化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6338-6349.DOI: 10.16085/j.issn.1000-6613.2022-0285
载体表面羟基种类对DMO加氢用Cu/SiO2催化剂性能的影响
- 兰州理工大学石油化工学院,甘肃 兰州 730050
-
收稿日期:2022-02-25修回日期:2022-04-24出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:赵鹬 -
作者简介:贠宏飞(1989—),男,博士研究生,研究方向为工业催化。E-mail:yzyeduc@163.com。 -
基金资助:甘肃省重点研发项目(18YF1GA062);国家自然科学基金(21763016);甘肃省高等学校产业支撑引导项目(2020C-06)
Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation
YUN Hongfei( ), ZHAO Yu(
), ZHAO Yu( ), LI Guixian
), LI Guixian
- School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
-
Received:2022-02-25Revised:2022-04-24Online:2022-12-20Published:2022-12-29 -
Contact:ZHAO Yu
摘要:
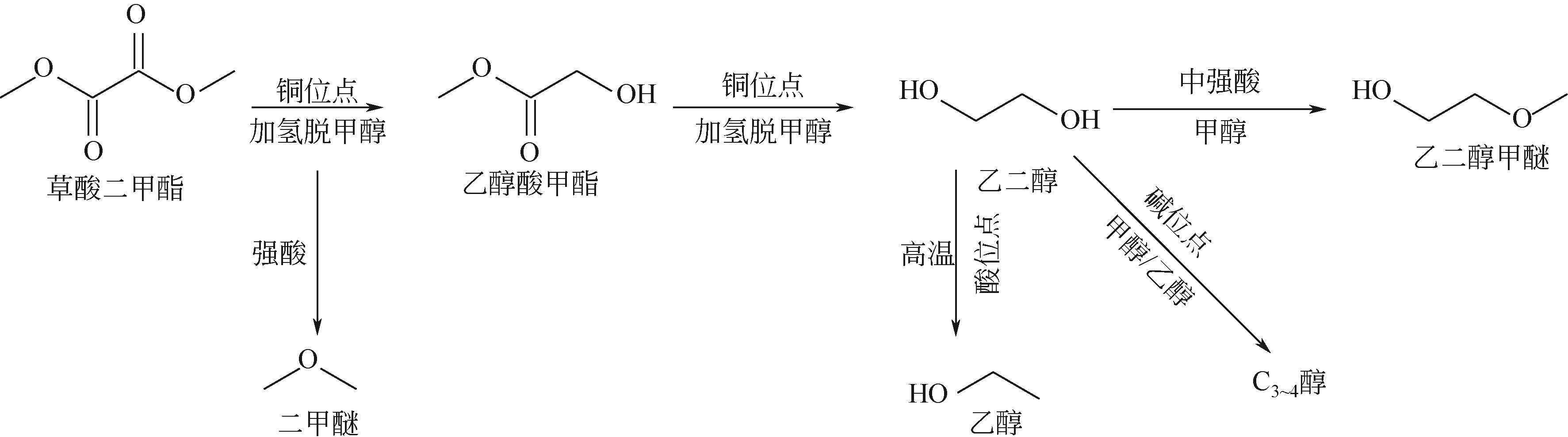
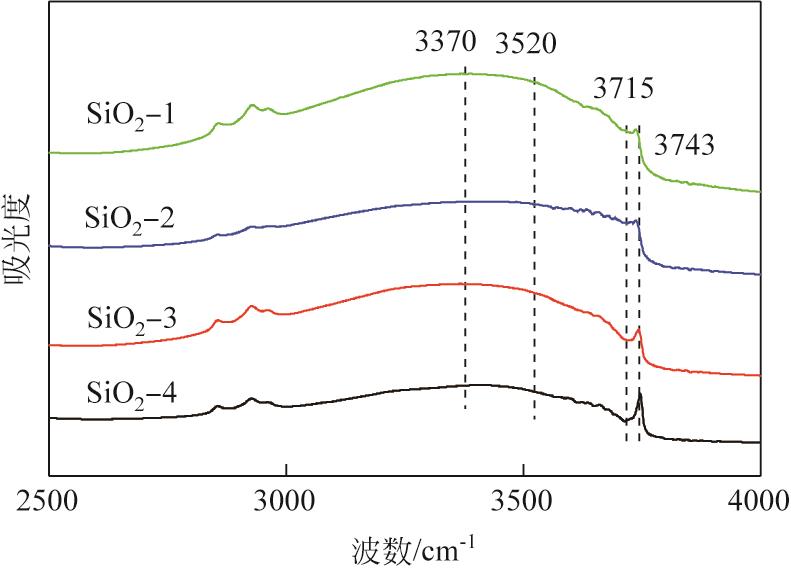
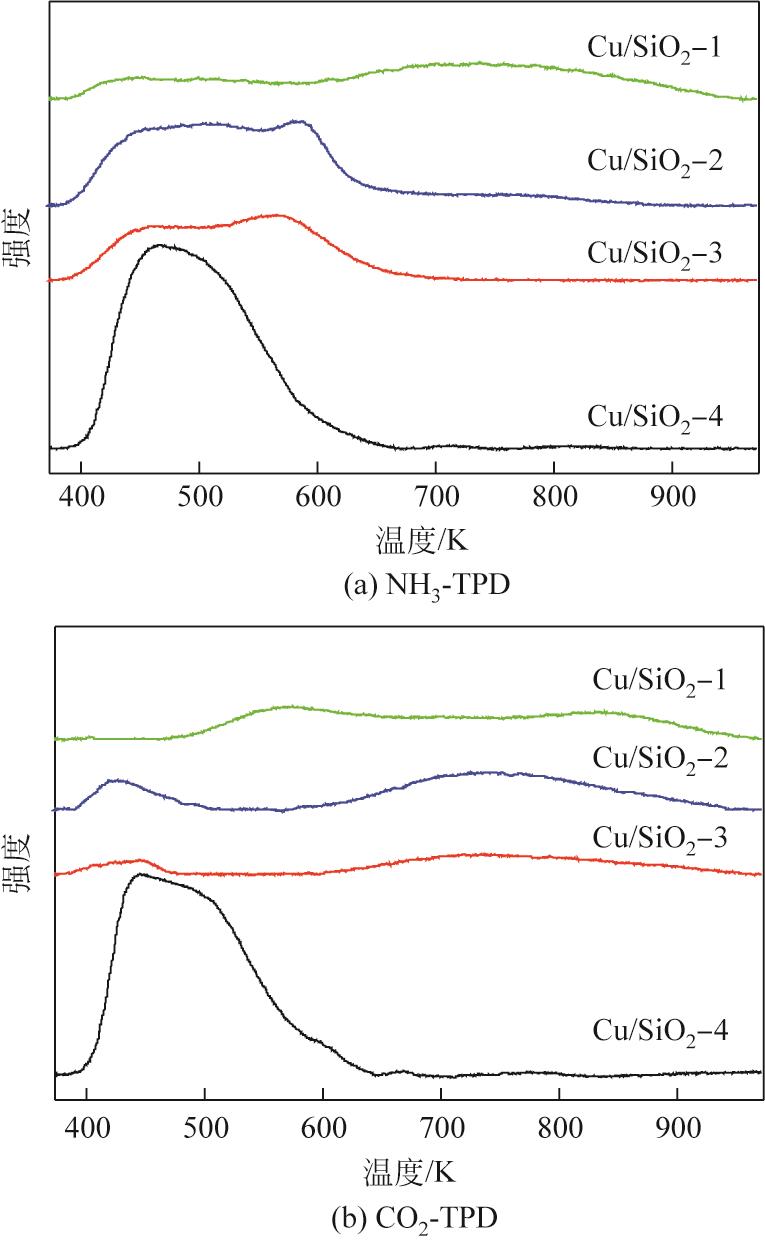
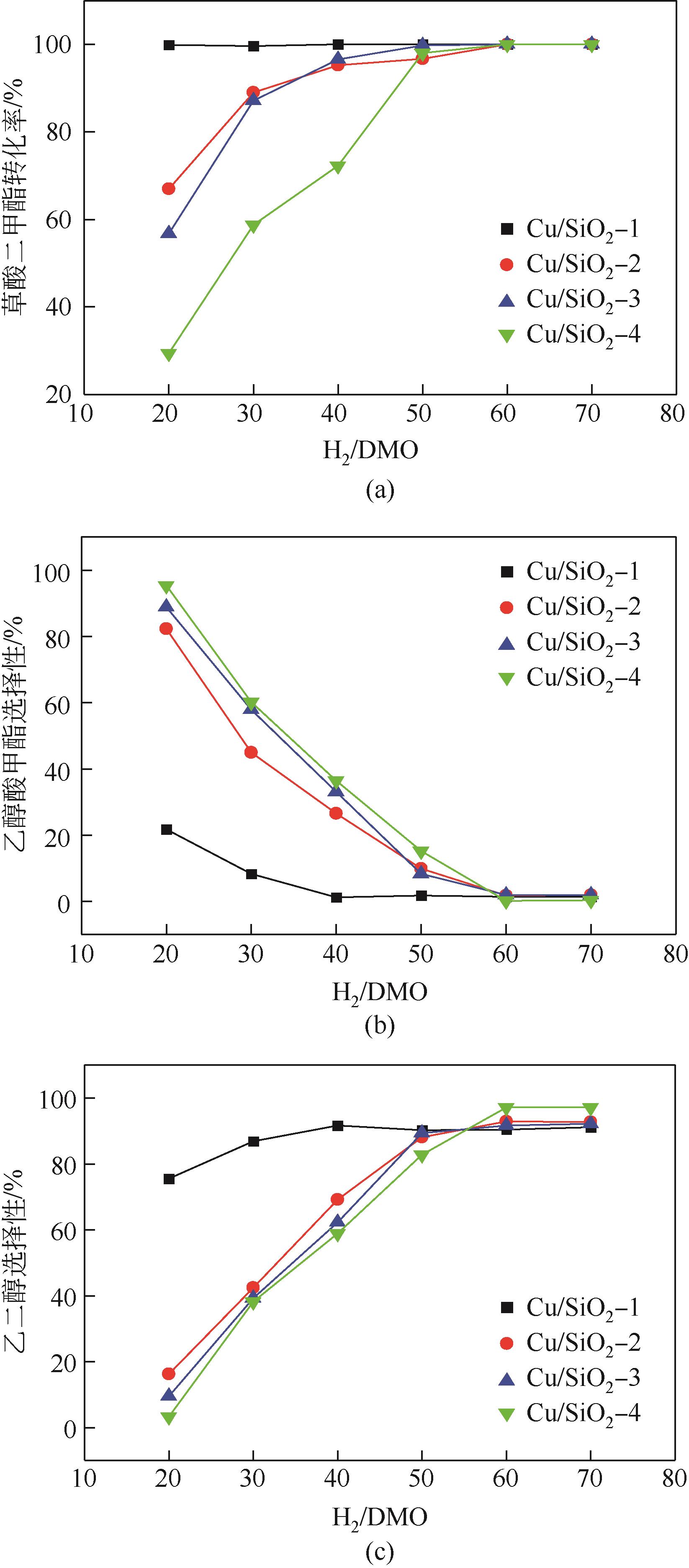
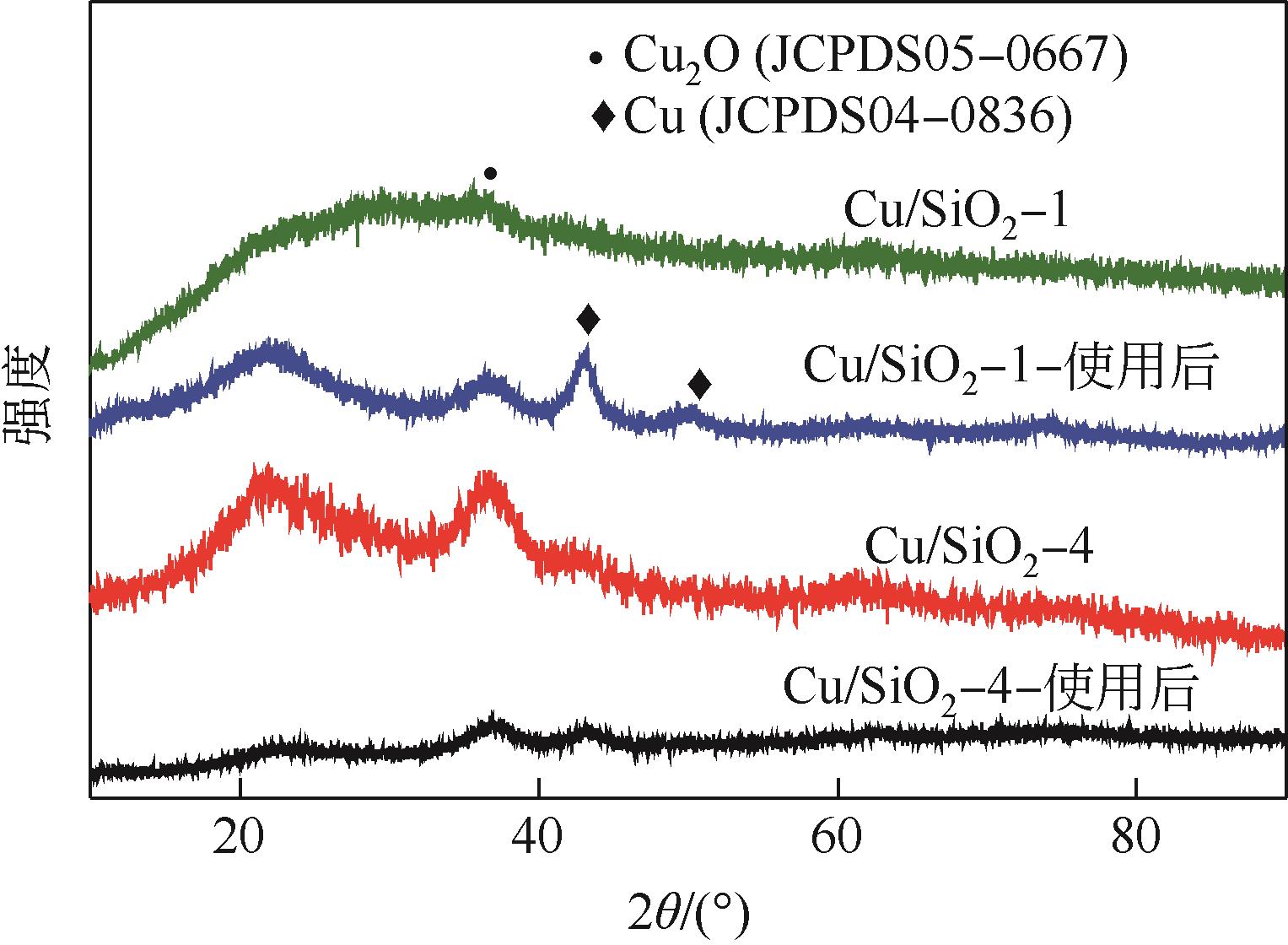
通过程序升温焙烧改变气相纳米二氧化硅表面的羟基含量及种类,并以其为载体,采用蒸氨法制备了Cu/SiO2催化剂,使用比表面积测试(BET)、傅里叶变换红外光谱(FTIR)、漫反射傅里叶变换红外光谱(DRIFT)、X射线衍射(XRD)、透射电子显微镜(TEM)、H2程序升温还原(H2-TPR)、NH3/CO2程序升温脱附(NH3/CO2-TPD)、X射线光电子能谱(XPS)、俄歇电子能谱(AES)等方法研究了Cu/SiO2催化剂的结构和酸碱性,采用固定床反应器在低温(448K)、低压(1.5MPa)的反应条件下进行草酸二甲酯加氢制备乙二醇的反应,评价其催化活性。结果表明,高温焙烧二氧化硅载体可显著改变后续合成Cu/SiO2催化剂的结构并降低其酸碱性,对提高乙二醇选择性和降低草酸二甲酯加氢过程中醇类或醚类副产物的选择性具有明显的促进作用。但同时该过程会导致催化剂的活性降低,载体焙烧(473K)后合成的催化剂均需要提高氢酯比方能获得最佳反应结果。其中经873K焙烧的二氧化硅制备的Cu/SiO2-4催化剂,在最佳反应条件下乙二醇的选择性由低温焙烧后的92%左右提升到97%以上,草酸二甲酯转化率保持在100%。
中图分类号:
引用本文
贠宏飞, 赵鹬, 李贵贤. 载体表面羟基种类对DMO加氢用Cu/SiO2催化剂性能的影响[J]. 化工进展, 2022, 41(12): 6338-6349.
YUN Hongfei, ZHAO Yu, LI Guixian. Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6338-6349.
| 样品 | 铜质量分数 /% | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm | I670/I800 |
|---|---|---|---|---|---|
| Cu/SiO2-1 | 23.69 | 484.5 | 1.15 | 7.51 | 0.32 |
| Cu/SiO2-2 | 22.17 | 460.7 | 1.02 | 6.97 | 0.31 |
| Cu/SiO2-3 | 23.07 | 457.2 | 0.98 | 6.78 | 0.28 |
| Cu/SiO2-4 | 22.21 | 440.6 | 1.03 | 9.4 | 0.38 |
表1 焙烧样品的质构特性
| 样品 | 铜质量分数 /% | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm | I670/I800 |
|---|---|---|---|---|---|
| Cu/SiO2-1 | 23.69 | 484.5 | 1.15 | 7.51 | 0.32 |
| Cu/SiO2-2 | 22.17 | 460.7 | 1.02 | 6.97 | 0.31 |
| Cu/SiO2-3 | 23.07 | 457.2 | 0.98 | 6.78 | 0.28 |
| Cu/SiO2-4 | 22.21 | 440.6 | 1.03 | 9.4 | 0.38 |
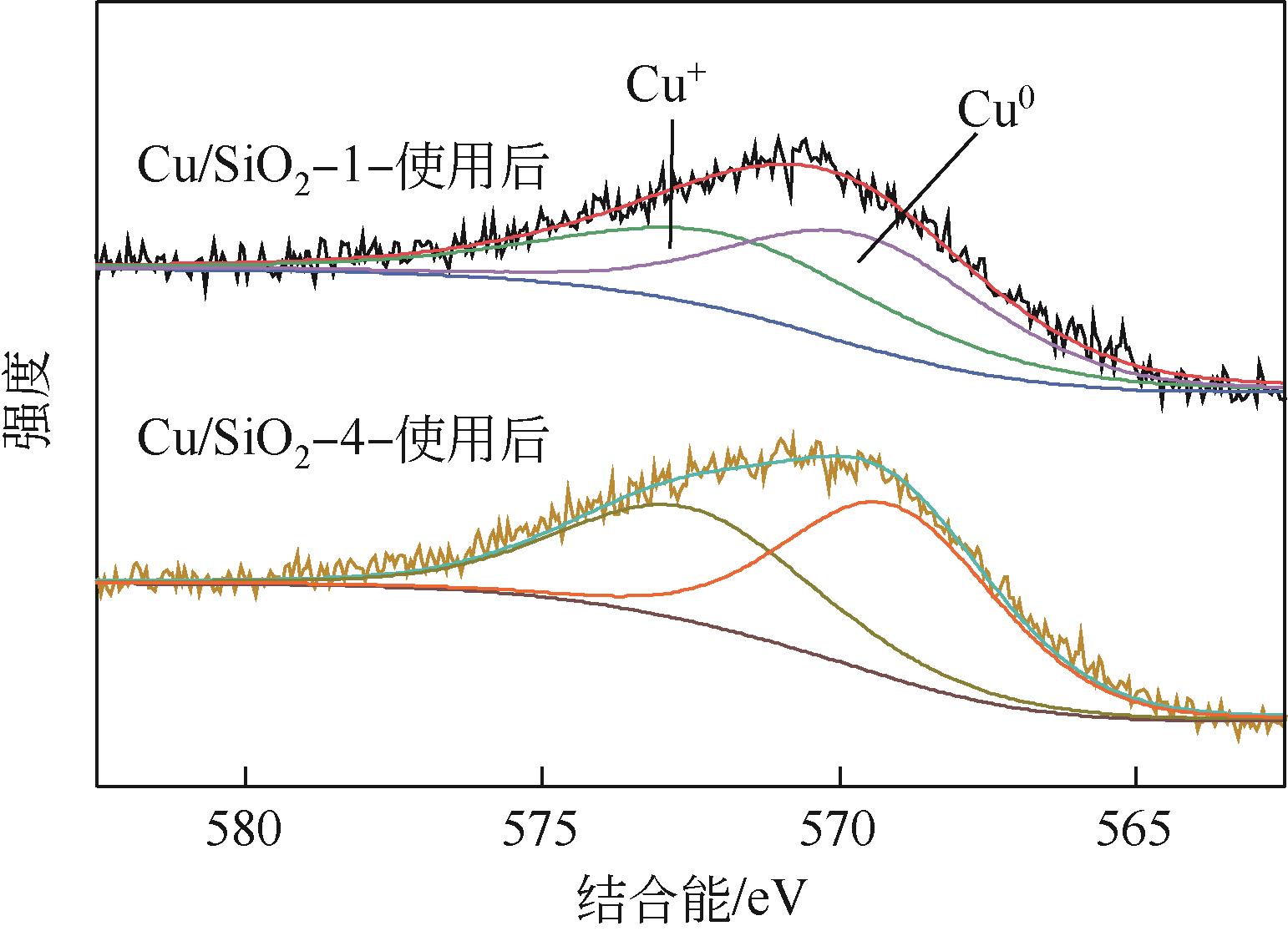
| 样品 | 铜分散度 /% | 铜比表面积 /m2·g-1 | XCu+ /% | XCu+(使用后) /% |
|---|---|---|---|---|
| Cu/SiO2-1 | 58.49 | 16.7 | 52.36 | 48.36 |
| Cu/SiO2-2 | 52.25 | 15.9 | 47.61 | — |
| Cu/SiO2-3 | 52.22 | 15.3 | 44.41 | — |
| Cu/SiO2-4 | 55.01 | 16.5 | 45.86 | 44.42 |
表2 Cu/SiO2-N催化剂样品中的铜种类
| 样品 | 铜分散度 /% | 铜比表面积 /m2·g-1 | XCu+ /% | XCu+(使用后) /% |
|---|---|---|---|---|
| Cu/SiO2-1 | 58.49 | 16.7 | 52.36 | 48.36 |
| Cu/SiO2-2 | 52.25 | 15.9 | 47.61 | — |
| Cu/SiO2-3 | 52.22 | 15.3 | 44.41 | — |
| Cu/SiO2-4 | 55.01 | 16.5 | 45.86 | 44.42 |
| 样品 | 酸浓度/mmol·g-1 | 碱浓度/mmol·g-1 | ||||
|---|---|---|---|---|---|---|
| 弱酸 | 中强酸 | 强酸 | 弱碱 | 中强碱 | 强碱 | |
| Cu/SiO2-1 | 0.069 | 0.074 | 0.368 | 0.183 | 0.064 | 0.171 |
| Cu/SiO2-2 | 0.474 | 0.243 | 0.066 | 0.077 | 0.325 | 0 |
| Cu/SiO2-3 | 0.188 | 0.305 | 0 | 0.033 | 0.179 | 0 |
| Cu/SiO2-4 | 0.178 | 0 | 0 | 0.174 | 0 | 0 |
表3 Cu/SiO2-N催化剂样品中酸碱浓度汇总
| 样品 | 酸浓度/mmol·g-1 | 碱浓度/mmol·g-1 | ||||
|---|---|---|---|---|---|---|
| 弱酸 | 中强酸 | 强酸 | 弱碱 | 中强碱 | 强碱 | |
| Cu/SiO2-1 | 0.069 | 0.074 | 0.368 | 0.183 | 0.064 | 0.171 |
| Cu/SiO2-2 | 0.474 | 0.243 | 0.066 | 0.077 | 0.325 | 0 |
| Cu/SiO2-3 | 0.188 | 0.305 | 0 | 0.033 | 0.179 | 0 |
| Cu/SiO2-4 | 0.178 | 0 | 0 | 0.174 | 0 | 0 |
| 样品 | 草酸二甲酯转化率/% | 乙醇含量/% | 乙醇酸甲酯选择性/% | 乙二醇选择性/% | 乙二醇甲醚含量/% | C3~4OH含量/% | 其他产物含量/% |
|---|---|---|---|---|---|---|---|
| Cu/SiO2-1 | 100 | 1.06 | 1.14 | 91.55 | 0.31 | 4.76 | 5.94 |
| Cu/SiO2-2 | 100 | 0.48 | 1.72 | 92.76 | 1.48 | 1.74 | 1.82 |
| Cu/SiO2-3 | 100 | 0.53 | 1.83 | 91.68 | 2.52 | 0.55 | 2.89 |
| Cu/SiO2-4 | 100 | 0.76 | 0.15 | 97.4 | 0.15 | 0.7 | 0.84 |
表4 最佳反应条件下催化剂Cu/SiO2-N催化DMO加氢性能对比
| 样品 | 草酸二甲酯转化率/% | 乙醇含量/% | 乙醇酸甲酯选择性/% | 乙二醇选择性/% | 乙二醇甲醚含量/% | C3~4OH含量/% | 其他产物含量/% |
|---|---|---|---|---|---|---|---|
| Cu/SiO2-1 | 100 | 1.06 | 1.14 | 91.55 | 0.31 | 4.76 | 5.94 |
| Cu/SiO2-2 | 100 | 0.48 | 1.72 | 92.76 | 1.48 | 1.74 | 1.82 |
| Cu/SiO2-3 | 100 | 0.53 | 1.83 | 91.68 | 2.52 | 0.55 | 2.89 |
| Cu/SiO2-4 | 100 | 0.76 | 0.15 | 97.4 | 0.15 | 0.7 | 0.84 |
| 1 | YUE Hairong, ZHAO Yujun, MA Xinbin, et al. Ethylene glycol: properties, synthesis, and applications[J]. Chemical Society Reviews, 2012, 41(11): 4218-4244. |
| 2 | JIN Erlei, HE Leilei, ZHANG Yulong, et al. A nanostructured CeO2 promoted Pd/α-alumina diethyl oxalate catalyst with high activity and stability[J]. RSC Advances, 2014, 4(90): 48901-48904. |
| 3 | YANG Qing, YANG Qingchun, XU Simin, et al. Technoeconomic and environmental analysis of ethylene glycol production from coal and natural gas compared with oil-based production[J]. Journal of Cleaner Production, 2020, 273: 123120-123135. |
| 4 | WANG Zhiqiao, XU Zhongning, PENG Siyan, et al. New catalysts for coal to ethylene glycol[J]. Chinese Journal of Chemistry, 2017, 35(6): 759-768. |
| 5 | LIN Haiqiang, ZHENG Xinlei, HE Zhe, et al. Cu/SiO2 hybrid catalysts containing HZSM-5 with enhanced activity and stability for selective hydrogenation of dimethyl oxalate to ethylene glycol[J]. Applied Catalysis A: General, 2012, 445: 287-296. |
| 6 | WANG Shurong, LI Xinbao, YIN Qianqian, et al. Highly active and selective Cu/SiO2 catalysts prepared by the urea hydrolysis method in dimethyl oxalate hydrogenation[J]. Catalysis Communications, 2011, 12(13): 1246-1250. |
| 7 | YIN Anyuan, GUO Xiuying, DAI Weilin, et al. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: new insights on the synergetic effect between Cu0 and Cu+ [J]. The Journal of Physical Chemistry C, 2009, 113(25): 11003-11013. |
| 8 | ZHANG Yajing, ZHENG Na, WANG Kangjun, et al. Effect of copper nanoparticles dispersion on catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Nanomaterials, 2013(1): 629375-1-629375-8. |
| 9 | ZHANG Shaoyan, LIU Quanyao, FAN Guoli, et al. Highly-dispersed copper-based catalysts from Cu-Zn-Al layered double hydroxide precursor for gas-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. Catalysis Letters, 2012, 142(9): 1121-1127. |
| 10 | WANG Bin, WEN Chao, CUI Yuanyuan, et al. Remarkable crystal phase effect of Cu/TiO2 catalysts on the selective hydrogenation of dimethyl oxalate[J]. RSC Advances, 2015, 5(37): 29040-29047. |
| 11 | ZHANG Chuancai, WANG Denghao, ZHU Mingyuan, et al. Plasma-enhanced copper dispersion and activity performance of Cu-Ni/ZrO2 catalyst for dimethyl oxalate hydrogenation[J]. Catalysis Communications, 2017, 102: 31-34. |
| 12 | Xiaoguang SAN, ZHAO Guodong, WANG Guosheng, et al. Synthesis of Cu/ZnO flower-like hierarchical porous structures and investigation of their catalytic performance for dimethyl oxalate hydrogenation[J]. China Petroleum Processing& Petrochemical Technology, 2017, 19(2): 40-47. |
| 13 | KONG Xiangpeng, CHEN Zheng, WU Yuehuan, et al. Synthesis of Cu-Mg/ZnO catalysts and catalysis in dimethyl oxalate hydrogenation to ethylene glycol: enhanced catalytic behavior in the presence of a Mg2+ dopant[J]. RSC Advances, 2017, 7(78): 49548-49561. |
| 14 | WANG Yue, SHEN Yongli, ZHAO Yujun, et al. Insight into the balancing effect of active Cu species for hydrogenation of carbon-oxygen bonds[J]. ACS Catalysis, 2015, 5(10): 6200-6208. |
| 15 | LI Siming, WANG Yue, ZHANG Jian, et al. Kinetics study of hydrogenation of dimethyl oxalate over Cu/SiO2 catalyst[J]. Industrial & Engineering Chemistry Research, 2015, 54(4): 1243-1250. |
| 16 | ZHU Yifeng, ZHU Yulei, DING Guoqiang, et al. Highly selective synthesis of ethylene glycol and ethanol via hydrogenation of dimethyl oxalate on Cu catalysts: influence of support[J]. Applied Catalysis A: General, 2013, 468: 296-304. |
| 17 | CHEN Liangfeng, GUO Pingjun, QIAO Minghua, et al. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Catalysis, 2008, 257(1): 172-180. |
| 18 | KOHLER M A, CURRY-HYDE H E, HUGHES A E, et al. The structure of CuSiO2 catalysts prepared by the ion-exchange technique[J]. Journal of Catalysis, 1987, 108(2): 323-333. |
| 19 | TOUPANCE T, KERMAREC M, LAMBERT J F, et al. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption[J]. Journal of Physical Chemistry B, 2002, 106(9): 2277-2286. |
| 20 | YIN Anyuan, GUO Xiuying, FAN Kangnian, et al. Ion-exchange temperature effect on Cu/HMS catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. ChemCatChem, 2010, 2(2): 206-213. |
| 21 | WANG Meilin, YAO Dawei, LI Antai, et al. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification[J]. Industrial & Engineering Chemistry Research, 2020, 59(20): 9414-9422. |
| 22 | CRÉPEAU G, Montouillout V, VIMONT A, et al. Nature, structure and strength of the acidic sites of amorphous silica alumina: an IR and NMR study[J]. The Journal of Physical Chemistry B, 2006, 110(31): 15172-15185. |
| 23 | SONG Yanbo, ZHANG Jian, Jing LYU, et al. Hydrogenation of dimethyl oxalate over copper-based catalysts: acid-base properties and reaction paths[J]. Industrial & Engineering Chemistry Research, 2015, 54(40): 9699-9707. |
| 24 | BURNEAU André, CARTERET Cédric. Near infrared and ab initio study of the vibrational modes of isolated silanol on silica[J]. Physical Chemistry Chemical Physics, 2000, 2(14): 3217-3226. |
| 25 | BUSH S G, JORGENSON J W. Confirmation and application of transmission near infrared absorption technique for absolute quantitation of functional groups on silica gel[J]. Journal of Chromatography A, 1990, 503(1): 69-91. |
| 26 | CHRISTY A A. New insights into the surface functionalities and adsorption evolution of water molecules on silica gel surface: a study by second derivative near infrared spectroscopy[J]. Vibrational Spectroscopy, 2010, 54(1): 42-49. |
| 27 | ZHAO Yujun, ZHANG Yaqing, WANG Yue, et al. Structure evolution of mesoporous silica supported copper catalyst for dimethyl oxalate hydrogenation[J]. Applied Catalysis A: General, 2017, 539: 59-69. |
| 28 | D'SOUZA A S, PANTANO C G. Mechanisms for silanol formation on amorphous silica fracture surfaces[J]. Journal of the American Ceramic Society, 1999, 82(5): 1289-1293. |
| 29 | WANG Rongwei, WUNDER S L. Effects of silanol density, distribution, and hydration state of fumed silica on the formation of self-assembled monolayers of n-octadecyltrichlorosilane[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2000, 16(11): 5008-5016. |
| 30 | SAUER J, HILL J R. The acidity of surface silanol groups: a theoretical estimate based on ab initio calculations on a model surface[J]. Chemical Physics Letters, 1994, 218(4): 333-337. |
| 31 | SINDORF D W, MACIEL G E. 29Si NMR study of dehydrated/rehydrated silica-gel using cross-polarization and magic-angle spinning[J]. Journal of the American Chemical Society, 1983, 105(6): 1487-1493. |
| 32 | YOUNG G J. Interaction of water vapor with silica surfaces[J]. Journal of Colloid Science, 1958, 13(1): 67-85. |
| 33 | WANG Yue, YANG Wenlong, YAO Dawei, et al. Effect of surface hydroxyl group of ultra-small silica on the chemical states of copper catalyst for dimethyl oxalate hydrogenation[J]. Catalysis Today, 2020, 350: 127-135. |
| 34 | MUSTER T H, PRESTIDGE C A, HAYES R A. Water adsorption kinetics and contact angles of silica particles[J]. Colloids & Surfaces A: Physicochemical & Engineering Aspects, 2001, 176(2/3): 253-266. |
| 35 | DING Jie, POPA Tiberiu, TANG Jinke, et al. Highly selective and stable Cu/SiO2 catalysts prepared with a green method for hydrogenation of diethyl oxalate into ethylene glycol[J]. Applied Catalysis B: Environmental, 2017, 209: 530-542. |
| 36 | ZHENG Jianwei, ZHOU Junfu, LIN Haiqiang, et al. CO-mediated deactivation mechanism of SiO2-supported copper catalysts during dimethyl oxalate hydrogenation to ethylene glycol[J]. The Journal of Physical Chemistry C, 2015, 119(24): 13758-13766. |
| 37 | VAN DER GRIFT C J G, WIELERS A F H, MULDER A, et al. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation[J]. Thermochimica Acta, 1990, 171: 95-113. |
| 38 | SULPIZI M, GAIGEOT M P, SPRIK M. The silica-water interface: how the silanols determine the surface acidity and modulate the water properties[J]. Journal of Chemical Theory and Computation, 2012, 8(3): 1037-1047. |
| 39 | MA Xiangang, YANG Zhiqiang, LIU Xuebin, et al. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation[J]. RSC Advances, 2015, 5(47): 37581-37584. |
| 40 | LI Feng, LU Chunshan, LI Xiaonian. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chinese Chemical Letters, 2014, 25(11): 1461-1465. |
| 41 | YIN Anyuan, GUO Xiuying, FAN Kangnian, et al. Influence of copper precursors on the structure evolution and catalytic performance of Cu/HMS catalysts in the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Applied Catalysis A: General, 2010, 377(1/2): 128-133. |
| [1] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [2] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [5] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||