化工进展 ›› 2019, Vol. 38 ›› Issue (07): 3207-3226.DOI: 10.16085/j.issn.1000-6613.2018-1858
基于金属氧化物的乙醇检测气敏材料的研究进展
- 北京有色金属研究总院智能传感功能材料国家重点实验室,北京101407
-
收稿日期:2018-09-14出版日期:2019-07-05发布日期:2019-07-05 -
作者简介:张晓(1991—),女,硕士,助理工程师,研究方向为金属-有机框架材料、金属氧化物材料及其催化性能。E-mail:<email>zhangxiao@grinm.com</email>。
Recent advances of ethanol detection materials based on metal oxides
Xiao ZHANG( ),Yaohua XU,Hao LIU,Feng WEI,Peng YUAN
),Yaohua XU,Hao LIU,Feng WEI,Peng YUAN
- State Key Laboratory of Advanced Materials for Smart Sensing, General Research Institute for Nonferrous Metals, Beijing 101407, China
-
Received:2018-09-14Online:2019-07-05Published:2019-07-05
摘要:
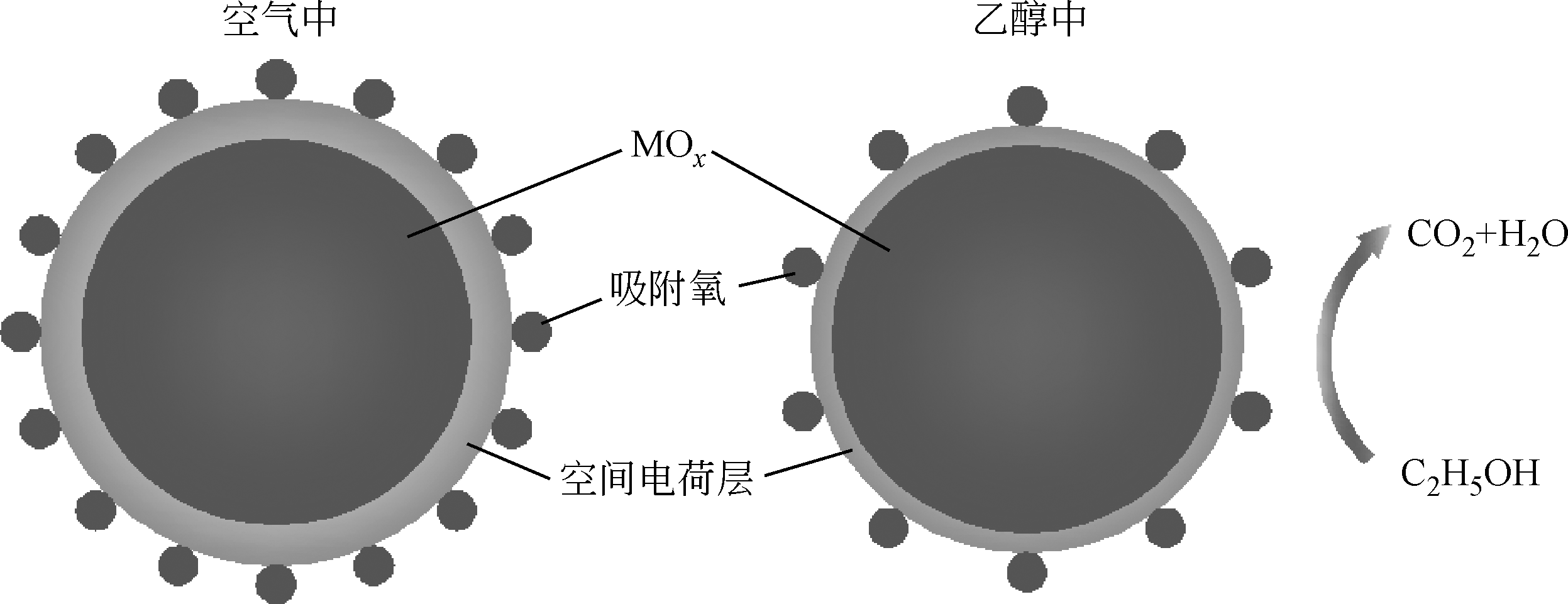
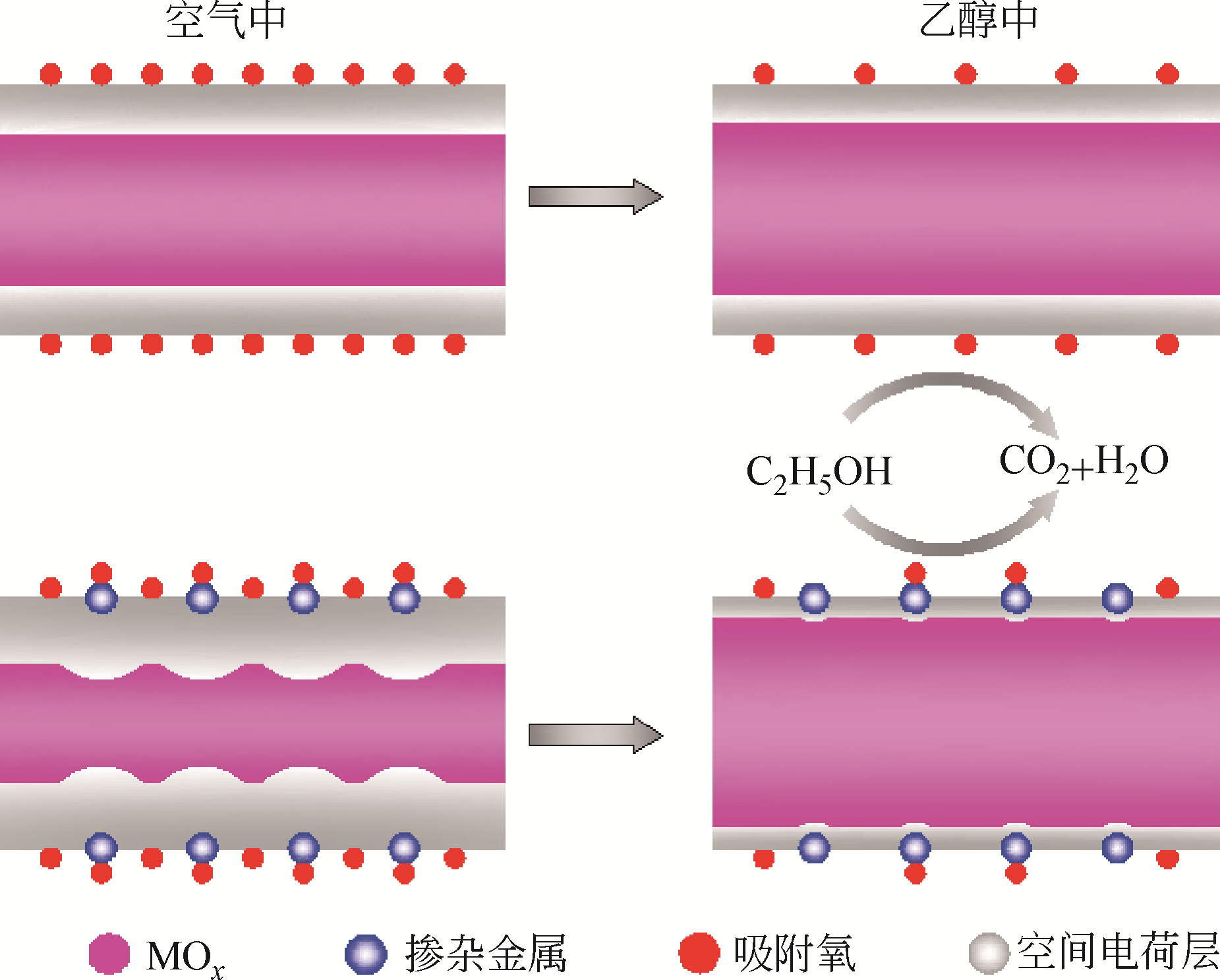
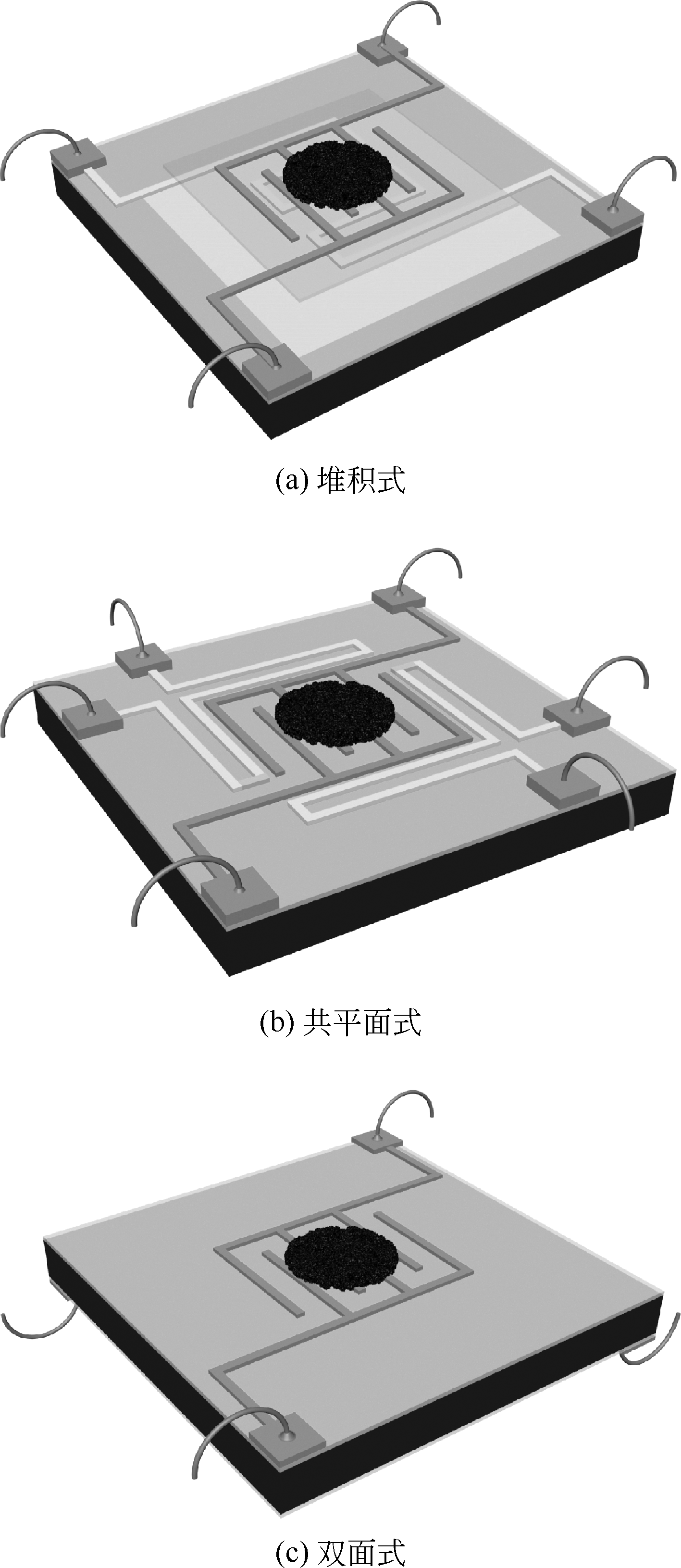
金属氧化物型半导体气体传感器是目前常用的乙醇检测手段,深入研究和改进金属氧化物型半导体材料是提升传感器性能的重要方式。本文首先论述了气敏检测的机理和影响因素,并综述了近年来发展的主要金属氧化物型半导体气敏材料,重点介绍了不同微观结构的Co3O4、ZnO、SnO2及掺杂金属氧化物材料、氧化物异质结等的研究和发展情况,对它们的合成方法、结构特点以及结构与乙醇气敏性能之间的关系进行了探讨。分析表明,减小材料颗粒尺寸、构建大比表面积多孔结构、掺杂和复合改性,是提升金属氧化物材料气敏性能的有效措施。此外,基于传感器微小化的趋势,以微机电系统(MEMS)工艺为基础的微型传感器成为气体传感器的发展趋势。然而,目前针对金属氧化物气敏材料的制备依然缺乏一定的理论指导,气体检测缺乏相应的机理研究,亟需物理、化学、材料等多学科的相互结合,促进乙醇等半导体气体传感器的进一步发展。
中图分类号:
引用本文
张晓, 徐瑶华, 刘皓, 魏峰, 苑鹏. 基于金属氧化物的乙醇检测气敏材料的研究进展[J]. 化工进展, 2019, 38(07): 3207-3226.
Xiao ZHANG, Yaohua XU, Hao LIU, Feng WEI, Peng YUAN. Recent advances of ethanol detection materials based on metal oxides[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3207-3226.
| 材料 | 形貌 | 材料 | 温度/℃ | 检测浓度 /μg?g-1 | 响应度 | 响应/恢复(时间)/s | 选择性(响应度) | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氢气 | ||||||||
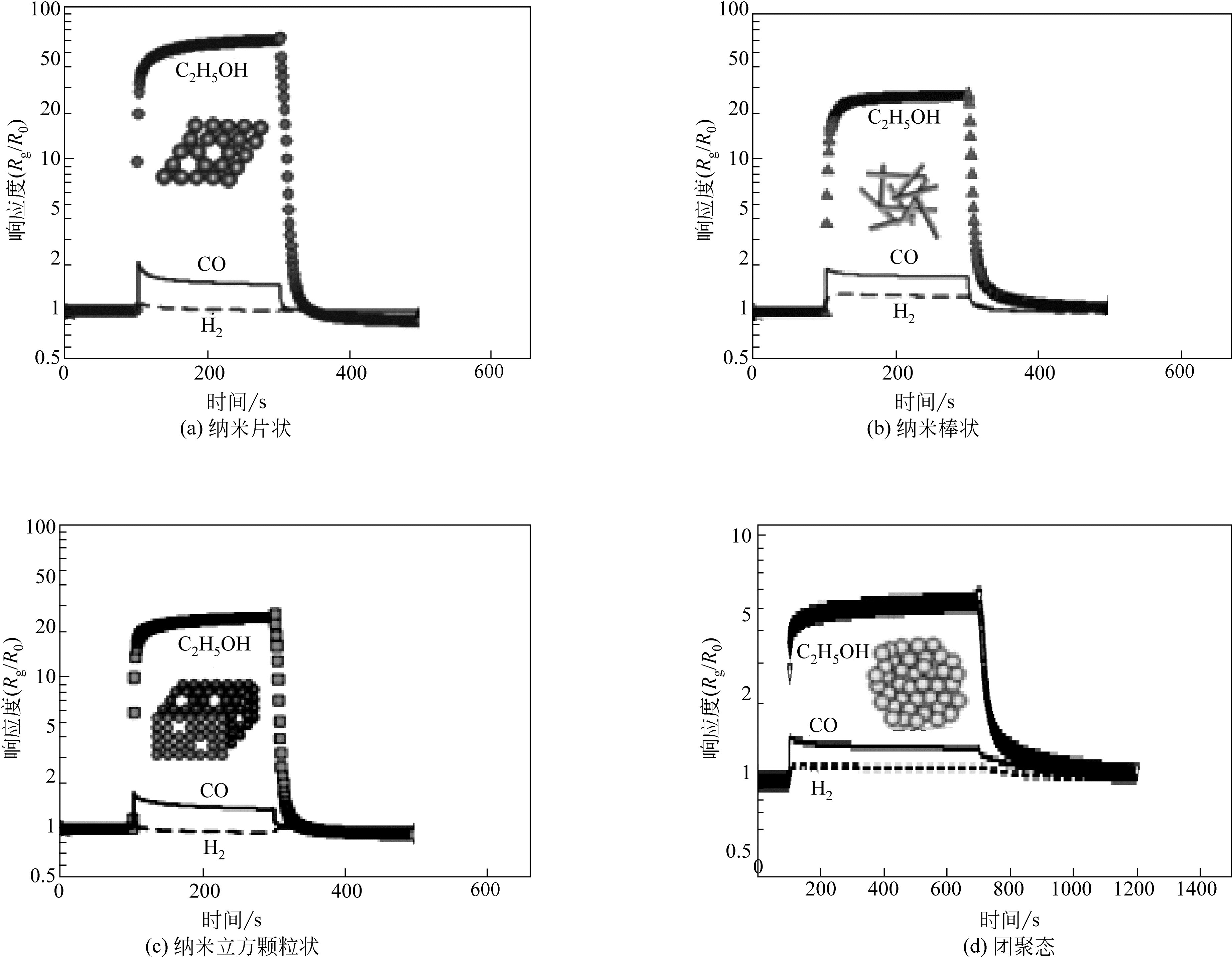
| 零维材料 | 立方颗粒 | Co3O4 | 200 | 100 | 5.0 | 无 | 3.0 | [ | |||
| 团聚颗粒 | Co3O4 | 300 | 100 | 5.5 | 150/55 | 5.1 | [ | ||||
| 纳米颗粒 | ZnO | 350 | 400 | 20.3 | 12/14 | 无 | [ | ||||
| 立方颗粒 | In2O3 | 200 | 100 | 17.0 | 无 | 5.0 | [ | ||||
| 一维材料 | 纳米纤维 | SnO2 | 330 | 10 | 4.5 | 13/13.9 | 无 | [ | |||
| 纳米线 | SnO2 | 380 | 100 | 17.0 | 22/18 | 无 | [ | ||||
| 纳米棒 | ZnO | 400 | 100 | 149.2 | 9/15 | 无 | [ | ||||
| 纳米线 | Co3O4 | 350 | 300 | 5.1 | 无 | 无 | [ | ||||
| 纳米棒 | Co3O4 | 160 | 500 | 71.0 | 90/60 | 38.8 | 20.1 | [ | |||
| 纳米棒 | Co3O4 | 300 | 100 | 25.7 | 29/13 | 20.1 | [ | ||||
| 纳米管 | Co3O4 | 300 | 100 | 24.7 | 49/13 | 26.3 | [ | ||||
| 纳米纤维 | TiO2 | 300 | 100 | 14.0 | 3/5 | 3.5 | [ | ||||
| 二维材料 | 纳米片 | ZnO | 350 | 400 | 23.3 | 12/5 | 无 | [ | |||
| 纳米片 | Co3O4 | 100 | 300 | 419.0 | 44/328 | 94.0 | 36.4 | 234.0 | [ | ||
| 纳米片 | Co3O4 | 300 | 100 | 57.5 | 66/10 | 56.0 | [ | ||||
| 层状结构 | CuO | 250 | 5 | 3.1 | 11.9/8.4 | 3.0 | 1.6 | 2.5 | [ | ||
| 三维材料 | 分级结构 | SnO2 | 350 | 100 | 10.5 | 5 | 无 | [ | |||
| 介孔材料 | SnO2 | 225 | 100 | 17.3 | 8/780 | 2.5 | 2.5 | 3.0 | [ | ||
| 分级多孔 | SnO2 | 240 | 500 | 72.0 | 10/15 | 12.0 | 9.0 | 7.0 | [ | ||
| (20μg?g-1) | |||||||||||
| 空心笼状 | ZnO | 300 | 1 | 8.0 | 无 | 14.8 | [ | ||||
| 介孔材料 | ZnO | 室温 | 300 | 1.4 | 42/40 | 无 | [ | ||||
| 分级多孔 | ZnO | 250 | 50 | 36.6 | 14/8 | 3.0 | [ | ||||
| 分级多孔 | ZnO | 220 | 100 | 8.5 | 10/80 | 5.0 | [ | ||||
| 分级结构 | ZnO | 370 | 100 | 340.0 | 12/约50 | 362.0 | [ | ||||
| 分级结构 | ZnO | 260 | 50 | 110.0 | 4/12 | 12.0 | 40.0 | 2.0 | [ | ||
| 纳米花 | ZnO | 350 | 400 | 30.4 | 10/4 | 无 | [ | ||||
| 三维微孔 | Co3O4@C | 170 | 100 | 14.7 | 无 | 11.0 | 7.8 | [ | |||
| 三维介孔 | Co3O4@CF | 320 | 100 | 4.2 | 44/31 | 无 | [ | ||||
| 介孔纳米片 | Co3O4/NCF | 100 | 100 | 10.4 | 45/140 | 1.7 | 1.6 | 1.3 | [ | ||
| 薄膜 | 薄膜 | SnO2 | 300 | 300 | 1.84 | 8/340 | 70.0 | [ | |||
| 薄膜 | ZnO | 室温 | 30 | 6.0 | 28/49 | 5.1 (10μg?g-1) | [ | ||||
| 薄膜 | ZnO | 400 | 50 | 55.0 | 362/147 | 无 | [ | ||||
| 阵列 | Co3O4 | 130 | 100 | 89.6 | 无 | 25.8 | 55.2 | [ | |||
| 薄膜 | CuO | 室温 | 200 | 1.24 | 15/15 | 无 | [ | ||||
表1 不同形貌MO x 乙醇性能对比
| 材料 | 形貌 | 材料 | 温度/℃ | 检测浓度 /μg?g-1 | 响应度 | 响应/恢复(时间)/s | 选择性(响应度) | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氢气 | ||||||||
| 零维材料 | 立方颗粒 | Co3O4 | 200 | 100 | 5.0 | 无 | 3.0 | [ | |||
| 团聚颗粒 | Co3O4 | 300 | 100 | 5.5 | 150/55 | 5.1 | [ | ||||
| 纳米颗粒 | ZnO | 350 | 400 | 20.3 | 12/14 | 无 | [ | ||||
| 立方颗粒 | In2O3 | 200 | 100 | 17.0 | 无 | 5.0 | [ | ||||
| 一维材料 | 纳米纤维 | SnO2 | 330 | 10 | 4.5 | 13/13.9 | 无 | [ | |||
| 纳米线 | SnO2 | 380 | 100 | 17.0 | 22/18 | 无 | [ | ||||
| 纳米棒 | ZnO | 400 | 100 | 149.2 | 9/15 | 无 | [ | ||||
| 纳米线 | Co3O4 | 350 | 300 | 5.1 | 无 | 无 | [ | ||||
| 纳米棒 | Co3O4 | 160 | 500 | 71.0 | 90/60 | 38.8 | 20.1 | [ | |||
| 纳米棒 | Co3O4 | 300 | 100 | 25.7 | 29/13 | 20.1 | [ | ||||
| 纳米管 | Co3O4 | 300 | 100 | 24.7 | 49/13 | 26.3 | [ | ||||
| 纳米纤维 | TiO2 | 300 | 100 | 14.0 | 3/5 | 3.5 | [ | ||||
| 二维材料 | 纳米片 | ZnO | 350 | 400 | 23.3 | 12/5 | 无 | [ | |||
| 纳米片 | Co3O4 | 100 | 300 | 419.0 | 44/328 | 94.0 | 36.4 | 234.0 | [ | ||
| 纳米片 | Co3O4 | 300 | 100 | 57.5 | 66/10 | 56.0 | [ | ||||
| 层状结构 | CuO | 250 | 5 | 3.1 | 11.9/8.4 | 3.0 | 1.6 | 2.5 | [ | ||
| 三维材料 | 分级结构 | SnO2 | 350 | 100 | 10.5 | 5 | 无 | [ | |||
| 介孔材料 | SnO2 | 225 | 100 | 17.3 | 8/780 | 2.5 | 2.5 | 3.0 | [ | ||
| 分级多孔 | SnO2 | 240 | 500 | 72.0 | 10/15 | 12.0 | 9.0 | 7.0 | [ | ||
| (20μg?g-1) | |||||||||||
| 空心笼状 | ZnO | 300 | 1 | 8.0 | 无 | 14.8 | [ | ||||
| 介孔材料 | ZnO | 室温 | 300 | 1.4 | 42/40 | 无 | [ | ||||
| 分级多孔 | ZnO | 250 | 50 | 36.6 | 14/8 | 3.0 | [ | ||||
| 分级多孔 | ZnO | 220 | 100 | 8.5 | 10/80 | 5.0 | [ | ||||
| 分级结构 | ZnO | 370 | 100 | 340.0 | 12/约50 | 362.0 | [ | ||||
| 分级结构 | ZnO | 260 | 50 | 110.0 | 4/12 | 12.0 | 40.0 | 2.0 | [ | ||
| 纳米花 | ZnO | 350 | 400 | 30.4 | 10/4 | 无 | [ | ||||
| 三维微孔 | Co3O4@C | 170 | 100 | 14.7 | 无 | 11.0 | 7.8 | [ | |||
| 三维介孔 | Co3O4@CF | 320 | 100 | 4.2 | 44/31 | 无 | [ | ||||
| 介孔纳米片 | Co3O4/NCF | 100 | 100 | 10.4 | 45/140 | 1.7 | 1.6 | 1.3 | [ | ||
| 薄膜 | 薄膜 | SnO2 | 300 | 300 | 1.84 | 8/340 | 70.0 | [ | |||
| 薄膜 | ZnO | 室温 | 30 | 6.0 | 28/49 | 5.1 (10μg?g-1) | [ | ||||
| 薄膜 | ZnO | 400 | 50 | 55.0 | 362/147 | 无 | [ | ||||
| 阵列 | Co3O4 | 130 | 100 | 89.6 | 无 | 25.8 | 55.2 | [ | |||
| 薄膜 | CuO | 室温 | 200 | 1.24 | 15/15 | 无 | [ | ||||
| 掺杂物 | 形貌 | 材料 | 温度 /℃ | 检测浓度 /μg?g-1 | 响应度 | 响应/恢复(时间)/s | 选择性(响应度) | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氨气 | ||||||||
| 贵金属 | 中空纳米球 | Pt-SnO2 | 325 | 5 | 1399.9 | 1/525 | 600.0 | 700.0 | 200 | [ | |
| 纳米棒 | Pt-SnO2 | 320 | 200 | 8.3 | 3~5/5~15 | 无 | [ | ||||
| 纳米颗粒 | Pt-SnO2 | 275 | 100 | 1.9 | 1/5 | 无 | [ | ||||
| 空心材料 | Pd-SnO2 | 300 | 50 | 2.0 | 1.5/18 | 0.9 | 0.9 | 1.0 | [ | ||
| 纳米花 | Au-SnO2 | 340 | 150 | 29.3 | 5/10 | 13.3 | 20.3 | [ | |||
| (100μg·g-1) | |||||||||||
| 薄膜 | Pt-In2O3 | 室温 | 0.095 | 12.2 | 1/2 | 无 | [ | ||||
| 纳米颗粒 | Pt-In2O3 | 250 | 100 | 32.6 | 2.2/0.7 | 4.0 | [ | ||||
| 纳米片 | Au-WO3 | 320 | 100 | 97.2 | 无 | 8.0 | 3.0 | 11.0 | [ | ||
| 非贵金属 | 纳米颗粒 | CuZn-SnO2 | 110 | 50 | 210.0 | 无 | 10.0 | [ | |||
| 纳米棒 | Ni-SnO2 | 450 | 50 | 2000.0 | 30/600 | 无 | [ | ||||
| 纳米微球 | Ni-SnO2 | 260 | 100 | 28.9 | 11/54 | 6.0 | 4.0 | [ | |||
| 纳米颗粒 | Ce-SnO2 | 室温 | 400 | 4.8 | 2~25/30~60 | 无 | [ | ||||
| 立方中空 | ZnSnO3 | 260 | 100 | 34.1 | 2/276 | 11.0 | 3 | [ | |||
| 纳米棒 | Cr-ZnO | 300 | 400 | 45.0 | 无 | 无 | [ | ||||
| 海绵状 | Co-ZnO | 220 | 100 | 120.0 | 10/5 | 18.0 | 24.0 | [ | |||
| 六边形 | Cr-Co3O4 | 300 | 100 | 28.9 | 1/7 | 4.0 | 3.0 | [ | |||
| 纳米球 | Co3O4/pCNH | 210 | 500 | 30.2 | 无 | 2.0 | 7.0 | [ | |||
表2 不同金属掺杂MO x 性能对比
| 掺杂物 | 形貌 | 材料 | 温度 /℃ | 检测浓度 /μg?g-1 | 响应度 | 响应/恢复(时间)/s | 选择性(响应度) | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氨气 | ||||||||
| 贵金属 | 中空纳米球 | Pt-SnO2 | 325 | 5 | 1399.9 | 1/525 | 600.0 | 700.0 | 200 | [ | |
| 纳米棒 | Pt-SnO2 | 320 | 200 | 8.3 | 3~5/5~15 | 无 | [ | ||||
| 纳米颗粒 | Pt-SnO2 | 275 | 100 | 1.9 | 1/5 | 无 | [ | ||||
| 空心材料 | Pd-SnO2 | 300 | 50 | 2.0 | 1.5/18 | 0.9 | 0.9 | 1.0 | [ | ||
| 纳米花 | Au-SnO2 | 340 | 150 | 29.3 | 5/10 | 13.3 | 20.3 | [ | |||
| (100μg·g-1) | |||||||||||
| 薄膜 | Pt-In2O3 | 室温 | 0.095 | 12.2 | 1/2 | 无 | [ | ||||
| 纳米颗粒 | Pt-In2O3 | 250 | 100 | 32.6 | 2.2/0.7 | 4.0 | [ | ||||
| 纳米片 | Au-WO3 | 320 | 100 | 97.2 | 无 | 8.0 | 3.0 | 11.0 | [ | ||
| 非贵金属 | 纳米颗粒 | CuZn-SnO2 | 110 | 50 | 210.0 | 无 | 10.0 | [ | |||
| 纳米棒 | Ni-SnO2 | 450 | 50 | 2000.0 | 30/600 | 无 | [ | ||||
| 纳米微球 | Ni-SnO2 | 260 | 100 | 28.9 | 11/54 | 6.0 | 4.0 | [ | |||
| 纳米颗粒 | Ce-SnO2 | 室温 | 400 | 4.8 | 2~25/30~60 | 无 | [ | ||||
| 立方中空 | ZnSnO3 | 260 | 100 | 34.1 | 2/276 | 11.0 | 3 | [ | |||
| 纳米棒 | Cr-ZnO | 300 | 400 | 45.0 | 无 | 无 | [ | ||||
| 海绵状 | Co-ZnO | 220 | 100 | 120.0 | 10/5 | 18.0 | 24.0 | [ | |||
| 六边形 | Cr-Co3O4 | 300 | 100 | 28.9 | 1/7 | 4.0 | 3.0 | [ | |||
| 纳米球 | Co3O4/pCNH | 210 | 500 | 30.2 | 无 | 2.0 | 7.0 | [ | |||
| 异质结 | 材料 | 温度/℃ | 检测浓度/μg?g-1 | 响应度 | 响应/恢复时间/s | 选择性(响应度) | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氨气 | |||||||
| MO x -MO x | ZnO/SnO2 | 225 | 30 | 34.8 | 1/120 | 53.7 | 8.9 | 52.1 | [ | |
| (100μg?g-1, 此时乙醇响应度为78.2) | ||||||||||
| NiO/SnO2 | 250 | 100 | 7.9 | 15/100 | 无 | [ | ||||
| Fe2O3/SnO2 | 300 | 200 | 57.6 | 无 | 20.0 | 15.0 | 35.0 | [ | ||
| CoO/SnO2 | 250 | 100 | 145.0 | 无 | 56.0 | 15.0 | 54.0 | [ | ||
| SnO2/ZnO | 350 | 100 | 13.3 | 无 | 4.0 | 4.0 | 1.4 | [ | ||
| SnO2/Fe2O3 | 260 | 100 | 41.7 | 3/4 | 9.0 | 5.0 | [ | |||
| SnO2/Fe2O3 | 240 | 100 | 5.9 | 1/5 | 1.0 | 2.5 | 1.5 | [ | ||
| ZnO/CuO | 300 | 10 | 2.2 | 22/26 | 1.2 | 0.7 | [ | |||
| ZnO/Co3O4 | 200 | 1000 | 106.0 | 7/236 | 无 | [ | ||||
| LaMnO3/ZnO | 300 | 50 | 6.0 | 8/17 | 无 | [ | ||||
| CuO/ZnO | 320 | 200 | 97.0 | 5/25 | 57.0 | 20.0 | [ | |||
| CdO/ZnO | 250 | 100 | 65.5 | 2/136 | 10.0 | 9.0 | 15.0 | [ | ||
| CdO/Mn-ZnO | 340 | 20 | 11.4 | 11.4/12.4 | 2.0 | 1.2 | [ | |||
| α-Fe2O3/ZnO/Au | 280 | 100 | 170.0 | 4/5 | 无 | [ | ||||
| MoO3/WO3 | 320 | 100 | 28.5 | 13/10 | 18.2 | [ | ||||
| Cr2O3/WO3 | 300 | 200 | 5.6 | 约46/57 | 4.1 | [ | ||||
| MoO3/In2O3 | 185 | 100 | 7.0 | 11/94 | 2.5 | [ | ||||
| 有机材料&石墨烯/MO x | PANI/TiO2 | 室温 | 200 | 约47.0 | 115/340 | 无 | [ | |||
| Zn2SnO4/rGO | 275 | 100 | 38.0 | 11/18 | 15.0 | 3.5 | 16 | [ | ||
| rGO-SnO2 | 300 | 100 | 70.4 | 11/ | 42.0 | 13 | [ | |||
| Fe3O4/rGO | 400 | 100 | 9.0 | 3/138 | 1.100 | [ | ||||
| TiO2[/多孔硅 | 150 | 100 | 1.4 | 无 | 1.03 | [ | ||||
表3 不同异质结MO x 性能对比
| 异质结 | 材料 | 温度/℃ | 检测浓度/μg?g-1 | 响应度 | 响应/恢复时间/s | 选择性(响应度) | 参考 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 甲醇 | 甲醛 | 丙酮 | 氨气 | |||||||
| MO x -MO x | ZnO/SnO2 | 225 | 30 | 34.8 | 1/120 | 53.7 | 8.9 | 52.1 | [ | |
| (100μg?g-1, 此时乙醇响应度为78.2) | ||||||||||
| NiO/SnO2 | 250 | 100 | 7.9 | 15/100 | 无 | [ | ||||
| Fe2O3/SnO2 | 300 | 200 | 57.6 | 无 | 20.0 | 15.0 | 35.0 | [ | ||
| CoO/SnO2 | 250 | 100 | 145.0 | 无 | 56.0 | 15.0 | 54.0 | [ | ||
| SnO2/ZnO | 350 | 100 | 13.3 | 无 | 4.0 | 4.0 | 1.4 | [ | ||
| SnO2/Fe2O3 | 260 | 100 | 41.7 | 3/4 | 9.0 | 5.0 | [ | |||
| SnO2/Fe2O3 | 240 | 100 | 5.9 | 1/5 | 1.0 | 2.5 | 1.5 | [ | ||
| ZnO/CuO | 300 | 10 | 2.2 | 22/26 | 1.2 | 0.7 | [ | |||
| ZnO/Co3O4 | 200 | 1000 | 106.0 | 7/236 | 无 | [ | ||||
| LaMnO3/ZnO | 300 | 50 | 6.0 | 8/17 | 无 | [ | ||||
| CuO/ZnO | 320 | 200 | 97.0 | 5/25 | 57.0 | 20.0 | [ | |||
| CdO/ZnO | 250 | 100 | 65.5 | 2/136 | 10.0 | 9.0 | 15.0 | [ | ||
| CdO/Mn-ZnO | 340 | 20 | 11.4 | 11.4/12.4 | 2.0 | 1.2 | [ | |||
| α-Fe2O3/ZnO/Au | 280 | 100 | 170.0 | 4/5 | 无 | [ | ||||
| MoO3/WO3 | 320 | 100 | 28.5 | 13/10 | 18.2 | [ | ||||
| Cr2O3/WO3 | 300 | 200 | 5.6 | 约46/57 | 4.1 | [ | ||||
| MoO3/In2O3 | 185 | 100 | 7.0 | 11/94 | 2.5 | [ | ||||
| 有机材料&石墨烯/MO x | PANI/TiO2 | 室温 | 200 | 约47.0 | 115/340 | 无 | [ | |||
| Zn2SnO4/rGO | 275 | 100 | 38.0 | 11/18 | 15.0 | 3.5 | 16 | [ | ||
| rGO-SnO2 | 300 | 100 | 70.4 | 11/ | 42.0 | 13 | [ | |||
| Fe3O4/rGO | 400 | 100 | 9.0 | 3/138 | 1.100 | [ | ||||
| TiO2[/多孔硅 | 150 | 100 | 1.4 | 无 | 1.03 | [ | ||||
| 1 | MANGAS J J , RODRÍGUEZ R , SUÁREZ B . Validation of a gas chromatography-flame ionization method for quality control and spoilage detection in wine and cider[J]. Acta Alimentaria, 2018, 47(1): 17-25. |
| 2 | RONG Q , ZHANG Y , LV T , et al . Highly selective and sensitive methanol gas sensor based on molecular imprinted silver-doped LaFeO3 core-shell and cage structures[J]. Nanotechnology, 2018, 29(14): 145503. |
| 3 | DOLAI S , BHUNIA S K , BEGLARYAN S S , et al . Colorimetric polydiacetylene-aerogel detector for volatile organic compounds (VOCs)[J]. ACS Applied Materials & Interfaces, 2017, 9(3): 2891-2898. |
| 4 | LIU X , CHENG S , LIU H , et al . A survey on gas sensing technology[J]. Sensors, 2012, 12(7): 9635-9665. |
| 5 | HÜBERT T , BOON-BRETT L , BLACK G , et al . Hydrogen sensors-a review[J]. Sensors and Actuators B: Chemical, 2011, 157(2): 329-352. |
| 6 | YAMAZOE N , SHIMANOE K . New perspectives of gas sensor technology[J]. Sensors and Actuators B: Chemical, 2009, 138(1): 100-107. |
| 7 | ZHU Z , KAO C T, WU R J . A highly sensitive ethanol sensor based on Ag@TiO2 nanoparticles at room temperature[J]. Applied Surface Science, 2014, 320: 348-355. |
| 8 | RAUT B T , GODSE P R , PAWAR S G , et al . Novel method for fabrication of polyaniline-CdS sensor for H2S gas detection[J]. Measurement, 2012, 45(1): 94-100. |
| 9 | KARMAOUI M , LEONARDI S G , LATINO M , et al . Pt-decorated In2O3 nanoparticles and their ability as a highly sensitive (<10ppb) acetone sensor for biomedical applications[J]. Sensors and Actuators B: Chemical, 2016, 230: 697-705. |
| 10 | JIN C , PARK S , KIM H, et al . Ultrasensitive multiple networked Ga2O3-core/ZnO-shell nanorod gas sensors[J]. Sensors and Actuators B: Chemical, 2012, 161(1): 223-228. |
| 11 | WANG C , YIN L , ZHANG L , et al . Metal oxide gas sensors: sensitivity and influencing factors[J]. Sensors, 2010, 10(3): 2088-2106. |
| 12 | MIRZAEI A , JANGHORBAN K , HASHEMI B , et al . Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by pechini sol-gel method[J]. Ceramics International, 2016, 42(5): 6136-6144. |
| 13 | SUN Y F , LIU S B , MENG F L , et al . Metal oxide nanostructures and their gas sensing properties: a review[J]. Sensors, 2012, 12(3): 2610-2631. |
| 14 | YAMAZOE N , SHIMANOE K . Basic approach to the transducer function of oxide semiconductor gas sensors[J]. Sensors & Actuators B: Chemical, 2011, 160(1):1352-1362. |
| 15 | ZHANG J , LIU X , NERI G , et al . Nanostructured materials for room-temperature gas sensors[J]. Advanced Materials, 2016, 28(5): 795-831. |
| 16 | JIN C , GE C , XU G , et al . Influence of nanoparticle size on ethanol gas sensing performance of mesoporous α-Fe2O3 hollow spheres[J]. Materials Science and Engineering B, 2017, 224: 158-162. |
| 17 | AMBARDEKAR V , BANDYOPADHYAY P P , MAJUMDER S B . Atmospheric plasma sprayed SnO2 coating for ethanol detection[J]. Journal of Alloys and Compounds, 2018, 752: 440-447. |
| 18 | TANG W , WANG J , QIAO Q , et al . Mechanism for acetone sensing property of Pd-loaded SnO2 nanofibers prepared by electrospinning: Fermi-level effects[J]. Journal of Materials Science, 2015, 50(6): 2605-2615. |
| 19 | ZHANG J , LIANG Y , MAO J , et al . 3D microporous Co3O4-carbon hybrids biotemplated from butterfly wings as high performance VOCs gas sensor[J]. Sensors and Actuators B: Chemical, 2016, 235: 420-431. |
| 20 | CHUNG J S , HUR S H . A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis[J]. Sensors and Actuators B: Chemical, 2016, 223: 76-82. |
| 21 | ZHANG M L , SONG J P , YUAN Z H , et al . Response improvement for In2O3-TiO2 thick film gas sensors[J]. Current Applied Physics, 2012, 12(3): 678-683. |
| 22 | MONTAZERI A , JAMALI-SHEINI F . Enhanced ethanol gas-sensing performance of Pb-doped In2O3 nanostructures prepared by sonochemical method[J]. Sensors and Actuators B: Chemical, 2017, 242: 778-791. |
| 23 | XU J J , LI S J , LI L , et al . Facile fabrication and superior gas sensing properties of spongelike Co-doped ZnO microspheres for ethanol sensors[J]. Ceramics International, 2018, 44(14): 16773-16780. |
| 24 | XING L L , YUAN S , CHEN Z H , et al . Enhanced gas sensing performance of SnO2/α -MoO3 heterostructure nanobelts[J]. Nanotechnology, 2011, 22(22): 225502. |
| 25 | 唐伟, 王兢 . 金属氧化物异质结气体传感器气敏增强机理[J]. 物理化学学报, 2016, 32(5): 1087-1104. |
| TANG W , WANG J . Enhanced gas sensing mechanisms of metal oxide heterojunction gas sensors[J]. Acta Physico-Chimica Sinica, 2016, 32(5): 1087-1104. | |
| 26 | PARK S , KIM S, SUN G J , et al . Ethanol sensing properties of networked In2O3, nanorods decorated with Cr2O3-nanoparticles[J]. Ceramics International, 2015, 41(8):9823-9827. |
| 27 | KATOCH A , CHOI S W , KIM J H, et al . Importance of the nanograin size on the H2S-sensing properties of ZnO-CuO composite nanofibers[J]. Sensors and Actuators B: Chemical, 2015, 214: 111-116. |
| 28 | NAYAK A K , GHOSH R , SANTRA S , et al . Hierarchical nanostructured WO3-SnO2 for selective sensing of volatile organic compounds[J]. Nanoscale, 2015, 7(29): 12460-12473. |
| 29 | KUMAR M , BHATI V S , RANWA S , et al . Pd/ZnO nanorods based sensor for highly selective detection of extremely low concentration hydrogen[J]. Scientific Reports, 2017, 7(1): 236. |
| 30 | PRADES J D , JIMENEZ-DIAZ R , HERNANDEZ-RAMIREZ F , et al . Ultralow power consumption gas sensors based on self-heated individual nanowires[J]. Applied Physics Letters, 2008, 93(12): 123110. |
| 31 | LUPAN O , POSTICA V , LABAT F , et al . Ultra-sensitive and selective hydrogen nanosensor with fast response at room temperature based on a single Pd/ZnO nanowire[J]. Sensors and Actuators B: Chemical, 2018, 254: 1259-1270. |
| 32 | GANESH R S , DURGADEVI E , NAVANEETHAN M , et al . Low temperature ammonia gas sensor based on Mn-doped ZnO nanoparticle decorated microspheres[J]. Journal of Alloys and Compounds, 2017, 721: 182-190. |
| 33 | KIM H J, LEE J H . Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview[J]. Sensors and Actuators B: Chemical, 2014, 192: 607-627. |
| 34 | SAN X, WANG G , LIANG B , et al . Flower-like NiO hierarchical microspheres self-assembled with nanosheets: surfactant-free solvothermal synthesis and their gas sensing properties[J]. Journal of Alloys and Compounds, 2015, 636: 357-362. |
| 35 | YOON J W , KIM H J, JEONG H M , et al . Gas sensing characteristics of p-type Cr2O3 and Co3O4 nanofibers depending on inter-particle connectivity[J]. Sensors and Actuators B: Chemical, 2014, 202: 263-271. |
| 36 | SUN C , SU X , XIAO F , et al . Synthesis of nearly monodisperse Co3O4 nanocubes via a microwave-assisted solvothermal process and their gas sensing properties[J]. Sensors and Actuators B: Chemical, 2011, 157(2): 681-685. |
| 37 | ZHOU X , QU F , ZHANG B , et al . Facile synthesis of In2O3 microcubes with exposed {100} facets as gas sensing material for selective detection of ethanol vapor[J]. Materials Letters, 2017, 209: 618-621. |
| 38 | ZHANG Y , HE X , LI J , et al . Fabrication and ethanol-sensing properties of micro gas sensor based on electrospun SnO2 nanofibers[J]. Sensors and Actuators B: Chemical, 2008, 132(1): 67-73. |
| 39 | LI R , CHEN S , LOU Z , et al . Fabrication of porous SnO2 nanowires gas sensors with enhanced sensitivity[J]. Sensors and Actuators B: Chemical, 2017, 252: 79-85. |
| 40 | ZHANG L , YIN Y . Large-scale synthesis of flower-like ZnO nanorods via a wet-chemical route and the defect-enhanced ethanol-sensing properties[J]. Sensors and Actuators B: Chemical, 2013, 183: 110-116. |
| 41 | MA M X, PAN Z Y , GUO L , et al . Porous cobalt oxide nanowires: notable improved gas sensing performances[J]. Chinese Science Bulletin, 2012, 57(31): 4019-4023. |
| 42 | WEN Z , ZHU L , MEI W , et al . Rhombus-shaped Co3O4 nanorod arrays for high-performance gas sensor[J]. Sensors and Actuators B: Chemical, 2013, 186: 172-179. |
| 43 | LIU L , ZHANG T , WANG Z J , et al . High performance micro-structure sensor based on TiO2 nanofibers for ethanol detection[J]. Chinese Physics Letters, 2009, 26(9): 77-80. |
| 44 | ZHU L , LI Y , ZENG W . Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties[J]. Applied Surface Science, 2018, 427: 281-287. |
| 45 | DENG S , LIU X , CHEN N , et al . A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method[J]. Sensors and Actuators B: Chemical, 2016, 233: 615-623. |
| 46 | CHOI K I , KIM H R, KIM K M, et al . C2H5OH sensing characteristics of various Co3O4 nanostructures prepared by solvothermal reaction[J]. Sensors and Actuators B: Chemical, 2010, 146(1): 183-189. |
| 47 | DENG H , LI H , WANG F , et al . A high sensitive and low detection limit of formaldehyde gas sensor based on hierarchical flower-like CuO nanostructure fabricated by sol-gel method[J]. Journal of Materials Science: Materials in Electronics, 2016, 27(7): 6766-6772. |
| 48 | WANG B , SUN L , WANG Y . Template-free synthesis of nanosheets-assembled SnO2 hollow spheres for enhanced ethanol gas sensing[J]. Materials Letters, 2018, 218: 290-294. |
| 49 | LI W , WU X , HAN N , et al . MOF-derived hierarchical hollow ZnO nanocages with enhanced low-concentration VOCs gas-sensing performance[J]. Sensors and Actuators B: Chemical, 2016, 225: 158-166. |
| 50 | CHITRA M , UTHAYARANI K , RAJASEKARAN N , et al . Rice husk templated mesoporous ZnO nanostructures for ethanol sensing at room temperature[J]. Chinese Physics Letters, 2015, 32(7): 078101. |
| 51 | LI L , LIU M , HE S , et al . Freestanding 3D mesoporous Co3O4@carbon foam nanostructures for ethanol gas sensing[J]. Analytical Chemistry, 2014, 86(15): 7996-8002. |
| 52 | LI L , ZHANG C , ZHANG R , et al . 2D ultrathin Co3O4 nanosheet array deposited on 3D carbon foam for enhanced ethanol gas sensing application[J]. Sensors and Actuators B: Chemical, 2017, 244: 664-672. |
| 53 | WANG Y , LIU C , WANG L , et al . Horseshoe-shaped SnO2 with annulus-like mesoporous for ethanol gas sensing application[J]. Sensors and Actuators B: Chemical, 2017, 240: 1321-1329. |
| 54 | ZHANG B , FU W , LI H , et al . Synthesis and characterization of hierarchical porous SnO2 for enhancing ethanol sensing properties[J]. Applied Surface Science, 2016, 363: 560-565. |
| 55 | FAN F , TANG P , WANG Y , et al . Facile synthesis and gas sensing properties of tubular hierarchical ZnO self-assembled by porous nanosheets[J]. Sensors and Actuators B: Chemical, 2015, 215: 231-240. |
| 56 | MENG F , HOU N , GE S , et al . Flower-like hierarchical structures consisting of porous single-crystalline ZnO nanosheets and their gas sensing properties to volatile organic compounds (VOCs)[J]. Journal of Alloys and Compounds, 2015, 626: 124-130. |
| 57 | JIN Z , ZHANG Y X , MENG F L , et al . Facile synthesis of porous single crystalline ZnO nanoplates and their application in photocatalytic reduction of Cr(Ⅵ) in the presence of phenol[J]. Journal of Hazardous Materials, 2014, 276: 400-407. |
| 58 | MENG F , GE S , JIA Y , et al . Interlaced nanoflake-assembled flower-like hierarchical ZnO microspheres prepared by bisolvents and their sensing properties to ethanol[J]. Journal of Alloys and Compounds, 2015, 632: 645-650. |
| 59 | XIE X , WANG X , TIAN J , et al . Growth of porous ZnO single crystal hierarchical architectures with ultrahigh sensing performances to ethanol and acetone gases[J]. Ceramics International, 2017, 43(1): 1121-1128. |
| 60 | SONG L , YUE H , LI H , et al . Hierarchical porous ZnO microflowers with ultra-high ethanol gas-sensing at low concentration[J]. Chemical Physics Letters, 2018, 699: 1-7. |
| 61 | CHENG X L , ZHAO H , HUO L H , et al . ZnO nanoparticulate thin film: preparation, characterization and gas-sensing property[J]. Sensors and Actuators B: Chemical, 2004, 102(2): 248-252. |
| 62 | TAMVAKOS A , CALESTANI D , TAMVAKOS D , et al . Effect of grain-size on the ethanol vapor sensing properties of room-temperature sputtered ZnO thin films[J]. Microchimica Acta, 2015, 182(11/12): 1991-1999. |
| 63 | WEN Z , ZHU L , LI Y , et al . Mesoporous Co3O4 nanoneedle arrays for high-performance gas sensor[J]. Sensors and Actuators B: Chemical, 2014, 203: 873-879. |
| 64 | GOPALAKRISHNA D , VIJAYALAKSHMI K , RAVIDHAS C . Effect of pyrolytic temperature on the properties of nano-structured CuO optimized for ethanol sensing applications[J]. Journal of Materials Science: Materials in Electronics, 2013, 24(3): 1004-1011. |
| 65 | KIM B Y, CHO J S, YOON J W , et al . Extremely sensitive ethanol sensor using Pt-doped SnO2 hollow nanospheres prepared by Kirkendall diffusion[J]. Sensors and Actuators B: Chemical, 2016, 234: 353-360. |
| 66 | LIU Y , HUANG J , YANG J , et al . Pt nanoparticles functionalized 3D SnO2 nanoflowers for gas sensor application[J]. Solid-State Electronics, 2017, 130: 20-27. |
| 67 | SHAIKH F I , CHIKHALE L P , MULLA I S , et al . Facile Co-precipitation synthesis and ethanol sensing performance of Pd loaded Sr doped SnO2 nanoparticles[J]. Powder Technology, 2018, 326: 479-487. |
| 68 | XIAO L , XU S , YU G , et al . Efficient hierarchical mixed Pd/SnO2 porous architecture deposited microheater for low power ethanol gas sensor[J]. Sensors and Actuators B: Chemical, 2018, 255: 2002-2010. |
| 69 | GUO J , ZHANG J , GONG H , et al . Au nanoparticle-functionalized 3D SnO2 microstructures for high performance gas sensor[J]. Sensors and Actuators B: Chemical, 2016, 226: 266-272. |
| 70 | KIM S Y, KIM J, CHEONG W H , et al . Alcohol gas sensors capable of wireless detection using In2O3/Pt nanoparticles and Ag nanowires[J]. Sensors and Actuators B: Chemical, 2018, 259: 825-832. |
| 71 | DAI E , WU S , YE Y , et al . Highly dispersed Au nanoparticles decorated WO3 nanoplatelets: laser-assisted synthesis and superior performance for detecting ethanol vapor[J]. Journal of Colloid and Interface Science, 2018, 514: 165-171. |
| 72 | ZHANG W , YANG B , LIU J , et al . Highly sensitive and low operating temperature SnO2 gas sensor doped by Cu and Zn two elements[J]. Sensors and Actuators B: Chemical, 2017, 243: 982-989. |
| 73 | INDERAN V , ARAFAT M M , KUMAR S , et al . Study of structural properties and defects of Ni-doped SnO2 nanorods as ethanol gas sensors[J]. Nanotechnology, 2017, 28(26): 265702. |
| 74 | LI Z , YI J . Enhanced ethanol sensing of Ni-doped SnO2 hollow spheres synthesized by a one-pot hydrothermal method[J]. Sensors and Actuators B: Chemical, 2017, 243: 96-103. |
| 75 | KUMAR M , BHATT V , ABHYANKAR A C , et al . New insights towards strikingly improved room temperature ethanol sensing properties of p-type Ce-doped SnO2 sensors[J]. Scientific Reports, 2018, 8(1): 8079. |
| 76 | ZHOU T , ZHANG T , ZHANG R , et al . Highly sensitive sensing platform based on ZnSnO3 hollow cubes for detection of ethanol[J]. Applied Surface Science, 2017, 400:262-268. |
| 77 | ZHU L , LI Y , ZENG W . Enhanced ethanol sensing and mechanism of Cr-doped ZnO nanorods: experimental and computational study[J]. Ceramics International, 2017, 43(17): 14873-14879. |
| 78 | ZHANG P , WANG J , LV X , et al . Facile synthesis of Cr-decorated hexagonal Co3O4 nanosheets for ultrasensitive ethanol detection[J]. Nanotechnology, 2015, 26(27): 275501. |
| 79 | GONG Y , WANG Y , SUN G , et al . Carbon nitride decorated ball-flower like Co3O4 hybrid composite: hydrothermal synthesis and ethanol gas sensing application[J]. Nanomaterials, 2018, 8(3): 132. |
| 80 | LIU J , WANG T , WANG B , et al . Highly sensitive and low detection limit of ethanol gas sensor based on hollow ZnO/SnO2 spheres composite material[J]. Sensors and Actuators B: Chemical, 2017, 245: 551-559. |
| 81 | FANG J , ZHU Y , WU D , et al . Gas sensing properties of NiO/SnO2 heterojunction thin film[J]. Sensors & Actuators B: Chemical, 2017, 252: 1163-1168. |
| 82 | CHOI K S , PARK S , CHANG S P . Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles[J]. Sensors and Actuators B: Chemical, 2017, 238: 871-879. |
| 83 | WANG Q , KOU X , LIU C , et al . Hydrothermal synthesis of hierarchical CoO/SnO2 nanostructures for ethanol gas sensor[J]. Journal of Colloid and Interface Science, 2018, 513: 760-766. |
| 84 | GONG H M , ZHAO C H , WANG F . On-chip growth of SnO2/ZnO core-shell nanosheet arrays for ethanol detection[J]. IEEE Electron Device Letters, 2018,39(7): 1065-1068. |
| 85 | WANG H , WEI S , ZHANG F , et al . Sea urchin-like SnO2/Fe2O3 microspheres for an ethanol gas sensor with high sensitivity and fast response/recovery[J]. Journal of Materials Science Materials in Electronics, 2017, 28(13):9969-9973. |
| 86 | LIU L , SONG P , YANG Z , et al . Ultra-fast responding C2H5OH sensors based on hierarchical assembly of SnO2 nanorods on cube-like α-Fe2O3 [J]. Journal of Materials Science Materials in Electronics, 2018, 29(7):1-8. |
| 87 | BEHERA B , CHANDRA S . An innovative gas sensor incorporating ZnO-CuO nanoflakes in planar MEMS technology[J]. Sensors and Actuators B: Chemical, 2016, 229: 414-424. |
| 88 | XIONG Y , XU W , ZHU Z , et al . ZIF-derived porous ZnO-Co3O4 hollow polyhedrons heterostructure with highly enhanced ethanol detection performance[J]. Sensors & Actuators B: Chemical, 2017, 253:523-532. |
| 89 | ZHANG H , YI J . Enhanced ethanol gas sensing performance of ZnO nanoflowers decorated with LaMnO3 perovskite nanoparticles[J]. Materials Letters, 2018, 216: 196-198. |
| 90 | LIU X , SUN Y , YU M , et al . Enhanced ethanol sensing properties of ultrathin ZnO nanosheets decorated with CuO nanoparticles[J]. Sensors and Actuators B: Chemical, 2018, 255: 3384-3390. |
| 91 | WANG T , KOU X , ZHAO L , et al . Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors[J]. Sensors and Actuators B: Chemical, 2017, 250: 692-702. |
| 92 | WANG J , YANG J , HAN N , et al . Highly sensitive and selective ethanol and acetone gas sensors based on modified ZnO nanomaterials[J]. Materials & Design, 2017, 121: 69-76. |
| 93 | CHEN Y , LI H , MA Q, et al . ZIF-8 derived hexagonal-like α-Fe2O3/ZnO/Au nanoplates with tunable surface heterostructures for superior ethanol gas-sensing performance[J]. Applied Surface Science, 2018, 439: 649-659. |
| 94 | SUN Y , CHEN L , WANG Y , et al . Synthesis of MoO3/WO3 composite nanostructures for highly sensitive ethanol and acetone detection[J]. Journal of Materials Science, 2017, 52(3):1561-1572. |
| 95 | CHOI S , BONYANI M , SUN G J , et al . Cr2O3 nanoparticle-functionalized WO3 nanorods for ethanol gas sensors[J]. Applied Surface Science, 2018, 432: 241-249. |
| 96 | HU J , WANG X , ZHANG M , et al . Synthesis and characterization of flower-like MoO3/In2O3 microstructures for highly sensitive ethanol detection[J]. RSC Advances, 2017, 7(38):23478-23485. |
| 97 | GENG L , WANG S , ZHAO Y , et al . Study of the primary sensitivity of polypyrrole/γ-Fe2O3 to toxic gases[J]. Materials Chemistry and Physics, 2006, 99(1): 15-19. |
| 98 | HAYNES A , GOUMA P I . Sensors for environment, health and security[M]. Dordrecht: Springer, 2009: 451-459. |
| 99 | DAS M, SARKAR D . Development of room temperature ethanol sensor from polypyrrole (PPy) embedded in polyvinyl alcohol (PVA) matrix[J]. Polymer Bulletin, 2018, 75(7): 3109-3125. |
| 100 | CUI S , YANG L , WANG J , et al . Fabrication of a sensitive gas sensor based on PPy/TiO2 nanocomposites films by layer-by-layer self-assembly and its application in food storage[J]. Sensors and Actuators B: Chemical, 2016, 233: 337-346. |
| 101 | GAWRI I , RIDHI R , SINGH K P , et al . Chemically synthesized TiO2 and PANI/TiO2 thin films for ethanol sensing applications[J]. Materials Research Express, 2018, 5(2): 025303. |
| 102 | KANNAPIRAN N , MUTHUSAMY A , RENGANATHAN B , et al . Investigation of magnetic, dielectric and ethanol sensing properties of poly(o-phenylenediamine)/NiFe2O4 nanocomposites[J]. Journal of Materials Science Materials in Electronics, 2017, 29(4):1-11. |
| 103 | CHATTERJEE S G , CHATTERJEE S , RAY A K, et al . Graphene-metal oxide nanohybrids for toxic gas sensor: a review[J]. Sensors and Actuators B: Chemical, 2015, 221: 1170-1181. |
| 104 | LI Y , LUO N , SUN G , et al . In situ decoration of Zn2SnO4 nanoparticles on reduced graphene oxide for high performance ethanol sensor[J]. Ceramics International, 2018, 44(6): 6836-6842. |
| 105 | ZITO C A , PERFECTO T M , VOLANTI D P . Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere[J]. Sensors and Actuators B: Chemical, 2017, 244: 466-474. |
| 106 | THU N T A, CUONG N D , KHIEU D Q , et al . Fe3O4 nanoporous network fabricated from Fe3O4/reduced graphene oxide for high-performance ethanol gas sensor[J]. Sensors and Actuators B: Chemical, 2018, 255: 3275-3283. |
| 107 | DWIVEDI P , CHAUHAN N , VIVEKANANDAN P , et al . Scalable fabrication of prototype sensor for selective and sub-ppm level ethanol sensing based on TiO2 nanotubes decorated porous silicon[J]. Sensors and Actuators B: Chemical, 2017, 249: 602-610. |
| 108 | SEMANCIK S , CAVICCHI R E , GAITAN M , et al . Temperature-controlled, micromachined arrays for chemical sensor fabrication and operation: US 5345213[P]. 1994-09-06. |
| 109 | 朱斌, 殷晨波, 陶春曼,等 . 气体传感器叉指电极结构设计及电极间分布电阻计算[J]. 仪表技术与传感器, 2011(8):14-16. |
| ZHU B , YIN C B , TAO C M , et al . Structural design and calculation of distributed film resistance of gas sensor’s interdigital electrodes[J]. Instrument Technique and Sensor, 2011(8): 14-16. | |
| 110 | JOHNSON C L , WISE K D , SCHWANK J W . A thin-film gas detector for semiconductor process gases[C]// San Francisco, CA, USA:Electron Devices Meeting, 1988. 1988: 662-665. |
| 111 | SUEHLE J S , CAVICCHI R E , GAITAN M , et al . Tin oxide gas sensor fabricated using CMOS micro-hotplates and in-situ processing[J]. IEEE Electron Device Letters, 1993, 14(3): 118-120. |
| 112 | 张子立, 殷晨波, 朱斌,等 . 基于MEMS工艺微气体传感器结构与工艺设计[J]. 南京工业大学学报(自然科学版), 2012, 34(3):31-35. |
| ZHANG Z L , YIN C B , ZHU B , et al . Design of structure and process for micro-gas sensor based on MEMS technology[J]. Journal of Nanjing University of Technology(Natural Science Edition), 2012, 34(3): 31-35. | |
| 113 | TAO C M , HE M X , TU S . Thermal analysis and design of a micro-hotplate for Si-substrated micro-structural gas sensor[C]//Sanya, China:Nano/Micro Engineered and Molecular Systems, 2008. 2008: 284-287. |
| 114 | KIM I, CHOI W Y . Hybrid gas sensor having TiO2 nanotube arrays and SnO2 nanoparticles[J]. International Journal of Nanotechnology, 2017, 14(1/2/3/4/5/6): 155-165. |
| 115 | XU J M , CHENG J P . The advances of Co3O4 as gas sensing materials: a review[J]. Journal of Alloys and Compounds, 2016, 686: 753-768. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 张婷婷, 左旭乾, 田玲娣, 王世猛. 化工园区挥发性有机物排放清单及因子库构建方法[J]. 化工进展, 2023, 42(S1): 549-557. |
| [11] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [12] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [13] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [14] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [15] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||