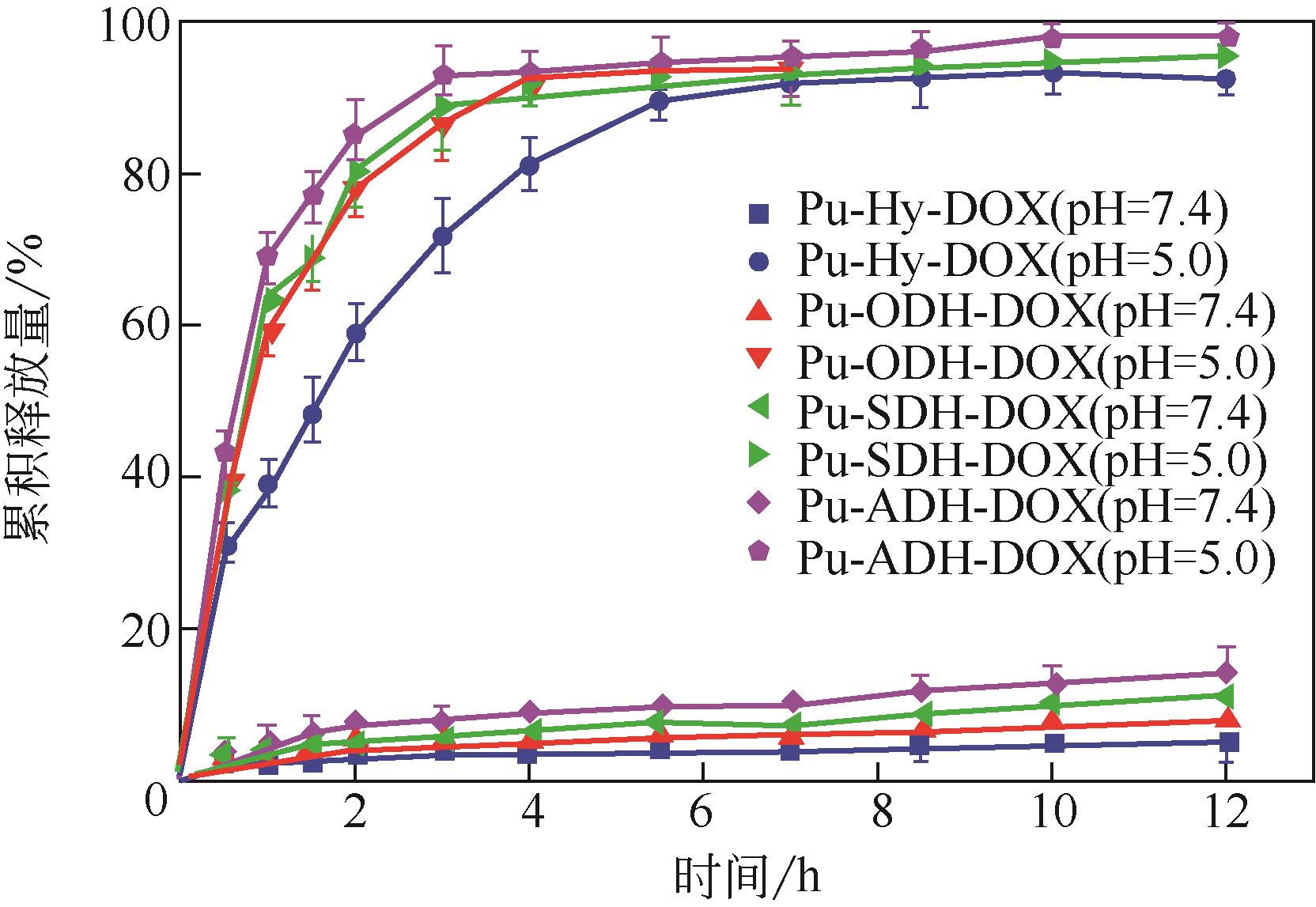
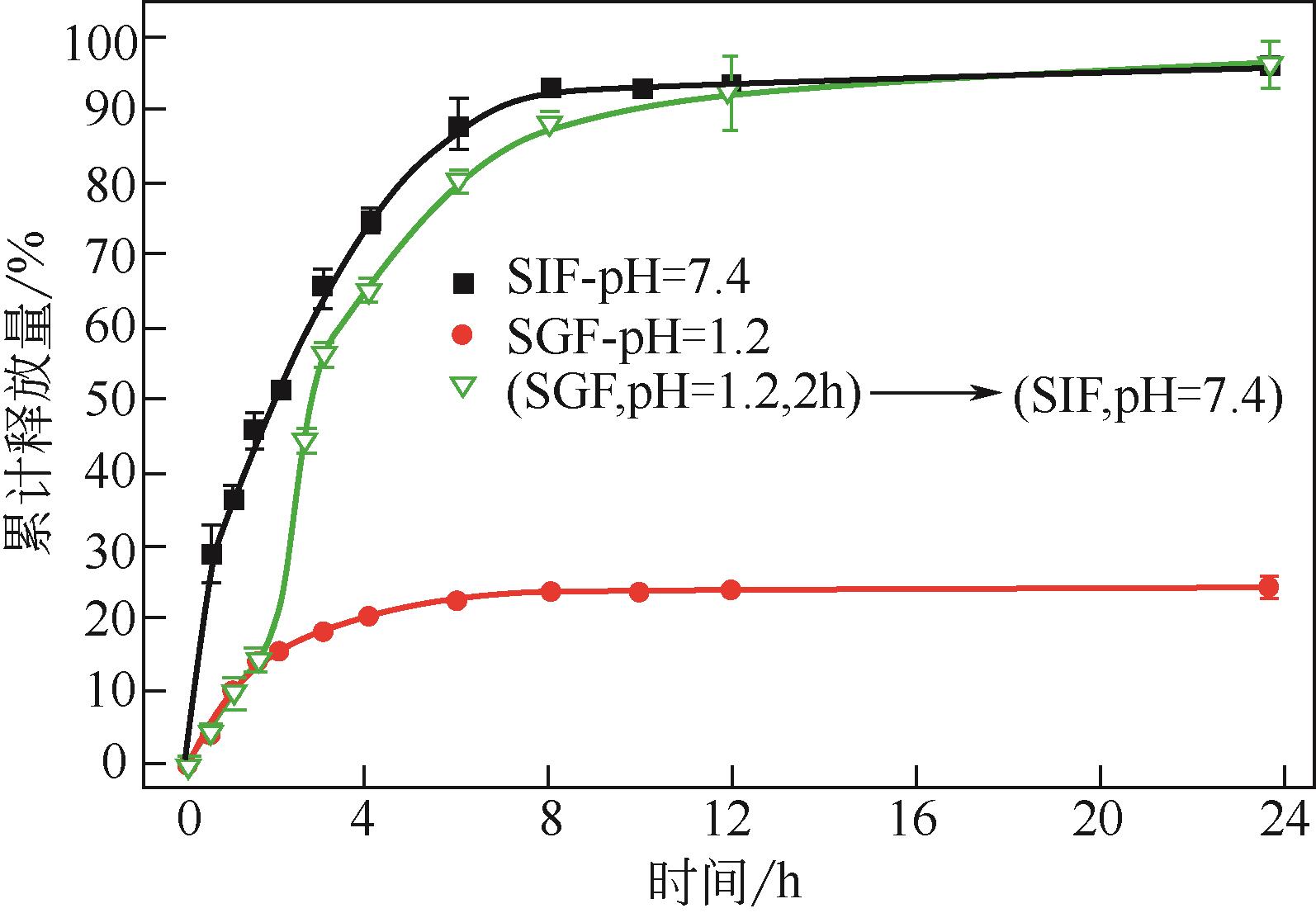
化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3411-3420.DOI: 10.16085/j.issn.1000-6613.2020-1423
pH敏感性生物基纳米载药粒子的研究进展
- 华南理工大学化学与化工学院,广东 广州 510640
-
收稿日期:2020-07-23修回日期:2020-09-20出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:易聪华 -
作者简介:易聪华(1979—),女,博士,副教授,研究方向为生物质资源的高值化利用。E-mail:chyi@scut.edu.cn 。
Research progress of pH-sensitive biopolymer nanocarriers
YI Conghua( ), XU Qinghe, WANG Miao, YANG Dongjie
), XU Qinghe, WANG Miao, YANG Dongjie
- School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, Guangdong, China
-
Received:2020-07-23Revised:2020-09-20Online:2021-06-06Published:2021-06-22 -
Contact:YI Conghua
摘要:
生物基来源的聚合物具有生物相容性高、无毒易降解等优势,近些年来作为药物载体在生物医药领域受到了广泛的关注。人体内的生理环境存在pH差异,利用pH作为刺激响应的信号,可以赋予聚合物纳米载药系统理想的靶向释药性能。本综述着眼于pH敏感性的生物基聚合物纳米粒子,揭示了纳米载药粒子中化学键断裂与质子化作用两种pH响应的控释机制,并针对两者的控释特点进行了分析总结。在此基础上,介绍了几种生物基药物载体的pH控释研究及其在生物医药领域的应用进展,并提出了目前利用各种生物基材料作为药物载体存在的问题。最后,针对目前存在的载药量低、敏感性不强等问题,提出了可采用多种方式联合载药、多重刺激响应结合等方式进行深入研究的展望。
中图分类号:
引用本文
易聪华, 徐青荷, 王淼, 杨东杰. pH敏感性生物基纳米载药粒子的研究进展[J]. 化工进展, 2021, 40(6): 3411-3420.
YI Conghua, XU Qinghe, WANG Miao, YANG Dongjie. Research progress of pH-sensitive biopolymer nanocarriers[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3411-3420.
| 类型 | pH敏感范围 | 化学结构 | 降解产物 |
|---|---|---|---|
| 腙键(hydrazone) | 5.0 |  |  |
| β-羧基酰胺键(amide) | 4.5~6.0 |  |  |
| 亚胺键(imine) | 6.8 |  |  |
表1 pH敏感的化学键和相应降解产物
| 类型 | pH敏感范围 | 化学结构 | 降解产物 |
|---|---|---|---|
| 腙键(hydrazone) | 5.0 |  |  |
| β-羧基酰胺键(amide) | 4.5~6.0 |  |  |
| 亚胺键(imine) | 6.8 |  |  |
| 类型 | pH敏感值 | 化学结构 | 质子化/ 离子化作用 |
|---|---|---|---|
| 咪唑基(imidazole) | 6.5 |  |  |
| 氨基(amino) | 约6.5 |  |  |
| 叔氨基(tertiary amino) | 约6.5 |  |  |
| 羧基(carboxyl) | 约7.4 |  |  |
表2 pH敏感的基团质子化/离子化作用示例
| 类型 | pH敏感值 | 化学结构 | 质子化/ 离子化作用 |
|---|---|---|---|
| 咪唑基(imidazole) | 6.5 |  |  |
| 氨基(amino) | 约6.5 |  |  |
| 叔氨基(tertiary amino) | 约6.5 |  |  |
| 羧基(carboxyl) | 约7.4 |  |  |
| 聚合物骨架 | 药物 | pH敏感化学键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 光交联壳聚糖-香豆素 | 槲皮素 | 亚胺键 | 10.2 | 78.4 | 肿瘤化疗 | [ |
| 壳聚糖/硫酸葡聚糖/壳聚糖 | 紫杉醇和5-氟尿嘧啶 | 氨基 | — | 66.3、75.2 | 肿瘤化疗 | [ |
| 羧甲基壳聚糖 | 阿霉素 | 氨基和羧基 | 53.02 | 16.77 | 肿瘤化疗 | [ |
| 聚乙二醇化羧甲基壳聚糖 | 阿霉素 | 叔氨基、氨基和羧基 | >36 | — | 肿瘤化疗 | [ |
| 海藻酸-精氨酸-壳聚糖 | 吡喹酮或伊维菌素 | 氨基和羧基 | — | — | 口服给药 | [ |
| 海藻酸-壳聚糖 | 甲苯达唑或伊维菌素 | 氨基和羧基 | — | — | 口服抗寄生虫给药 | [ |
表3 pH敏感性壳聚糖基聚合物纳米粒子的载药性质
| 聚合物骨架 | 药物 | pH敏感化学键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 光交联壳聚糖-香豆素 | 槲皮素 | 亚胺键 | 10.2 | 78.4 | 肿瘤化疗 | [ |
| 壳聚糖/硫酸葡聚糖/壳聚糖 | 紫杉醇和5-氟尿嘧啶 | 氨基 | — | 66.3、75.2 | 肿瘤化疗 | [ |
| 羧甲基壳聚糖 | 阿霉素 | 氨基和羧基 | 53.02 | 16.77 | 肿瘤化疗 | [ |
| 聚乙二醇化羧甲基壳聚糖 | 阿霉素 | 叔氨基、氨基和羧基 | >36 | — | 肿瘤化疗 | [ |
| 海藻酸-精氨酸-壳聚糖 | 吡喹酮或伊维菌素 | 氨基和羧基 | — | — | 口服给药 | [ |
| 海藻酸-壳聚糖 | 甲苯达唑或伊维菌素 | 氨基和羧基 | — | — | 口服抗寄生虫给药 | [ |
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 羧甲基淀粉/季铵盐淀粉 | 牛血清蛋白 | 羧基 | — | 45.52 | 口服给药 | [ |
| 柠檬酸交联淀粉 | 姜黄素 | 羧基 | — | — | 口服给药 | [ |
| 胆固醇-咪唑-氧化淀粉 | 姜黄素 | 咪唑基 | 4.16 | 17.84 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 咪唑基 | 5.37~20.3 | 32.7~76.5 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 咪唑基 | 8.6~19.8 | 49.5~90.5 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 腙键 | 13.59~47.67 | — | 肿瘤化疗 | [ |
表4 pH敏感性淀粉基聚合物纳米粒子的载药性质
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 羧甲基淀粉/季铵盐淀粉 | 牛血清蛋白 | 羧基 | — | 45.52 | 口服给药 | [ |
| 柠檬酸交联淀粉 | 姜黄素 | 羧基 | — | — | 口服给药 | [ |
| 胆固醇-咪唑-氧化淀粉 | 姜黄素 | 咪唑基 | 4.16 | 17.84 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 咪唑基 | 5.37~20.3 | 32.7~76.5 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 咪唑基 | 8.6~19.8 | 49.5~90.5 | 肿瘤化疗 | [ |
| 普鲁兰多糖 | 阿霉素 | 腙键 | 13.59~47.67 | — | 肿瘤化疗 | [ |
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 透明质酸修饰二氧化硅 | 阿霉素和光敏剂玫瑰红 | 亚胺键 | 15.30和12.78 | 76.67和95.85 | 化疗与光动力联合治疗 | [ |
| 透明质酸修饰二氧化硅 | 阿霉素 | 腙键 | 14 | — | 肿瘤化疗 | [ |
| 透明质酸 | 阿霉素 | 腙键 | 约11~14 | — | 肿瘤化疗 | [ |
| 透明质酸-脱氧胆酸-组氨酸 | 紫杉醇 | 咪唑基 | 81.9 | 91.0 | 肿瘤化疗 | [ |
| 透明质酸 | 阿霉素和光敏剂二氢卟吩 | 腙键 | 7.34 | 73.40 | 化疗与光动力联合治疗 | [ |
| 透明质酸 | 阿霉素 | 腙键 | 28.2 | 79.2 | 肿瘤化疗 | [ |
表5 利用透明质酸制备pH敏感性聚合物纳米粒子的载药性质(包括表面修饰及基体)
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 透明质酸修饰二氧化硅 | 阿霉素和光敏剂玫瑰红 | 亚胺键 | 15.30和12.78 | 76.67和95.85 | 化疗与光动力联合治疗 | [ |
| 透明质酸修饰二氧化硅 | 阿霉素 | 腙键 | 14 | — | 肿瘤化疗 | [ |
| 透明质酸 | 阿霉素 | 腙键 | 约11~14 | — | 肿瘤化疗 | [ |
| 透明质酸-脱氧胆酸-组氨酸 | 紫杉醇 | 咪唑基 | 81.9 | 91.0 | 肿瘤化疗 | [ |
| 透明质酸 | 阿霉素和光敏剂二氢卟吩 | 腙键 | 7.34 | 73.40 | 化疗与光动力联合治疗 | [ |
| 透明质酸 | 阿霉素 | 腙键 | 28.2 | 79.2 | 肿瘤化疗 | [ |
| 聚合物骨架 | 药物 | pH敏感键 /基团 | 负载率 /% | 封装率 /% | 应用 方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 叶酸偶联明胶 | 伊立替康 | 酰胺键和 羧基 | 11.2 | — | 肿瘤 化疗 | [ |
| 角蛋白 | 阿霉素 | 腙键 | 11.3 | 58.4 | 肿瘤 化疗 | [ |
| 聚乙二醇化角蛋白 | 阿霉素 | 腙键 | 29.4 | — | 肿瘤 化疗 | [ |
| 聚乙二醇化角蛋白 | 阿霉素 | 酰胺键和 氨基 | 24.8 | — | 肿瘤 化疗 | [ |
| 羧甲基角蛋白 | 阿霉素 | 羧基和氨基 | 5.7~13.0 | >90.0 | 肿瘤 化疗 | [ |
| 角蛋白 | 阿霉素 | 氨基 | 30.0 | 92.0 | 肿瘤 化疗 | [ |
表6 pH敏感性蛋白基聚合物纳米粒子的载药性质
| 聚合物骨架 | 药物 | pH敏感键 /基团 | 负载率 /% | 封装率 /% | 应用 方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 叶酸偶联明胶 | 伊立替康 | 酰胺键和 羧基 | 11.2 | — | 肿瘤 化疗 | [ |
| 角蛋白 | 阿霉素 | 腙键 | 11.3 | 58.4 | 肿瘤 化疗 | [ |
| 聚乙二醇化角蛋白 | 阿霉素 | 腙键 | 29.4 | — | 肿瘤 化疗 | [ |
| 聚乙二醇化角蛋白 | 阿霉素 | 酰胺键和 氨基 | 24.8 | — | 肿瘤 化疗 | [ |
| 羧甲基角蛋白 | 阿霉素 | 羧基和氨基 | 5.7~13.0 | >90.0 | 肿瘤 化疗 | [ |
| 角蛋白 | 阿霉素 | 氨基 | 30.0 | 92.0 | 肿瘤 化疗 | [ |
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 羧化木质素 | BZL | 咪唑基 | 9~11 | 50~57 | 肿瘤化疗 | [ |
| 胺化木质素 | 10-羟基喜树碱 | 咪唑基 | 15.6 | — | 肿瘤化疗 | [ |
| 碱木质素 | 布地奈德 | 羧基 | — | 34~37 | 口服给药 | [ |
| 季铵化木质素 | 布洛芬 | 羧基 | 46 | 74 | 口服给药 | [ |
| 酰化木质素-甲基丙烯酸甲酯 | 布洛芬 | 羧基 | 15.98 | 19.01 | 口服给药 | [ |
| 木质素-胆固醇 | 叶酸 | 羧基 | — | 约67 | — | [ |
表7 pH敏感性木质素基聚合物纳米粒子的载药研究
| 聚合物骨架 | 药物 | pH敏感键/基团 | 负载率/% | 封装率/% | 应用方向 | 参考文献 |
|---|---|---|---|---|---|---|
| 羧化木质素 | BZL | 咪唑基 | 9~11 | 50~57 | 肿瘤化疗 | [ |
| 胺化木质素 | 10-羟基喜树碱 | 咪唑基 | 15.6 | — | 肿瘤化疗 | [ |
| 碱木质素 | 布地奈德 | 羧基 | — | 34~37 | 口服给药 | [ |
| 季铵化木质素 | 布洛芬 | 羧基 | 46 | 74 | 口服给药 | [ |
| 酰化木质素-甲基丙烯酸甲酯 | 布洛芬 | 羧基 | 15.98 | 19.01 | 口服给药 | [ |
| 木质素-胆固醇 | 叶酸 | 羧基 | — | 约67 | — | [ |
| 11 | RASTEGARI B, KARBALAEI-HEIDARI H R, ZEINALI S, et al. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: synthesis, characterization and cytotoxicity studies[J]. Colloids and Surfaces B: Biointerfaces, 2017, 158: 589-601. |
| 12 | BHATTA A, KRISHNAMOORTHY G, MARIMUTHU N, et al. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery[J]. International Journal of Biological Macromolecules, 2019, 136: 951-961. |
| 13 | RIZWAN M, YAHYA R, HASSAN A, et al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications[J]. Polymers, 2017, 9(4): 137. |
| 14 | BAZBAN-SHOTORBANI S, HASANI-SADRABADI M M, KARKHANEH A, et al. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications[J]. Journal of Controlled Release, 2017, 253: 46-63. |
| 15 | XU Y, ZI Y, LEI J, et al. pH-Responsive nanoparticles based on cholesterol/imidazole modified oxidized-starch for targeted anticancer drug delivery[J]. Carbohydrate Polymers, 2020, 233: 115858. |
| 16 | FANG Z, PAN S, GAO P, et al. Stimuli-responsive charge-reversal nano drug delivery system: the promising targeted carriers for tumor therapy[J]. International Journal of Pharmaceutics, 2020, 575: 118841. |
| 17 | RAMASAMY T, RUTTALA H B, GUPTA B, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review[J]. Journal of Controlled Release, 2017, 258: 226-253. |
| 18 | SHI Z, LI Q, MEI L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy[J]. Chinese Chemical Letters, 2020, 31: 1345-1356. |
| 19 | BAHRAMI B, HOJJAT-FARSANGI M, MOHAMMADI H, et al. Nanoparticles and targeted drug delivery in cancer therapy[J]. Immunology Letters, 2017, 190: 64-83. |
| 20 | LI H, CUI Y, SUI J, et al. Efficient delivery of DOX to nuclei of hepatic carcinoma cells in the subcutaneous tumor model using pH-sensitive Pullulan-DOX conjugates[J]. ACS Applied Materials & Interfaces, 2015, 7(29): 15855-15865. |
| 21 | DENG H, LIU J, ZHAO X, et al. PEG-b-PCL copolymer micelles with the ability of pH-controlled negative-to-positive charge reversal for intracellular delivery of Doxorubicin[J]. Biomacromolecules, 2014, 15(11): 4281-4292. |
| 22 | ZHANG C, AN T, WANG D, et al. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(β-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma[J]. Journal of Controlled Release, 2016, 226: 193-204. |
| 1 | MASOOD F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy[J]. Materials Science and Engineering, 2016, 60: 569-578. |
| 2 | ZHANG D, WANG L, ZHANG X, et al. Polymeric micelles for pH-responsive lutein delivery[J]. Journal of Drug Delivery Science and Technology, 2018, 45: 281-286. |
| 3 | LI C, WANG X, LI R, et al. Resveratrol-loaded PLGA nanoparticles functionalized with red blood cell membranes as a biomimetic delivery system for prolonged circulation time[J]. Journal of Drug Delivery Science and Technology, 2019, 54: 101369. |
| 4 | KALYANE D, RAVAL N, MAHESHWARI R, et al. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer[J]. Materials Science and Engineering, 2019, 98: 1252-1276. |
| 5 | VRIGNAUD S, BENOIT J, SAULNIER P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles[J]. Biomaterials, 2011, 32(33): 8593-8604. |
| 6 | SUR S, RATHORE A, DAVE V, et al. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system[J]. Nano-Structures & Nano-Objects, 2019, 20: 100397. |
| 7 | RAO J P, GECKELER K E. Polymer nanoparticles: preparation techniques and size-control parameters[J]. Progress in Polymer Science, 2011, 36(7): 887-913. |
| 23 | WANG Y, KHAN A, LIU Y, et al. Chitosan oligosaccharide-based dual pH responsive nano-micelles for targeted delivery of hydrophobic drugs[J]. Carbohydrate Polymers, 2019, 223: 115061. |
| 24 | LIU Y, WANG W, YANG J, et al. pH-Sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery[J]. Asian Journal of Pharmaceutical Sciences, 2013, 8(3): 159-167. |
| 8 | GAO S, TANG G, HUA D, et al. Stimuli-responsive bio-based polymeric systems and their applications[J]. Journal of Materials Chemistry B, 2019, 7: 709-729. |
| 9 | WANG X, XU J, XU X, et al. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumor[J]. Materials Science and Engineering, 2020, 113: 111004. |
| 25 | JIA N, YE Y, WANG Q, et al. Preparation and evaluation of poly(L-histidine) based pH-sensitive micelles for intracellular delivery of doxorubicin against MCF-7/ADR cells[J]. Asian Journal of Pharmaceutical Sciences, 2017(5): 433-441. |
| 26 | ZHANG L, DING Y, WEN Q, et al. Synthesis of core-crosslinked zwitterionic polymer nano aggregates and pH/Redox responsiveness in drug controlled release[J]. Materials Science & Engineering, 2020, 106: 110288. |
| 27 | LI Y, QIU X, QIAN Y, et al. pH-Responsive lignin-based complex micelles: preparation, characterization and application in oral drug delivery[J]. Chemical Engineering Journal, 2017, 327: 1176-1183. |
| 28 | YU Z, MA L, YE S, et al. Construction of an environmentally friendly octenylsuccinic anhydride modified pH-sensitive chitosan nanoparticle drug delivery system to alleviate inflammation and oxidative stress[J]. Carbohydrate Polymers, 2020, 236: 115972. |
| 10 | ZHOU K, ZHU Y, CHEN X, et al. Redox- and MMP-2-sensitive drug delivery nanoparticles based on gelatin and albumin for tumor targeted delivery of paclitaxel[J]. Materials Science and Engineering, 2020, 114: 111006. |
| 29 | RAHIMI S, KHOEE S, GHANDI M. Development of photo and pH dual crosslinked coumarin-containing chitosan nanoparticles for controlled drug release[J]. Carbohydrate Polymers, 2018, 201: 236-245. |
| 30 | WANG F, LI J, TANG X, et al. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery[J]. Colloids and Surfaces B: Biointerfaces, 2020, 190: 110925. |
| 31 | LI T, YANG J, LIU R, et al. Efficient fabrication of reversible pH-induced carboxymethyl chitosan nanoparticles for antitumor drug delivery under weakly acidic microenvironment[J]. International Journal of Biological Macromolecules, 2019, 126: 68-73. |
| 32 | XIE P, LIU P. pH-Responsive surface charge reversal carboxymethyl chitosan-based drug delivery system for pH and reduction dual-responsive triggered DOX release[J]. Carbohydrate Polymers, 2020, 236: 116093. |
| 33 | FERNANDES PATTA A C M, MATHEWS P D, MADRID R R M, et al. Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications[J]. International Journal of Biological Macromolecules, 2020, 148: 550-564. |
| 34 | MATHEWS P D, FERNANDES P A, GONCALVES J V, et al. Targeted drug delivery and treatment of endoparasites with biocompatible particles of pH-responsive structure[J]. Biomacromolecules, 2018, 19(2): 499-510. |
| 35 | XIE P, LIU P. Core-shell-corona chitosan-based micelles for tumor intracellular pH-triggered drug delivery: improving performance by grafting polycation[J]. International Journal of Biological Macromolecules, 2019, 141: 161-170. |
| 36 | ZHANG Y, CHI C, HUANG X, et al. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract[J]. Carbohydrate Polymers, 2017, 171: 242-251. |
| 37 | SUFI-MARAGHEH P, NIKFARJAM N, DENG Y, et al. Pickering emulsion stabilized by amphiphilic pH-sensitive starch nanoparticles as therapeutic containers[J]. Colloids and Surfaces B: Biointerfaces, 2019, 181: 244-251. |
| 38 | RAYCHAUDHURI R, NAIK S, SHREYA A B, et al. Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: synthesis, nanoformulations and toxicological perspective[J]. International Journal of Biological Macromolecules, 2020, 161: 1189-1205. |
| 39 | WANG Y, LIU Y, LIU Y, et al. pH-sensitive pullulan-based nanoparticles for intracellular drug delivery[J]. Polym. Chem., 2014, 5(2): 423-432. |
| 40 | GUO H, LIU Y, WANG Y, et al. pH-Sensitive pullulan-based nanoparticle carrier for adriamycin to overcome drug-resistance of cancer cells[J]. Carbohydrate Polymers, 2014, 111: 908-917. |
| 41 | DENG B, XIA M, QIAN J, et al. Calcium phosphate-reinforced reduction-sensitive hyaluronic acid micelles for delivering paclitaxel in cancer therapy[J]. Molecular Pharmaceutics, 2017, 14(6): 1938-1949. |
| 42 | ZHAO X, JIA X, LIU L, et al. Double-cross-linked hyaluronic acid nanoparticles with pH/reduction dual-responsive triggered release and ph-modulated fluorescence for folate-receptor-mediated targeting visualized chemotherapy[J]. Biomacromolecules, 2016, 17(4): 1496-1505. |
| 43 | CHEN K, CHANG C, LIU Z, et al. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy[J]. Colloids and Surfaces B: Biointerfaces, 2020, 194: 111166. |
| 44 | CHEN C, SUN W, WANG X, et al. pH-Responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery[J]. International Journal of Biological Macromolecules, 2018, 111: 1106-1115. |
| 45 | LIAO J, ZHENG H, FEI Z, et al. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin[J]. International Journal of Biological Macromolecules, 2018, 113: 737-747. |
| 46 | LIU Y, ZHOU C, WANG W, et al. CD44 receptor targeting and endosomal pH-sensitive dual functional hyaluronic acid micelles for intracellular paclitaxel delivery[J]. Molecular Pharmaceutics, 2016, 13(12): 4209-4221. |
| 47 | REN Q, LIANG Z, JIANG X, et al. Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy[J]. International Journal of Biological Macromolecules, 2019, 130: 845-852. |
| 48 | YIN T, WANG Y, CHU X, et al. Free adriamycin-loaded pH/reduction dual-responsive hyaluronic acid-adriamycin prodrug micelles for efficient cancer therapy[J]. ACS Applied Materials & Interfaces, 2018, 10(42): 35693-35704. |
| 49 | ELZOGHBY A O, SAMY W M, ELGINDY N A. Protein-based nanocarriers as promising drug and gene delivery systems[J]. Journal of Controlled Release, 2012, 161(1): 38-49. |
| 50 | DAS R P, CHAKRAVARTI S, PATEL S S, et al. Tuning the pharmacokinetics and efficacy of irinotecan (IRI) loaded gelatin nanoparticles through folate conjugation[J]. International Journal of Pharmaceutics, 2020, 586: 119522. |
| 51 | LIU P, WU Q, LI Y, et al. DOX-conjugated keratin nanoparticles for pH-sensitive drug delivery[J]. Colloids and Surfaces B: Biointerfaces, 2019, 181: 1012-1018. |
| 52 | ZHANG H, PEI M, LIU P. Keratin-based drug-protein conjugate with acid-labile and reduction-cleavable linkages in series for tumor intracellular DOX delivery[J]. Journal of Industrial and Engineering Chemistry, 2019, 80: 739-748. |
| 53 | ZHANG H, LIU P. Bio-inspired keratin-based core-crosslinked micelles for pH and reduction dual-responsive triggered DOX delivery[J]. International Journal of Biological Macromolecules, 2019, 123: 1150-1156. |
| 54 | LI Y, ZHI X, LIN J, et al. Preparation and characterization of DOX loaded keratin nanoparticles for pH/GSH dual responsive release[J]. Materials Science and Engineering, 2017, 73: 189-197. |
| 55 | ALUIGI A, BALLESTRI M, GUERRINI A, et al. Organic solvent-free preparation of keratin nanoparticles as doxorubicin carriers for antitumour activity[J]. Materials Science and Engineering C, 2018, 90: 476-484. |
| 56 | ZHANG Y, SUN T, JIANG C. Biomacromolecules as carriers in drug delivery and tissue engineering[J]. Acta Pharmaceutica Sinica B, 2018, 8(1): 34-50. |
| 57 | 王才威, 张守玉, 杨东杰, 等. 木醋液制备及形成机理研究进展[J]. 化工进展, 2020, 39(9): 3723-3738. |
| WANG Caiwei, ZHANG Shouyu, YANG Dongjie, et al. Research advance in preparation and formation mechanism of wood vinegar[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3723-3738. | |
| 58 | FIGUEIREDO P, FERRO C, KEMELL M, et al. Functionalization of carboxylated lignin nanoparticles for targeted and pH-responsive delivery of anticancer drugs[J]. Nanomedicine, 2017, 12(21): 2581-2596. |
| 59 | ZHAO J, ZHENG D, TAO Y, et al. Self-assembled pH-responsive polymeric nanoparticles based on lignin-histidine conjugate with small particle size for efficient delivery of anti-tumor drugs[J]. Biochemical Engineering Journal, 2020, 156: 107526. |
| 60 | SIPPONEN M H, LANGE H, AGO M, et al. Understanding lignin aggregation processes. A case study: budesonide entrapment and stimuli controlled release from lignin nanoparticles[J]. ACS Sustainable Chemistry and Engineering, 2018, 6(7): 9342-9351. |
| 61 | CHENG L, DENG B, LUO W, et al. pH-Responsive lignin-based nanomicelles for oral drug delivery[J]. Journal of Agricultural and Food Chemistry, 2020, 68(18): 5249-5258. |
| 62 | POURMOAZZEN Z, SADEGHIFAR H, YANG G, et al. Cholesterol-modified lignin: a new avenue for green nanoparticles, meltable materials, and drug delivery[J]. Colloids and Surfaces B: Biointerfaces, 2020, 186: 110685. |
| [1] | 谈继淮, 余敏, 张彤彤, 黄能坤, 王梓雯, 朱新宝. 新型栲胶聚丙氧基醚酯的合成及增塑PVC性能[J]. 化工进展, 2023, 42(9): 4847-4855. |
| [2] | 刘淑琼, 吴芳芳, 刘瑞来, 许祯毅. 聚乳酸/壳聚糖/氧化石墨烯载阿司匹林仿生支架的制备与表征[J]. 化工进展, 2023, 42(8): 4362-4371. |
| [3] | 戴行, 高瑞雪, 李奕国, 朱锦, 王静刚. 高玻璃化转变温度抗冲击透明聚酯的研究进展[J]. 化工进展, 2023, 42(5): 2555-2565. |
| [4] | 文家新, 张欣, 刘云霞, 和志强, 屈琦超. 基于纳米容器BTA@MSNs-PAA的智能防腐涂层的制备及性能[J]. 化工进展, 2021, 40(5): 2685-2694. |
| [5] | 尹微虹, 巨晓洁, 谢锐, 汪伟, 刘壮, 褚良银. 具有梯级释药性能的核壳型双重载药微球[J]. 化工进展, 2021, 40(2): 998-1007. |
| [6] | 蒋波, 蔡飞鹏, 秦显忠, 王波, 姜桂林, 高金华. 生物基尼龙材料改性与应用进展[J]. 化工进展, 2020, 39(9): 3469-3477. |
| [7] | 赵海田,李旭东,曹凤芹,倪艳,姚磊. 基于壳聚糖纳米粒子载药体系的制备与应用研究进展[J]. 化工进展, 2019, 38(11): 5057-5065. |
| [8] | 陈光宇, 吴林波, 李伯耿. HMF路线合成生物基单体2,5-呋喃二甲酸的研究进展[J]. 化工进展, 2018, 37(08): 3146-3154. |
| [9] | 王静刚, 刘小青, 朱锦. 生物基芳香平台化合物2,5-呋喃二甲酸的合成研究进展[J]. 化工进展, 2017, 36(02): 672-682. |
| [10] | 周瑾洁, 王旭东, 孙亚琴, 修志龙. 生物基化学品的微生物电合成研究进展[J]. 化工进展, 2016, 35(10): 3005-3015. |
| [11] | 徐鑫, 陈骁, 咸漠. 面向资源与环境的生物基化学品技术创新与展望[J]. 化工进展, 2015, 34(11): 3825-3831. |
| [12] | 李素莲1,陈尔凡2. 丙烯酰胺-丙烯酸钠反相微乳液体系制备聚合物纳米粒子[J]. 化工进展, 2013, 32(09): 2180-2184. |
| [13] | 罗 琳1,2,王运灿1,王天强1,郝建原1,2,刘 钰1,2. 新型非浸润模板法制备抗癌载药微粒的研究进展[J]. 化工进展, 2013, 32(06): 1372-1376. |
| [14] | 王 军,李明春,辛梅华,张晓林,毛扬帆. N-mPEG-O-季铵化壳聚糖微球的制备及其载药性能[J]. 化工进展, 2013, 32(01): 140-144. |
| [15] | 蒋平平,张书源,冷 炎,董玉明,张萍波. 催化合成环保增塑剂的研究及其应用进展[J]. 化工进展, 2012, 31(05): 953-964. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||