化工进展 ›› 2024, Vol. 43 ›› Issue (12): 7115-7124.DOI: 10.16085/j.issn.1000-6613.2023-2129
• 资源与环境化工 • 上一篇
胺化改性树脂脱二硫化碳吸附剂的制备及吸附动力学
仇玉静1,2( ), 刘畅1,2, 林杰1,2, 罗国华1,2(
), 刘畅1,2, 林杰1,2, 罗国华1,2( ), 戴晓兵3
), 戴晓兵3
- 1.北京石油化工学院,北京 102600
2.燃料清洁化及高效催化减排技术北京市重点实验室,北京 102600
3.珠海市赛纬电子材料股份有限公司,广东 珠海 519000
-
收稿日期:2023-12-01修回日期:2024-08-19出版日期:2024-12-15发布日期:2025-01-11 -
通讯作者:罗国华 -
作者简介:仇玉静(1997—),女,硕士研究生,研究方向为芳烃精制脱硫。E-mail:2413121739@qq.com。
Preparation and adsorption kinetics of resin adsorbent modified by amine for removing carbon disulfide
QIU Yujing1,2( ), LIU Chang1,2, LIN Jie1,2, LUO Guohua1,2(
), LIU Chang1,2, LIN Jie1,2, LUO Guohua1,2( ), DAI Xiaobing3
), DAI Xiaobing3
- 1.Beijing Institute of Petrochemical Technology, Beijing 102600, China
2.Beijing Key Laboratory of Fuels Cleaning and Advanced Catalytic Emission Reduction Technology, Beijing 102600, China
3.Zhuhai Saiwei Electronic Materials Co. , Ltd. , Zhuhai 519000, Guangdong, China
-
Received:2023-12-01Revised:2024-08-19Online:2024-12-15Published:2025-01-11 -
Contact:LUO Guohua
摘要:
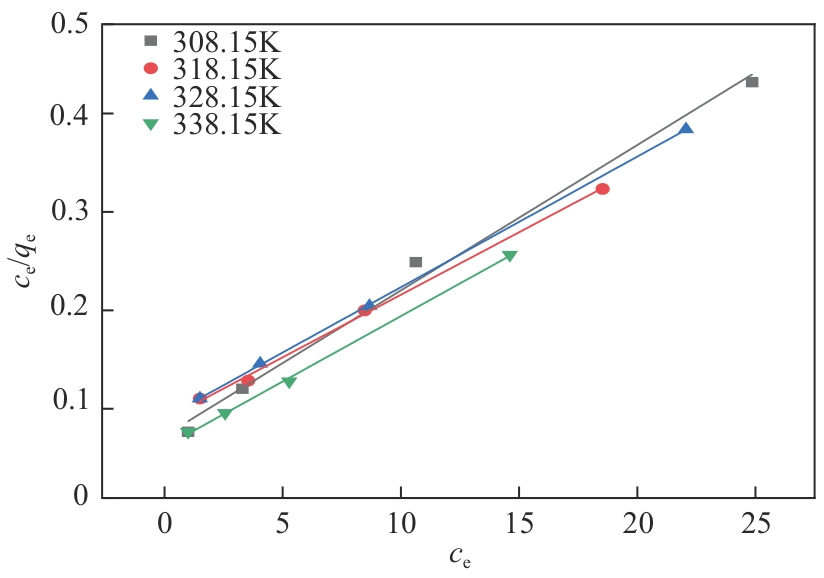
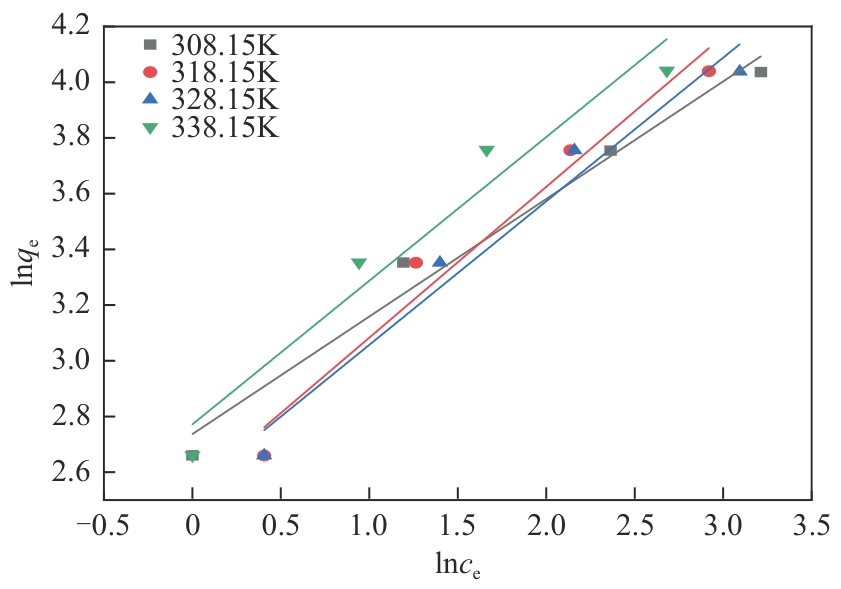
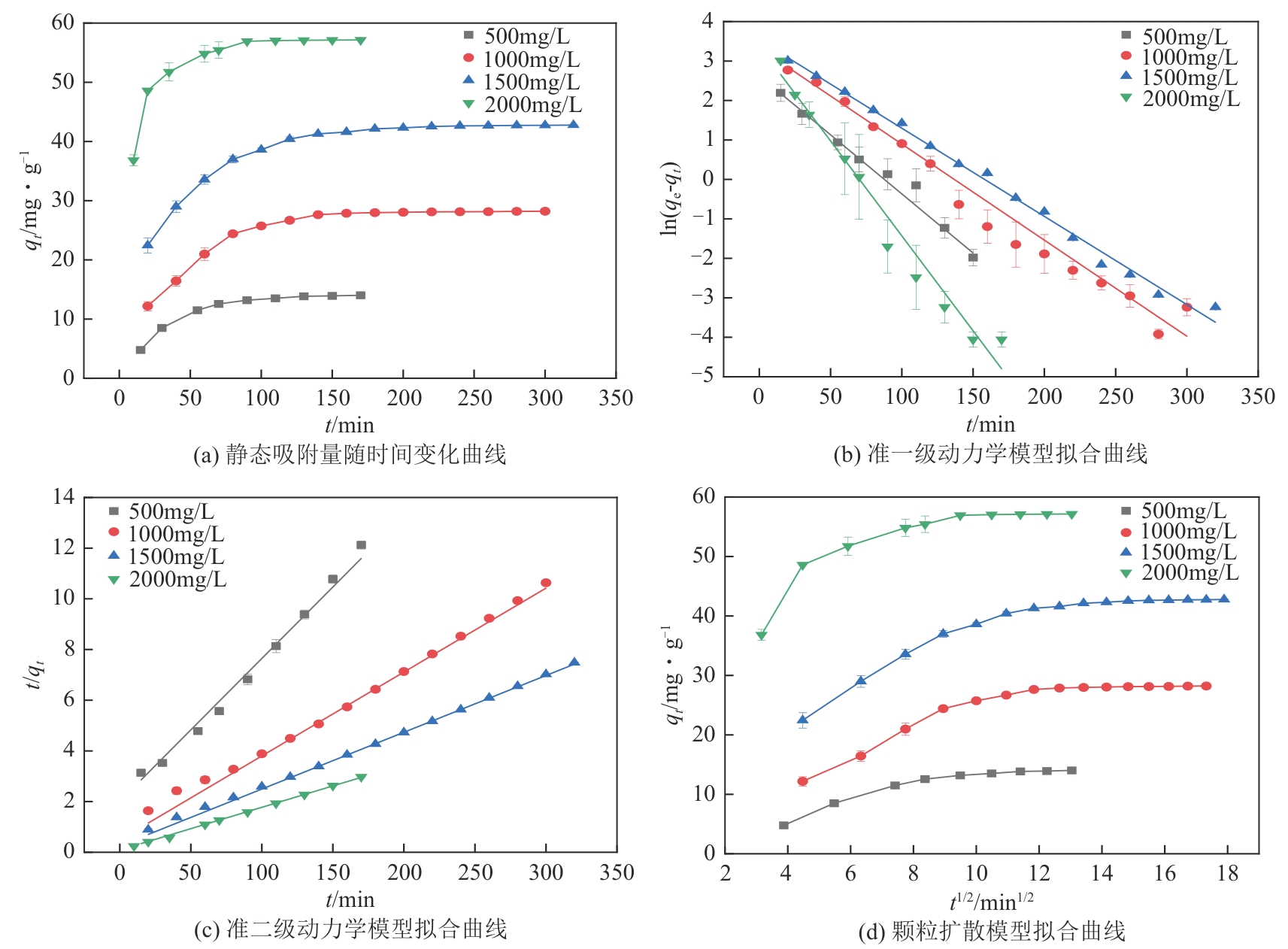
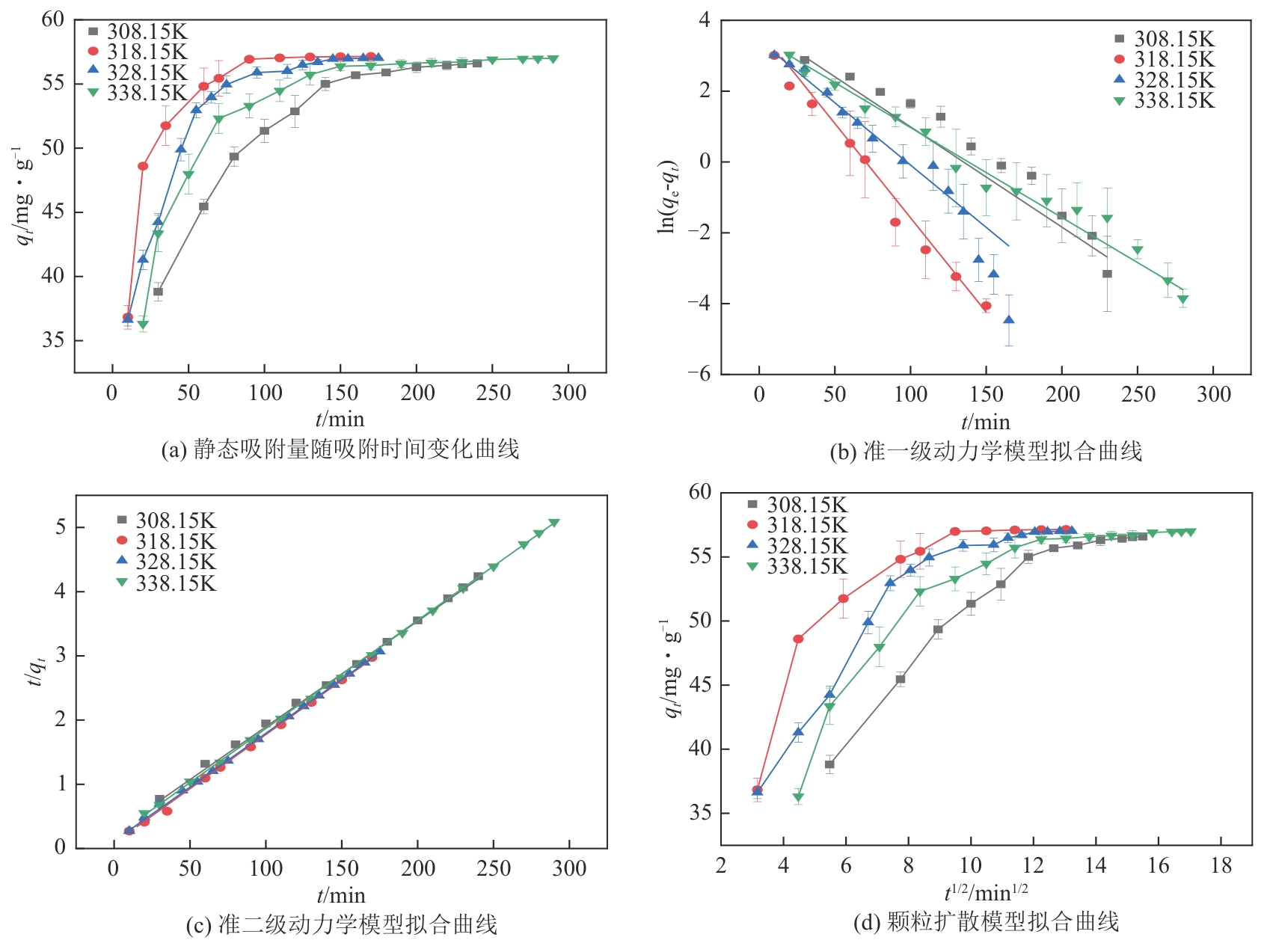
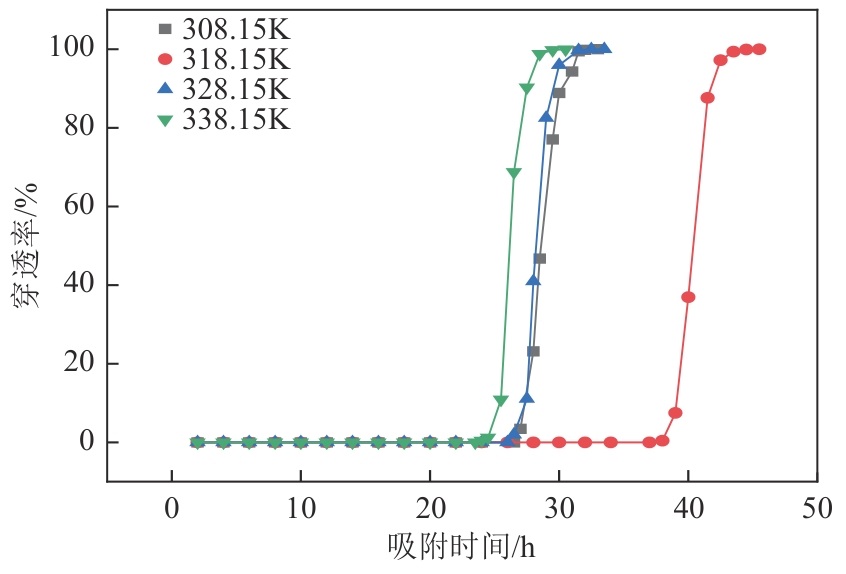
以氯甲基聚苯乙烯大孔树脂为载体,二乙烯三胺为胺化剂,通过取代反应将含伯胺和仲胺的活性官能团锚定到树脂链上制得CS2脱硫吸附剂,采用FTIR、元素分析仪、BET对改性树脂进行表征,并对吸附剂脱除苯中CS2吸附性能进行评价,得到不同温度及不同CS2初始浓度下的吸附穿透曲线,采用Langmuir和Freundlich模型对吸附剂吸附苯中CS2的吸附等温线进行分析,准一级、准二级动力学模型及颗粒扩散模型对吸附动力学进行拟合。结果表明:胺化改性的聚苯乙烯树脂具有优异的选择性吸附二硫化碳性能,其在常压、318.15K、1h-1、原料CS2浓度为2000mg/L条件下的穿透吸附容量高达154.167mg/g。同时,该吸附剂吸附CS2符合Langmuir模型和准二级动力学模型,吸附控制步骤为CS2与吸附剂表面活性胺基基团的反应,而非传质过程。通过建立动态吸附过程的Y-N吸附模型,并对吸附穿透曲线进行分析验证,拟合值与实验值十分吻合。
中图分类号:
引用本文
仇玉静, 刘畅, 林杰, 罗国华, 戴晓兵. 胺化改性树脂脱二硫化碳吸附剂的制备及吸附动力学[J]. 化工进展, 2024, 43(12): 7115-7124.
QIU Yujing, LIU Chang, LIN Jie, LUO Guohua, DAI Xiaobing. Preparation and adsorption kinetics of resin adsorbent modified by amine for removing carbon disulfide[J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7115-7124.
| 分析物 | 元素质量分数/% | |||
|---|---|---|---|---|
| N | C | H | S | |
| 胺化改性树脂 | 10.85 | 77.81 | 7.66 | 0 |
表1 胺化改性后树脂的元素分析结果
| 分析物 | 元素质量分数/% | |||
|---|---|---|---|---|
| N | C | H | S | |
| 胺化改性树脂 | 10.85 | 77.81 | 7.66 | 0 |
| 样品 | BET比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm |
|---|---|---|---|
| 氯甲基聚苯乙烯大孔树脂 | 11.668 | 0.081 | 27.710 |
| 二乙烯三胺胺化改性树脂 | 18.901 | 0.098 | 20.699 |
| 45℃吸附饱和后的树脂 | 19.520 | 0.095 | 19.518 |
表2 胺化改性及吸附前后树脂的BET参数
| 样品 | BET比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm |
|---|---|---|---|
| 氯甲基聚苯乙烯大孔树脂 | 11.668 | 0.081 | 27.710 |
| 二乙烯三胺胺化改性树脂 | 18.901 | 0.098 | 20.699 |
| 45℃吸附饱和后的树脂 | 19.520 | 0.095 | 19.518 |
| c0/mg·L-1 | 308.15K | 318.15K | 328.15K | 338.15K | ||||
|---|---|---|---|---|---|---|---|---|
| ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | |
| 500 | 1.000 | 14.306 | 1.500 | 14.292 | 1.500 | 14.292 | 1.000 | 14.306 |
| 1000 | 3.295 | 28.575 | 3.536 | 28.568 | 4.051 | 28.554 | 2.564 | 28.596 |
| 1500 | 10.631 | 42.700 | 8.467 | 42.762 | 8.667 | 42.756 | 5.277 | 42.853 |
| 2000 | 24.852 | 56.627 | 18.532 | 56.808 | 22.059 | 56.707 | 14.610 | 56.921 |
表3 不同温度下胺化树脂吸附二硫化碳的平衡等温吸附数据
| c0/mg·L-1 | 308.15K | 318.15K | 328.15K | 338.15K | ||||
|---|---|---|---|---|---|---|---|---|
| ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | ce/mg·L-1 | qe/mg·g-1 | |
| 500 | 1.000 | 14.306 | 1.500 | 14.292 | 1.500 | 14.292 | 1.000 | 14.306 |
| 1000 | 3.295 | 28.575 | 3.536 | 28.568 | 4.051 | 28.554 | 2.564 | 28.596 |
| 1500 | 10.631 | 42.700 | 8.467 | 42.762 | 8.667 | 42.756 | 5.277 | 42.853 |
| 2000 | 24.852 | 56.627 | 18.532 | 56.808 | 22.059 | 56.707 | 14.610 | 56.921 |
| T/K | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL/L·mg-1 | R2 | RL | KF/mg·g-1·L1/n ·mg-1/n | 1/n | R2 | |
| 308.15 | 60.606 | 0.242 | 0.999 | 0.002 | 15.436 | 0.422 | 0.986 |
| 318.15 | 67.568 | 0.180 | 0.998 | 0.003 | 12.699 | 0.542 | 0.983 |
| 328.15 | 65.359 | 0.175 | 1.000 | 0.003 | 12.701 | 0.515 | 0.983 |
| 338.15 | 64.516 | 0.274 | 0.999 | 0.002 | 15.985 | 0.516 | 0.976 |
表4 不同温度下胺化树脂吸附二硫化碳Langmuir模型和Freundlich模型拟合结果
| T/K | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL/L·mg-1 | R2 | RL | KF/mg·g-1·L1/n ·mg-1/n | 1/n | R2 | |
| 308.15 | 60.606 | 0.242 | 0.999 | 0.002 | 15.436 | 0.422 | 0.986 |
| 318.15 | 67.568 | 0.180 | 0.998 | 0.003 | 12.699 | 0.542 | 0.983 |
| 328.15 | 65.359 | 0.175 | 1.000 | 0.003 | 12.701 | 0.515 | 0.983 |
| 338.15 | 64.516 | 0.274 | 0.999 | 0.002 | 15.985 | 0.516 | 0.976 |
| c0/mg·L-1 | η/% | qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe,cal/mg·g-1 | k2/min-1 | R2 | qe,cal/mg·g-1 | |||
| 2000 | 99.66 | 57.147 | 0.048 | 0.968 | 31.869 | 0.004 | 1.000 | 58.651 |
| 1500 | 99.46 | 42.771 | 0.022 | 0.993 | 34.242 | 0.002 | 0.999 | 44.763 |
| 1000 | 98.40 | 28.212 | 0.024 | 0.993 | 27.988 | 0.002 | 0.996 | 30.553 |
| 500 | 97.79 | 14.019 | 0.037 | 0.968 | 13.849 | 0.002 | 0.992 | 16.714 |
表5 不同CS2浓度下胺化树脂吸附CS2准一级动力学模型和准二级动力学模型拟合参数
| c0/mg·L-1 | η/% | qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe,cal/mg·g-1 | k2/min-1 | R2 | qe,cal/mg·g-1 | |||
| 2000 | 99.66 | 57.147 | 0.048 | 0.968 | 31.869 | 0.004 | 1.000 | 58.651 |
| 1500 | 99.46 | 42.771 | 0.022 | 0.993 | 34.242 | 0.002 | 0.999 | 44.763 |
| 1000 | 98.40 | 28.212 | 0.024 | 0.993 | 27.988 | 0.002 | 0.996 | 30.553 |
| 500 | 97.79 | 14.019 | 0.037 | 0.968 | 13.849 | 0.002 | 0.992 | 16.714 |
| T/K | η/% | qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe,cal/mg·g-1 | k2/min-1 | R2 | qe,cal/mg·g-1 | |||
| 308.15 | 98.71 | 56.601 | 0.021 | 36.087 | 0.961 | 0.001 | 0.999 | 61.538 |
| 318.15 | 99.66 | 57.147 | 0.051 | 24.516 | 0.962 | 0.004 | 0.999 | 58.651 |
| 328.15 | 99.44 | 57.022 | 0.034 | 30.054 | 0.937 | 0.003 | 1.000 | 59.032 |
| 338.15 | 99.41 | 57.002 | 0.025 | 33.869 | 0.994 | 0.002 | 1.000 | 59.418 |
表6 不同温度下胺化树脂吸附苯中CS2准一级动力学模型和准二级动力学模型拟合参数
| T/K | η/% | qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe,cal/mg·g-1 | k2/min-1 | R2 | qe,cal/mg·g-1 | |||
| 308.15 | 98.71 | 56.601 | 0.021 | 36.087 | 0.961 | 0.001 | 0.999 | 61.538 |
| 318.15 | 99.66 | 57.147 | 0.051 | 24.516 | 0.962 | 0.004 | 0.999 | 58.651 |
| 328.15 | 99.44 | 57.022 | 0.034 | 30.054 | 0.937 | 0.003 | 1.000 | 59.032 |
| 338.15 | 99.41 | 57.002 | 0.025 | 33.869 | 0.994 | 0.002 | 1.000 | 59.418 |
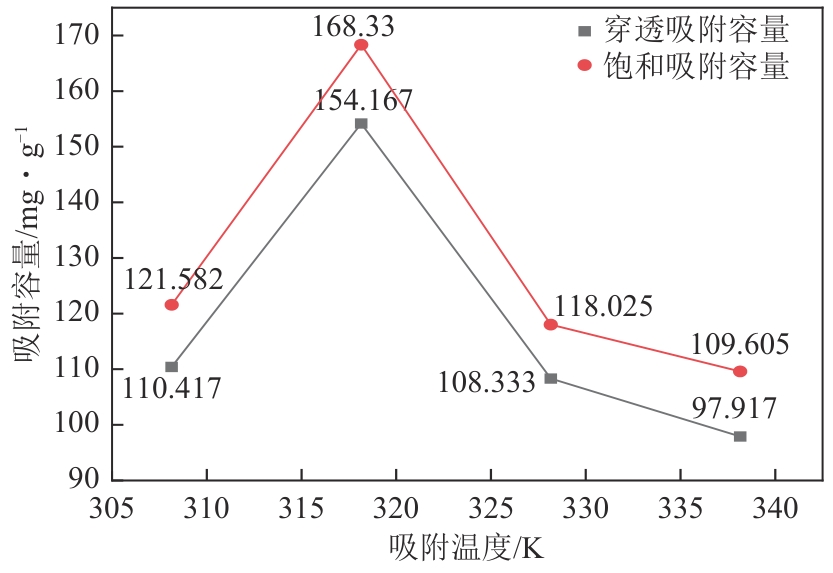
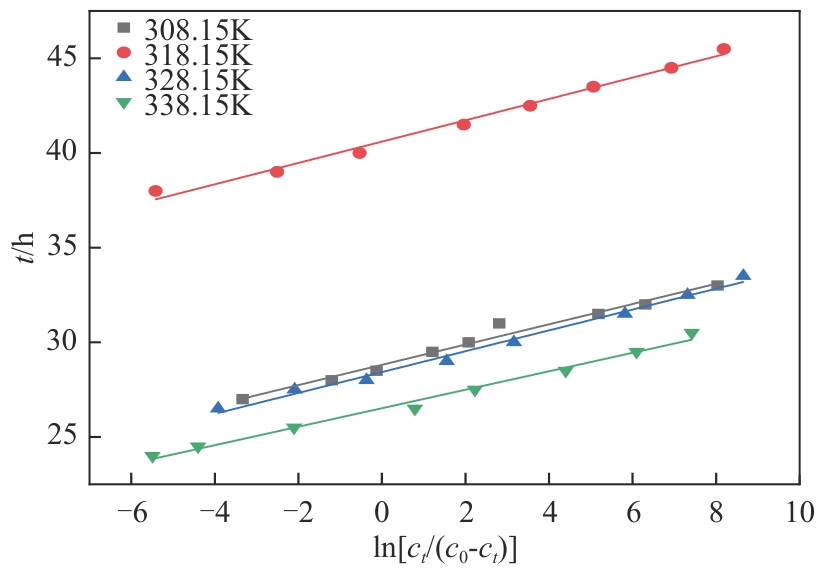
| T/K | texp/h | qexp/mg·g-1 | k/h-1 | τ/h | R2 | tcal/h | qe-YN/mg·g-1 |
|---|---|---|---|---|---|---|---|
308.15 318.15 328.15 338.15 | 26.5 37.0 26.0 23.5 | 121.582 168.330 118.025 109.603 | 1.801 1.775 1.817 2.049 | 28.810 40.700 28.428 26.518 | 0.993 0.991 0.991 0.990 | 25.331 36.418 24.244 22.809 | 122.492 169.583 118.450 110.492 |
表7 不同温度下CS2在胺化改性树脂吸附剂上的Y-N模型拟合结果及吸附穿透时间
| T/K | texp/h | qexp/mg·g-1 | k/h-1 | τ/h | R2 | tcal/h | qe-YN/mg·g-1 |
|---|---|---|---|---|---|---|---|
308.15 318.15 328.15 338.15 | 26.5 37.0 26.0 23.5 | 121.582 168.330 118.025 109.603 | 1.801 1.775 1.817 2.049 | 28.810 40.700 28.428 26.518 | 0.993 0.991 0.991 0.990 | 25.331 36.418 24.244 22.809 | 122.492 169.583 118.450 110.492 |
| 1 | 李怀柱. 黄原酸盐反应法脱除焦化粗苯中的二硫化碳[D]. 太原: 太原理工大学, 2012. |
| LI Huaizhu. The removal of carbon disulfide in crude benzene by using xanthogenate method[D]. Taiyuan: Taiyuan University of Technology, 2012. | |
| 2 | 江大好, 宿亮虎, 陆殿乔, 等. 焦化粗苯的组成及其加氢精制工艺技术的开发[J]. 现代化工, 2009, 29(5): 72-75. |
| JIANG Dahao, SU Lianghu, LU Dianqiao, et al. Crude benzol coking products and development of its hydro-refining technology[J]. Modern Chemical Industry, 2009, 29(5): 72-75. | |
| 3 | 党帅, 岳红. 粗苯深加工路线及焦化粗苯产业发展分析[J]. 煤化工, 2013, 41(2): 4-7. |
| DANG Shuai, YUE Hong. Route of deep processing of crude benzol and development of the coking crude benzol industry[J]. Coal Chemical Industry, 2013, 41(2): 4-7. | |
| 4 | 曾丹林, 胡义, 王可苗, 等. 焦化粗苯中噻吩分离回收的研究进展[J]. 精细石油化工进展, 2012, 13(1): 45-50. |
| ZENG Danlin, HU Yi, WANG Kemiao, et al. Progress on recovery of thiophene in coking crude benzene[J]. Advances in Fine Petrochemicals, 2012, 13(1): 45-50. | |
| 5 | 梁顺琴, 吕龙刚, 马好文, 等. 硫化物对裂解汽油一段加氢用Pd/Al2O3催化剂性能的影响[J]. 现代化工, 2014, 34(9): 85-87. |
| LIANG Shunqin, Longgang LYU, MA Haowen, et al. Effects of sulfur compounds on performance of Pd/Al2O3 catalyst for first-stage hydrogenation of pyrolysis gasoline[J]. Modern Chemical Industry, 2014, 34(9): 85-87. | |
| 6 | LIAO Junjie, BAO Lei, WANG Wenbo, et al. Preparation of AlCl3/silica gel catalyst for simultaneously removing thiophene and olefins from coking benzene by inclosed grafting method[J]. Fuel Processing Technology, 2014, 117: 38-43. |
| 7 | 王丽, 吴迪镛, 王树东, 等. 稀土氧化物CeO2脱除二硫化碳的研究[J]. 中国稀土学报, 2005, 23(S1): 35-39. |
| WANG Li, WU Diyong, WANG Shudong, et al. Study on removal of carbon disulfide by CeO2 [J]. Journal of the Chinese Society of Rare Earths, 2005, 23(S1): 35-39. | |
| 8 | HE Dan, YI Honghong, TANG Xiaolong, et al. Carbon disulfide hydrolysis over Fe-Cu/AC catalyst modified by cerium and lanthanum at low temperature[J]. Journal of Rare Earths, 2010, 28: 343-346. |
| 9 | 王丽, 李福林, 吴迪镛, 等. 催化水解-氧化耦合一步法脱除二硫化碳的研究[J]. 燃料化学学报, 2004, 32(4): 466-470. |
| WANG Li, LI Fulin, WU Diyong, et al. A novel desulfuring agent for CS2 removal by couple processing[J]. Journal of Fuel Chemistry and Technology, 2004, 32(4): 466-470. | |
| 10 | 贾丽丽, 胡典明, 孔渝华. 脱除二硫化碳的研究进展[J]. 辽宁化工, 2008, 37(3): 184-186. |
| JIA Lili, HU Dianming, KONG Yuhua. Development of removing carbon disulfide[J]. Liaoning Chemical Industry, 2008, 37(3): 184-186. | |
| 11 | YANG Ji, JUAN Peng, SHEN Zhemin, et al. Removal of carbon disulfide (CS2) from water via adsorption on active carbon fiber (ACF)[J]. Carbon, 2006, 44(8): 1367-1375. |
| 12 | 李敏, 张智宏, 魏燕. 活性炭基π络合吸附剂的制备及其脱除二硫化碳性能[J]. 精细化工, 2016, 33(10): 1130-1134. |
| LI Min, ZHANG Zhihong, WEI Yan. Preparation of π complexation adsorbent based on activated carbon and its removal performance for carbon disulfide[J]. Fine Chemicals, 2016, 33(10): 1130-1134. | |
| 13 | 孟令刚, 张现策, 李芹, 等. 焙烧气氛对K2CO3/γ-Al2O3吸附剂脱除异戊二烯中二硫化碳性能的影响[J]. 化工进展, 2021, 40(1): 221-226. |
| MENG Linggang, ZHANG Xiance, LI Qin, et al. Effect of calcination atmosphere on K2CO3/γ-Al2O3 adsorbent for removing carbon disulfide from isoprene[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 221-226. | |
| 14 | 王亚宁, 熊春华, 王槐三. 树脂吸附法去除水及空气中的CS2 [J]. 化工环保, 2006, 26(3): 174-177. |
| WANG Yaning, XIONG Chunhua, WANG Huaisan. Removal of CS2 from water and air by resin adsorption process[J]. Environmental Protection of Chemical Industry, 2006, 26(3): 174-177. | |
| 15 | 曲宏昌, 马佳凯, 龙超. 吸附树脂和活性炭吸附气体中二硫化碳的性能差异及成因研究[J]. 中国环境科学, 2023, 43(9): 4534-4541. |
| QU Hongchang, MA Jiakai, LONG Chao. An investigation of the causes of different adsorption properties of carbon disulfide vapor in gas on polymeric resin and activated carbon[J]. China Environmental Science, 2023, 43(9): 4534-4541. | |
| 16 | WANG Shuangshuang, ZHANG Liang, LONG Chao, et al. Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents[J]. Journal of Colloid and Interface Science, 2014, 428: 185-190. |
| 17 | 杨新玉, 史秋怡, 龙超. 吸附树脂吸附多组分VOCs的动力学特性及预测[J]. 中国环境科学, 2019, 39(5): 1830-1837. |
| YANG Xinyu, SHI Qiuyi, LONG Chao. Adsorption kinetics and prediction of multicomponent VOCs on polymeric resins[J]. China Environmental Science, 2019, 39(5): 1830-1837. | |
| 18 | 孟启, 肖网琴, 戎志峰, 等. 二(β-氨基乙基)胺型聚苯乙烯树脂的合成和性能研究[J]. 离子交换与吸附, 2014, 30(3): 258-266. |
| MENG Qi, XIAO Wangqin, RONG Zhifeng, et al. Synthesis and adsorption properties of di(β-aminoethyl) amine polystyrene resins[J]. Ion Exchange and Adsorption, 2014, 30(3): 258-266. | |
| 19 | 郑子苹, 沈健, 邓桂春. ZSM-5-SBA-15复合分子筛吸附脱除碱性氮动力学数学模型[J]. 精细石油化工, 2022, 39(5): 48-51. |
| ZHENG Ziping, SHEN Jian, DENG Guichun. Mathematic model of adsorption denitrogenation kinetics of ZSM-5-SBA-15 composite molecular sieve[J]. Speciality Petrochemicals, 2022, 39(5): 48-51. | |
| 20 | 赵子科, 陈春亮, 柯盛, 等. 榴莲壳和不同炭材料对低汞溶液的吸附动力学[J]. 岩矿测试, 2022, 41(1): 90-98. |
| ZHAO Zike, CHEN Chunliang, KE Sheng, et al. Adsorption kinetics of low mercury solution with durian shell and activated carbon[J]. Rock and Mineral Analysis, 2022, 41(1): 90-98. | |
| 21 | 刘新辙, 陈娟, 张明阳, 等. CO2在栗子壳基碳材料上的吸附动力学及热力学研究[J]. 应用化工, 2023, 52(2): 445-450. |
| LIU Xinzhe, CHEN Juan, ZHANG Mingyang, et al. Kinetics and thermodynamics of CO2 on chestnut shell-based carbon materials[J]. Applied Chemical Industry, 2023, 52(2): 445-450. | |
| 22 | 孟冠华, 张林森, 刘宝河, 等. 可见光下Cu2O/TiO2催化臭氧氧化头孢曲松钠性能研究[J]. 过程工程学报, 2023, 23(2): 311-322. |
| MENG Guanhua, ZHANG Linsen, LIU Baohe, et al. Photocatalytic ozonation of ceftriaxone sodium by Cu2O/TiO2 under visible light[J]. The Chinese Journal of Process Engineering, 2023, 23(2): 311-322. | |
| 23 | 曹仕文, 张鸿, 孟驰涵, 等. 海藻酸钠/二氧化硅杂化微球结构与吸附性能[J]. 化工进展, 2018, 37(9): 3512-3519. |
| CAO Shiwen, ZHANG Hong, MENG Chihan, et al. Structure and properties of sodium alginate/silica hybrid microspheres[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3512-3519. | |
| 24 | 郭可心, 田佳一, 孙煜璨, 等. 磁性污泥基生物炭对Pb2+的吸附性能[J]. 环境工程学报, 2022, 16(5): 1416-1428. |
| GUO Kexin, TIAN Jiayi, SUN Yucan, et al. Adsorption performance of magnetic sludge-derived biochar towards Pb2+ [J]. Chinese Journal of Environmental Engineering, 2022, 16(5): 1416-1428. | |
| 25 | LI Tingting, LIU Yunguo, PENG Qingqing, et al. Removal of lead(Ⅱ) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling[J]. Chemical Engineering Journal, 2013, 214: 189-197. |
| 26 | Abuzer ÇELEKLI, Gizem İLGÜN, Hüseyin BOZKURT. Sorption equilibrium, kinetic, thermodynamic, and desorption studies of Reactive Red 120 on Chara contraria [J]. Chemical Engineering Journal, 2012, 191: 228-235. |
| 27 | ZHAO Yafei, ZHANG Bing, ZHANG Xiang, et al. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions[J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 658-664. |
| 28 | 杨静, 沈健, 石薇薇. Cu-β/SBA-15的吸附脱硫性能及吸附动力学[J]. 精细化工, 2017, 34(10): 1153-1160. |
| YANG Jing, SHEN Jian, SHI Weiwei. Adsorption desulfurization and adsorption kinetics of Cu-β/SBA-15[J]. Fine Chemicals, 2017, 34(10): 1153-1160. | |
| 29 | 岳晓晴. 醇胺基二硫化碳储集材料的合成及制备金属硫化物的研究[D]. 呼和浩特: 内蒙古工业大学, 2019. |
| YUE Xiaoqing. Study on synthesis of alcoholamine-based carbon disulfide storage materials and preparation of metal sulfides[D]. Hohhot: Inner Mongolia University of Tehchnology, 2019. | |
| 30 | 马佳凯, 许博文, 梁晓珊, 等. CS2在超高交联树脂上的吸附脱附特性研究[J]. 应用化工, 2018, 47(8): 1684-1687, 1694. |
| MA Jiakai, XU Bowen, LIANG Xiaoshan, et al. Adsorption and desorption behavior of carbon disulfide on hypercrosslinked resin[J]. Applied Chemical Industry, 2018, 47(8): 1684-1687, 1694. | |
| 31 | 霍宇辰, 靳岩, 张茜, 等. 分子筛吸附脱除2-甲基四氢呋喃中水的研究[J]. 现代化工, 2021, 41(3): 134-139. |
| HUO Yuchen, JIN Yan, ZHANG Xi, et al. Study on removing water from 2-methyltetrahydrofuran by molecular sieves adsorption[J]. Modern Chemical Industry, 2021, 41(3): 134-139. |
| [1] | 宋财城, 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 碳基载体负载加氢脱硫催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 305-314. |
| [2] | 杨新衡, 纪志永, 郭志远, 刘萁, 张盼盼, 汪婧, 刘杰, 毕京涛, 赵颖颖, 袁俊生. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 2024, 43(9): 5262-5274. |
| [3] | 马红周, 党煜博, 王耀宁, 曾劲阳, 赵小军, 史建伟. 废锌基脱硫剂与铜锌基催化剂协同真空碳热提取锌[J]. 化工进展, 2024, 43(9): 5275-5281. |
| [4] | 李一, 梁李斯, 张立兴, 乔江鱼, 崔忠宜, 陈进, 徐强, 赵晨. 次氯酸盐氧化剂同时脱硫脱硝一体化[J]. 化工进展, 2024, 43(9): 5282-5289. |
| [5] | 高昕玥, 范高峰, 刘爱平, 王长安, 侯育杰, 张津铭, 徐杰, 车得福. 湿法脱硫后烟气和浆液余热回收技术研究进展[J]. 化工进展, 2024, 43(8): 4307-4319. |
| [6] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [7] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [8] | 武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693. |
| [9] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [10] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [11] | 郑锁祺, 詹凌霄, 陈恒, 李志浩, 王禹瑞, 赵宁, 吴昊, 杨林军. 基于混合模型的脱硫废水旁路蒸发系统能耗特性[J]. 化工进展, 2024, 43(6): 2968-2976. |
| [12] | 刘京都, 余关龙, 龙志奇, 周璐, 包璞瑞, 滕骏毅, 杜春艳. 纳米球状LaAlO3的制备及其在酸性条件下的除氟性能[J]. 化工进展, 2024, 43(6): 3199-3208. |
| [13] | 屈超, 刘俊红, 贾彬, 黄勇. 水性丙烯酸树脂对PBAT/淀粉复合材料性能影响[J]. 化工进展, 2024, 43(6): 3268-3276. |
| [14] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [15] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||