化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3421-3433.DOI: 10.16085/j.issn.1000-6613.2020-1446
CO2插入C—H(sp)键制备丙炔酸衍生物的研究进展
李民康1,2( ), 张莉娜2, 张阿方1, 赵永慧2, 孙楠楠2(
), 张莉娜2, 张阿方1, 赵永慧2, 孙楠楠2( ), 魏伟2(
), 魏伟2( )
)
- 1.上海大学材料科学与工程学院,上海 200444
2.中国科学院上海高等研究院低碳转化科学与工程重点实验室,上海 201210
-
收稿日期:2020-07-27修回日期:2020-09-05出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:孙楠楠,魏伟 -
作者简介:李民康(1995—),男,硕士研究生,研究方向为CO2气体催化转化。E-mail:liminkang2018@sari.ac.cn 。
Research advances on the carboxylation of terminal alkynes with CO2
LI Minkang1,2( ), ZHANG Lina2, ZHANG Afang1, ZHAO Yonghui2, SUN Nannan2(
), ZHANG Lina2, ZHANG Afang1, ZHAO Yonghui2, SUN Nannan2( ), WEI Wei2(
), WEI Wei2( )
)
- 1.School of Materials Science & Engineering, Shanghai University, Shanghai 200444, China
2.CAS Key Laboratory of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
-
Received:2020-07-27Revised:2020-09-05Online:2021-06-06Published:2021-06-22 -
Contact:SUN Nannan,WEI Wei
摘要:
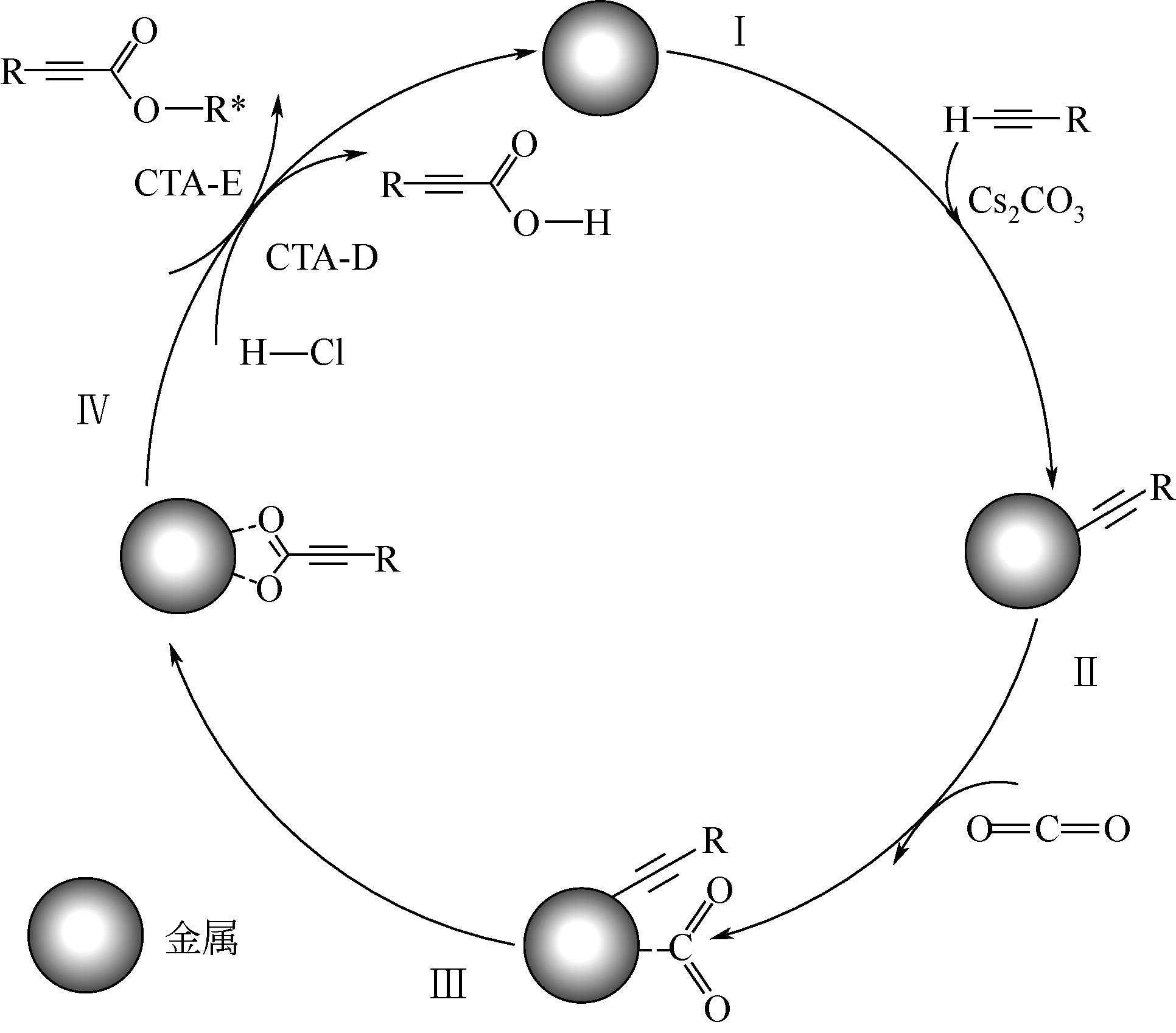
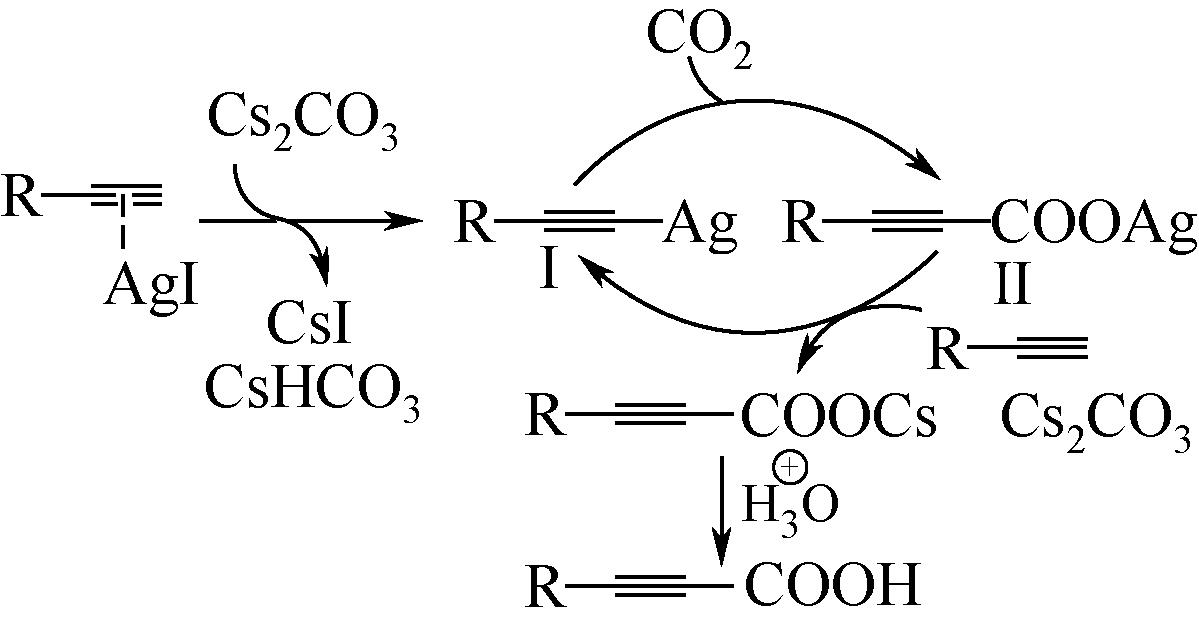
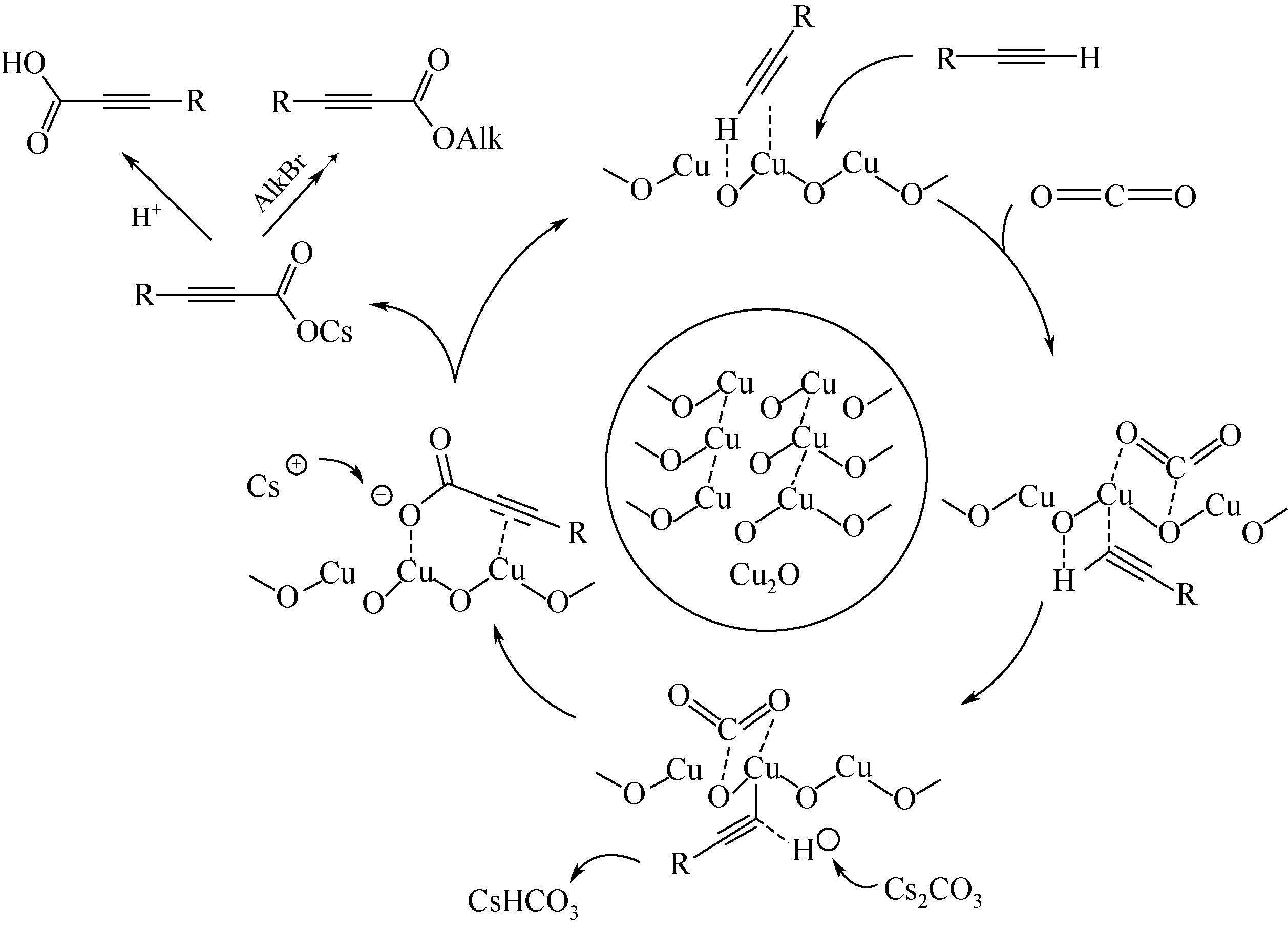
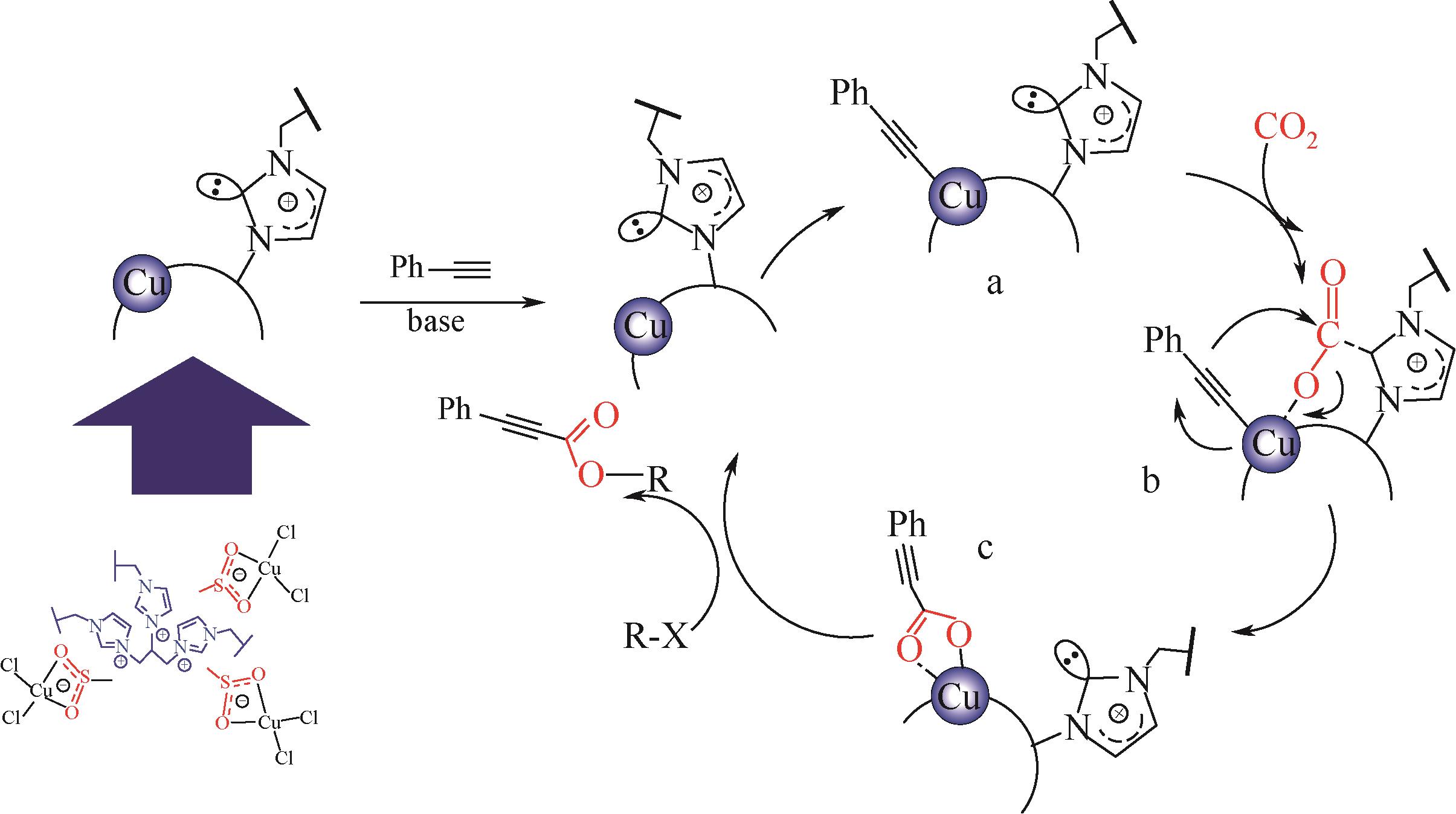
丙炔酸类化合物是一类用途广泛的有机中间体,以CO2为羧基化试剂,将其插入到端基炔烃的C—H(sp)键是制备丙炔酸类化合物的一条新型路线,其绿色化程度显著高于传统方法。本文在系统分析CO2路线制备丙炔酸类化合物反应机理的基础上,梳理了该反应催化体系的研究进展,并总结了近年来与该反应实际应用密切相关的前瞻研究。基于上述总结,本文认为后续研究应开展的工作包括:①从实际工况角度(耐强碱性反应体系、耐强极性溶剂等)出发进行催化剂的设计;②强化反应机理研究,明确不同催化剂上的反应路径和中间体形态差异,夯实理论基础;③强化潜在技术瓶颈问题的突破,尤其是湿度敏感、碱助剂循环、下游合成网络衔接等。
中图分类号:
引用本文
李民康, 张莉娜, 张阿方, 赵永慧, 孙楠楠, 魏伟. CO2插入C—H(sp)键制备丙炔酸衍生物的研究进展[J]. 化工进展, 2021, 40(6): 3421-3433.
LI Minkang, ZHANG Lina, ZHANG Afang, ZHAO Yonghui, SUN Nannan, WEI Wei. Research advances on the carboxylation of terminal alkynes with CO2[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3421-3433.
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| (IPr)CuCl | 60 | 1.5 | 24 | 91 | 9 | 0.38 | CTA-E | [ |
| Cu-NHC | 25 | 0.1 | 16 | 90 | 45 | 2.81 | CTA-D | [ |
| bis-(NHC)-Ag | 室温 | 0.1 | 16 | 85 | 85 | 5.31 | CTA-D | [ |
| L3/Ag | 35 | 0.1 | 24 | 98 | 392 | 16.33 | CTA-D | [ |
| Ag-NHC化合物 | 室温 | 0.1 | 16 | 82 | 82 | 5.13 | CTA-D | [ |
| Ag-NHC化合物 | 40 | 0.1 | 48 | 92 | 46 | 0.96 | CTA-E | [ |
| Ag-NHC化合物 | 60 | 0.2 | 12 | 47 | 5 | 0.39 | CTA-D | [ |
| CuI+PEt3 | 室温 | 0.1 | 24 | 90 | 11 | 0.47 | CTA-E | [ |
| [CuI(dtbpf)] | 25 | 0.1 | 24 | 96 | 48 | 2.00 | CTA-D | [ |
| [Cu2(μ-CN)2(k2-P,P-dppet)2] | 25 | 0.1 | 12 | 97 | 97 | 8.08 | CTA-D | [ |
| AgI | 50 | 0.2 | 12 | 94 | 94 | 7.83 | CTA-D | [ |
| AgI | 60 | 1.5 | 24 | 91 | 910 | 37.92 | CTA-E | [ |
| CuI | 50 | 8 | 12 | 92 | 46 | 3.83 | CTA-E | [ |
| AgBF4 | 50 | 0.1 | 16 | 99 | 1980 | 123.75 | CTA-D | [ |
| CuI | 80 | 0.1 | 18 | 99 | 10 | 0.55 | CTA-E | [ |
| AgI | 40 | 0.1 | 48 | 88 | 18 | 0.37 | CTA-E | [ |
| CuCl | 室温 | 0.1 | 16 | 90 | 18 | 1.13 | CTA-D | [ |
| Ag2WO4 | 室温 | 0.1 | 24 | 99 | 40 | 1.65 | CTA-E | [ |
| Ag(O2CNMe2) | 50 | 0.1 | 24 | 77 | 39 | 1.60 | CTA-E | [ |
表1 一价铜盐/银盐催化剂体系CTA反应催化性能对比
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| (IPr)CuCl | 60 | 1.5 | 24 | 91 | 9 | 0.38 | CTA-E | [ |
| Cu-NHC | 25 | 0.1 | 16 | 90 | 45 | 2.81 | CTA-D | [ |
| bis-(NHC)-Ag | 室温 | 0.1 | 16 | 85 | 85 | 5.31 | CTA-D | [ |
| L3/Ag | 35 | 0.1 | 24 | 98 | 392 | 16.33 | CTA-D | [ |
| Ag-NHC化合物 | 室温 | 0.1 | 16 | 82 | 82 | 5.13 | CTA-D | [ |
| Ag-NHC化合物 | 40 | 0.1 | 48 | 92 | 46 | 0.96 | CTA-E | [ |
| Ag-NHC化合物 | 60 | 0.2 | 12 | 47 | 5 | 0.39 | CTA-D | [ |
| CuI+PEt3 | 室温 | 0.1 | 24 | 90 | 11 | 0.47 | CTA-E | [ |
| [CuI(dtbpf)] | 25 | 0.1 | 24 | 96 | 48 | 2.00 | CTA-D | [ |
| [Cu2(μ-CN)2(k2-P,P-dppet)2] | 25 | 0.1 | 12 | 97 | 97 | 8.08 | CTA-D | [ |
| AgI | 50 | 0.2 | 12 | 94 | 94 | 7.83 | CTA-D | [ |
| AgI | 60 | 1.5 | 24 | 91 | 910 | 37.92 | CTA-E | [ |
| CuI | 50 | 8 | 12 | 92 | 46 | 3.83 | CTA-E | [ |
| AgBF4 | 50 | 0.1 | 16 | 99 | 1980 | 123.75 | CTA-D | [ |
| CuI | 80 | 0.1 | 18 | 99 | 10 | 0.55 | CTA-E | [ |
| AgI | 40 | 0.1 | 48 | 88 | 18 | 0.37 | CTA-E | [ |
| CuCl | 室温 | 0.1 | 16 | 90 | 18 | 1.13 | CTA-D | [ |
| Ag2WO4 | 室温 | 0.1 | 24 | 99 | 40 | 1.65 | CTA-E | [ |
| Ag(O2CNMe2) | 50 | 0.1 | 24 | 77 | 39 | 1.60 | CTA-E | [ |
| 催化剂 | 温度 /℃ | 压力 /MPa | 时间 /h | 收率 /% | TON | TOF /h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@P-NHC | 25 | 0.1 | 20 | 98 | 327 | 16.35 | CTA-D | [ |
| CuBr@C | 80 | 0.1 | 2 | 90 | 18 | 9.00 | CTA-E | [ |
| Ag/PCNF | 25 | 0.1 | 18 | 98 | 71 | 3.94 | CTA-D | [ |
| Ag@PHNCT | 50 | 0.1 | 20 | 98 | 94 | 4.68 | CTA-D | [ |
| Cu-CN | 80 | 0.1 | 10 | 97 | 97 | 9.70 | CTA-D | [ |
| AgNPs@m-MgO | 70 | 0.1 | 12 | 98 | 47 | 3.89 | CTA-D | [ |
| Ag/M-CeO2 | 60 | 0.5 | 24 | 91 | 26 | 1.09 | CTA-E | [ |
| CuNPs/Al2O3 | 60 | 0.2 | 16 | 92 | 18 | 1.15 | CTA-E | [ |
| Ag/F-Al2O3 | 50 | 6 | 18 | 62 | 12 | 0.67 | CTA-D | [ |
| Ag/Schiff-SiO2 | 60 | 0.1 | 24 | 98 | 705 | 29.38 | CTA-D | [ |
表2 负载型催化剂CTA反应催化性能对比
| 催化剂 | 温度 /℃ | 压力 /MPa | 时间 /h | 收率 /% | TON | TOF /h-1 | CTA-D/CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@P-NHC | 25 | 0.1 | 20 | 98 | 327 | 16.35 | CTA-D | [ |
| CuBr@C | 80 | 0.1 | 2 | 90 | 18 | 9.00 | CTA-E | [ |
| Ag/PCNF | 25 | 0.1 | 18 | 98 | 71 | 3.94 | CTA-D | [ |
| Ag@PHNCT | 50 | 0.1 | 20 | 98 | 94 | 4.68 | CTA-D | [ |
| Cu-CN | 80 | 0.1 | 10 | 97 | 97 | 9.70 | CTA-D | [ |
| AgNPs@m-MgO | 70 | 0.1 | 12 | 98 | 47 | 3.89 | CTA-D | [ |
| Ag/M-CeO2 | 60 | 0.5 | 24 | 91 | 26 | 1.09 | CTA-E | [ |
| CuNPs/Al2O3 | 60 | 0.2 | 16 | 92 | 18 | 1.15 | CTA-E | [ |
| Ag/F-Al2O3 | 50 | 6 | 18 | 62 | 12 | 0.67 | CTA-D | [ |
| Ag/Schiff-SiO2 | 60 | 0.1 | 24 | 98 | 705 | 29.38 | CTA-D | [ |
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/ CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@MIL-101(Fe) | 50 | 0.1 | 15 | 97 | 36 | 2.40 | CTA-D | [ |
| Ag@UIO-66(Zr) | 50 | 0.1 | 15 | 96.5 | 21 | 1.42 | CTA-D | [ |
| AgNPs/Co-MOF | 80 | 0.1 | 14 | 98 | 49 | 3.50 | CTA-D | [ |
| Pd-Cu/MIL-101 | 25 | 0.1 | 24 | 96 | 691 | 28.79 | CTA-D | [ |
| Ag@1 | 60 | 0.1 | 6 | 91 | 18 | 3.03 | CTA-E | [ |
| Ag@ZIF-8 | 40 | 0.1 | 20 | 97 | 86 | 4.31 | CTA-D | [ |
| UiO-66@UiO-67-BPY-Ag | 50 | 0.1 | 24 | 97 | 80 | 3.31 | CTA-D | [ |
| ZIF-8@Au25@ZIF-67 | 50 | 0.1 | 12 | 99 | 4433 | 369.42 | CTA-D | [ |
| Cu-MOFs | 80 | 0.1 | 4 | 80 | 20 | 5.00 | CTA-E | [ |
| Ag/KAPs-P | 60 | 0.1 | 10 | 92 | 9936 | 993.60 | CTA-D | [ |
| Ag@CTFN | 60 | 0.1 | 24 | 97 | 128 | 5.34 | CTA-D | [ |
| CTF-DCE-Ag | 50 | 0.1 | 24 | 90.2 | 226 | 9.42 | CTA-D | [ |
| Ag@NOMP | 50 | 0.1 | 12 | 96 | 960 | 80.00 | CTA-D | [ |
| Ag-HMP | 80 | 0.1 | 12 | 98 | 82 | 6.86 | CTA-D | [ |
| AgNPs@m-PS-PC | 70 | 0.1 | 10 | 91 | 779 | 77.93 | CTA-D | [ |
| [Cu(Im12)2][CuBr2] | 25 | 0.1 | 12 | 96 | 10 | 0.83 | CTA-E | [ |
| CuCl2@poly-GLY(1-vim)3(OMs)3 | 40 | 4 | 12 | 96 | 5 | 0.40 | CTA-E | [ |
表3 新型催化剂CTA反应催化性能对比
| 催化剂 | 温度/℃ | 压力/MPa | 时间/h | 收率/% | TON | TOF/h-1 | CTA-D/ CTA-E | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ag@MIL-101(Fe) | 50 | 0.1 | 15 | 97 | 36 | 2.40 | CTA-D | [ |
| Ag@UIO-66(Zr) | 50 | 0.1 | 15 | 96.5 | 21 | 1.42 | CTA-D | [ |
| AgNPs/Co-MOF | 80 | 0.1 | 14 | 98 | 49 | 3.50 | CTA-D | [ |
| Pd-Cu/MIL-101 | 25 | 0.1 | 24 | 96 | 691 | 28.79 | CTA-D | [ |
| Ag@1 | 60 | 0.1 | 6 | 91 | 18 | 3.03 | CTA-E | [ |
| Ag@ZIF-8 | 40 | 0.1 | 20 | 97 | 86 | 4.31 | CTA-D | [ |
| UiO-66@UiO-67-BPY-Ag | 50 | 0.1 | 24 | 97 | 80 | 3.31 | CTA-D | [ |
| ZIF-8@Au25@ZIF-67 | 50 | 0.1 | 12 | 99 | 4433 | 369.42 | CTA-D | [ |
| Cu-MOFs | 80 | 0.1 | 4 | 80 | 20 | 5.00 | CTA-E | [ |
| Ag/KAPs-P | 60 | 0.1 | 10 | 92 | 9936 | 993.60 | CTA-D | [ |
| Ag@CTFN | 60 | 0.1 | 24 | 97 | 128 | 5.34 | CTA-D | [ |
| CTF-DCE-Ag | 50 | 0.1 | 24 | 90.2 | 226 | 9.42 | CTA-D | [ |
| Ag@NOMP | 50 | 0.1 | 12 | 96 | 960 | 80.00 | CTA-D | [ |
| Ag-HMP | 80 | 0.1 | 12 | 98 | 82 | 6.86 | CTA-D | [ |
| AgNPs@m-PS-PC | 70 | 0.1 | 10 | 91 | 779 | 77.93 | CTA-D | [ |
| [Cu(Im12)2][CuBr2] | 25 | 0.1 | 12 | 96 | 10 | 0.83 | CTA-E | [ |
| CuCl2@poly-GLY(1-vim)3(OMs)3 | 40 | 4 | 12 | 96 | 5 | 0.40 | CTA-E | [ |
| 1 | DECONTO R M, POLLARD D. Contribution of Antarctica to past and future sea-level rise[J]. Nature, 2016, 531(7596): 591-597. |
| 2 | CARLETON T A, HSIANG S M. Social and economic impacts of climate[J]. Science, 2016, 353(6304): add9837. |
| 3 | IPCC. Climate change 2013: the physical science basis. working group Ⅰ contribution to the fifth assessment report of the intergovernmental panel on climate change[R]. Cambridge: Cambridge University Press, 2013. |
| 4 | IEA. World energy outlook 2019[R]. Paris: IEA, 2019. |
| 5 | MANJOLINHOHO F, ARANT M, GOOßEN K, et al. Catalytic C—H carboxylation of terminal alkynes with carbon dioxide[J]. ChemInform, 2012, 43(44): 2014-2021. |
| 6 | QIAO C, CAO Y, HE L N. Transition metal-catalyzed carboxylation of terminal alkynes with CO2[J]. Mini-Reviews in Organic Chemistry, 2018, 15: 283-290. |
| 7 | YU B, DIAO Z F, GUO C X, et al. Carboxylation of terminal alkynes at ambient CO2 pressure in ethylene carbonate[J]. Green Chemistry, 2013, 15(9): 2401-2407. |
| 8 | GOOßEN L J, RODRÍGUEZ N, MANJOLINHO F, et al. Synthesis of propiolic acids via copper-catalyzed insertion of carbon dioxide into the C—H bond of terminal alkynes[J]. Advanced Synthesis & Catalysis, 2010, 352(17): 2913-2917. |
| 9 | LIU C, LUO Y, ZHANG W Z, et al. DFT studies on the silver-catalyzed carboxylation of terminal alkynes with CO2: an insight into the catalytically activespecies [J]. Organometallics, 2014, 33(12): 2984-2989. |
| 10 | YASUO FUKUE S O, INOUE YOSHIO. Direct synthesis of alkyl 2-alkynoates from alklynes, CO2, and bromoalkanes catalysed by copper(Ⅰ) or silver(Ⅰ) salt[J]. Journal of the Chemical Society, Chemical Communications, 1994, 18: 2091. |
| 11 | ZHANG W Z, LI W J, ZHANG X, et al. Cu(Ⅰ)-catalyzed carboxylative coupling of terminal alkynes, allylic chlorides, and CO2[J]. Organic Letters, 2010, 12(21): 4748-4751. |
| 12 | YU D, ZHANG Y. Copper-and copper-N-heterocyclic carbene-catalyzed C—H activating carboxylation of terminal alkynes with CO2 at ambient conditions[J]. Proceeding of the Royal Society of Sciences of the UnitedStates of America, 2010, 107(47): 20184-20189. |
| 13 | DÍAZ VELÁZQUEZ H, WU Z X, VANDICHEL M, et al. Inserting CO2 into terminal alkynes via bis-(NHC)-metal complexes [J]. Catalysis Letters, 2017, 147(2): 463-471. |
| 14 | YUAN Y, CHEN C, ZENG C, et al. Carboxylation of terminal alkynes with carbon dioxide catalyzed by an in situ Ag2O/N-heterocyclic carbene precursor system [J]. ChemCatChem, 2017, 9(5): 882-887. |
| 15 | LI S S, SUN J, ZHANG Z Z, et al. Carboxylation of terminal alkynes with CO2 using novel silver N-heterocyclic carbene complexes[J]. Dalton Transacitions, 2016, 45(26): 10577-10584. |
| 16 | ZHANG Z Z, MI R J, GUO F J, et al. 1,3-bis(4-methylbenzyl)imidazol-2-ylidene silver(Ⅰ) chloride catalyzed carboxylative coupling of terminal alkynes, butyl iodide and carbon dioxide[J]. Journal of Saudi Chemical Society, 2017, 21(6): 685-690. |
| 17 | WANG W, ZHANG G, LANG R, et al. pH-responsive N-heterocyclic carbene copper(Ⅰ) complexes: syntheses and recoverable applications in the carboxylation of arylboronic esters and benzoxazole with carbon dioxide[J]. Green Chemistry, 2013, 15(3): 635-640. |
| 18 | PAPASTAVROU A T, PAUZE M, GÓMEZ BENGOA E, et al. Unprecedented multicomponent organocatalytic synthesis of propargylic esters via CO2 activation[J]. ChemCatChem, 2019, 11(21): 5379-5386. |
| 19 | INAMOTO K, ASANO N, KOBAYASHI K, et al. A copper-based catalytic system for carboxylation of terminal alkynes: synthesis of alkyl 2-alkynoates[J]. Organic & Biomolecular Chemistry, 2012, 10(8): 1514-1516. |
| 20 | TRIVEDI M, SINGH G, KUMAR A, et al. 1,1'-bis(di-tert-butylphosphino) ferrocene copper(Ⅰ) complex catalyzed C—H activation and carboxylation of terminal alkynes[J]. Dalton Transactions, 2015, 44(48): 20874-20882. |
| 21 | TRIVEDI M, SMREKER J R, SINGH G, et al. Cis-1,2-bis(diphenylphosphino)ethylene copper(Ⅰ) catalyzed C—H activation and carboxylation of terminal alkynes[J]. New Journal of Chemistry, 2017, 41(23): 14145-14151. |
| 22 | ZHANG X, ZHANG W Z, REN X, et al. Ligand-free Ag(Ⅰ)-catalyzed carboxylation of terminal alkynes with CO2[J]. Organic Letters, 2011, 40(5): 2435-2452. |
| 23 | ZHANG X, ZHANG W Z, SHI L L, et al. Ligand-free Ag(Ⅰ)-catalyzed carboxylative coupling of terminal alkynes, chloride compounds, and CO2[J]. Tetrahedron, 2012, 68(44): 9085-9089. |
| 24 | LI F W, SUO Q L, HONG H L, et al. DBU and copper(Ⅰ) mediated carboxylation of terminal alkynes using supercritical CO2 as a reactant and solvent[J]. Tetrahedron Letters, 2014, 55(29): 3878-3880. |
| 25 | ARNDT M, RISTO E, KRAUSE T, et al. C—H carboxylation of terminal alkynescatalyzed by low loadings of silver(Ⅰ)/DMSO at ambient CO2 pressure[J]. ChemCatChem, 2012, 4(4): 484-487. |
| 26 | GUO F J, ZHANG Z Z, WANG J Y, et al. Silver-catalyzed one-pot synthesis of benzyl 2-alkynoates under ambient pressure of CO2 and ligand-free conditions[J]. Tetrahedron, 2017, 73(7): 900-906. |
| 27 | WANG W H, JIA L H, FENG X J, et al. Efficient carboxylation of terminal alkynes with carbon dioxide catalyzed by ligand-free copper catalyst under ambient conditions[J]. Asian Journal of Organic Chemistry, 2019, 8(8): 1501-1505. |
| 28 | GUO C X, YU B, XIE J N, et al. Silver tungstate: a single-component bifunctional catalyst for carboxylation of terminal alkynes with CO2 in ambient conditions[J]. Green Chemistry, 2015, 17(1): 474-479. |
| 29 | BRESCIANI G, MARCHETTI F, PAMPALONI G. Carboxylation of terminal alkynes promoted by silver carbamate at ambient pressure[J]. New Journal of Chemistry, 2019, 43(27): 10821-10825. |
| 30 | YU D Y, TAN M X, ZHANG Y G. Carboxylation of terminal alkynes with carbon dioxide catalyzed by poly(N-heterocyclic carbene)-supported silver nanoparticles[J]. Advanced Synthesis & Catalysis, 2012, 354(6): 969-974. |
| 31 |
YU B, XIE J N, ZHONG C L, et al. Copper( )@carbon-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. ACS Catalysis, 2015, 5(7): 3940-3944. )@carbon-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. ACS Catalysis, 2015, 5(7): 3940-3944.
|
| 32 | LAN X W, LI Y M, DU C, et al. Porous carbon nitride frameworks derived from covalent triazine framework anchored Ag nanoparticles for catalytic CO2 conversion[J]. Chemistry, 2019, 25(36): 8560-8569. |
| 33 | LAN X W, LI Q, CAO L L, et al. Rebuilding supramolecular aggregates to porous hollow N-doped carbon tube inlaid with ultrasmall Ag nanoparticles: a highly efficient catalyst for CO2 conversion[J]. Applied Surface Science, 2020, 508: 45220-45229. |
| 34 | YANG P, ZUO S W, ZHANG F T, et al. Carbon nitride-based single-atom Cu catalysts for highly efficient carboxylation of alkynes with atmospheric CO2[J]. Industrial & Engineering Chemistry Research, 2020, 59(16): 7327-7335. |
| 35 | CHOWDHURY A H, GHOSH S, ISLAM S M. Flower-like AgNPs@ m-MgO as an excellent catalyst for CO2 fixation and acylation reactions under ambient conditions[J]. New Journal of Chemistry, 2018, 42(17): 14194-14202. |
| 36 | ZHANG X, WANG D K, JING M Z, et al. Ordered mesoporous CeO2-supported Ag as an effective catalyst for carboxylative coupling reaction using CO2[J]. ChemCatChem, 2019, 11(8): 2089-2098. |
| 37 | CHOWDHURY A H, KAYAL U, CHOWDHURY I H, et al. Nanoporous ZnO supported CuBr (CuBr/ZnO): an efficient catalyst for CO2 fixation reactions[J]. ChemistrySelect, 2019, 4(3): 1069-1077. |
| 38 | BONDARENKO G N, DVURECHENSKAYA E G, MAGOMMEDOV E S, et al. Copper(0) nanoparticles supported on Al2O3 as catalyst for carboxylation of terminal alkynes[J]. Catalysis Letters, 2017, 147(10): 2570-2580. |
| 39 | FINASHINA E D, KUSTOV L M, TKACHENKO O P, et al. Carboxylation of phenylacetylene by carbon dioxide on heterogeneous Ag-containing catalysts[J]. Russian Chemical Bulletin: International Edition, 2014, 63(12): 2652-2656. |
| 40 | WU Z L, SUN L, LIU Q G, et al. A Schiff base-modified silver catalyst for efficient fixation of CO2 as carboxylic acid at ambient pressure[J]. Green Chemistry, 2017, 19(9): 2080-2085. |
| 41 | LIU X H, MA J G, NIU Z, et al. An efficient nanoscale heterogeneous catalyst for the capture and conversion of carbon dioxide at ambient pressure[J]. Angewandte Chemie: International Edition, 2015, 54(3): 988-991. |
| 42 | ZHU N N, LIU X H, LI T, et al. Composite system of Ag nanoparticles and metal-organic frameworks for the capture and conversion of carbon dioxide under mild conditions[J]. Inorganic Chemistry, 2017, 56(6): 3414-3420. |
| 43 | MOLLA R A, GHOSH K, BANERJEE B, et al. Silver nanoparticles embedded over porous metal organic frameworks for carbon dioxide fixation via carboxylation of terminal alkynes at ambient pressure[J]. Journal of Colloid and Interface Science, 2016, 477: 20-29. |
| 44 | TRIVEDI M, BHASKARAN B, KUMAR A, et al. Metal-organic framework MIL-101 supported bimetallic Pd-Cu nanocrystals as efficient catalysts for chromium reduction and conversion of carbon dioxide at room temperature[J]. New Journal of Chemistry, 2016, 40(4): 3109-3118. |
| 45 | DUTTA G, JANA A K, SINGH D K, et al. Encapsulation of silver nanoparticles in an amine-functionalized porphyrin metal-organic framework and its use as a heterogeneous catalyst for CO2 fixation under atmospheric pressure[J]. Chemistry—An Asian Journal, 2018, 13(18): 2677-2684. |
| 46 | SHI J L, ZHANG L N, SUN N N, et al. Facile and rapid preparation of Ag@ZIF-8 for carboxylation of terminal alkynes with CO2 in mild conditions[J]. ACS Applied Materials & Interfaces, 2019, 11(32): 28858-28867. |
| 47 | GONG Y Y, YUAN Y, CHEN C, et al. Core-shell metal-organic frameworks and metal functionalization to access highest efficiency in catalytic carboxylation[J]. Journal of Catalysis, 2019, 371: 106-115. |
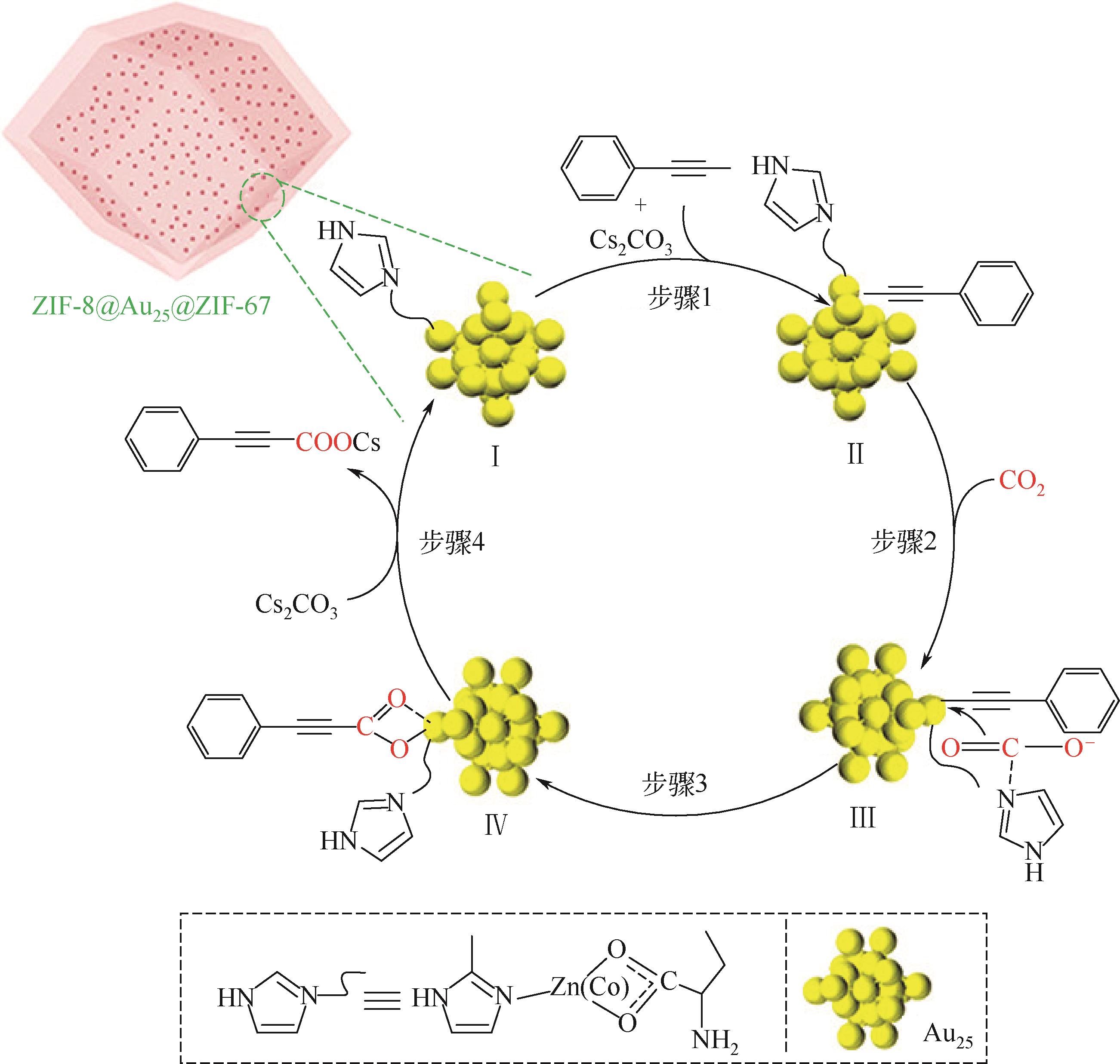
| 48 | YUN Y P, SHENG H T, BAO K, et al. Design and remarkable efficiency of the robust sandwich cluster composite nanocatalysts ZIF-8@Au25@ZIF-67[J]. Journal of the American Chemical Society, 2020, 142(9): 4126-4130. |
| 49 | XIONG G, YU B, DONG J, et al. Cluster-based MOFs with accelerated chemical conversion of CO2 through C—C bond formation[J]. Chemical Communications, 2017, 53(44): 6013-6016. |
| 50 | GANINA O G, BONDARENKO G N, ISAEVA V I, et al. Cu-MOF-catalyzed carboxylation of alkynes and epoxides[J]. Russian Journal of Organic Chemistry, 2019, 55(12): 1813-1820. |
| 51 | WU Z L, LIU Q G, YANG X F, et al. Knitting aryl network polymers-incorporated Ag nanoparticles: a mild and efficient catalyst for the fixation of CO2 as carboxylic acid[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9634-9639. |
| 52 | LAN X W, DU C, CAO L L, et al. Ultrafine Ag nanoparticles encapsulated by covalent triazine framework nanosheets for CO2 conversion[J]. ACS Applied Materials & Interfaces, 2018, 10(45): 38953-38962. |
| 53 | DANG Q Q, LIU C Y, WANG X M, et al. Novel covalent triazine framework for high-performance CO2 capture and alkyne carboxylation reaction[J]. ACS Applied Materials & Interfaces, 2018, 10(33): 27972-27978. |
| 54 | ZHANG W, MEI Y, HUANG X, et al. Size-controlled growth of silver nanoparticles onto functionalized ordered mesoporous polymers for efficient CO2 upgrading[J]. ACS Applied Materials & Interfaces, 2019, 11(47): 44241-44248. |
| 55 | GHOSH S, GHOSH A, RIYAJUDDIN S, et al. Silver nanoparticles architectured HMP as a recyclable catalyst for tetramic acid and propiolic acid synthesis through CO2 capture at atmospheric pressure[J]. ChemCatChem, 2020, 12(4): 1055-1067. |
| 56 | SALAM N, PAUL P, GHOSH S, et al. AgNPs encapsulated by an amine-functionalized polymer nanocatalyst for CO2 fixation as a carboxylic acid and the oxidation of cyclohexane under ambient conditions[J]. New Journal of Chemistry, 2020, 44(14): 5448-5456. |
| 57 | XIE J N, YU B, ZHOU Z H, et al. Copper(Ⅰ)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure[J]. Tetrahedron Letters, 2015, 56(50): 7059-7062. |
| 58 | CHAUGULE A A, TAMBOLI A H, KIM H. CuCl2@poly-IL catalyzed carboxylation of terminal alkynes through CO2 utilization[J]. Chemical Engineering Journal, 2017, 326: 1009-1019. |
| 59 | WANG X, LIM Y N, LEE C, et al. 1,5,7-triazabicyclo[4.4.0]dec-1-ene-mediated acetylene dicarboxylation and alkyne carboxylation using carbon dioxide[J]. European Journal of Organic Chemistry, 2013(10): 1867-1871. |
| 60 | TONIOLO D, BOBBINK F D, DYSON P J, et al. Anhydrous conditions enable the catalyst-free carboxylation of aromatic alkynes with CO2 under mild conditions[J]. Helvetica Chimica Acta, 2020, 103(2): e1900258. |
| 61 | YU D Y, ZHANG Y G. The direct carboxylation of terminal alkynes with carbon dioxide[J]. Green Chemistry, 2011, 13(5): 1275-1279. |
| 62 | WANG W H, FENG X J, SUI K, et al. Transition metal-free carboxylation of terminal alkynes with carbon dioxide through dual activation: synthesis of propiolic acids[J]. Journal of CO2 Utilization, 2019, 32: 140-145. |
| 63 | YU D Y, ZHOU F, LIM D S, et al. NHC-Ag/Pd-catalyzed reductive carboxylation of terminal alkynes with CO2 and H2: a combined experimental and computational study for fine-tuned selectivity [J]. ChemSusChem, 2017, 10(5): 836-841. |
| 64 | SONG B, HE B Z, QIN A J, et al. Direct polymerization of carbon dioxide, diynes, and alkyl dihalides under mild reaction conditions[J]. Macromolecules, 2017, 51(1): 42-48. |
| 65 | KUGE K, LUO Y, FUJITA Y, et al. Copper-catalyzed stereodefined construction of acrylic acid derivatives from terminal alkynes via CO2 insertion[J]. Organic Letters, 2017, 19(4): 854-857. |
| 66 | WENDLING T, RISTO E, KRAUSE T, et al. Salt-free strategy for the insertion of CO2 into C—H bonds: catalytic hydroxymethylation of alkynes[J]. Chemistry, 2018, 24(23): 6019-6024. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [8] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||