| 1 |
PENG Fangwei, MU Deying, LI Ruhong, et al. Impurity removal with highly selective and efficient methods and the recycling of transition metals from spent lithium-ion batteries[J]. RSC Advances, 2019, 9(38): 21922-21930.
|
| 2 |
SONG Weu, LIU Jianwen, YOU Lei, et al. Re-synthesis of nano-structured LiFePO4/graphene composite derived from spent lithium-ion battery for booming electric vehicle application[J]. Journal of Power Sources, 2019, 419: 192-202.
|
| 3 |
ZHANG Xiaoxiao, LI Li, FAN Ersha, et al. Toward sustainable and systematic recycling of spent rechargeable batteries[J]. Chemical Society Reviews, 2018, 47(19): 7239-7302.
|
| 4 |
SONG Yingjie, ZHONG Shuqi, LI Yingjiao, et al. Study on the catalytic degradation of sodium lignosulfonate to aromatic aldehydes over nano-CuO: Process optimization and reaction kinetics[J]. Chinese Journal of Chemical Engineering, 2023, 53: 300-309.
|
| 5 |
张笑笑, 王鸯鸯, 刘媛, 等. 废旧锂离子电池回收处理技术与资源化再生技术进展[J]. 化工进展, 2016, 35(12): 4026-4032.
|
|
ZHANG Xiaoxiao, WANG Yangyang, LIU Yuan, et al. Recent progress in disposal and recycling of spent lithium-ion batteries[J]. Chemical Industry and Engineering Progress, 2016, 35(12): 4026-4032.
|
| 6 |
RITCHIE Andrew, HOWARD Wilmont. Recent developments and likely advances in lithium-ion batteries[J]. Journal of Power Sources, 2006, 162(2): 809-812.
|
| 7 |
DENG Sixu, WANG Hao, LIU Hao, et al. Research progress in improving the rate performance of LiFePO4 cathode materials[J]. Nano-Micro Letters, 2014, 6(3): 209-226.
|
| 8 |
曹玲, 刘雅丽, 康铎之, 等. 废旧锂电池中有价金属回收及三元正极材料的再制备[J]. 化工进展, 2019, 38(5): 2499-2505.
|
|
CAO Ling, LIU Yali, KANG Duozhi, et al. Recovery of valuable metals from spent lithium ion battery and the resynthesis of Li(Ni1/3Co1/3Mn1/3)O2 materials[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2499-2505.
|
| 9 |
ZHENG Rujuan, ZHAO Li, WANG Wenjui, et al. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method[J]. RSC Advances, 2016, 6(49): 43613-43625.
|
| 10 |
YUN Liu, LINH Duy, SHUI Li, et al. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles[J]. Resources, Conservation and Recycling, 2018, 136: 198-208.
|
| 11 |
HE Kai, ZHANG Zhiyuan, ALAI Lagu, et al. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2019, 375: 43-51.
|
| 12 |
XU Panpan, DAI Q, GAO Hongpeng, et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing[J]. Joule, 2020, 4(12): 2609-2626.
|
| 13 |
LIU Yan, LV Weiguang, ZHENG Xiaohong, et al. Near-to-stoichiometric acidic recovery of spent lithium-ion batteries through induced crystallization[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(8): 3183-3194.
|
| 14 |
LI Jingkun, MA Zifeng. Past and present of LiFePO4: From fundamental research to industrial applications[J]. Chem, 2019, 5(1): 3-6.
|
| 15 |
黎华玲, 陈永珍, 宋文吉, 等. 湿法回收退役三元锂离子电池有价金属的研究进展[J]. 化工进展, 2019, 38(2): 921-932.
|
|
LI Hualing, CHEN Yongzhen, SONG Wenji, et al. Research progress on the recovery of valuable matals in retired LiNi x Co y Mn z O2batteries by wet process[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 921-932.
|
| 16 |
华政, 梁风, 姚耀春. 电动汽车电池的发展现状与趋势[J]. 化工进展, 2017, 36(8): 2874-2881.
|
|
HUA Zheng, LIANG Feng, YAO Yaochun. Status and development trend for battery of electric vehicles [J]. Chemical Industry and Engineering Progress, 2017, 36(8): 2874-2881.
|
| 17 |
XU Xueqing, MU Wenning, XIAO Tengfei, et al. A clean and efficient process for simultaneous extraction of Li, Co, Ni and Mn from spent Lithium-ion batteries by low-temperature NH4Cl roasting and water leaching[J]. Waste Management, 2022, 153: 61-71.
|
| 18 |
HARPER Gavin, SOMMERVILLE Roberto, KENDRICK Emma, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86
|
| 19 |
YANG Yongxia, MENG Xiangqi, CAO Hongbin, et al. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process[J]. Green Chemistry, 2018, 20(13): 3121-3133.
|
| 20 |
孟奇, 张英杰, 董鹏, 等. 废旧锂离子电池中钴、锂的回收研究进展[J]. 化工进展, 2017, 36(9): 3485-3491.
|
|
MENG Qi, ZHANG Yingjie, DONG Peng, et al. Recovery of Co and Li from spent lithium ion batteries[J]. Chemical Industry and Engineering Progress, 2017, 36(9): 3485-3491.
|
| 21 |
刘肖贝, 张西华, 熊梅, 等. 退役锂电池放电废水特征有机污染物解析[J]. 化工进展, 2022, 41(10): 5619-5629.
|
|
LIU Xiaobei, ZHANG Xihua, XIONG Mei, et al. Analysis on the characteristic organic pollutants from discharge wastewater of spent lithium batteries[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5619-5629.
|
| 22 |
于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768.
|
|
YU Jie, ZHANG Wenlong. Development status and progress of lithium ion battery separator[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1760-1768.
|
| 23 |
BIAN Doucheng, SUN Yonghui, LI Sheng, et al. A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers[J]. Electrochimica Acta, 2016, 190: 134-140.
|
| 24 |
KUMAR Jai, SHEN Xing, LI Bo, et al. Selective recovery of Li and FePO4from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4 [J]. Waste Management, 2020, 113: 32-40.
|
| 25 |
王特, 蒋立, 田晓录, 等. 锂离子电池安全材料的研究进展[J]. 化工进展, 2021, 40(6): 3132-3142.
|
|
WANG Te, JIANG Li, TIAN Xiaolu, et al. Research progress of lithium ion batteries safety materials[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3132-3142.
|
| 26 |
LI Huan, XING Shengzhou, LIU Yu, et al. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024.
|
| 27 |
WU Deyou, WANG Dongxing, LIU Zhiqiang, et al. Selective recovery of lithium from spent lithium iron phosphate batteries using oxidation pressure sulfuric acid leaching system[J]. Transactions of Nonferrous Metals Society of China, 2022, 32(6): 2071-2079.
|
| 28 |
周吉奎, 刘牡丹, 刘勇, 等. 硫酸-双氧水浸出废弃磷酸铁锂中锂的实验研究[J]. 矿冶工程, 2020, 40(6): 79-81.
|
|
ZHOU Jikui, LIU Mudan, LIU Yong, et al. Experimental study on leaching of lithium from waste lithium iron phosphate with sulfuric acid and hydrogen peroxide[J]. Mining and Metallurgical Engineering, 2020, 40(6): 79-81.
|
| 29 |
张恒, 李海浩, 王梦娇, 等. 退役动力电池材料再生利用综述[J]. 电池, 2022, 52(1): 105-109.
|
|
ZHANG Heng, LI Haihao, WANG Mengjiao, et al. Review on the regeneration and utilization of retired power battery[J]. Battery Bimonthly, 2022, 52(1): 105-109.
|
| 30 |
万青珂, 张洋, 郑诗礼, 等.废旧磷酸铁锂正极粉磷酸浸出过程的优化及宏观动力学[J]. 化工进展, 2020, 39(6): 2495-2502.
|
|
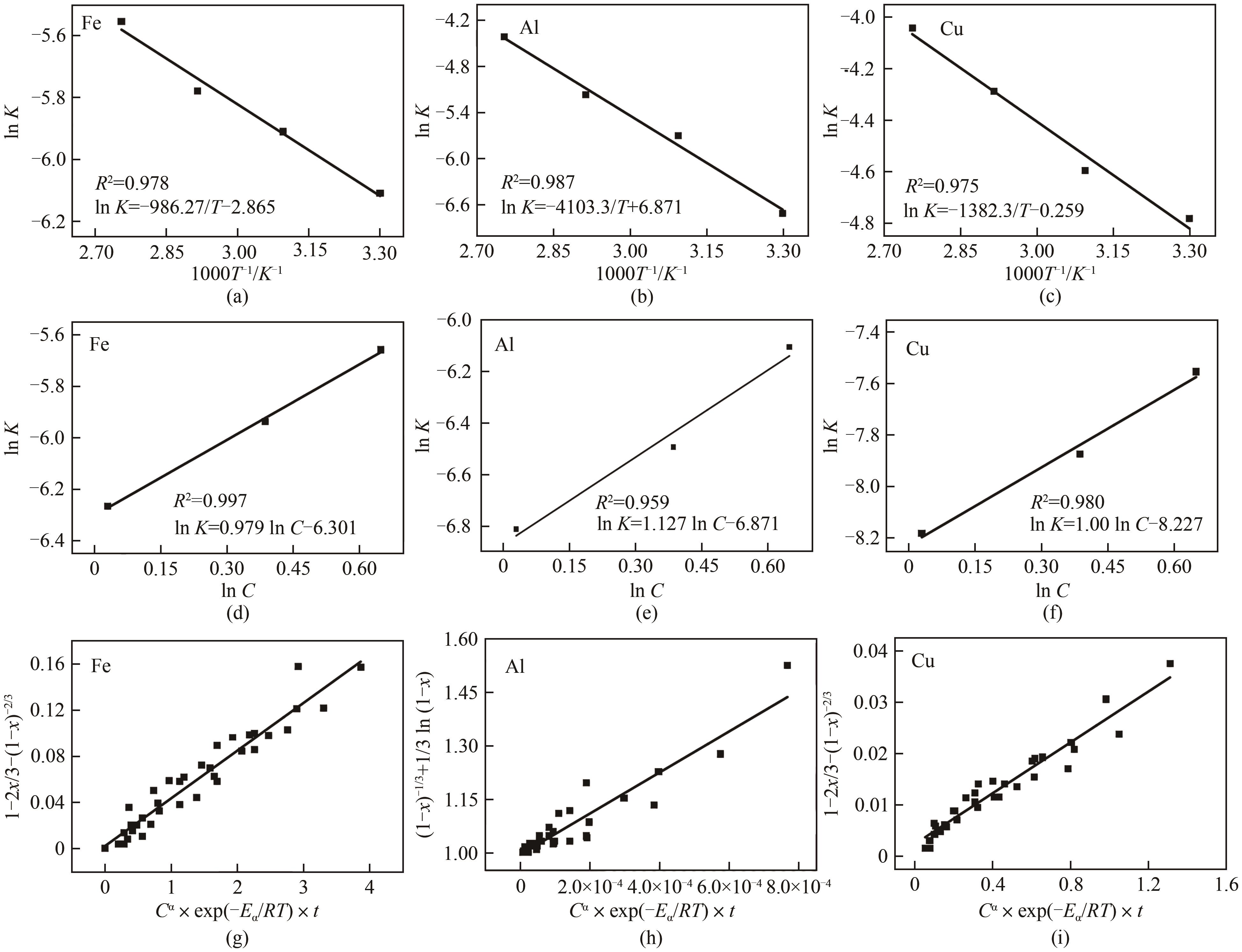
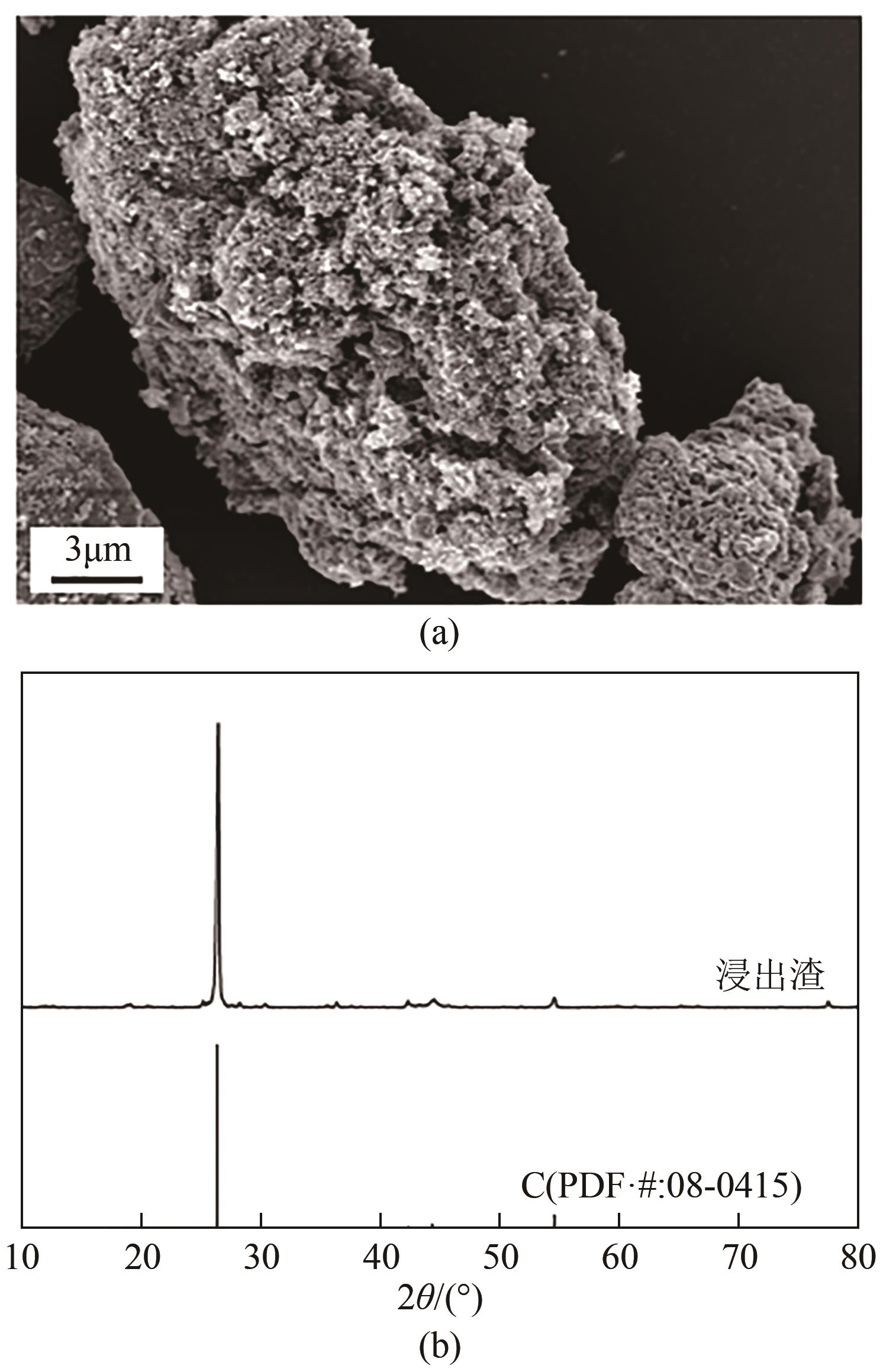
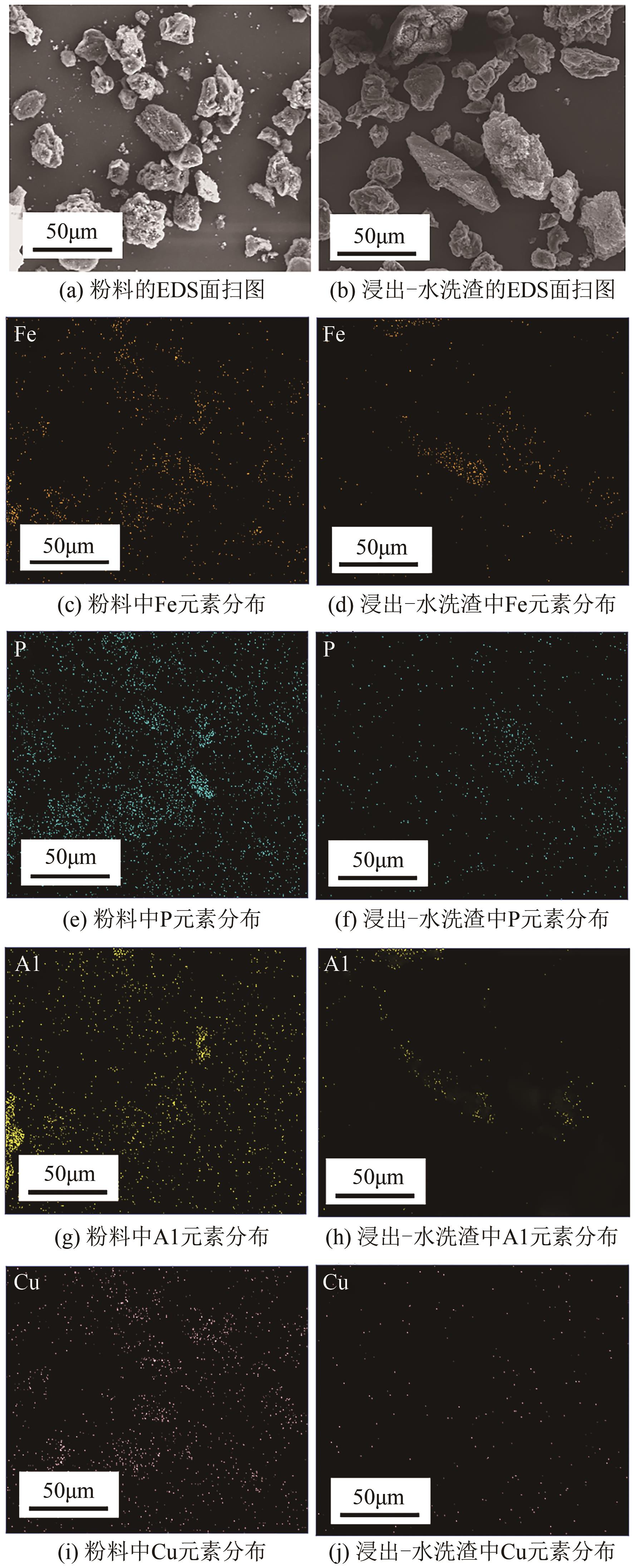
WAN Qingke, ZHANG Yang, ZHENG Shili, et al. Process optimization and kinetics for leaching spent lithium iron phosphate cathode powder by phosphate acid[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2495-2502.
|
| 31 |
李劲, 邵威, 毛洪仁. 废弃锂离子电池回收处理的污染物分析[J]. 化工进展, 2016, 35(5): 1529-1538.
|
|
LI Jin, SHAO Wei, MAO Hongren. Analysis of pollutants in the recycling of waste lithium batteries[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1529-1538.
|
 ), 彭长宏2, 林金秀1, 江洋2, 邱在容2, 周豪2, 胡振光1(
), 彭长宏2, 林金秀1, 江洋2, 邱在容2, 周豪2, 胡振光1( )
)
 ), PENG Changhong2, LIN Jinxiu1, JIANG Yang2, QIU Zairong2, ZHOU Hao2, HU Zhenguang1(
), PENG Changhong2, LIN Jinxiu1, JIANG Yang2, QIU Zairong2, ZHOU Hao2, HU Zhenguang1( )
)