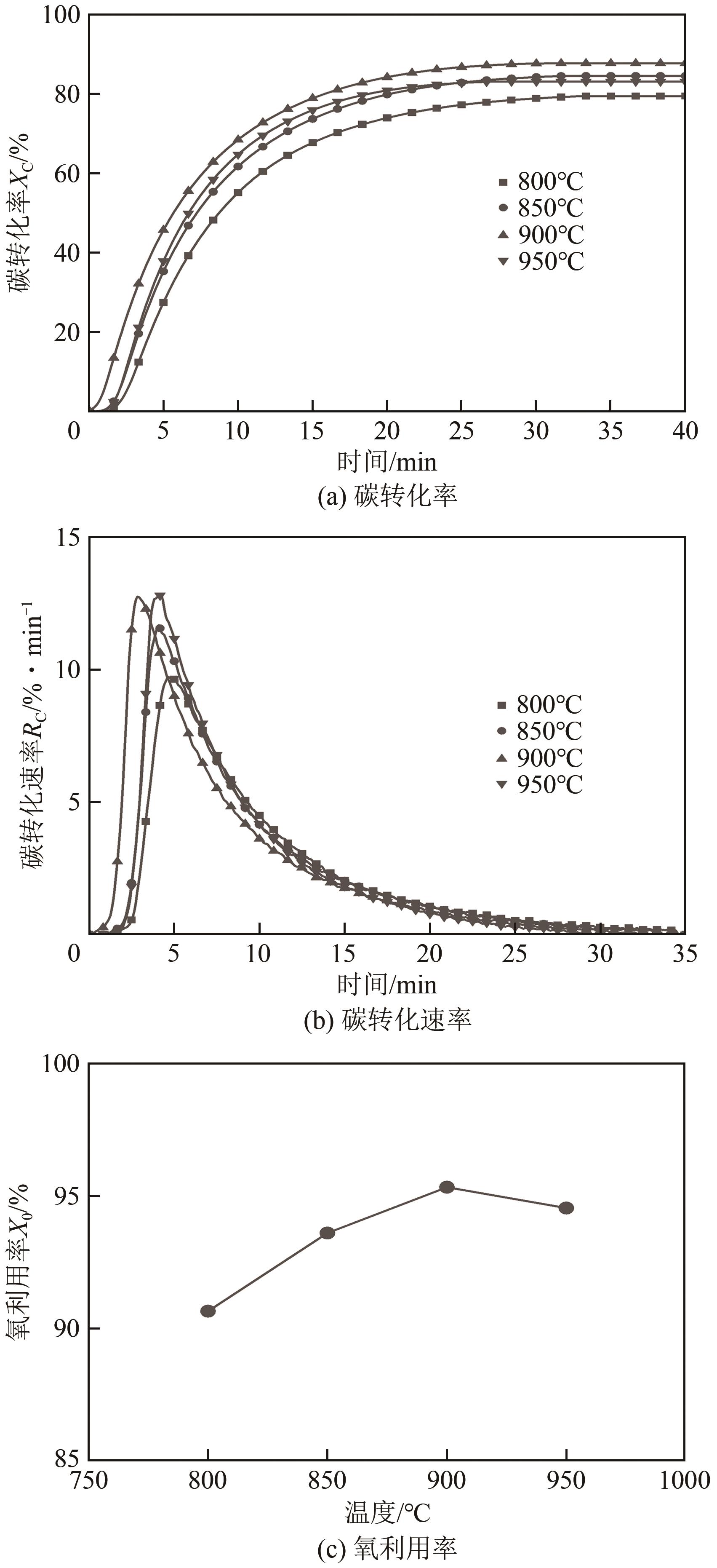
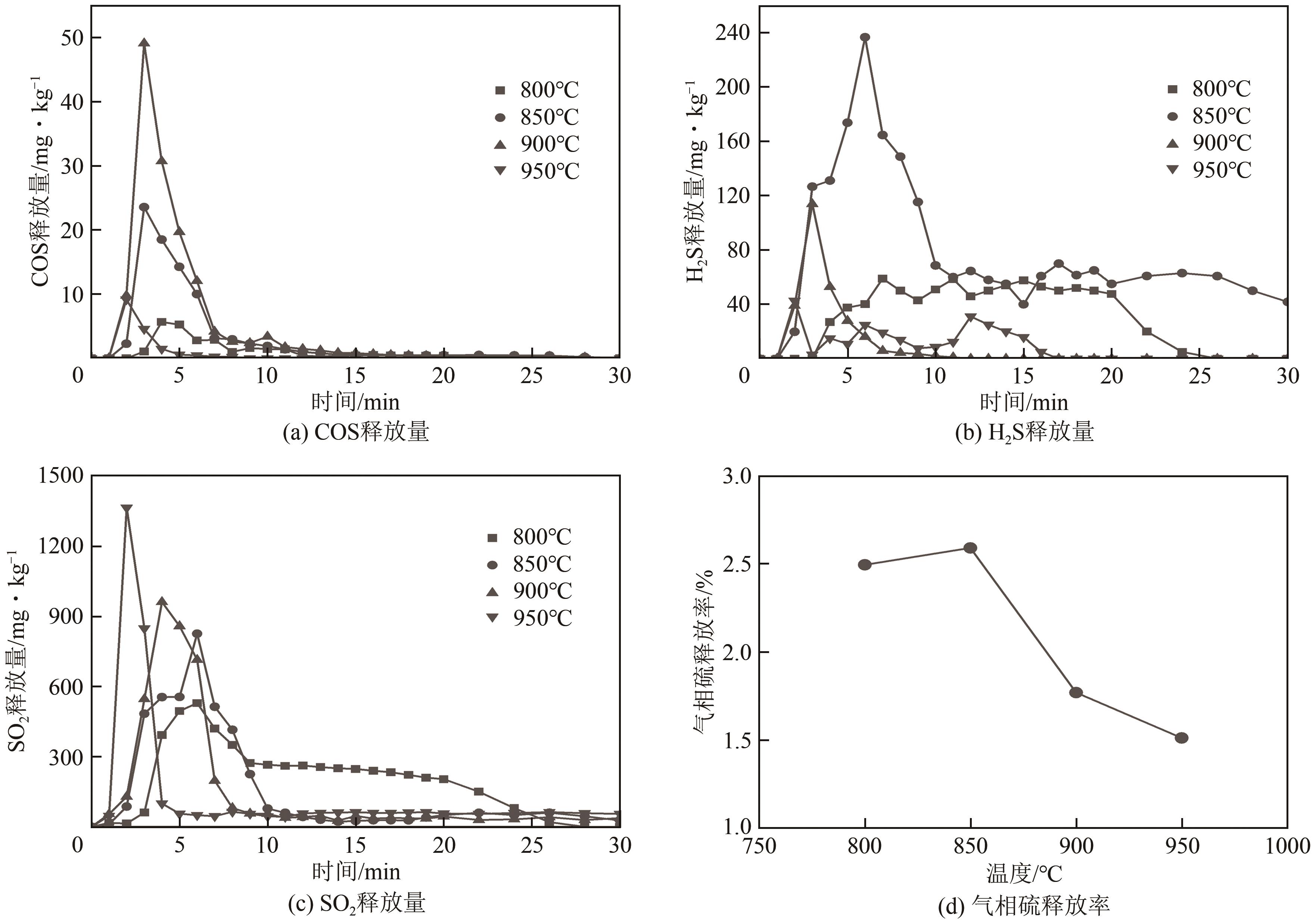
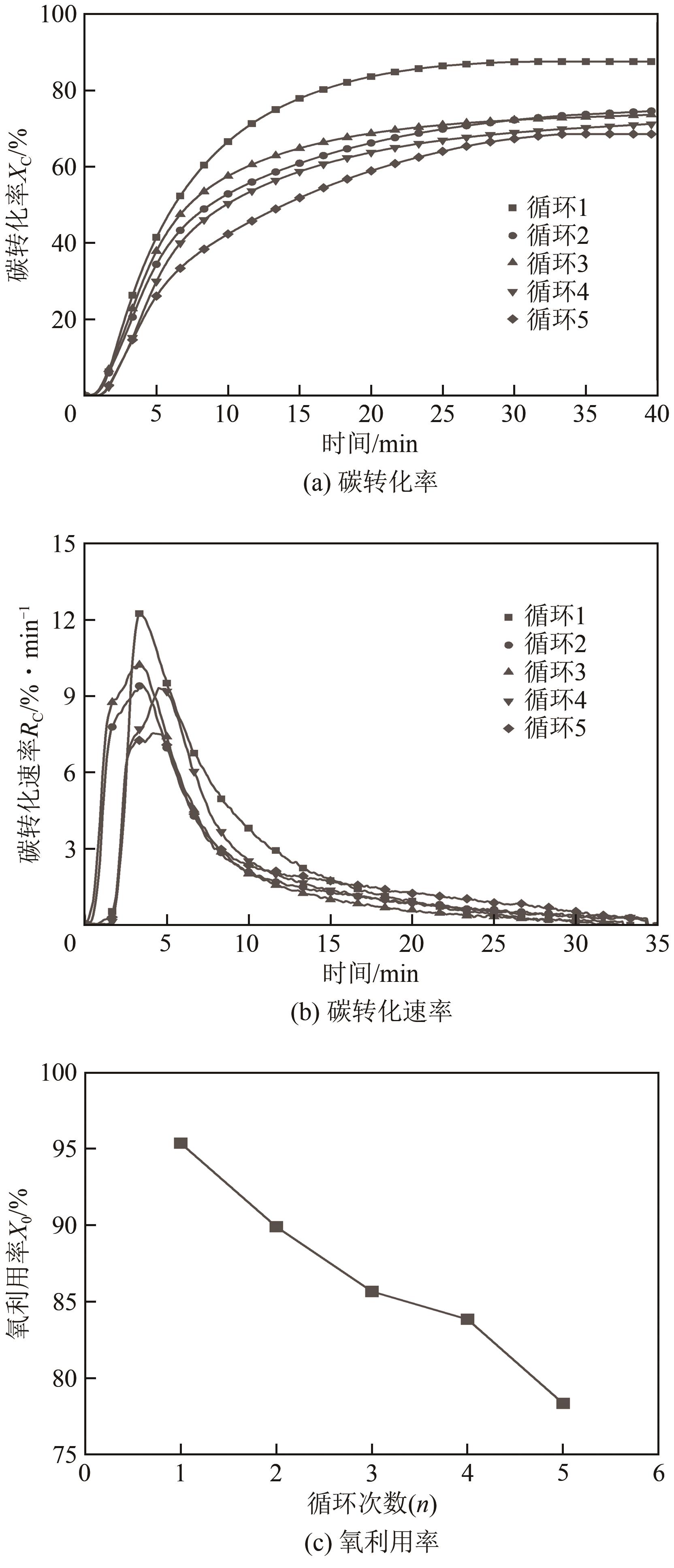
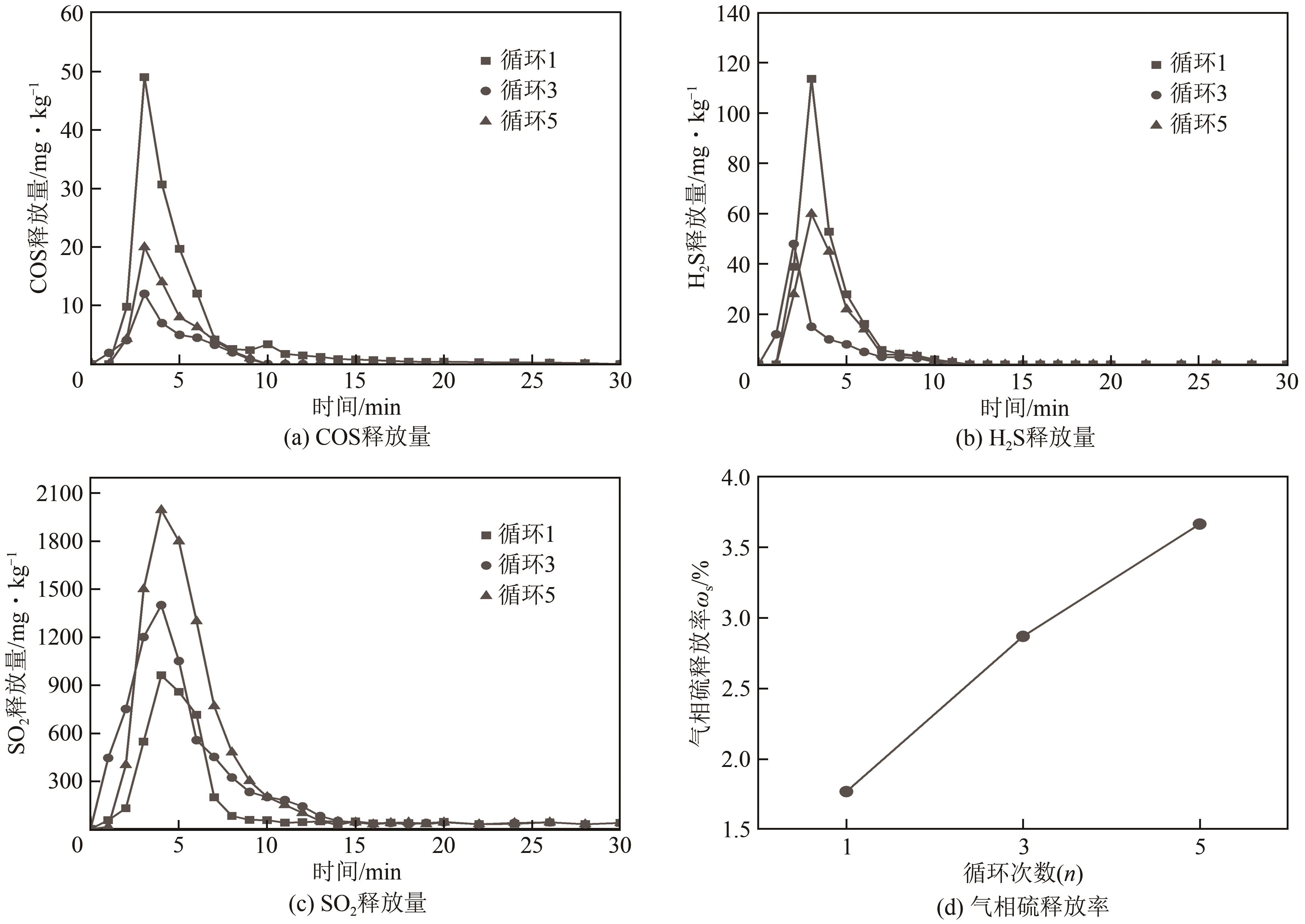
| 11 |
王保文, 王维, 李贺宇, 等. 提纯烟气脱硫渣载氧体煤化学链燃烧性能研究[J]. 燃料化学学报, 2020, 48(8): 908-919.
|
|
WANG Baowen, WANG Wei, LI Heyu, et al. Study on the performance of the purified CaSO4 oxygen carrier derived from wet flue gas desulphurization slag in coal chemical looping combustion[J]. Journal of Fuel Chemistry and Technology, 2020, 48(8): 908-919.
|
| 12 |
王璐璐, 冯璇, 沈来宏. 高硫石油焦化学链燃烧特性及硫的转化[J]. 东南大学学报(自然科学版), 2019, 49(2): 288-295.
|
|
WANG Lulu, FENG Xuan, SHEN Laihong. Chemical looping combustion of petroleum coke and conversion of sulfur[J]. Journal of Southeast University (Natural Science Edition), 2019, 49(2): 288-295.
|
| 13 |
陈国平, 马琎晨, 赵海波. 化学链中失活铁矿石载氧体的改性提质研究[J]. 工程热物理学报, 2020, 41(5): 1270-1278.
|
|
CHEN Guoping, MA Jinchen, ZHAO Haibo. Modification of deactivated iron ore as oxygen carriers in chemical looping combustion[J]. Journal of Engineering Thermophysics, 2020, 41(5): 1270-1278.
|
| 14 |
王保文, 李贺宇, 王维, 等. 部分氧解耦煤化学链燃烧中硫的演化与分布[J]. 石油学报(石油加工), 2020, 36(6): 1129-1139.
|
|
WANG Baowen, LI Heyu, WANG Wei, et al. Evolution and distribution of sulfur in chemical looping combustion with partial oxygen uncoupling of coal[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(6): 1129-1139.
|
| 15 |
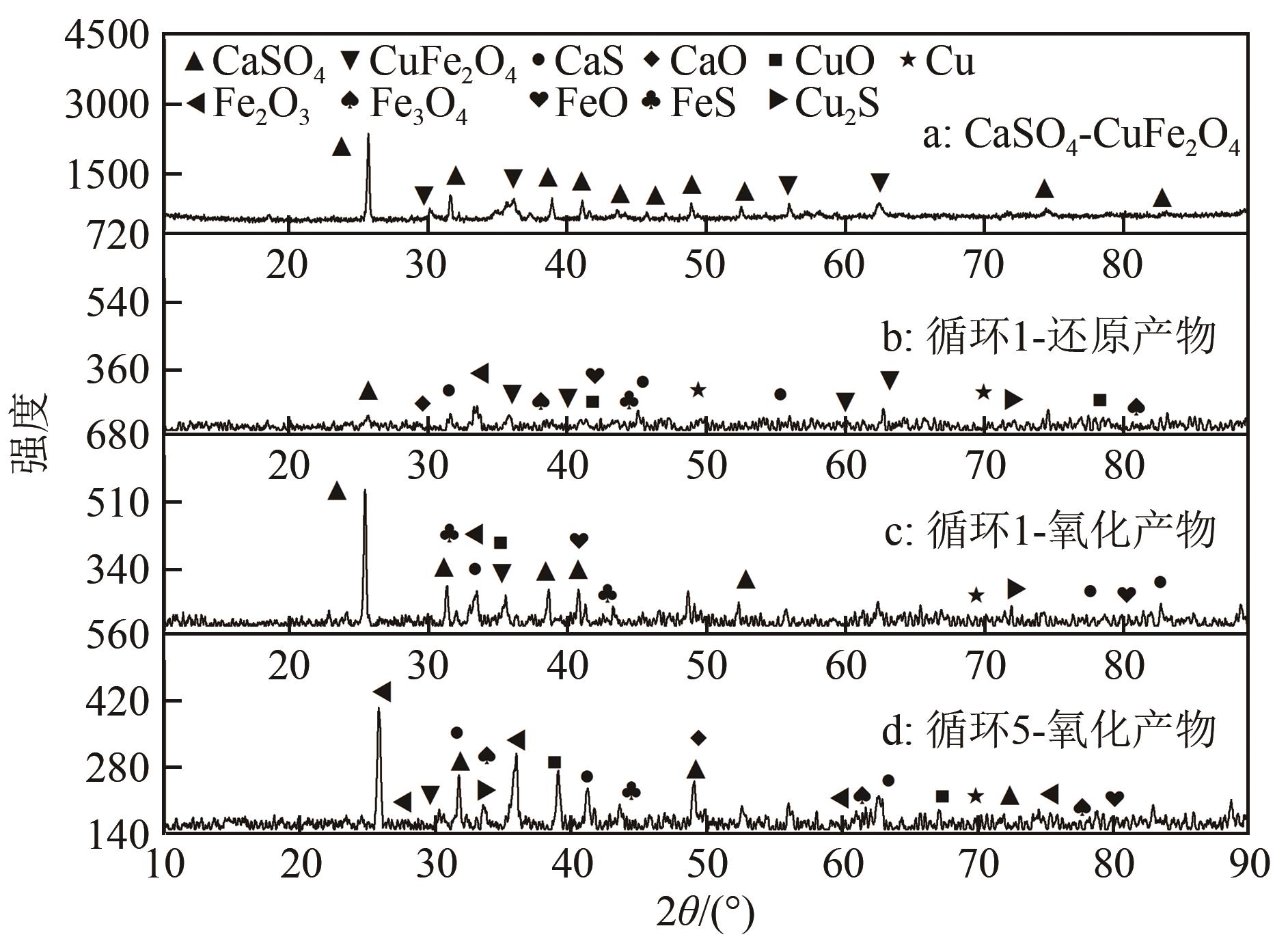
李旭刚, 王维, 李君, 等. 脱硫渣-CuFe2O4混合载氧体煤化学链燃烧性能研究[J]. 洁净煤技术, 2022. DOI:10.13226/j.issn.1006-6772.F21081301 .
|
|
LI Xugang, WANG Wei, LI Jun, et al. Study on the performance of desulfurized slag-CuFe2O4 mixed oxygen carrier in coal chemical looping combustion[J]. Clean Coal Technol, 2022. DOI:10.13226/j.issn.1006-6772.F21081301 .
|
| 16 |
崔晓婧, 马丽萍, 杨杰, 等. Fe2O3, SiO2, Al2O3对磷石膏化学链燃烧反应的影响研究[J]. 硅酸盐通报, 2018, 37(5): 1589-1594.
|
|
CUI Xiaojing, MA Liping, YANG Jie, et al. Effect of Fe2O3, SiO2 and Al2O3 in phosphogypsum on chemical looping combustion[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(5): 1589-1594.
|
| 1 |
Juan ADÁNEZ, ABAD Alberto, GARCÍA-LABIANO F, et al. Progress in chemical-looping combustion and reforming technologies[J]. Fuel and Energy Abstracts, 2012, 38(2): 215-282.
|
| 2 |
王志轩, 潘荔, 杨帆. 火电厂脱硫石膏资源综合利用[M]. 北京: 化学工业出版社, 2018.
|
|
WANG Zhixuan, PAN Li, YANG Fan. Comprehensive utilization of desulfurization gypsum resources in thermal power plants[M]. Beijing: Chemical Industry Press, 2018.
|
| 3 |
郑瑛, 王保文, 宋侃, 等. 化学链燃烧技术中新型氧载体CaSO4的特性研究[J]. 工程热物理学报, 2006, 27(3): 531-533.
|
|
ZHENG Ying, WANG Baowen, SONG Kan, et al. The performance research on new oxygen carrier CaSO4 used in chemical-looping combustion[J]. Journal of Engineering Thermophysics, 2006, 27(3): 531-533.
|
| 4 |
ZHANG S, XIAO R, YANG Y, et al. CO2 capture and desulfurization in chemical looping combustion of coal with a CaSO4 oxygen carrier[J]. Chemical Engineering & Technology, 2013, 36(9): 1469-1478.
|
| 5 |
ZHENG Min, SHEN Laihong, XIAO Jun. Reduction of CaSO4 oxygen carrier with coal in chemical-looping combustion: Effects of temperature and gasification intermediate[J]. International Journal of Greenhouse Gas Control, 2010, 4(5): 716-728.
|
| 6 |
狄子琛, 巩盼, 杨凤玲, 等. 化学链燃烧钙基载氧体的研究进展[J]. 洁净煤技术, 2022. .
|
|
GENG Zichen, GANG Pan, YANG Fengling, et al. Recent advances of calcium-based oxygen carriers for the chemicallooping combustion process: a review[J]. Clean Coal Technology, 2022. .
|
| 7 |
GUO Qingjie, HU Xiude, LIU Yongzhuo, et al. Coal chemical-looping gasification of Ca-based oxygen carriers decorated by CaO[J]. Powder Technology, 2015, 275: 60-68.
|
| 8 |
SONG Tao, ZHENG Min, SHEN Laihong, et al. Mechanism investigation of enhancing reaction performance with CaSO4/Fe2O3 oxygen carrier in chemical-looping combustion of coal[J]. Industrial & Engineering Chemistry Research, 2013, 52(11): 4059-4071.
|
| 9 |
CHEAH Singfoong, CARPENTER Daniel, MAGRINI-BAIR K A. Review of mid- to high-temperature sulfur sorbents for desulfurization of biomass- and coal-derived syngas[J]. Energy & Fuels, 2009, 23(6): 5291-5307.
|
| 17 |
YANG Jing, REN Yujie, LU Jinsuo, et al. Chemical looping gasification with a CuFe2O4-enhanced phosphogypsum oxygen carrier during reduction in a fluidized bed reactor[J]. Chemical Engineering Journal, 2021, 426: 131346.
|
| 18 |
WANG Baowen, YAN Rong, ZHENG Ying, et al. Mechanistic investigation of chemical looping combustion of coal with Fe2O3 oxygen carrier[J]. Fuel, 2011, 90(7): 2359-2366.
|
| 19 |
WANG Baowen, LI Jun, DING Ning, et al. Chemical looping combustion of a typical lignite with a CaSO4-CuO mixed oxygen carrier[J]. Energy & Fuels, 2017, 31(12): 13942-13954.
|
| 20 |
ZHANG Shuai, XIAO Rui, LIU Jian, et al. Performance of Fe2O3/CaSO4 composite oxygen carrier on inhibition of sulfur release in calcium-based chemical looping combustion[J]. International Journal of Greenhouse Gas Control, 2013, 17: 1-12.
|
| 10 |
汪潇, 杨留栓, 朱新峰, 等. 湿法脱硫石膏颗粒特性与杂质赋存状况分析[J]. 环境科学与技术, 2013, 36(9): 135-138.
|
|
WANG Xiao, YANG Liushuan, ZHU Xinfeng, et al. Analysis of particle characteristics and existing formation of the impurities in wet FGD gypsum[J]. Environmental Science & Technology, 2013, 36(9): 135-138.
|
 ), 刘同庆1, 张港1, 李炜光1, 林德顺1, 王梦家1, 马晶晶2
), 刘同庆1, 张港1, 李炜光1, 林德顺1, 王梦家1, 马晶晶2
 ), LIU Tongqing1, ZHANG Gang1, LI Weiguang1, LIN Deshun1, WANG Mengjia1, MA Jingjing2
), LIU Tongqing1, ZHANG Gang1, LI Weiguang1, LIN Deshun1, WANG Mengjia1, MA Jingjing2