化工进展 ›› 2021, Vol. 40 ›› Issue (8): 4600-4609.DOI: 10.16085/j.issn.1000-6613.2020-2303
用于水中有机污染物处理的Co3O4/TiO2/多孔炭复合材料
- 福州大学至诚学院,福建 福州 350002
-
收稿日期:2020-11-18出版日期:2021-08-05发布日期:2021-08-12 -
通讯作者:张书泉 -
作者简介:张书泉(1984—),男,博士,副教授,主要从事功能复合材料在能源与环境方面应用的研究。E-mail:sqzhang@fzu.edu.cn 。 -
基金资助:福建省自然科学基金(2018J01431);福建省教育厅中青年教师教育科研项目(JT180814);国家级大学生创新训练计划(202013470002);福建省高校杰出青年科研人才培育计划(ZJ1751)
Co3O4/TiO2/porous carbon for organic wastewater treatment
ZHANG Shuquan( ), XU Min, WANG Guotao
), XU Min, WANG Guotao
- College of Zhicheng, Fuzhou University, Fuzhou 350002, Fujian, China
-
Received:2020-11-18Online:2021-08-05Published:2021-08-12 -
Contact:ZHANG Shuquan
摘要:
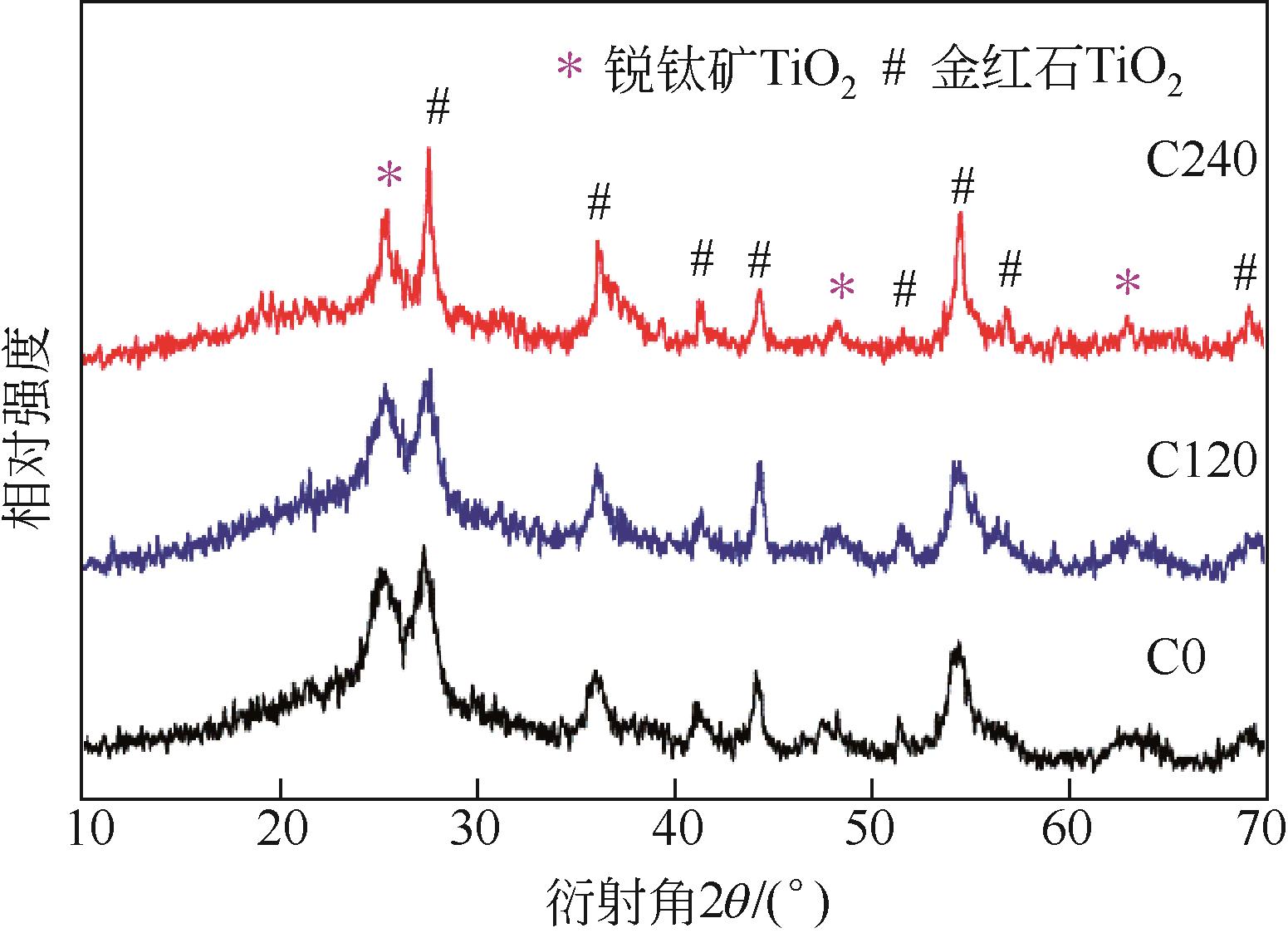
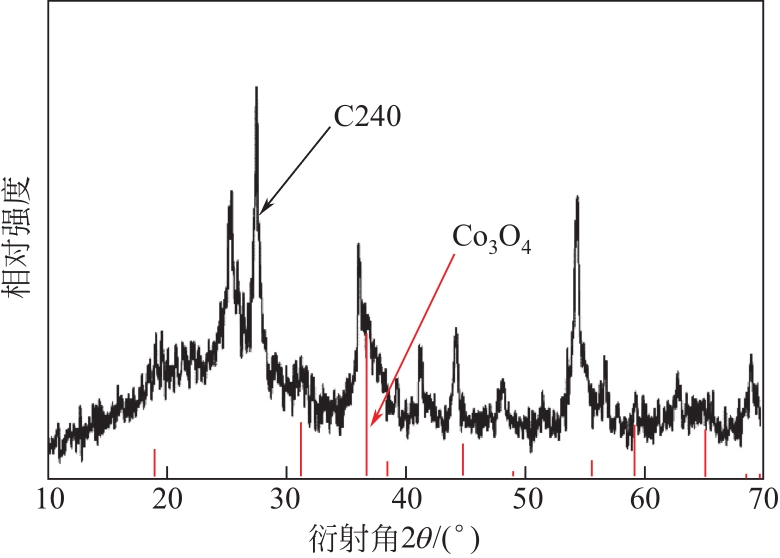
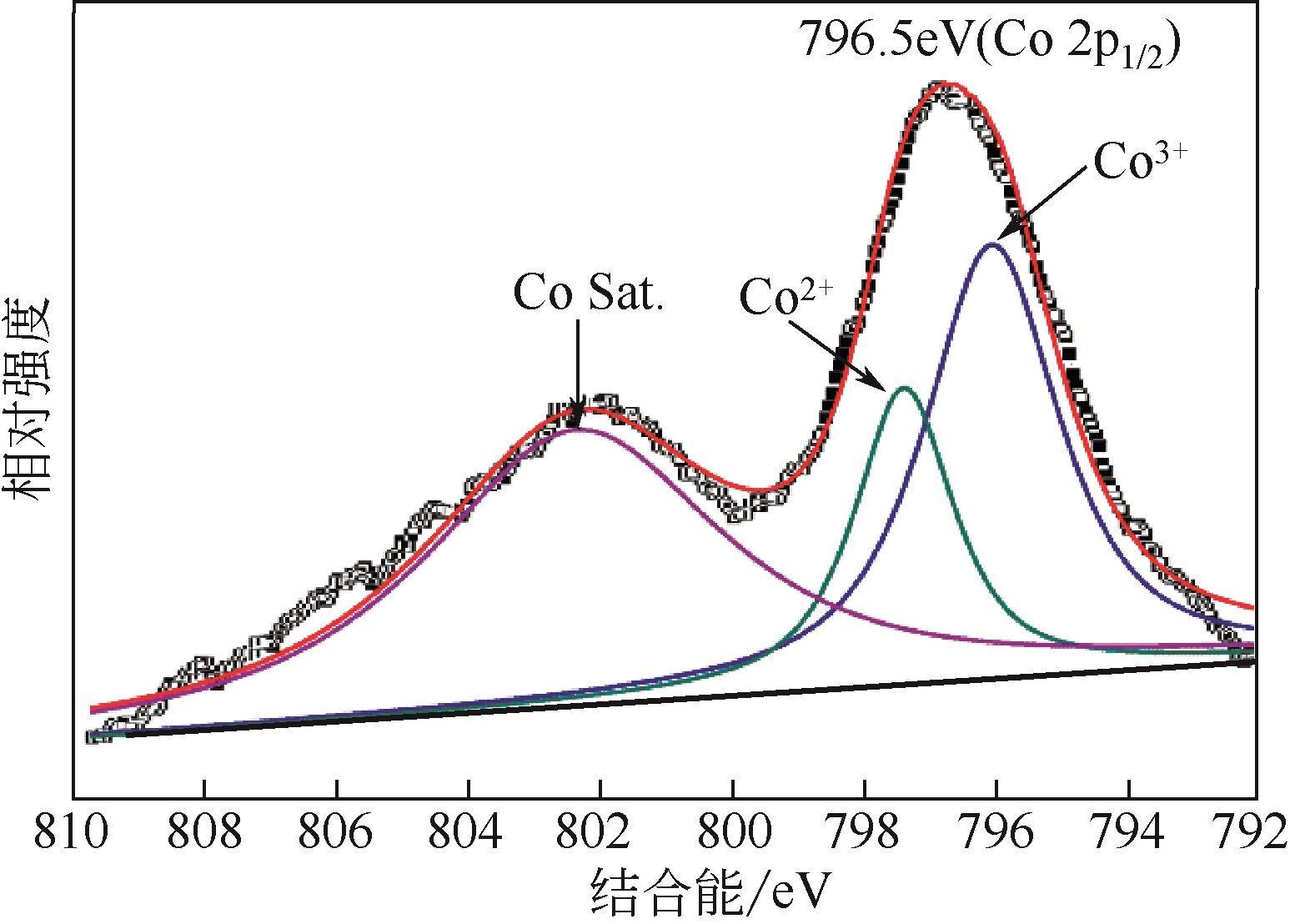
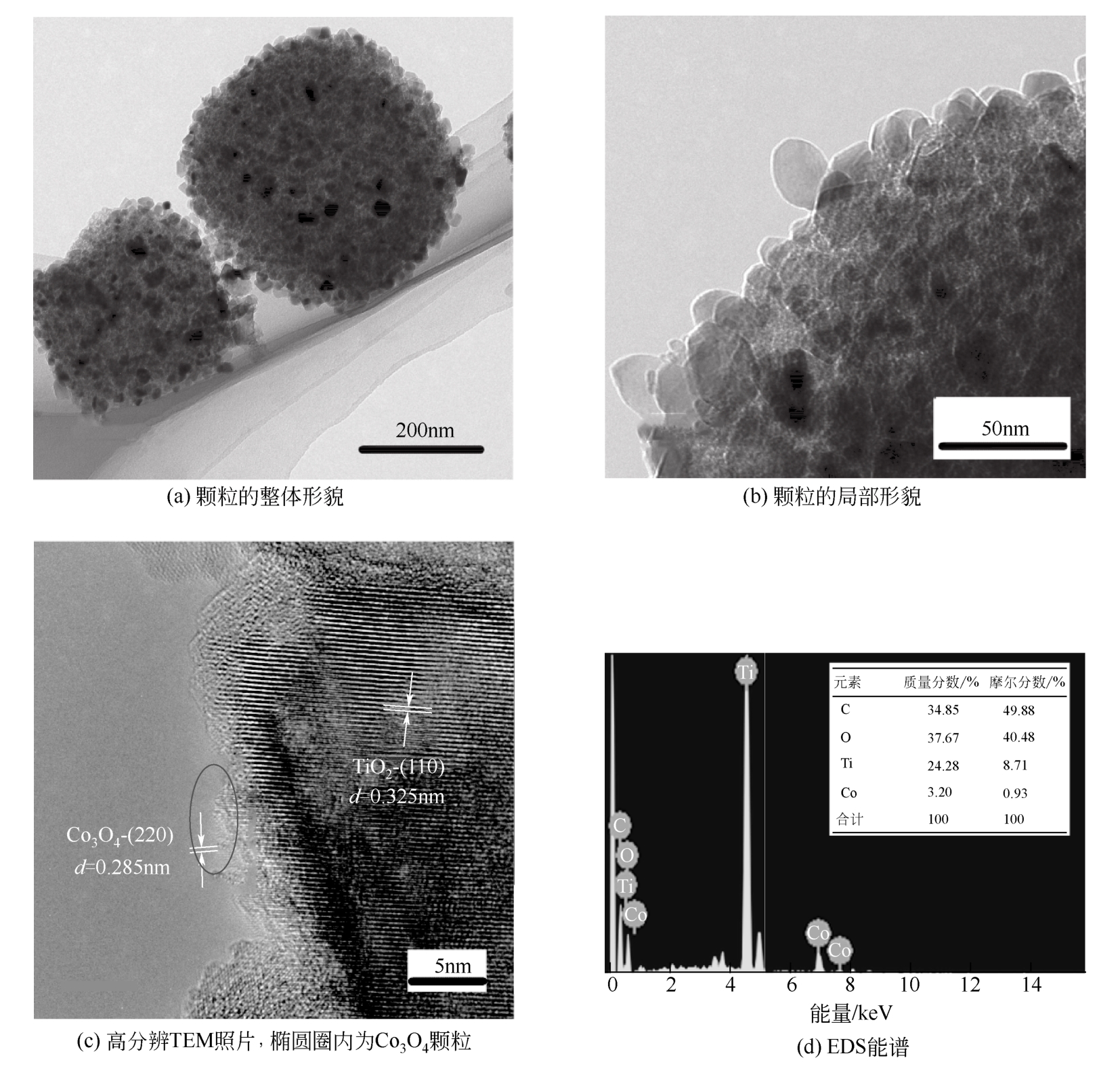
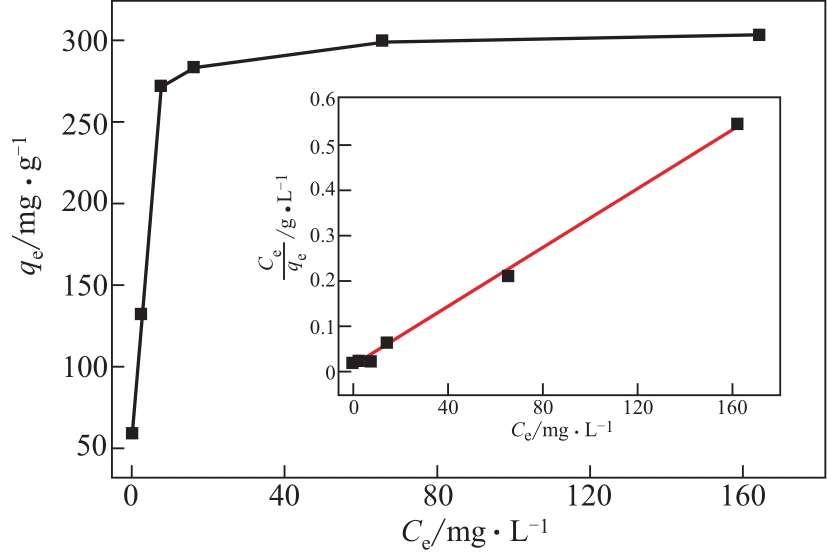
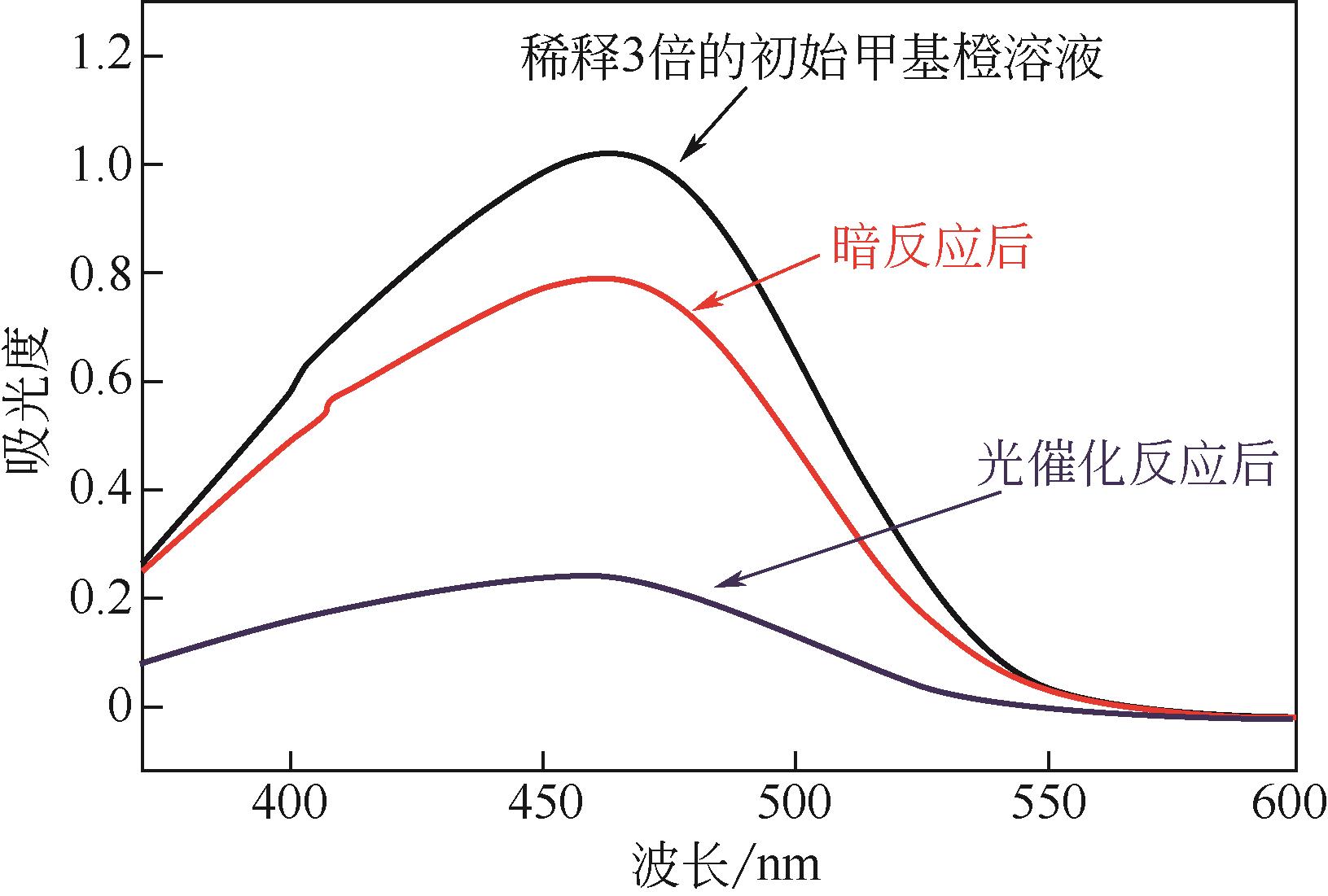
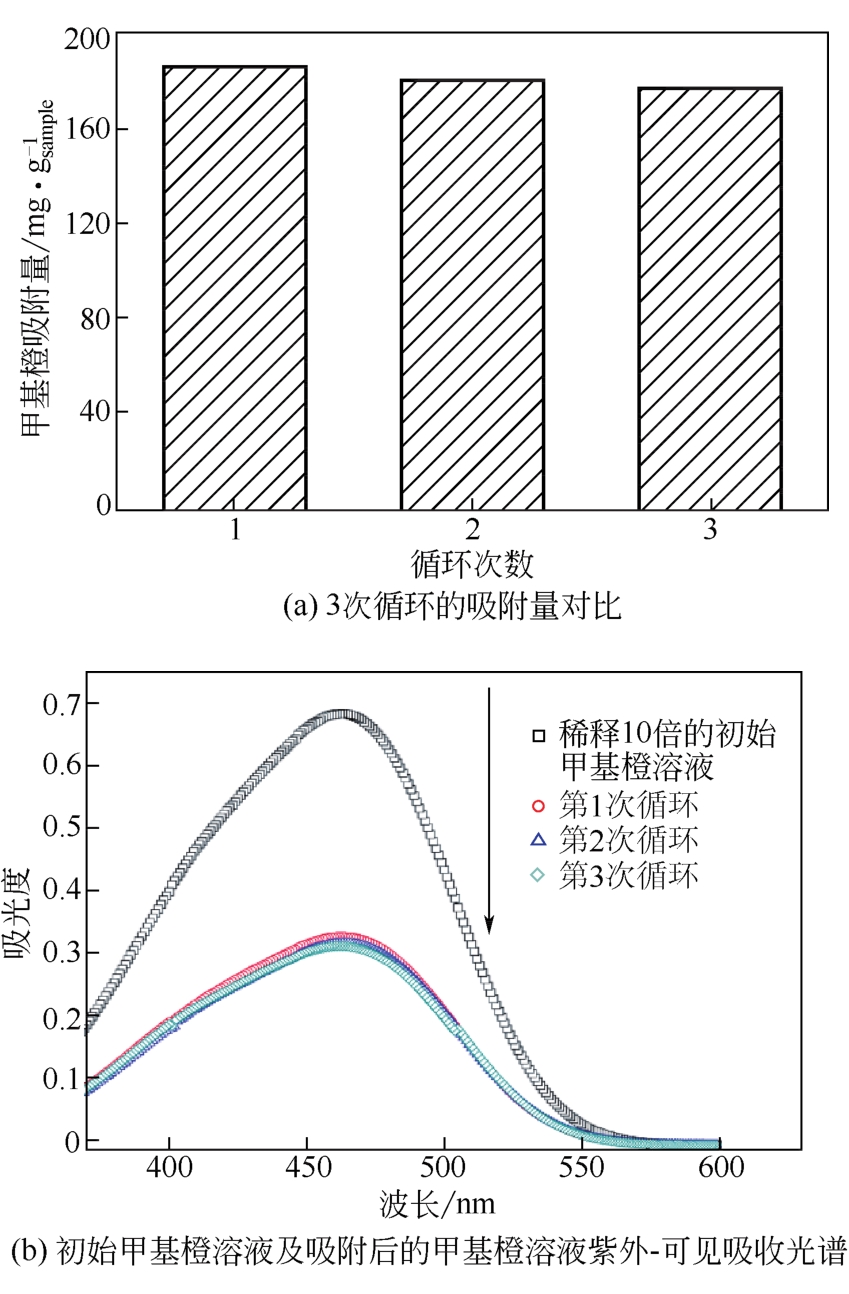
具有高吸附、强降解、易回收特性的新型功能材料在治理水中有机污染物上具有广泛的应用前景。本研究以多孔Ti基MOF材料NH2-MIL-125(Ti)为前体,通过后修饰方法在孔洞中引入Co2+,再通过高温炭化制备得到Co3O4/TiO2/多孔炭复合材料,并通过多种手段对复合材料进行了表征。实验用甲基橙模拟水中有机污染物,研究了复合材料对甲基橙的吸附性能、光催化降解性能及回收再利用能力。研究结果表明复合材料对甲基橙的吸附过程符合准二级动力学方程,其吸附能力随着复合材料中Co3O4含量先增加后减小,C120(5.23% Co3O4/48.72%TiO2/多孔炭)具有最强的平衡吸附量,达到273.22mg/gsample。同时该样品对甲基橙也具有较强的光催化降解能力,并且由于Co3O4的强磁性,可以很容易地通过外磁场从水中分离。本工作为应用于有机废水处理的新型功能材料的开发提供了实验依据。
中图分类号:
引用本文
张书泉, 徐敏, 王国滔. 用于水中有机污染物处理的Co3O4/TiO2/多孔炭复合材料[J]. 化工进展, 2021, 40(8): 4600-4609.
ZHANG Shuquan, XU Min, WANG Guotao. Co3O4/TiO2/porous carbon for organic wastewater treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4600-4609.
| 样品 | 合成时加入的NH2-MIL-125(Ti) 质量/mg | 合成时加入的Co(NO3)2·6H2O质量/mg | 实测样品中TiO2的 质量分数/% | 实测样品中Co3O4的 质量分数/% |
|---|---|---|---|---|
| C0 | 800 | 0 | 52.48 | 0 |
| C80 | 800 | 80 | 49.66 | 3.49 |
| C120 | 800 | 120 | 48.72 | 5.23 |
| C160 | 800 | 160 | 48.87 | 6.91 |
| C200 | 800 | 200 | 46..69 | 8.56 |
| C240 | 800 | 240 | 45.73 | 9.87 |
表1 样品制备时加入各原料含量以及实测样品中各组分含量
| 样品 | 合成时加入的NH2-MIL-125(Ti) 质量/mg | 合成时加入的Co(NO3)2·6H2O质量/mg | 实测样品中TiO2的 质量分数/% | 实测样品中Co3O4的 质量分数/% |
|---|---|---|---|---|
| C0 | 800 | 0 | 52.48 | 0 |
| C80 | 800 | 80 | 49.66 | 3.49 |
| C120 | 800 | 120 | 48.72 | 5.23 |
| C160 | 800 | 160 | 48.87 | 6.91 |
| C200 | 800 | 200 | 46..69 | 8.56 |
| C240 | 800 | 240 | 45.73 | 9.87 |
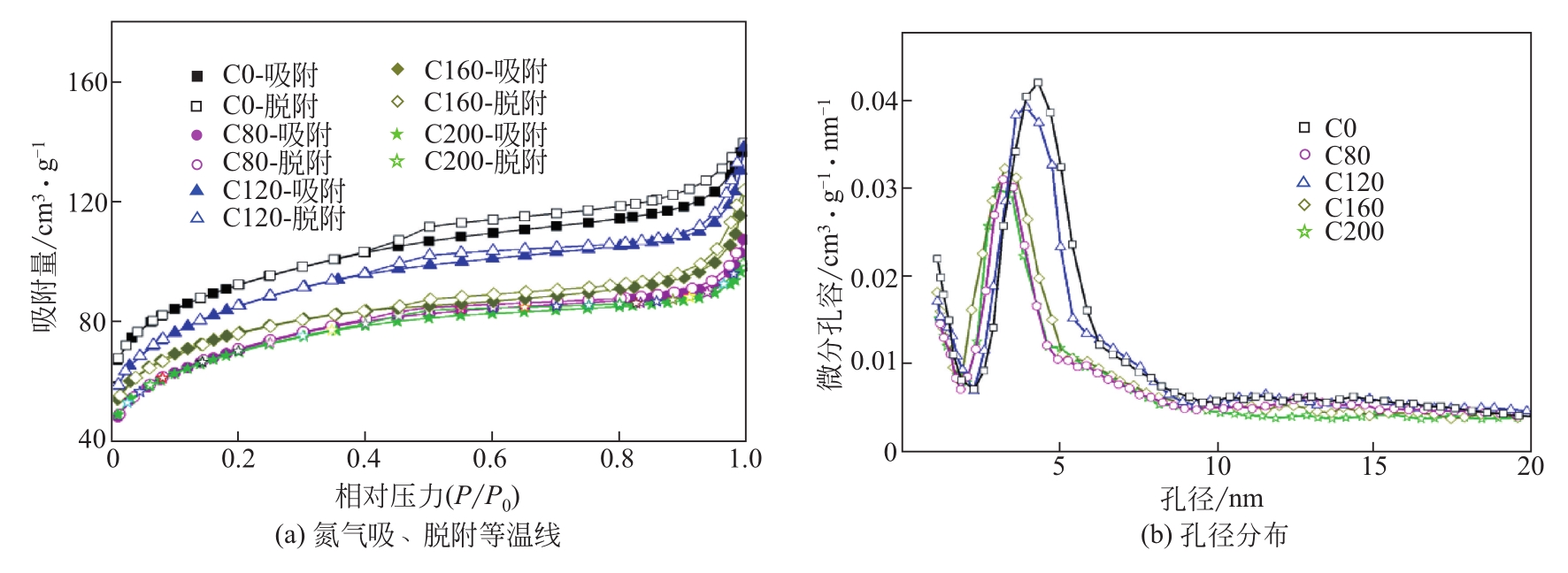
| 样品 | 比表面积/m2?g-1 | 孔容/cm3?g-1 |
|---|---|---|
| C0 | 298 | 0.217 |
| C80 | 238 | 0.172 |
| C120 | 279 | 0.213 |
| C160 | 247 | 0.190 |
| C200 | 230 | 0.154 |
表2 不同样品的比表面积及孔容
| 样品 | 比表面积/m2?g-1 | 孔容/cm3?g-1 |
|---|---|---|
| C0 | 298 | 0.217 |
| C80 | 238 | 0.172 |
| C120 | 279 | 0.213 |
| C160 | 247 | 0.190 |
| C200 | 230 | 0.154 |
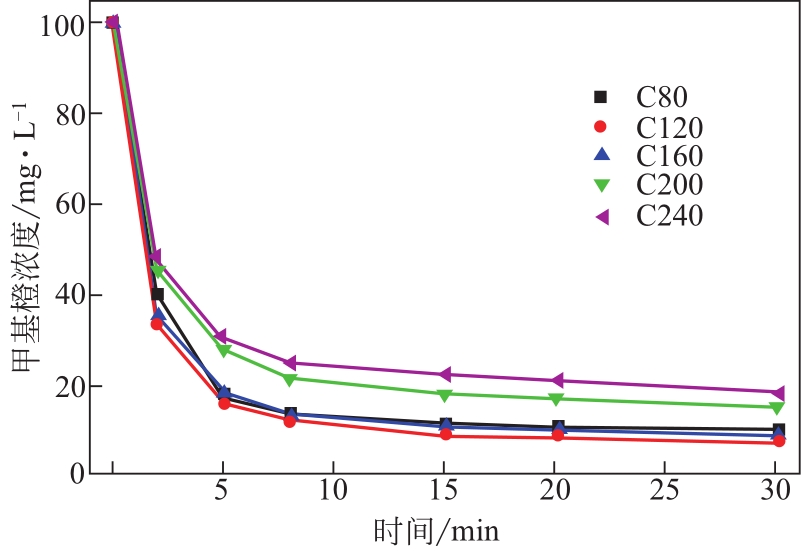
| 样品 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附速率常数k2/g sample·mg-1·min-1 | 相关系数R2/% |
|---|---|---|---|
| C80 | 252.53 | 2.91×10-3 | 99.7 |
| C120 | 273.22 | 2.55×10-3 | 99.9 |
| C160 | 253.81 | 2.91×10-3 | 99.9 |
| C200 | 164.47 | 6.50×10-3 | 99.5 |
| C240 | 144.09 | 8.16×10-3 | 99.7 |
表3 Co3O4/TiO2/多孔炭对甲基橙吸附的准二级动力学常数
| 样品 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附速率常数k2/g sample·mg-1·min-1 | 相关系数R2/% |
|---|---|---|---|
| C80 | 252.53 | 2.91×10-3 | 99.7 |
| C120 | 273.22 | 2.55×10-3 | 99.9 |
| C160 | 253.81 | 2.91×10-3 | 99.9 |
| C200 | 164.47 | 6.50×10-3 | 99.5 |
| C240 | 144.09 | 8.16×10-3 | 99.7 |
| 吸附剂 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附条件 | 参考文献 |
|---|---|---|---|
| Ni/多孔炭/碳纳米管 | 271 | 5mg吸附剂于10mL MO水溶液(20~350mg·L-1);吸附时间60min;吸附温度室温 | [ |
| (Fe2O3,Fe3C,Fe)@多孔炭 | 183 | 10mg吸附剂于20mL MO水溶液(12.5~400mg·L-1);吸附时间90min;吸附温度室温 | [ |
| ZnFe2O4/木质素衍生炭 | 113 | 20mg吸附剂于20mL MO水溶液(20~180mg·L-1);吸附时间160min;吸附温度293K;pH=5 | [ |
| Fe负载多孔炭 | 187 | 20mg吸附剂于50mL MO水溶液(10~100mg·L-1);吸附时间60min;吸附温度室温;pH=7 | [ |
| Fe2O3/Mn3O4 | 333 | 200mg吸附剂于200mL MO水溶液(60~1000mg·L-1);吸附时间45min;吸附温度室温;pH=2 | [ |
| SiO2@Fe3O4 | 240 | 5mg吸附剂于10mL MO水溶液(0~100mg·L-1);吸附时间180min;吸附温度293K;pH=5.5 | [ |
| NiFe2O4/活性炭 | 183 | 300mg吸附剂于100mL MO水溶液(0~200mg·L-1);吸附时间30min;吸附温度303K;pH=3 | [ |
| Fe3O4/季铵壳聚糖衍生物 | 267 | 100mg吸附剂于100mL MO水溶液(50~300mg·L-1);吸附时间60min;吸附温度293K | [ |
| Fe3O4/PPY | 149 | 10mg吸附剂于10mL MO水溶液(60~320mg·L-1);吸附时间90min;吸附温度298K | [ |
| LnBDC/活性炭 | 125 | 5mg吸附剂于10mL MO水溶液(0~200mg·L-1);吸附时间1440min;吸附温度303K;pH=5 | [ |
| Co3O4/TiO2/多孔炭 | 273 | 50mg吸附剂于100mL MO水溶液(30~300mg·L-1);吸附时间30min;吸附温度室温 | 本工作 |
表4 不同磁性吸附剂对甲基橙的吸附能力
| 吸附剂 | 甲基橙的平衡吸附量qe/mg·g-1sample | 吸附条件 | 参考文献 |
|---|---|---|---|
| Ni/多孔炭/碳纳米管 | 271 | 5mg吸附剂于10mL MO水溶液(20~350mg·L-1);吸附时间60min;吸附温度室温 | [ |
| (Fe2O3,Fe3C,Fe)@多孔炭 | 183 | 10mg吸附剂于20mL MO水溶液(12.5~400mg·L-1);吸附时间90min;吸附温度室温 | [ |
| ZnFe2O4/木质素衍生炭 | 113 | 20mg吸附剂于20mL MO水溶液(20~180mg·L-1);吸附时间160min;吸附温度293K;pH=5 | [ |
| Fe负载多孔炭 | 187 | 20mg吸附剂于50mL MO水溶液(10~100mg·L-1);吸附时间60min;吸附温度室温;pH=7 | [ |
| Fe2O3/Mn3O4 | 333 | 200mg吸附剂于200mL MO水溶液(60~1000mg·L-1);吸附时间45min;吸附温度室温;pH=2 | [ |
| SiO2@Fe3O4 | 240 | 5mg吸附剂于10mL MO水溶液(0~100mg·L-1);吸附时间180min;吸附温度293K;pH=5.5 | [ |
| NiFe2O4/活性炭 | 183 | 300mg吸附剂于100mL MO水溶液(0~200mg·L-1);吸附时间30min;吸附温度303K;pH=3 | [ |
| Fe3O4/季铵壳聚糖衍生物 | 267 | 100mg吸附剂于100mL MO水溶液(50~300mg·L-1);吸附时间60min;吸附温度293K | [ |
| Fe3O4/PPY | 149 | 10mg吸附剂于10mL MO水溶液(60~320mg·L-1);吸附时间90min;吸附温度298K | [ |
| LnBDC/活性炭 | 125 | 5mg吸附剂于10mL MO水溶液(0~200mg·L-1);吸附时间1440min;吸附温度303K;pH=5 | [ |
| Co3O4/TiO2/多孔炭 | 273 | 50mg吸附剂于100mL MO水溶液(30~300mg·L-1);吸附时间30min;吸附温度室温 | 本工作 |
| 样品 | 降解率/% |
|---|---|
| C80 | 70 |
| C120 | 68 |
| C160 | 72 |
| C200 | 61 |
| C240 | 58 |
表5 不同样品对MO的降解效果
| 样品 | 降解率/% |
|---|---|
| C80 | 70 |
| C120 | 68 |
| C160 | 72 |
| C200 | 61 |
| C240 | 58 |
| 1 | KUMAR REDDY D H, LEE S M. Water pollution and treatment technologies[J]. Journal of Environmental & Analytical Toxicology, 2012, 2(5): 1000e103. |
| 2 | NIU L J, WEI T, LI Q G, et al. Ce-based catalysts used in advanced oxidation processes for organic wastewater treatment: a review[J]. Journal of Environmental Sciences, 2020, 96: 109-116. |
| 3 | DONG L J, WU S Y, LI S B, et al. Sorption behaviors and mechanisms of Eu(III) on rice straw-derived biochar[J]. Journal of Inorganic Materials, 2020, 35(3): 390-398.. |
| 4 | HAN H W, RAFIQ M K, ZHOU T Y, et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants[J]. Journal of Hazardous Materials, 2019, 369: 780-796. |
| 5 | OUKIL S, BALI F, HALLICHE D. Adsorption and kinetic studies of methylene blue on modified HUSY zeolite and an amorphous mixture of γ-alumina and silica[J]. Separation Science and Technology, 2020, 55(15): 2642-2658. |
| 6 | ROJAS S, HORCAJADA P. Metal-organic frameworks for the removal of emerging organic contaminants in water[J]. Chemical Reviews, 2020, 120(16): 8378-8415. |
| 7 | BŮŽEK D, ONDRUŠOVÁ S, HYNEK J, et al. Robust aluminum and iron phosphinate metal-organic frameworks for efficient removal of bisphenol A[J]. Inorganic Chemistry, 2020, 59(8): 5538-5545. |
| 8 | LIN K Y A, CHANG H A, CHEN R C. MOF-derived magnetic carbonaceous nanocomposite as a heterogeneous catalyst to activate oxone for decolorization of Rhodamine B in water[J]. Chemosphere, 2015, 130: 66-72. |
| 9 | ZHANG S Q, HAN L, LI L N, et al. A highly symmetric metal-organic framework based on a propeller-like Ru-organic metalloligand for photocatalysis and explosives detection[J]. Crystal Growth & Design, 2013, 13(12): 5466-5472. |
| 10 | ANI I J, AKPAN U G, OLUTOYE M A, et al. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: recent development[J]. Journal of Cleaner Production, 2018, 205: 930-954. |
| 11 | LI Y F, ZHOU M H, CHENG B, et al. Recent advances in g-C3N4-based heterojunction photocatalysts[J]. Journal of Materials Science & Technology, 2020, 56: 1-17. |
| 12 | KAMEGAWA T, KUWAHARA Y, YAMASHITA H. Design of TiO2-loaded porous siliceous materials and application to photocatalytic environmental purification[J]. Journal of the Japan Petroleum Institute, 2016, 59(5): 165-173. |
| 13 | ZHANG D S, CAI H, GAO K Y, et al. Preparation and visible-light photocatalytic degradation on metronidazole of Zn2SiO4-ZnO-biochar composites[J]. Journal of Inorganic Materials, 2020, 35(8): 923-930. |
| 14 | ESRAFILI L, MORSALI A, DEHGHANI F F, et al. Development of porous cobalt-/copper-doped carbon nanohybrids derived from functionalized MOFs as efficient catalysts for the ullmann cross-coupling reaction: insights into the active centers[J]. ACS Applied Materials & Interfaces, 2020, 12(38): 43115-43124. |
| 15 | SUN T T, XU L B, WANG D S, et al. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion[J]. Nano Research, 2019, 12(9): 2067-2080. |
| 16 | RASHEED T, HASSAN A A, BILAL M, et al. Metal-organic frameworks based adsorbents: a review from removal perspective of various environmental contaminants from wastewater[J]. Chemosphere, 2020, 259: 127369. |
| 17 | FU Y, SUN D, CHEN Y, et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction[J]. Angewandte Chemie:International Edition, 2012, 51(14): 3364-3367. |
| 18 | LIU D S, LI M N, LI X C, et al. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation[J]. Chemical Engineering Journal, 2020, 387: 124008. |
| 19 | NIE R F, SHI J J, DU W C, et al. A sandwich N-doped graphene/Co3O4 hybrid: an efficient catalyst for selective oxidation of olefins and alcohols[J]. Journal of Materials Chemistry A, 2013, 1(32): 9037-9045. |
| 20 | DAN-HARDI M, SERRE C, FROT T, et al. A new photoactive crystalline highly porous titanium(Ⅳ) dicarboxylate[J]. Journal of the American Chemical Society, 2009, 131(31): 10857-10859. |
| 21 | ZHU Q L, XIA W, AKITA T, et al. Metal-organic framework-derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction[J]. Advanced Materials, 2016, 28(30): 6391-6398. |
| 22 | HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. |
| 23 | JIN L N, ZHAO X S, QIAN X Y, et al. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes[J]. Journal of Colloid and Interface Science, 2018, 509: 245-253. |
| 24 | WANG L H, KE F, ZHU J F. Metal-organic gel templated synthesis of magnetic porous carbon for highly efficient removal of organic dyes[J]. Dalton Transactions, 2016, 45(11): 4541-4547. |
| 25 | MA Y Z, ZHENG D F, MO Z Y, et al. Magnetic lignin-based carbon nanoparticles and the adsorption for removal of methyl orange[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 559: 226-234. |
| 26 | JIANG T S, FANG W B, ZHAO Q, et al. Synthesis of Fe (Co or Ni) loaded mesoporous carbon composites and their adsorption behaviors for methyl orange[J]. Journal of Nanoscience and Nanotechnology, 2017, 17(8): 5261-5270. |
| 27 | BHOWMIK M, DEB K, DEBNATH A, et al. Mixed phase Fe2O3 /Mn3O4 magnetic nanocomposite for enhanced adsorption of methyl orange dye: neural network modeling and response surface methodology optimization[J]. Applied Organometallic Chemistry, 2018, 32(3): e4186. DOI:10.1002/aoc.4186. |
| 28 | GALLO-CORDOVA A, LEMUS J, PALOMARES F J, et al. Superparamagnetic nanosorbent for water purification: assessment of the adsorptive removal of lead and methyl orange from aqueous solutions[J]. Science of the Total Environment, 2020, 711: 134644. |
| 29 | JIANG T, LIANG Y D, HE Y J, et al. Activated carbon/NiFe2O4 magnetic composite: a magnetic adsorbent for the adsorption of methyl orange[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1740-1751. |
| 30 | ZHAO B, SUN X J, WANG L, et al. Adsorption of methyl orange from aqueous solution by composite magnetic microspheres of chitosan and quaternary ammonium chitosan derivative[J]. Chinese Journal of Chemical Engineering, 2019, 27(8): 1973-1980. |
| 31 | ZHANG M M, YU Z H, YU H C. Adsorption of Eosin Y, methyl orange and brilliant green from aqueous solution using ferroferric oxide/polypyrrole magnetic composite[J]. Polymer Bulletin, 2020, 77(2): 1049-1066. |
| 32 | SANTOS G D C, BARROS A L, DE OLIVEIRA C A F, et al. New composites LnBDC@AC and CB[6]@AC: from design toward selective adsorption of methylene blue or methyl orange[J]. PLoS One, 2017, 12(1): e0170026. |
| 33 | SIYASUKH A, CHIMUPALA Y, TONANON N. Preparation of magnetic hierarchical porous carbon spheres with graphitic features for high methyl orange adsorption capacity[J]. Carbon, 2018, 134: 207-221. |
| 34 | ZHAO X R, CAO Y Q, CHEN J, et al. Photocatalytic properties of Co3O4-coated TiO2 powders prepared by plasma-enhanced atomic layer deposition[J]. Nanoscale Research Letters, 2017, 12(1): 1-9. |
| 35 | SAEED M, USMAN M, IBRAHIM M, et al. Enhanced photo catalytic degradation of methyl orange using p-n Co3O4-TiO2 hetero-junction as catalyst[J]. International Journal of Chemical Reactor Engineering, 2020, 18(5/6). DOI: 10.1515/ijcre-2020-0004. |
| 36 | 张金龙,陈锋,田宝柱, 等. 光催化[M]. 2版. 上海: 华东理工大学出版社, 2015: 3-4. |
| ZHANG Jinlong, CHEN Feng, TIAN Baozhu, et al. Photocatalysis[M]. 2rd edition. Shanghai: East China University of Science and Technology Press, 2015: 3-4. | |
| 37 | HEIDARI-ASIL S A, ZINATLOO-AJABSHIR S, AMIRI O, et al. Amino acid assisted-synthesis and characterization of magnetically retrievable ZnCo2O4-Co3O4 nanostructures as high activity visible-light-driven photocatalyst[J]. International Journal of Hydrogen Energy, 2020, 45(43): 22761-22774. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [6] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [7] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [8] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [9] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [10] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [11] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [12] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [15] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||