化工进展 ›› 2021, Vol. 40 ›› Issue (7): 3736-3746.DOI: 10.16085/j.issn.1000-6613.2020-1649
结构化铜基催化剂电化学还原CO2为多碳产物研究进展
张轩1( ), 黄耀桢1, 邵秀丽1, 李晶1(
), 黄耀桢1, 邵秀丽1, 李晶1( ), 李丰1, 岳秦2, 王政1(
), 李丰1, 岳秦2, 王政1( )
)
- 1.宁夏大学化学化工学院,省部共建煤炭高效利用与绿色化工国家重点实验室,宁夏 银川 750021
2.电子科技大学基础与前沿研究院,四川 成都 610051
-
收稿日期:2020-08-18修回日期:2020-11-30出版日期:2021-07-06发布日期:2021-07-19 -
通讯作者:李晶,王政 -
作者简介:张轩(1985—),男,博士研究生,研究方向为能源催化材料。E-mail:anguszhang@yeah.net 。 -
基金资助:国家自然科学基金(21766026);宁夏科技领军人才项目(KJT2016001);宁夏回族自治区国内一流学科建设项目(NXYLXK2017A04)
Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products
ZHANG Xuan1( ), HUANG Yaozhen1, SHAO Xiuli1, LI Jing1(
), HUANG Yaozhen1, SHAO Xiuli1, LI Jing1( ), LI Feng1, YUE Qin2, WANG Zheng1(
), LI Feng1, YUE Qin2, WANG Zheng1( )
)
- 1.State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, Ningxia, China
2.Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610051, Sichuan, China
-
Received:2020-08-18Revised:2020-11-30Online:2021-07-06Published:2021-07-19 -
Contact:LI Jing,WANG Zheng
摘要:
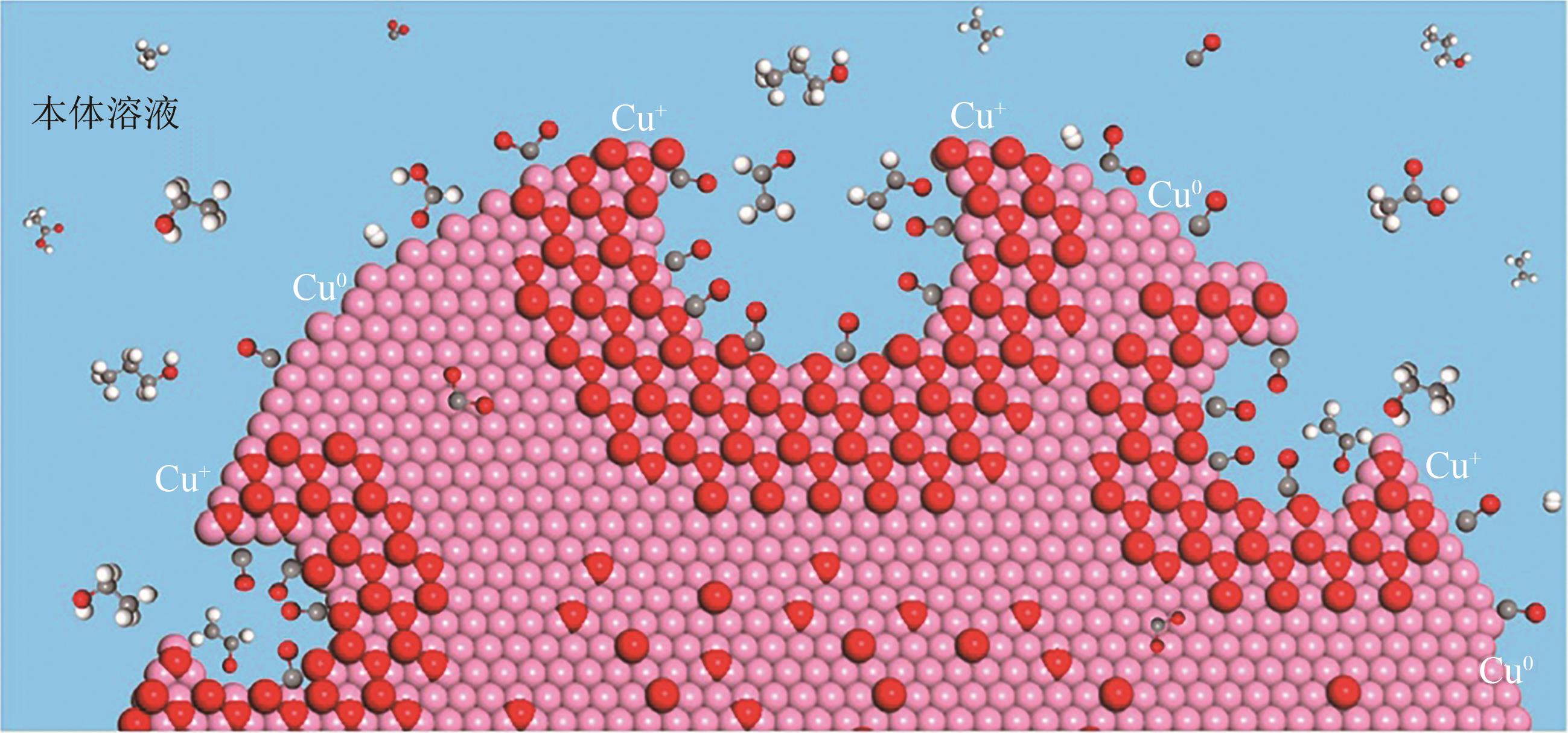
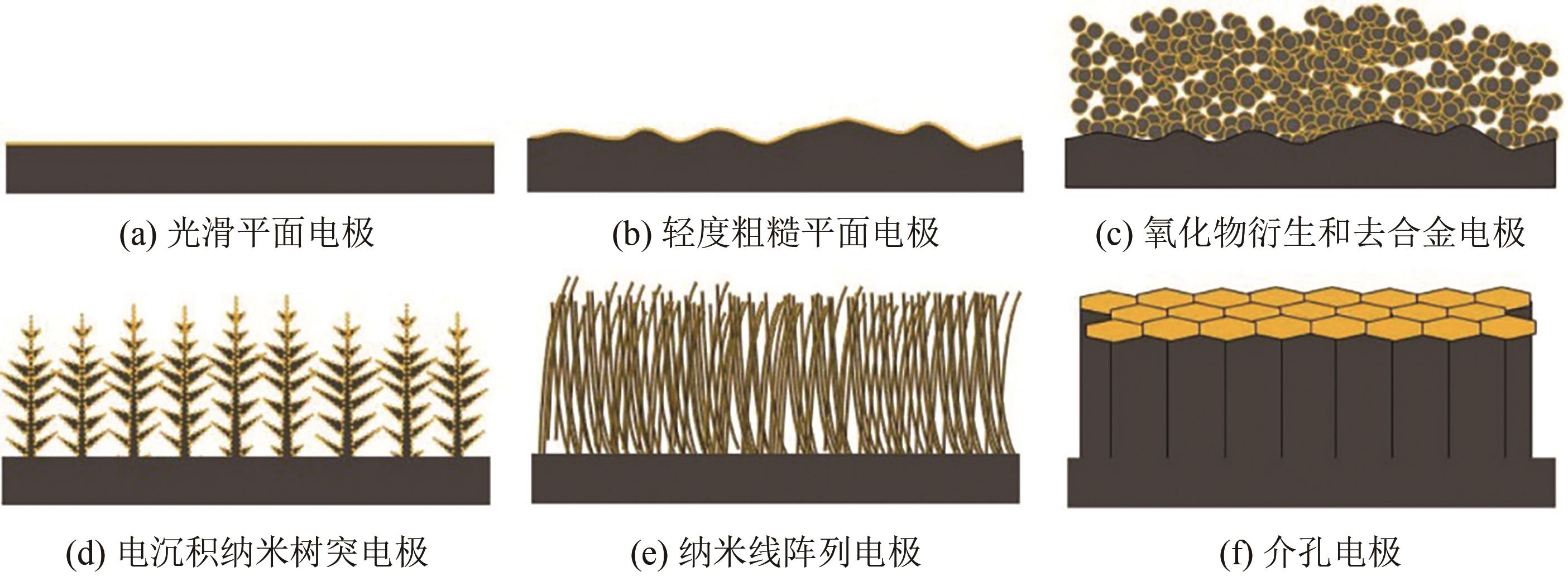
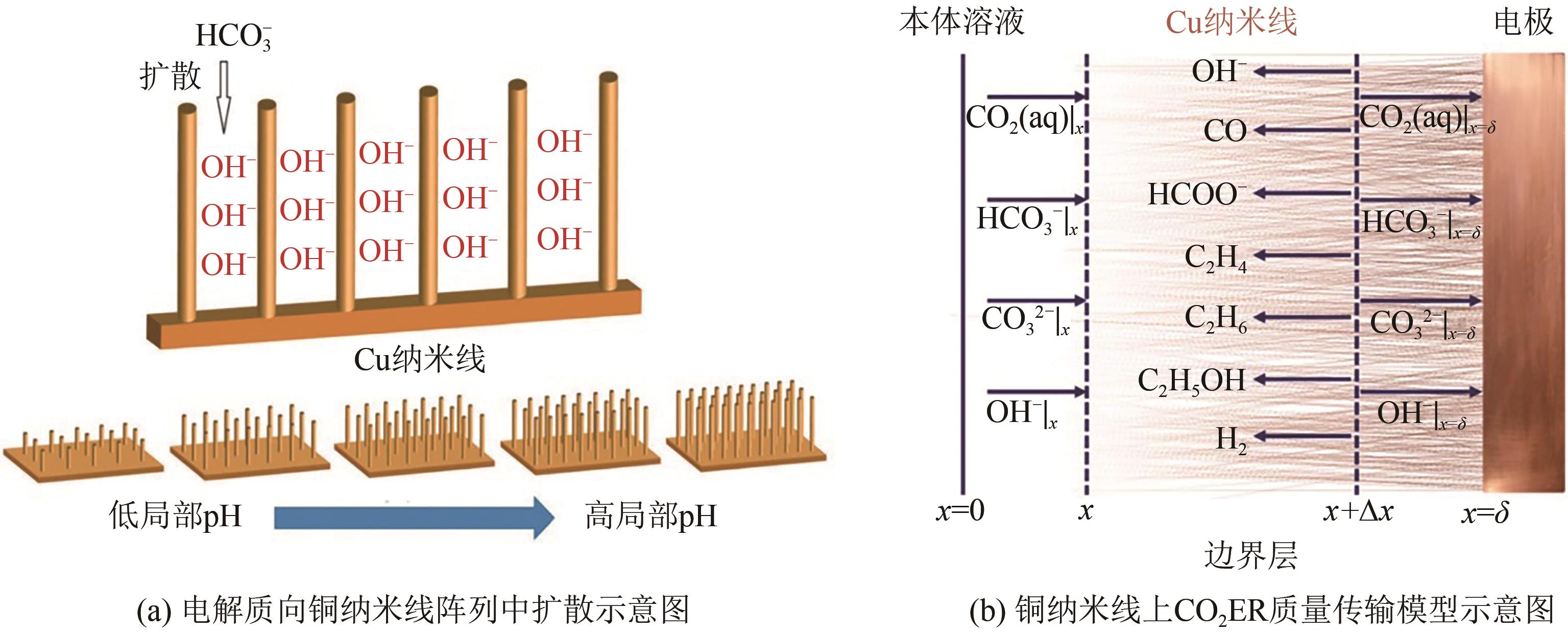
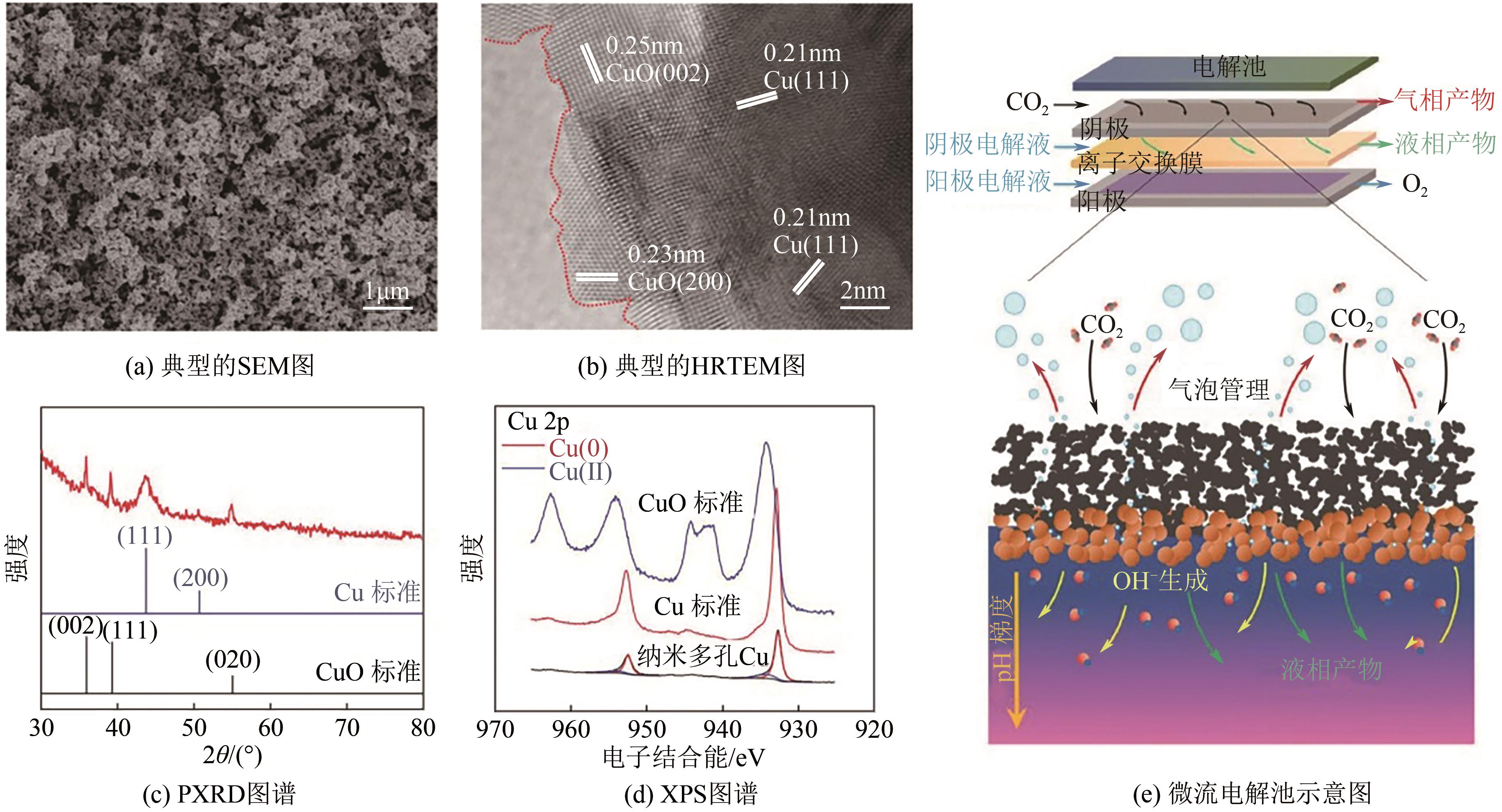
近年来,随着有关铜基催化剂价态、晶面、微观形态等结构化因素对其催化性能影响的研究不断深入,铜基催化剂电化学还原CO2高选择性制备高附加值多碳(C2+)产物取得长足进展。本文系统综述了近五年来结构化铜基催化剂电化学还原CO2生成C2+产物的研究报道,并分析总结了铜基催化剂表面混合价态、高活性晶面和丰富晶界的存在,以及富含限域空间的形态学结构(纳米线阵列、纳米树突和纳米多孔结构等)的构建与其电化学还原CO2生成C2+产物的活性和选择性之间的构效关系。进一步提出了CO2电化学还原领域发展的新趋势,即充分发挥各个结构化因素的协同作用,原位制备具有混合价态和丰富晶界的纳米多孔结构铜基催化剂,并在流通池中高效还原CO2持续生成C2+产物。
中图分类号:
引用本文
张轩, 黄耀桢, 邵秀丽, 李晶, 李丰, 岳秦, 王政. 结构化铜基催化剂电化学还原CO2为多碳产物研究进展[J]. 化工进展, 2021, 40(7): 3736-3746.
ZHANG Xuan, HUANG Yaozhen, SHAO Xiuli, LI Jing, LI Feng, YUE Qin, WANG Zheng. Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3736-3746.
| 催化剂 | 主要结构化调控因素 | 电解池 | C2+产物法拉第效率 | 电位(vs.RHE)/V | 电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| Cu-Cu3N | 价态 Cu+/Cu0 | H型电解池 | C2H4(39%±2%) C2H5OH(19%±1%) C3H7OH(6%±1%) | -0.95 | 14 | [ |
| Cu(B) | 价态 Cuδ+/Cu0 | H型电解池 | C2H4(52%±2%) C2H5OH(27%±1%) | -1.1 | 70 | [ |
| Cu-SCP | 价态 Cu+ | H型电解池 | C2H4(19.7%) C2H5OH(33.5%) | -1.34 | — | [ |
| Cu | 价态 Cu+/Cu0 | H型电解池 | C2H4(38%) | -1.2 | 22.2 | [ |
| Cu2O | 价态 Cu+ | 流通池 | C2H4(38.0%±1.4%) CH3COOH(4.8%±1.1%) C2H5OH(26.9%±2.0%) C3H7OH(5.5%±1.1%) | -0.61 | 267±13 | [ |
| Cu2O | 晶面 Cu2O(111) | H型电解池 | C2H4(51%) | -0.76 | 15.7 | [ |
| Cu | 晶面 Cu(111) | 流通池 | C2+(73.1%) | -1.2 | 96.62 | [ |
| Cu | 晶面 Cu(100)/Cu(110) | 流通池 | C2H4(31.22%) C2H5OH(35.73%) CH3COO-(1.59%) | -1.05 | 240.8 | [ |
| Cu2O | 晶面 Cu2O{100}/{111} | H型电解池 | C2H4(59%) | -1.1 | 22 | [ |
| Cu2O/Cu | 晶面 Cu2O(110)/Cu(111) | H型电解池 | C2+(64.5%) | -0.9 | 26.2 | [ |
| Cu | 晶面 Cu(100)/Cu(111) | H型电解池 | C2+(72.1%) | -1.1 | 25.2 | [ |
| Cu | 形态:纳米线阵列 | 隔膜电解池 | C2H4(17.4%) C2H6(2.4%) C2H5OH(3.8%) C3H7OH(7.8%) | -1.1 | — | [ |
| Cu | 形态:纳米线阵列 | 隔膜电解池 | C2H4(3.0%) C2H6(6.8%) C2H5OH(15.2%) | -0.695 | — | [ |
| Cu3Au | 形态:纳米线阵列 | H型电解池 | C2H5OH(48%) | -0.5 | 0.4 | [ |
| Cu2O | 形态:纳米线阵列 | 气密反应器 | C2H4(65%) | -0.8 | — | [ |
| Cu | 形态:纳米树突 | 流通池 | C2H4(57%) | — | 170 | [ |
| Cu | 形态:纳米树突 | 隔膜电解池 | C2H4(56%) C2H5OH(17%) | -1.4 | 30 | [ |
| Cu | 形态:纳米树突 | 常规电解池 | C2H4(22.3%) n-C3H7OH(3.1%) C2H5CHO(2.9%) | -1.2 | — | [ |
| Cu-Cu2O/Cu | 形态:纳米树突 | H型电解池 | CH3COOH(48%) C2H5OH(32%) | -0.4 | 11.5 | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(35%) | -1.3 | — | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(38%) | -1.7(vs.NHE) | — | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(30%) C2H5OH(10%) | -0.81 | 4.3+1.43 | [ |
| Cu4O | 形态:多孔结构 | H型电解池 | C2H4(45%) | -1.0 | 44.7 | [ |
| Cu | 形态:多孔结构 | 微流电解池 | C2+(62%) | -0.67 | 411 | [ |
表1 不同铜基催化剂CO2ER催化性能汇总
| 催化剂 | 主要结构化调控因素 | 电解池 | C2+产物法拉第效率 | 电位(vs.RHE)/V | 电流密度/mA·cm-2 | 参考文献 |
|---|---|---|---|---|---|---|
| Cu-Cu3N | 价态 Cu+/Cu0 | H型电解池 | C2H4(39%±2%) C2H5OH(19%±1%) C3H7OH(6%±1%) | -0.95 | 14 | [ |
| Cu(B) | 价态 Cuδ+/Cu0 | H型电解池 | C2H4(52%±2%) C2H5OH(27%±1%) | -1.1 | 70 | [ |
| Cu-SCP | 价态 Cu+ | H型电解池 | C2H4(19.7%) C2H5OH(33.5%) | -1.34 | — | [ |
| Cu | 价态 Cu+/Cu0 | H型电解池 | C2H4(38%) | -1.2 | 22.2 | [ |
| Cu2O | 价态 Cu+ | 流通池 | C2H4(38.0%±1.4%) CH3COOH(4.8%±1.1%) C2H5OH(26.9%±2.0%) C3H7OH(5.5%±1.1%) | -0.61 | 267±13 | [ |
| Cu2O | 晶面 Cu2O(111) | H型电解池 | C2H4(51%) | -0.76 | 15.7 | [ |
| Cu | 晶面 Cu(111) | 流通池 | C2+(73.1%) | -1.2 | 96.62 | [ |
| Cu | 晶面 Cu(100)/Cu(110) | 流通池 | C2H4(31.22%) C2H5OH(35.73%) CH3COO-(1.59%) | -1.05 | 240.8 | [ |
| Cu2O | 晶面 Cu2O{100}/{111} | H型电解池 | C2H4(59%) | -1.1 | 22 | [ |
| Cu2O/Cu | 晶面 Cu2O(110)/Cu(111) | H型电解池 | C2+(64.5%) | -0.9 | 26.2 | [ |
| Cu | 晶面 Cu(100)/Cu(111) | H型电解池 | C2+(72.1%) | -1.1 | 25.2 | [ |
| Cu | 形态:纳米线阵列 | 隔膜电解池 | C2H4(17.4%) C2H6(2.4%) C2H5OH(3.8%) C3H7OH(7.8%) | -1.1 | — | [ |
| Cu | 形态:纳米线阵列 | 隔膜电解池 | C2H4(3.0%) C2H6(6.8%) C2H5OH(15.2%) | -0.695 | — | [ |
| Cu3Au | 形态:纳米线阵列 | H型电解池 | C2H5OH(48%) | -0.5 | 0.4 | [ |
| Cu2O | 形态:纳米线阵列 | 气密反应器 | C2H4(65%) | -0.8 | — | [ |
| Cu | 形态:纳米树突 | 流通池 | C2H4(57%) | — | 170 | [ |
| Cu | 形态:纳米树突 | 隔膜电解池 | C2H4(56%) C2H5OH(17%) | -1.4 | 30 | [ |
| Cu | 形态:纳米树突 | 常规电解池 | C2H4(22.3%) n-C3H7OH(3.1%) C2H5CHO(2.9%) | -1.2 | — | [ |
| Cu-Cu2O/Cu | 形态:纳米树突 | H型电解池 | CH3COOH(48%) C2H5OH(32%) | -0.4 | 11.5 | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(35%) | -1.3 | — | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(38%) | -1.7(vs.NHE) | — | [ |
| Cu | 形态:多孔结构 | H型电解池 | C2H4(30%) C2H5OH(10%) | -0.81 | 4.3+1.43 | [ |
| Cu4O | 形态:多孔结构 | H型电解池 | C2H4(45%) | -1.0 | 44.7 | [ |
| Cu | 形态:多孔结构 | 微流电解池 | C2+(62%) | -0.67 | 411 | [ |
| 1 | LIU X Y, XIAO J P, PENG H J, et al. Understanding trends in electrochemical carbon dioxide reduction rates[J]. Nature Communications, 2017, 8: 15438. |
| 2 | LI J, GUO S X, LI F, et al. Electrohydrogenation of carbon dioxide using a ternary Pd/Cu2O-Cu catalyst[J]. ChemSusChem, 2019, 12(19): 4471-4479. |
| 3 | YILIGUMA, WANG Z J, YANG C, et al. Sub-5nm SnO2 chemically coupled hollow carbon spheres for efficient electrocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2018, 6(41): 20121-20127. |
| 4 | LIANG Y, ZHOU W, SHI Y M, et al. Unveiling in situ evolved In/In2O3-xheterostructure as the active phase of In2O3 toward efficient electroreduction of CO2 to formate[J]. Science Bulletin, 2020, 65(18): 1547-1554. |
| 5 | SONG Y F, WANG S B, CHEN W, et al. Enhanced ethanol production from CO2 electroreduction at micropores in nitrogen-doped mesoporous carbon[J]. ChemSusChem, 2020, 13(2): 293-297. |
| 6 | ZHENG Y, VASILEFF A, ZHOU X L, et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts[J]. Journal of the American Chemical Society, 2019, 141(19): 7646-7659. |
| 7 | GAO D F, ARÁN-AIS R M, JEON H S, et al. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products[J]. Nature Catalysis, 2019, 2(3): 198-210. |
| 8 | BIRDJA Y Y, PÉREZ-GALLENT E, FIGUEIREDO M C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nature Energy, 2019, 4(9): 732-745. |
| 9 | FAN Q, ZHANG M L, JIA M W, et al. Electrochemical CO2 reduction to C2+ species: heterogeneous electrocatalysts, reaction pathways, and optimization strategies[J]. Materials Today Energy, 2018, 10: 280-301. |
| 10 | FAN L, XIA C, YANG F Q, et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products[J]. Science Advances, 2020, 6(8): eaay3111. |
| 11 | NITOPI S, BERTHEUSSEN E, SCOTT S B, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J]. Chemical Reviews, 2019, 119(12): 7610-7672. |
| 12 | HOANG T T H, VERMA S, MA S C, et al. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol[J]. Journal of the American Chemical Society, 2018, 140(17): 5791-5797. |
| 13 | TOMBOC G M, CHOI S, KWON T, et al. Potential link between Cu surface and selective CO2 electroreduction: perspective on future electrocatalyst designs[J]. Advanced Materials, 2020, 32(17): 1908398. |
| 14 | CHOU T C, CHANG C C, YU H L, et al. Controlling the oxidation state of the Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene[J]. Journal of the American Chemical Society, 2020, 142(6): 2857-2867. |
| 15 | LIN S C, CHANG C C, CHIU S Y, et al. Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction[J]. Nature Communications, 2020, 11(1): 3525. |
| 16 | XIAO H, GODDARD W A, CHENG T, et al. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(26): 6685-6688. |
| 17 | GROSSE P, GAO D F, SCHOLTEN F, et al. Dynamic changes in the structure, chemical state and catalytic selectivity of Cu nanocubes during CO2 electroreduction: size and support effects[J]. Angewandte Chemie International Edition, 2018, 57(21): 6192-6197. |
| 18 | LIANG Z Q, ZHUANG T T, SEIFITOKALDANI A, et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2[J]. Nature Communications, 2018, 9: 3828. |
| 19 | ZHOU Y S, CHE F L, LIU M, et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons[J]. Nature Chemistry, 2018, 10(9): 974-980. |
| 20 | SAKAMOTO N, NISHIMURA Y F, NONAKA T, et al. Self-assembled cuprous coordination polymer as a catalyst for CO2 electrochemical reduction into C2 products[J]. ACS Catalysis, 2020, 10(18): 10412-10419. |
| 21 | BAI X W, LI Q, SHI L, et al. Hybrid Cu0 and Cux+ as atomic interfaces promote high-selectivity conversion of CO2 to C2H5OH at low potential[J]. Small, 2020, 16(12): 1901981. |
| 22 | VASILEFF A, ZHU Y P, ZHI X, et al. Electrochemical reduction of CO2 to ethane through stabilization of an ethoxy intermediate[J]. Angewandte Chemie International Edition, 2020, 59(44): 19649-19653. |
| 23 | SHAH A H, WANG Y J, HUSSAIN S, et al. New aspects of C2 selectivity in electrochemical CO2 reduction over oxide-derived copper[J]. Physical Chemistry Chemical Physics, 2020, 22(4): 2046-2053. |
| 24 | ZHAO J, XUE S, BARBER J, et al. An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction[J]. Journal of Materials Chemistry A, 2020, 8(9): 4700-4734. |
| 25 | DE LUNA P, QUINTERO-BERMUDEZ R, DINH C T, et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction[J]. Nature Catalysis, 2018, 1(2): 103-110. |
| 26 | KAUFFMAN D R, ALFONSO D. Directing CO2 conversion with copper nanoneedles[J]. Nature Catalysis, 2018, 1(2): 99-100. |
| 27 | YANG P P, ZHANG X L, GAO F Y, et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels[J]. Journal of the American Chemical Society, 2020, 142(13): 6400-6408. |
| 28 | ARÁN-AIS R M, SCHOLTEN F, KUNZE S, et al. The role of in situ generated morphological motifs and Cu(Ⅰ) species in C2+ product selectivity during CO2 pulsed electroreduction[J]. Nature Energy, 2020, 5(4): 317-325. |
| 29 | DE GREGORIO G L, BURDYNY T, LOIUDICE A, et al. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities[J]. ACS Catalysis, 2020, 10(9): 4854-4862. |
| 30 | BAGGER A, JU W, VARELA A S, et al. Electrochemical CO2 reduction: classifying Cu facets[J]. ACS Catalysis, 2019, 9(9): 7894-7899. |
| 31 | AJMAL S, YANG Y, TAHIR M A, et al. Boosting C2 products in electrochemical CO2 reduction over highly dense copper nanoplates[J]. Catalysis Science & Technology, 2020, 10(14): 4562-4570. |
| 32 | HORI Y, TAKAHASHI I, KOGA O, et al. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes[J]. The Journal of Physical Chemistry B, 2002, 106(1): 15-17. |
| 33 | WANG Y H, WANG Z Y, DINH C T, et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis[J]. Nature Catalysis, 2020, 3(2): 98-106. |
| 34 | ZHANG Y F, ZHAO Y, WANG C Y, et al. Zn-doped Cu(100) facet with efficient catalytic ability for the CO2 electroreduction to ethylene[J]. Physical Chemistry Chemical Physics, 2019, 21(38): 21341-21348. |
| 35 | REN X N, ZHANG X W, CAO X Z, et al. Efficient electrochemical reduction of carbon dioxide into ethylene boosted by copper vacancies on stepped cuprous oxide[J]. Journal of CO2 Utilization, 2020, 38: 125-131. |
| 36 | CHANG C C, LI E Y, TSAI M K. A computational exploration of CO2 reduction via CO dimerization on mixed-valence copper oxide surface[J]. Physical Chemistry Chemical Physics, 2018, 20(25): 16906-16909. |
| 37 | CHEN Z Q, WANG T, LIU B, et al. Grain-boundary-rich copper for efficient solar-driven electrochemical CO2 reduction to ethylene and ethanol[J]. Journal of the American Chemical Society, 2020, 142(15): 6878-6883. |
| 38 | YANG C, SHEN H C, GUAN A X, et al. Fast cooling induced grain-boundary-rich copper oxide for electrocatalytic carbon dioxide reduction to ethanol[J]. Journal of Colloid and Interface Science, 2020, 570: 375-381. |
| 39 | GAO Y G, WU Q, LIANG X Z, et al. Cu2O nanoparticles with both {100} and {111} facets for enhancing the selectivity and activity of CO2 electroreduction to ethylene[J]. Advanced Science, 2020, 7(6): 1902820. |
| 40 | CHEN C J, SUN X F, YAN X P, et al. A strategy to control the grain boundary density and Cu+/Cu0 ratio of Cu-based catalysts for efficient electroreduction of CO2 to C2 products[J]. Green Chemistry, 2020, 22(5): 1572-1576. |
| 41 | XU Z, WU T C, CAO Y, et al. Dynamic restructuring induced Cu nanoparticles with ideal nanostructure for selective multi-carbon compounds production via carbon dioxide electroreduction[J]. Journal of Catalysis, 2020, 383: 42-50. |
| 42 | KARAISKAKIS A N, GOLRU S S, BIDDINGER E J. Effect of electrode geometry on selectivity and activity in CO2 electroreduction[J]. Industrial & Engineering Chemistry Research, 2019, 58(50): 22506-22515. |
| 43 | GU Z X, SHEN H, SHANG L M, et al. Nanostructured copper-based electrocatalysts for CO2 reduction[J]. Small Methods, 2018, 2(11): 1800121. |
| 44 | KAS R, YANG K L, BOHRA D, et al. Electrochemical CO2 reduction on nanostructured metal electrodes: fact or defect?[J]. Chemical Science, 2020, 11(7): 1738-1749. |
| 45 | MA M, DJANASHVILI K, SMITH W A. Controllable hydrocarbon formation from the electrochemical reduction of CO2 over Cu nanowire arrays[J]. Angewandte Chemie International Edition, 2016, 55(23): 6680-6684. |
| 46 | LE DUFF C S, LAWRENCE M J, RODRIGUEZ P. Role of the adsorbed oxygen species in the selective electrochemical reduction of CO2 to alcohols and carbonyls on copper electrodes[J]. Angewandte Chemie: International Edition, 2017, 56(42): 12919-12924. |
| 47 | RACITI D, MAO M, WANG C. Mass transport modelling for the electroreduction of CO2 on Cu nanowires[J]. Nanotechnology, 2018, 29(4): 044001. |
| 48 | ZHU W W, ZHAO K M, LIU S Q, et al. Low-overpotential selective reduction of CO2 to ethanol on electrodeposited CuxAuy nanowire arrays[J]. Journal of Energy Chemistry, 2019, 37: 176-182. |
| 49 | WANG Y X, NIU C L, ZHU Y C. Copper-silver bimetallic nanowire arrays for electrochemical reduction of carbon dioxide[J]. Nanomaterials, 2019, 9(2): 173. |
| 50 | WANG Y X, ZHU Y C, NIU C. Surface and length effects for aqueous electrochemical reduction of CO2 as studied over copper nanowire arrays[J]. Journal of Physics and Chemistry of Solids, 2020, 144: 109507. |
| 51 | LYU Z H, ZHU S Q, XIE M H, et al. Controlling the surface oxidation of Cu nanowires improves their catalytic selectivity and stability toward C2+ products in CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(4): 1909-1915. |
| 52 | HOSSAIN M NUR, CHEN S, CHEN A C. Thermal-assisted synthesis of unique Cu nanodendrites for the efficient electrochemical reduction of CO2[J]. Applied Catalysis B: Environmental, 2019, 259: 118096. |
| 53 | MAREPALLY B C, AMPELLI C, GENOVESE C, et al. Electrocatalytic reduction of CO2 over dendritic-type Cu- and Fe-based electrodes prepared by electrodeposition[J]. Journal of CO2 Utilization, 2020, 35: 194-204. |
| 54 | RELLER C, KRAUSE R, VOLKOVA E, et al. Selective electroreduction of CO2 toward ethylene on nano dendritic copper catalysts at high current density[J]. Advanced Energy Materials, 2017, 7(12): 1602114. |
| 55 | WAKERLEY D, LAMAISON S, OZANAM F, et al. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface[J]. Nature Materials, 2019, 18(11): 1222-1227. |
| 56 | WU M F, ZHU C, WANG K, et al. Promotion of CO2 electrochemical reduction via Cu nanodendrites[J]. ACS Applied Materials & Interfaces, 2020, 12(10): 11562-11569. |
| 57 | ZHU Q G, SUN X F, YANG D X, et al. Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex[J]. Nature Communications, 2019, 10(1): 3851. |
| 58 | 刘志敏. 铜配合物衍生铜-氧化亚铜催化剂的原位电合成及其对二氧化碳电还原制备C2产物催化性能的研究[J]. 物理化学学报, 2019, 35(12): 1307-1308. |
| LIU Z M. Electroreduction of carbon dioxide to C2 products over copper-cuprous oxide prepared from in situ electrosynthesized copper complex[J]. Acta Physico-Chimica Sinica, 2019, 35(12): 1307-1308. | |
| 59 | HOANG T T H, MA S C, GOLD J I, et al. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis[J]. ACS Catalysis, 2017, 7(5): 3313-3321. |
| 60 | EBAID M, JIANG K, ZHANG Z M, et al. Production of C2/C3 oxygenates from planar copper nitride-derived mesoporous copper via electrochemical reduction of CO2[J]. Chemistry of Materials, 2020, 32(7): 3304-3311. |
| 61 | PENG Y C, WU T, SUN L B, et al. Selective electrochemical reduction of CO2 to ethylene on nanopores-modified copper electrodes in aqueous solution[J]. ACS Applied Materials & Interfaces, 2017, 9(38): 32782-32789. |
| 62 | YANG K D, KO W R, LEE J H, et al. Morphology-directed selective production of ethylene or ethane from CO2 on a Cu mesopore electrode[J]. Angewandte Chemie International Edition, 2017, 56(3): 796-800. |
| 63 | SONG H, IM M, SONG J T, et al. Effect of mass transfer and kinetics in ordered Cu-mesostructures for electrochemical CO2 reduction[J]. Applied Catalysis B: Environmental, 2018, 232: 391-396. |
| 64 | BELL D, RALL D, GROßEHEIDE M, et al. Tubular hollow fibre electrodes for CO2 reduction made from copper aluminum alloy with drastically increased intrinsic porosity[J]. Electrochemistry Communications, 2020, 111: 106645. |
| 65 | LIU J, FU J J, ZHOU Y, et al. Controlled synthesis of EDTA-modified porous hollow copper microspheres for high-efficiency conversion of CO2 to multicarbon products[J]. Nano Letters, 2020, 20(7): 4823-4828. |
| 66 | NAM D H, BUSHUYEV O S, LI J, et al. Metal-organic frameworks mediate Cu coordination for selective CO2 electroreduction[J]. Journal of the American Chemical Society, 2018, 140(36): 11378-11386. |
| 67 | TAN X Y, YU C, ZHAO C T, et al. Restructuring of Cu2O to Cu2O@Cu-metal-organic frameworks for selective electrochemical reduction of CO2[J]. ACS Applied Materials & Interfaces, 2019, 11(10): 9904-9910. |
| 68 | YANG F, DENG P L, WANG Q Y, et al. Metal-organic framework-derived cupric oxide polycrystalline nanowires for selective carbon dioxide electroreduction to C2 valuables[J]. Journal of Materials Chemistry A, 2020, 8(25): 12418-12423. |
| 69 | YAO K L, XIA Y J, LI J, et al. Metal-organic framework derived copper catalysts for CO2 to ethylene conversion[J]. Journal of Materials Chemistry A, 2020, 8(22): 11117-11123. |
| 70 | ZHANG W, HUANG C Q, XIAO Q, et al. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2020, 142(26): 11417-11427. |
| 71 | ZHANG J, LUO W, ZÜTTEL A. Self-supported copper-based gas diffusion electrodes for CO2 electrochemical reduction[J]. Journal of Materials Chemistry A, 2019, 7(46): 26285-26292. |
| 72 | DINH C T, BURDYNY T, KIBRIA M G, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface[J]. Science, 2018, 360(6390): 783-787. |
| 73 | WEEKES D M, SALVATORE D A, REYES A, et al. Electrolytic CO2 reduction in a flow cell[J]. Accounts of Chemical Research, 2018, 51(4): 910-918. |
| 74 | ANASTASIADOU D, SCHELLEKENS M, DE HEER M, et al. Electrodeposited Cu2O films on gas diffusion layers for selective CO2 electroreduction to ethylene in an alkaline flow electrolyzer[J]. ChemElectroChem, 2019, 6(15): 3928-3932. |
| 75 | LYU J J, JOUNY M, LUC W, et al. A highly porous copper electrocatalyst for carbon dioxide reduction[J]. Advanced Materials, 2018, 30(49): 1803111. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [12] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [13] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [14] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [15] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||