化工进展 ›› 2019, Vol. 38 ›› Issue (11): 4941-4948.DOI: 10.16085/j.issn.1000-6613.2019-0195
金属氧化物催化CO还原NO的研究进展
- 北京科技大学能源与环境工程学院,北京 100083
-
收稿日期:2018-12-30出版日期:2019-11-05发布日期:2019-11-05 -
通讯作者:唐晓龙 -
作者简介:周远松(1985—),男,硕士,工程师,研究方向为大气污染控制。E-mail:zhouys@ustb.edu.cn 。 -
基金资助:国家重点研发计划重点专项(2017YFC0210605);国家自然科学基金(U1660109);中央高校基本科研业务费(FRF-TP-18-019A1);京津冀协同创新推动(Z161100002716025)
Research progress on NO reduction by CO over metal oxide catalysts
Yuansong ZHOU( ),Fengyu GAO,Xiaolong TANG(
),Fengyu GAO,Xiaolong TANG( ),Honghong YI,Jingxuan MENG
),Honghong YI,Jingxuan MENG
- School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China
-
Received:2018-12-30Online:2019-11-05Published:2019-11-05 -
Contact:Xiaolong TANG
摘要:
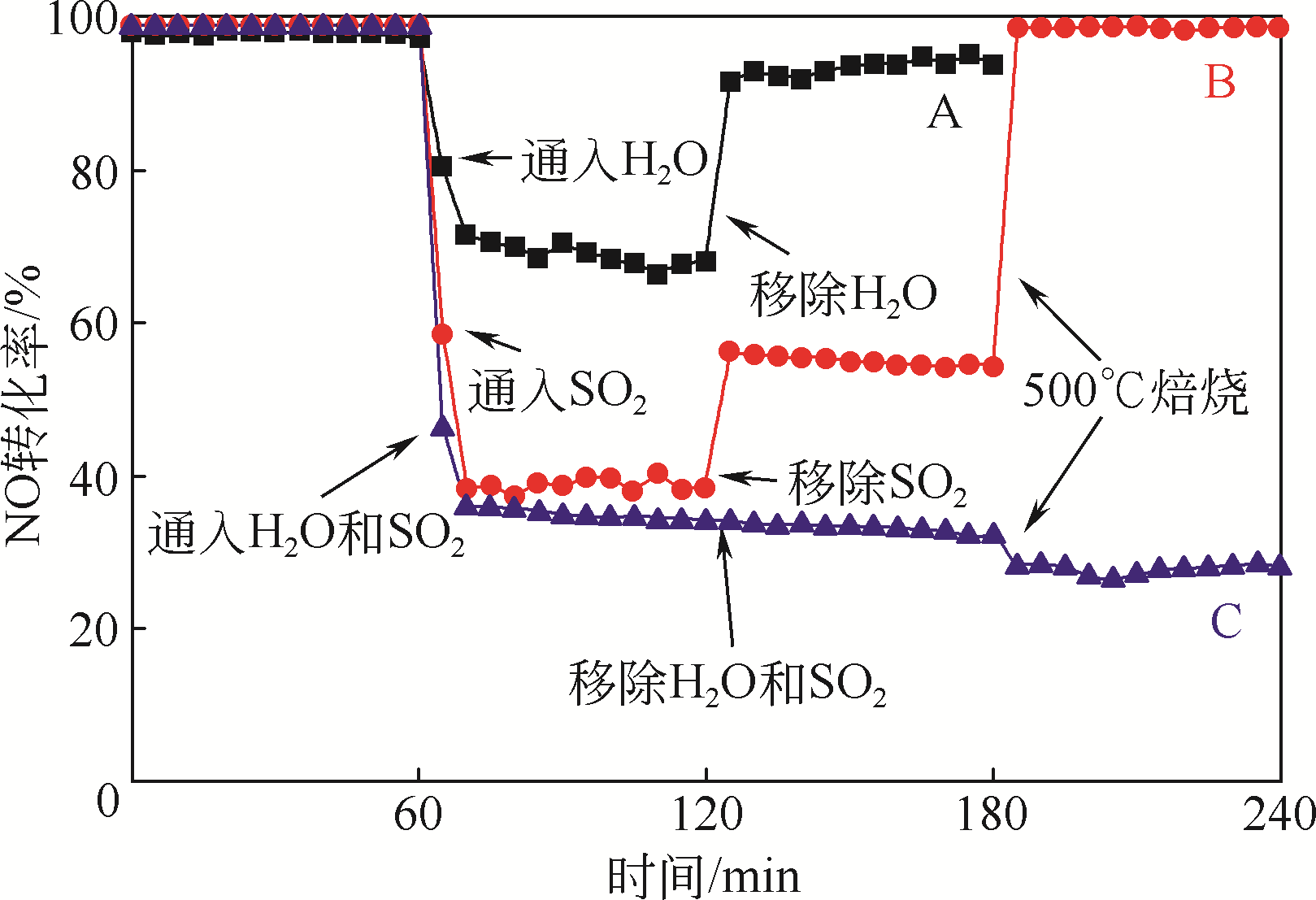
一氧化碳(CO)广泛存在于烧结/球团/焦化烟气或汽车尾气中,应用CO-选择性催化还原(SCR)技术同时脱除烟气中CO和NO是烟气治理的理想方案之一。目前,在NO-CO反应研究中较多的是贵金属催化剂,但由于其价格昂贵、高温失活、易中毒等问题难以在工业中实现应用。本文将近几年来金属氧化物催化CO还原NO的研究成果进行了系统的梳理与总结,重点介绍Fe基、Ce基、Co基、Cu基这4种金属氧化物催化剂的研究进展,分析催化剂的制备方法、掺杂助剂种类和比例、NO-CO反应条件等因素与催化活性之间的关系,总结催化剂抗水抗硫性能及可能的CO-SCR反应机理,并探讨O2存在的条件下对催化剂活性的影响,为提高金属氧化物催化剂抗氧性研究提供理论参考。
中图分类号:
引用本文
周远松,高凤雨,唐晓龙,易红宏,孟婧轩. 金属氧化物催化CO还原NO的研究进展[J]. 化工进展, 2019, 38(11): 4941-4948.
Yuansong ZHOU,Fengyu GAO,Xiaolong TANG,Honghong YI,Jingxuan MENG. Research progress on NO reduction by CO over metal oxide catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4941-4948.
| 催化剂 | 反应条件 | 反应 温度/℃ | NO 转化率/% | CO 转化率/% | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| NO/% | CO/% | O2/% | SO2/μg?g-1 | H2O/% | |||||
| Fe-Ba/ZSM-5 | 0.1 | 0.2 | 0 | 0 | 0 | 325 | 100 | — | [ |
| Fe0.8Co0.2/ASC | 0.1 | 0.2 | 0 | 0 | 0 | 200 | 100 | — | [ |
| Ce0.67Sn0.33O2 | 5 | 10 | 0 | 0 | 0 | 325 | 70 | — | [ |
| Cu0.1La0.1Ce0.8O | 5 | 10 | 0 | 0 | 10 | 250 | 90 | 42 | [ |
| Rh0.05Co2.95O4 | 5 | 5 | 2.5 | 0 | 2 | 250 | 80 | — | [ |
| Co2.9Cu0.1O4 | 5 | 5 | 2.5 | 0 | 2.5 | 200 | 70 | — | [ |
| Ag0.3Co2.7O4 | 5 | 5 | 2.5 | 0 | 2.5 | 120 | 90 | — | [ |
| Cu/CeMn-10∶1 | 5 | 5 | 0 | 0 | 0 | 225 | 98 | 48 | [ |
| Cu/TC-60∶1 | 5 | 10 | 0 | 0 | 0 | 300 | 99 | 53 | [ |
| CuO/CeO2/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 350 | 100 | — | [ |
| CuO-V2O5/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 350 | 100 | — | [ |
| CuO/NixOy/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 200 | 90 | — | [ |
表1 部分金属氧化物催化剂CO-SCR活性数据
| 催化剂 | 反应条件 | 反应 温度/℃ | NO 转化率/% | CO 转化率/% | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| NO/% | CO/% | O2/% | SO2/μg?g-1 | H2O/% | |||||
| Fe-Ba/ZSM-5 | 0.1 | 0.2 | 0 | 0 | 0 | 325 | 100 | — | [ |
| Fe0.8Co0.2/ASC | 0.1 | 0.2 | 0 | 0 | 0 | 200 | 100 | — | [ |
| Ce0.67Sn0.33O2 | 5 | 10 | 0 | 0 | 0 | 325 | 70 | — | [ |
| Cu0.1La0.1Ce0.8O | 5 | 10 | 0 | 0 | 10 | 250 | 90 | 42 | [ |
| Rh0.05Co2.95O4 | 5 | 5 | 2.5 | 0 | 2 | 250 | 80 | — | [ |
| Co2.9Cu0.1O4 | 5 | 5 | 2.5 | 0 | 2.5 | 200 | 70 | — | [ |
| Ag0.3Co2.7O4 | 5 | 5 | 2.5 | 0 | 2.5 | 120 | 90 | — | [ |
| Cu/CeMn-10∶1 | 5 | 5 | 0 | 0 | 0 | 225 | 98 | 48 | [ |
| Cu/TC-60∶1 | 5 | 10 | 0 | 0 | 0 | 300 | 99 | 53 | [ |
| CuO/CeO2/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 350 | 100 | — | [ |
| CuO-V2O5/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 350 | 100 | — | [ |
| CuO/NixOy/γ-Al2O3 | 5 | 10 | 0 | 0 | 0 | 200 | 90 | — | [ |
| 1 | YAOXiaojiang, TANGChangjin, GAOFei, et al. Research progress on the catalytic elimination of atmospheric molecular contaminants over supported metal-oxide catalysts[J]. Catalysis Science & Technology, 2014, 4(9): 2814-2829. |
| 2 | SKALSKAK, MILLERJ S, LEDAKOWICZS. Trends in NOx abatement: a review[J]. Science of the Total Environment, 2010, 408(19): 3976-3989. |
| 3 | ROY S, HEGDEM S, MADRASG. Catalysis for NOx abatement[J]. Applied Energy, 2009, 86(11): 2283-2297. |
| 4 | TWIGGM V. Progress and future challenges in controlling automotive exhaust gas emissions[J]. Applied Catalysis B: Environmental, 2007, 70(1/2/3/4): 2-15. |
| 5 | WANGLuyuan, CHENGXingxing, WANGZhiqiang, et al. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO[J]. Applied Catalysis B:Environmental, 2017, 201: 636-651. |
| 6 | CHENGX X, BIX T. A review of recent advances in selective catalytic NOx reduction reactor technologies[J]. Particuology, 2014, 16: 1-18. |
| 7 | IsabellaNOVA, CristianCIARDELLI, EnricoTRONCONI, et al. NH3-SCR of NO over a V-based catalyst: low-T redox kinetics with NH3 inhibition[J]. AIChE Journal, 2006, 52(9): 3222-3233. |
| 8 | CHENGX X, BIX T. Reaction kinetics of selective catalytic reduction of NOx by propylene over Fe/ZSM-5[J]. Chemical Engineering Journal, 2012, 211: 453-462. |
| 9 | YANGT T, BIH T, CHENGX X. Effects of O2, CO2 and H2O on NOx adsorption and selective catalytic reduction over Fe/ZSM-5[J]. Applied Catalysis B: Environmental, 2011, 102(1/2): 163-171. |
| 10 | CHENGX X, BIX T. Modeling NOx adsorption onto Fe/ZSM-5 catalysts in a eixed bed reactor[J]. International Journal of Chemical Reactor Engineering, 2013, 11(1):19-30. |
| 11 | LIULianjun, YAOZhijian, LIUBin, et al. Correlation of structural characteristics with catalytic performance of CuO/CexZr1-xO2 catalysts for NO reduction by CO[J]. Journal of Catalysis, 2010, 275(1): 45-60. |
| 12 | ILIOPOULOUE F, EFTHIMIADISE A, NALBANDIANL, et al. Ir-based additives for NO reduction and CO oxidation in the FCC regenerator: evaluation, characterization and mechanistic studies[J]. Applied Catalysis B: Environmental, 2005, 60(3/4): 277-288. |
| 13 | SUNChuanzhi, TANGYingjie, GAOFei, et al. Effects of different manganese precursors as promoters on catalytic performance of CuO-MnOx/TiO2 catalysts for NO removal by CO[J]. Physical Chemistry Chemical Physics, 2015, 17(24): 15996-16006. |
| 14 | KACIMIM, ZIYADM, LIOTTAL F. Cu on amorphous AlPO4: preparation, characterization and catalytic activity in NO reduction by CO in presence of oxygen[J]. Catalysis Today, 2015, 241: 151-158. |
| 15 | YAOXiaojiang, XIONGYan, ZOUWeixin, et al. Correlation between the physicochemical properties and catalytic performances of CexSn1-xO2 mixed oxides for NO reduction by CO[J]. Applied Catalysis B: Environmental, 2014, 144: 152-165. |
| 16 | YAOXiaojiang, TANGChangjin, JIZeyang, et al. Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2 (M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO[J]. Catalysis Science & Technology, 2013, 3(3): 688-698. |
| 17 | YUQiang, YAOXiaojiang, ZHANGHongliang, et al. Effect of ZrO2 addition method on the activity of Al2O3-supported CuO for NO reduction with CO: impregnation vs. coprecipitation[J]. Applied Catalysis A: General, 2012, 423: 42-51. |
| 18 | 孟婧轩, 高凤雨, 唐晓龙, 等. Ir基催化剂用于CO选择性催化还原 NO的研究进展[J]. 化工进展, 2019, 38(6): 2746-2755. |
| MENGJingxuan, GAOFengyu, TANGXiaolong, et al. Review of Ir-based catalyst in selective catalytic reduction of NO with CO[J] Chemistry Industry and Engineering Progress, 2019, 38(6): 2746-2755. | |
| 19 | CHAFIKT, KONDARIDESD I, VERYKIOSX E. Catalytic reduction of NO by CO over rhodium catalysts 1. Adsorption and displacement characteristics investigated by insitu FTIR and transient-MS techniques[J]. Journal of Catalysis, 2000, 190(2): 446-459. |
| 20 | ZHANGXingyu, MAChunyuan, CHENGXingxing, et al. Performance of Fe-Ba/ZSM-5 catalysts in NO+O2 adsorption and NO+CO reduction[J]. International Journal of Hydrogen Energy, 2017, 42(10): 7077-7088. |
| 21 | LIUTangkang, QIANJunning, YAOYanyan, et al. Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: effect of the grinding[J]. Molecular Catalysis, 2017, 430: 43-53. |
| 22 | SALKERA V, DESAIM S F. Catalytic activity and mechanistic approach of NO reduction by CO over M0.05Co2.95O4 (M = Rh, Pd & Ru) spinel system[J]. Applied Surface Science, 2016, 389: 344-353. |
| 23 | SALKERA V, DESAIM S F. CO-NO/O2 redox reactions over Cu substituted cobalt oxide spinels[J]. Catalysis Communications, 2016, 87: 116-119. |
| 24 | SALKERA V, DESAIM S F. Low-temperature nitric oxide reduction over silver-substituted spinels cobalt oxide [J]. Catalysis Science & Technology, 2016, 6(2): 430-433. |
| 25 | DENGChangshun, HUANGQingqing, ZHUXiying, et al. The influence of Mn-doped CeO2 on the activity of CuO/CeO2 in CO oxidation and NO+CO model reaction[J]. Applied Surface Science, 2016, 389: 1033-1049. |
| 26 | DENGChangshun, LIBin, DONGLihui, et al. NO reduction by CO over CuO supported on CeO2-doped TiO2: the effect of the amount of a few CeO2[J]. Physical Chemistry Chemical Physics, 2015, 17(24): 16092-16109. |
| 27 | GEChengyan, LIULichen, LIUZhuotong, et al. Improving the dispersion of CeO2 on gamma-Al2O3 to enhance the catalytic performances of CuO/CeO2/gamma-Al2O3 catalysts for NO removal by CO[J]. Catalysis Communications, 2014, 51: 95-99. |
| 28 | XIONGYan, YAOXiaojiang, TANGChangjin, et al. Effect of CO-pretreatment on the CuO-V2O5/gamma-Al2O3 catalyst for NO reduction by CO[J]. Catalysis Science & Technology, 2014, 4(12): 4416-4425. |
| 29 | GEChengyan, LIULianjun, YAOXiaojiang, et al. Treatment induced remarkable enhancement of low-temperature activity and selectivity of copper-based catalysts for NO reduction[J]. Catalysis Science & Technology, 2013, 3(6): 1547-1557. |
| 30 | DONGLihui, ZHANGBing, TANGChangjin, et al. Influence of CeO2 modification on the properties of Fe2O3-Ti0.5Sn0.5O2 catalyst for NO reduction by CO[J]. Catalysis Science & Technology, 2014, 4(2): 482-493. |
| 31 | LUOMengfei, CHENJun, CHENLinshen, et al. Structure and redox properties of CexTi1-xO2 solid solution[J]. Chemistry of Materials, 2001, 13(1): 197-202. |
| 32 | BAIDYAT, GUPTAA, DESHPANDEYP A, et al. High oxygen storage capacity and high rates of CO oxidation and NO reduction catalytic properties of Ce1-xSnxO2 and Ce0.78Sn0.2Pd0.02O2-delta[J]. Journal of Physical Chemistry C, 2009, 113(10): 4059-4068. |
| 33 | NGUYENT B, DELOUMEJ P, PERRICHONV. Study of the redox behaviour of high surface area CeO2-SnO2 solid solutions[J]. Applied Catalysis A: General, 2003, 249(2): 273-284. |
| 34 | LIUTangkang, QIANJunning, YAOYangyan, et al. Research on SCR of NO with CO over the Cu0.1La0.1Ce0.8O mixed-oxide catalysts: effect of the grinding[J]. Molecular Catalysis, 2017, 430: 43-53. |
| 35 | DAIXiaoxia, JIANGWeiyu, WANGWanglong, et al. Supercritical water syntheses of transition metal-doped CeO2 nano-catalysts for selective catalytic reduction of NO by CO: an in situ diffuse reflectance fourier transform infrared spectroscopy study[J]. Chinese Journal of Catalysis, 2018, 39(4): 728-735. |
| 36 | 郭磊, 张涛, 常化振, 等. Ce掺杂改性Ni-Al-Ox催化剂CO-NO反应性能[J]. 中国环境科学, 2018, 38(9): 3313-3321. |
| GUOLei, ZHANGTao, CHANGHuazhen, et al. Study on Ce-doped Ni-Al-Ox catalysts for reduction by CO[J]. China Environmenal Science, 2018, 38(9): 3313-3321. | |
| 37 | SONGW Q, POYRAZA S, MENGY T, et al. Mesoporous Co3O4 with controlled porosity: inverse micelle synthesis and high-performance catalytic CO oxidation at-60 degrees C[J]. Chemistry of Materials, 2014, 26(15): 4629-4639. |
| 38 | WANGLei, ZHANGShiran, ZHUYuan, et al. Catalysis and in situ studies of Rh-1/Co3O4 nanorods in reduction of NO with H-2[J]. ACS Catalysis, 2013, 3(5): 1011-1019. |
| 39 | ZHOUMinjie, CAILili, MichalBAJDICH, et al. Enhancing catalytic CO oxidation over Co3O4 nanowires by substituting Co2+ with Cu2+[J]. ACS Catalysis, 2015, 5(8): 4485-4491. |
| 40 | SamiBARKAOUI, HassounaDHAOUADI, SalahKOUASS, et al. Structural and optical proprieties of doped cobalt oxide: CuxCO3-xO4 (x=0.0; 0.1; 0.2; 0.4; and 0.6)[J]. Optik, 2015, 126(9/10): 1047-1051. |
| 41 | NICKOLOVR, STANKOVAN, KHRISTOVAM, et al. Copper oxide supported on carbon modified alumina as catalyst for reduction of NO with CO[J]. Journal of Colloid and Interface Science, 2003, 265(1): 121-128. |
| 42 | KHRISTOVAM, IVANOVB, SPASSOVAI, et al. NO reduction with CO on copper and ceria oxides supported on alumina[J]. Catalysis Letters, 2007, 119(1/2): 79-86. |
| 43 | WANHaiqin, LIDan, DAIYue, et al. Catalytic behaviors of CuO supported on Mn2O3 modified gamma-Al2O3 for NO reduction by CO[J]. Journal of Molecular Catalysis A: Chemical, 2010, 332(1/2): 32-44. |
| 44 | CHENGXingxing, ZHANGXingyu, SUDexin, et al. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: catalyst design and activity test[J]. Applied Catalysis B:Environmental, 2018, 239: 485-501. |
| 45 | 孔令朋, 苗杰, 李明航, 等. CuMnCeLa-O/γ-Al2O3催化剂助燃脱硝性能研究[J]. 分子催化, 2018, 32(4): 295-304. |
| KONGLingpeng, MIAOJie, LIMinghang, et al. Performances of selective catalytic reduction of NO with CO over CuMnCeLa-O/γ-Al2O3 catalyst[J]. Journal of Molecular Catalysis, 2018, 32(4): 295-304. | |
| 46 | WENBin, HEMingyuan, EthanSCHRUM, et al. NO reduction and CO oxidation over Cu/Ce/Mg/Al mixed oxide catalyst in FCC operation[J]. Journal of Molecular Catalysis A:Chemical, 2002, 180(1/2): 187-192. |
| 47 | LIJun, WANGShan, ZHOULi, et al. NO reduction by CO over a Fe-based catalyst in FCC regenerator conditions[J]. Chemical Engineering Journal, 2014, 255: 126-133. |
| 48 | ZahraGHOLAMI, LUOGuohua. Low-temperature selective catalytic reduction of NO by CO in the presence of O2 over Cu∶Ce catalysts supported by multiwalled carbon nanotubes[J]. Industrial & Engineering Chemistry Research, 2018, 57(27): 8871-8883. |
| 49 | SREEKANTHP M, SMIRNIOTISP G. Selective reduction of NO with CO over titania supported transition metal oxide catalysts[J]. Catalysis Letters, 2008, 122(1/2): 37-42. |
| 50 | BONINGARIT, PAVANIS M, ETTIREDDYP R, et al. Mechanistic investigations on NO reduction with CO over Mn/TiO2 catalyst at low temperatures[J]. Molecular Catalysis, 2018, 451: 33-42. |
| 51 | 付玉秀, 仲雪梅, 常化振, 等. 铈钴复合氧化物催化剂催化CO-SCR反应机理研究 [J]. 中国环境科学, 2018, 38(8): 2934-2940. |
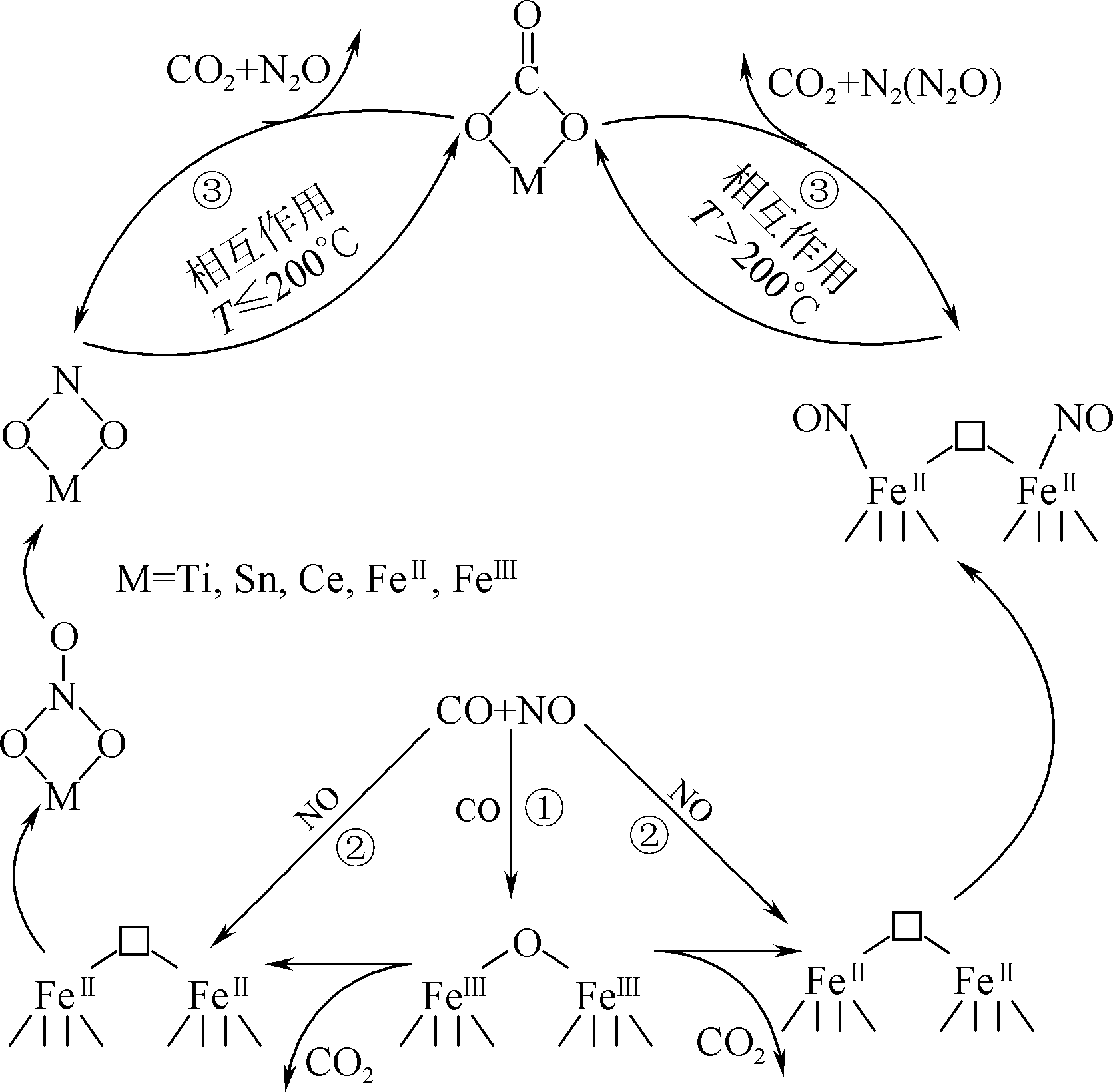
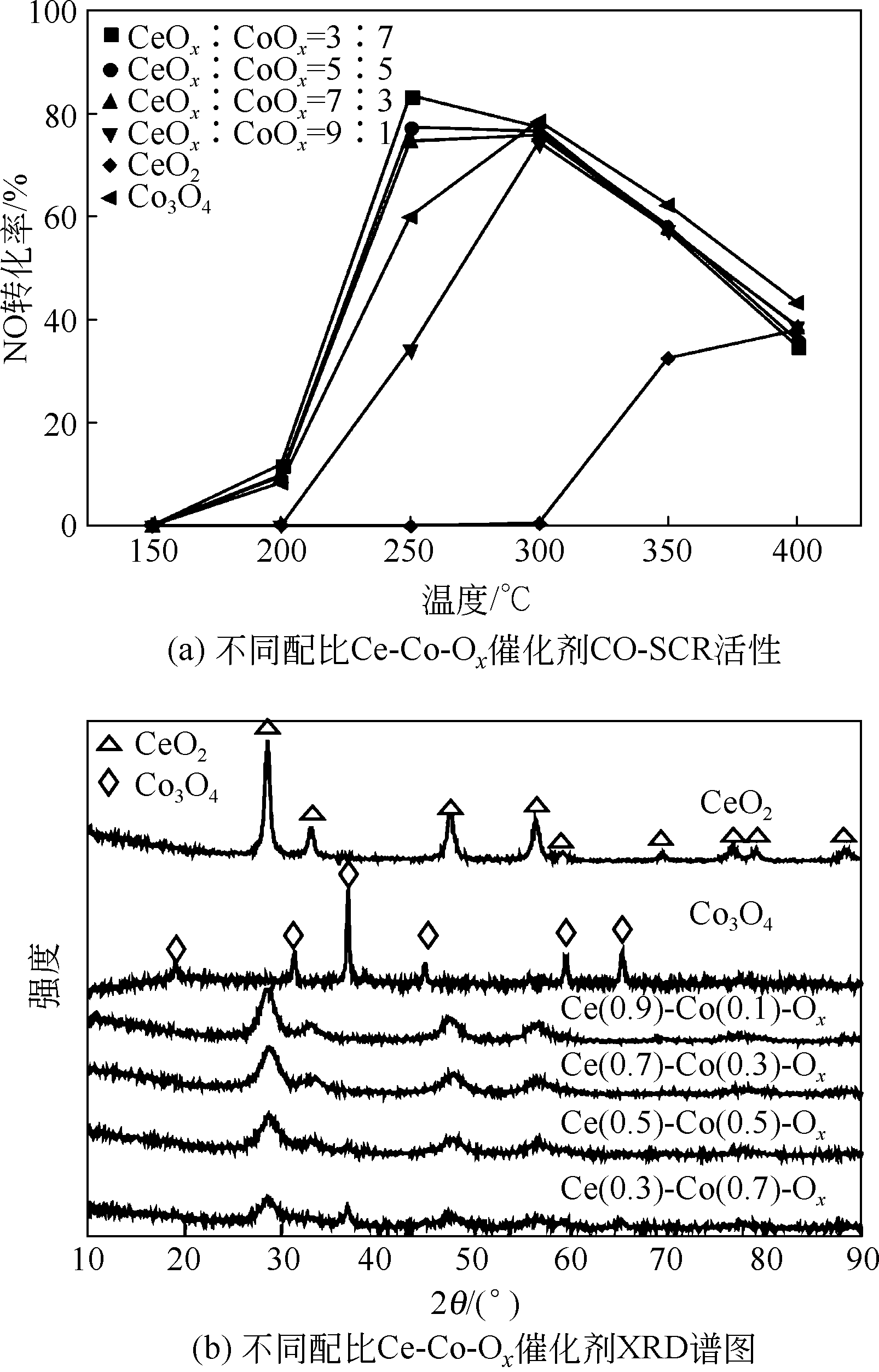
| FUYuxiu, ZHONGXuemei, CHANGHuazhen, et al. Mechanism study on CO-SCR over Ce-Co-Ox mixed oxides catalysts[J]. China Enverimantal Science, 2018, 38(8): 2934-2940. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 李东泽, 张祥, 田键, 胡攀, 姚杰, 朱林, 卜昌盛, 王昕晔. 基于水泥窑脱硝的碳基还原NO x 研究进展[J]. 化工进展, 2023, 42(9): 4882-4893. |
| [13] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [14] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||