化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6140-6154.DOI: 10.16085/j.issn.1000-6613.2023-1735
• 工业催化 • 上一篇
氢气辅助HC-SCR脱硝性能和作用机制的研究进展
周强1( ), 殷成阳2(
), 殷成阳2( ), 刘百军1(
), 刘百军1( ), 赵震1,2(
), 赵震1,2( )
)
- 1.中国石油大学(北京)重质油国家重点实验室,北京 102249
2.沈阳师范大学化学化工学院能源与环境催化研究所,辽宁 沈阳 110034
-
收稿日期:2023-10-07修回日期:2023-12-26出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:殷成阳,刘百军,赵震 -
作者简介:周强(1991—),男,博士研究生,研究方向为氢气选择催化还原氮氧化物。E-mail:1987107063@qq.com。 -
基金资助:国家重点研发计划(2021YFB3500603);国家自然科学基金(U1908204);沈阳市科技计划(23-407-3-14)
Research progress on the performance and mechanism of H2-assisted HC-SCR denitration
ZHOU Qiang1( ), YIN Chengyang2(
), YIN Chengyang2( ), LIU Baijun1(
), LIU Baijun1( ), ZHAO Zhen1,2(
), ZHAO Zhen1,2( )
)
- 1.State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
2.Institute of Catalysis for Energy and Environment, College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, Liaoning, China
-
Received:2023-10-07Revised:2023-12-26Online:2024-11-15Published:2024-12-07 -
Contact:YIN Chengyang, LIU Baijun, ZHAO Zhen
摘要:
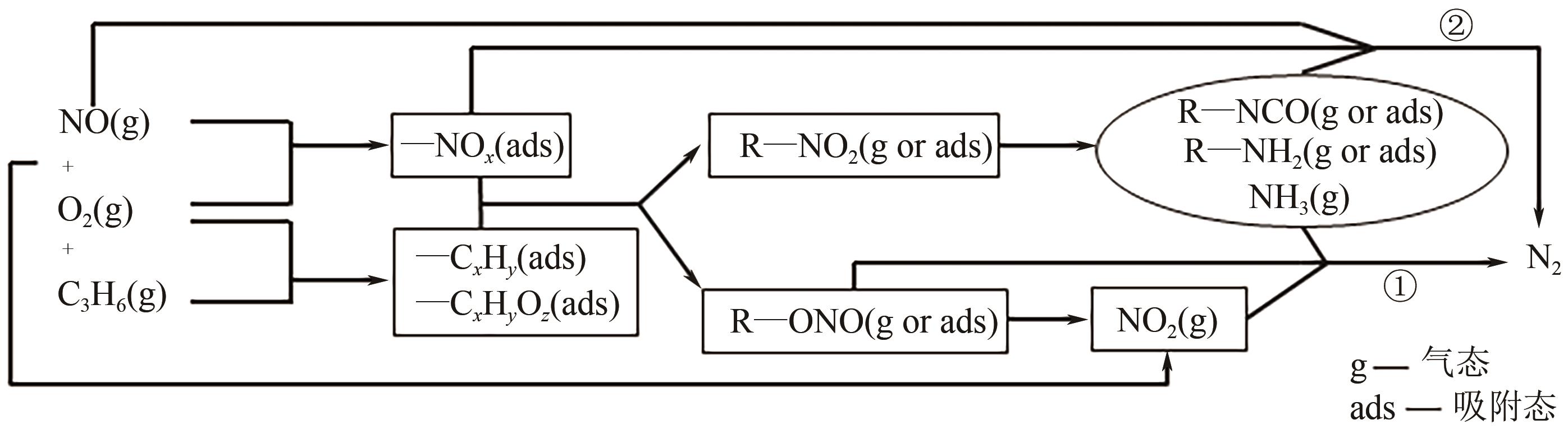
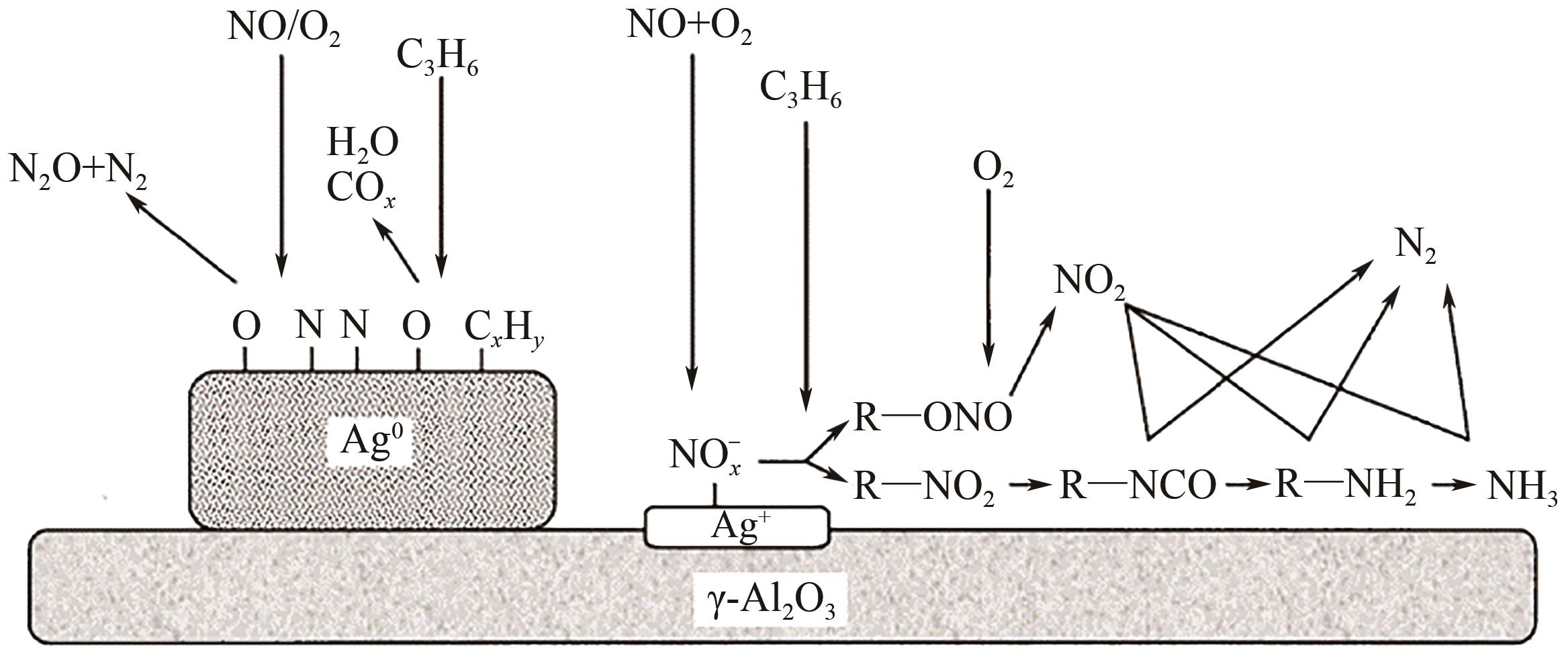
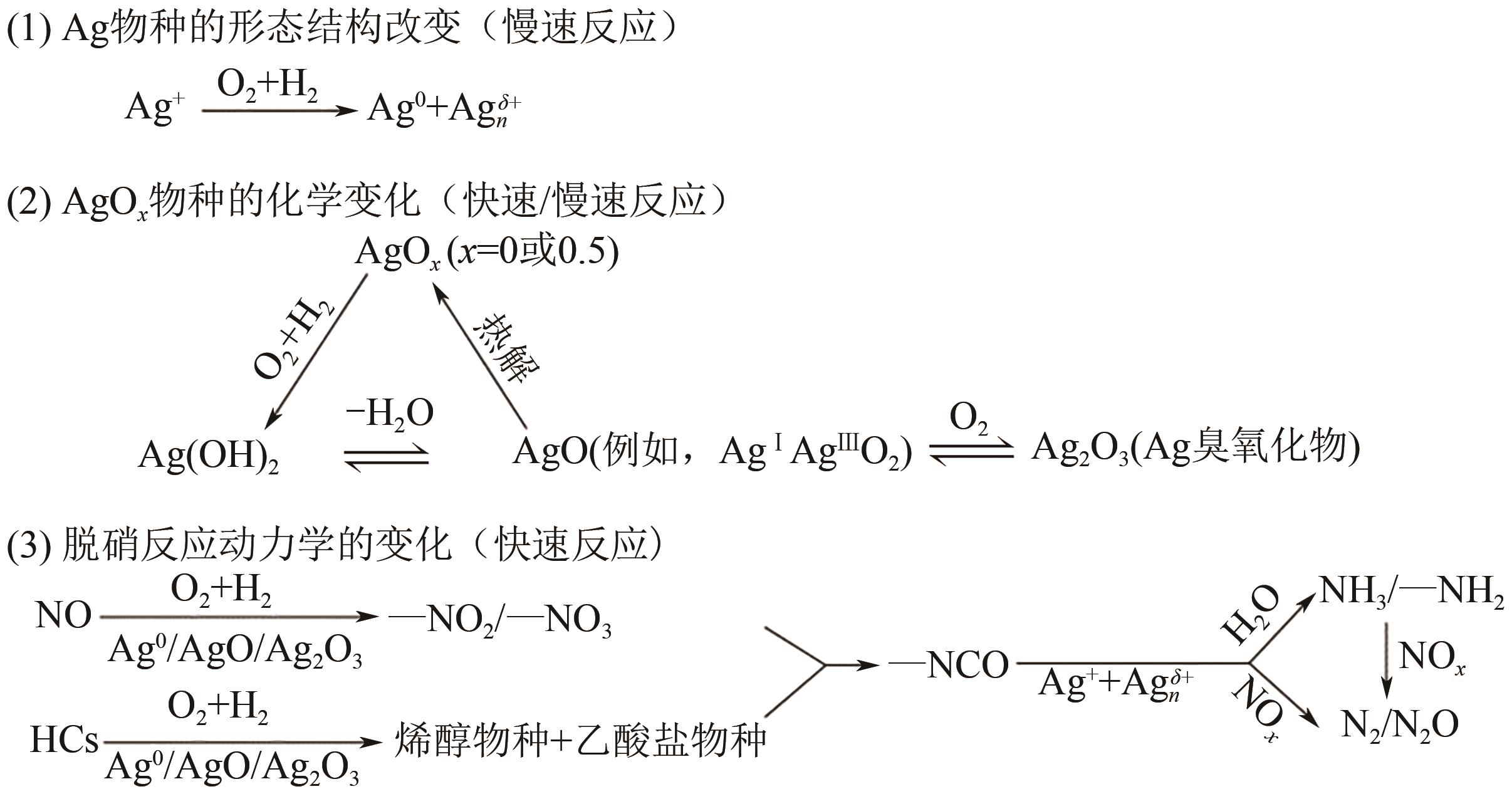
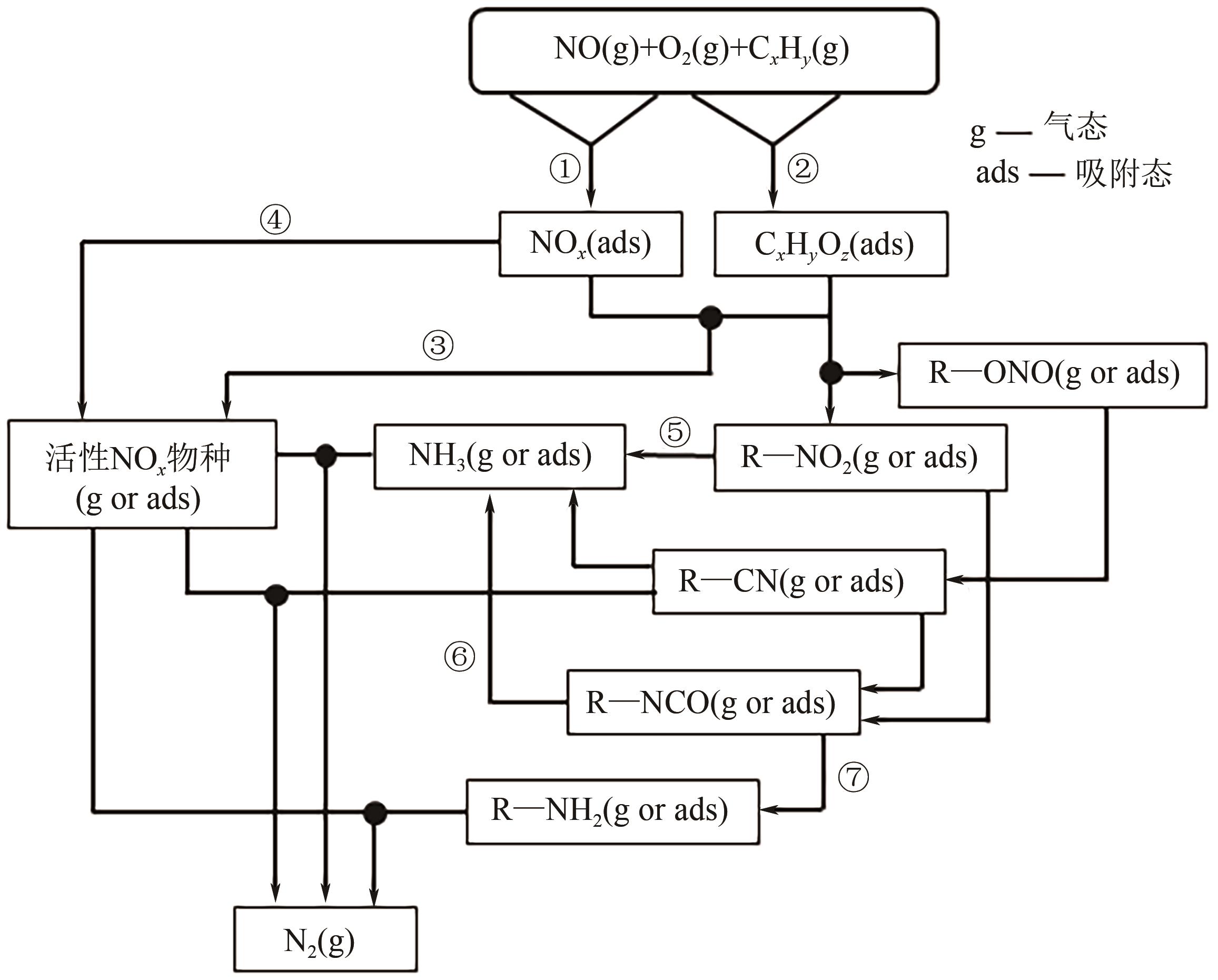
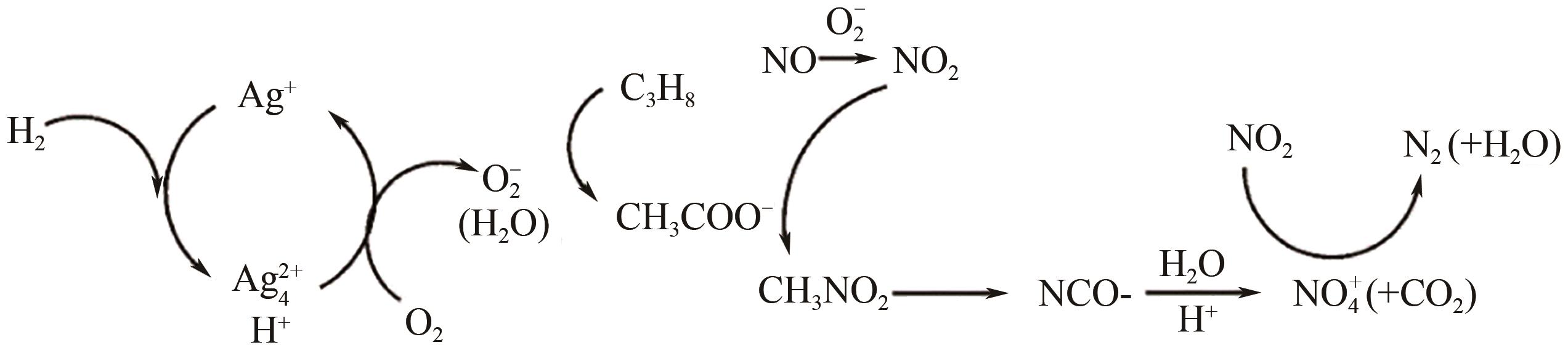
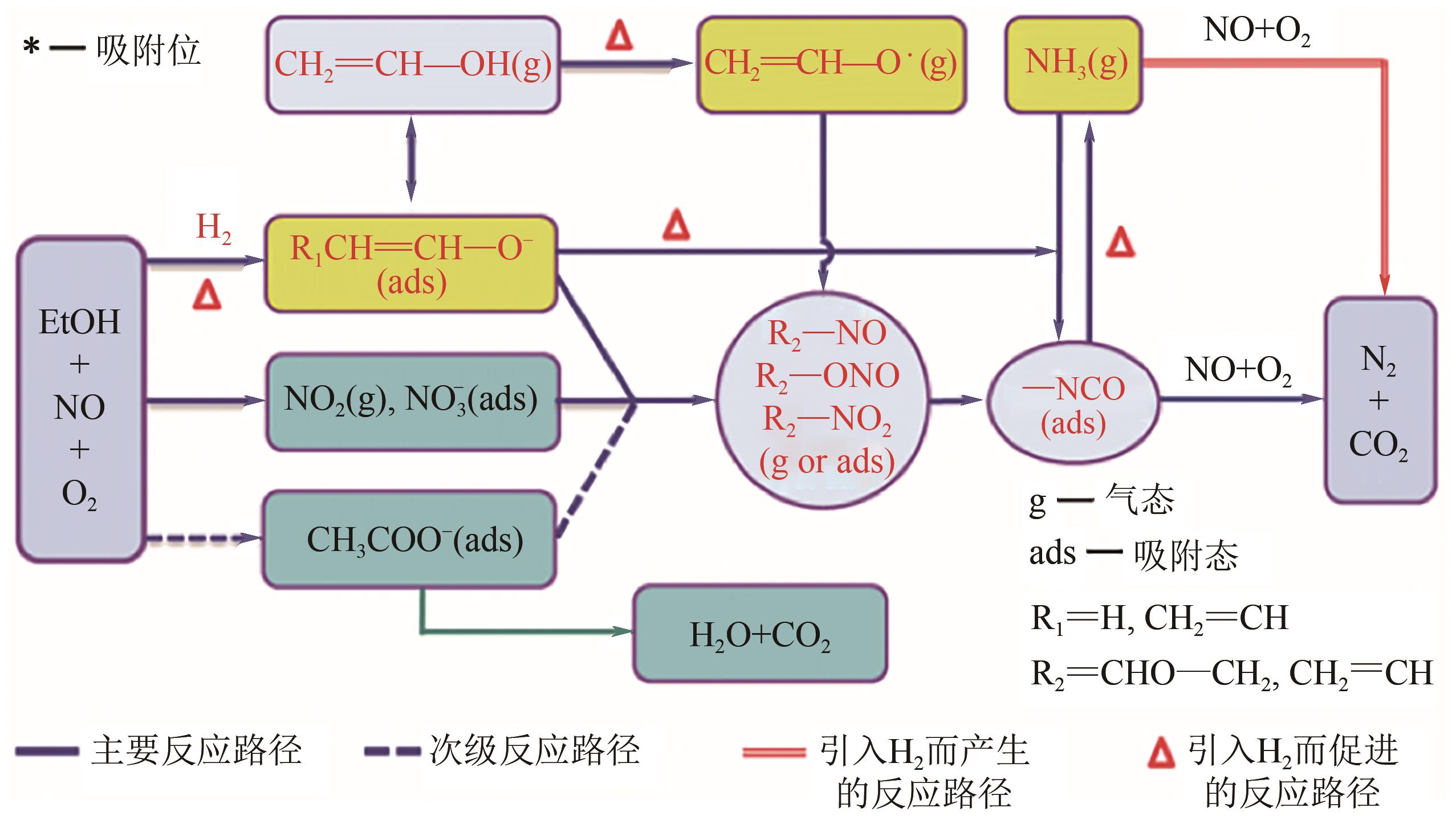
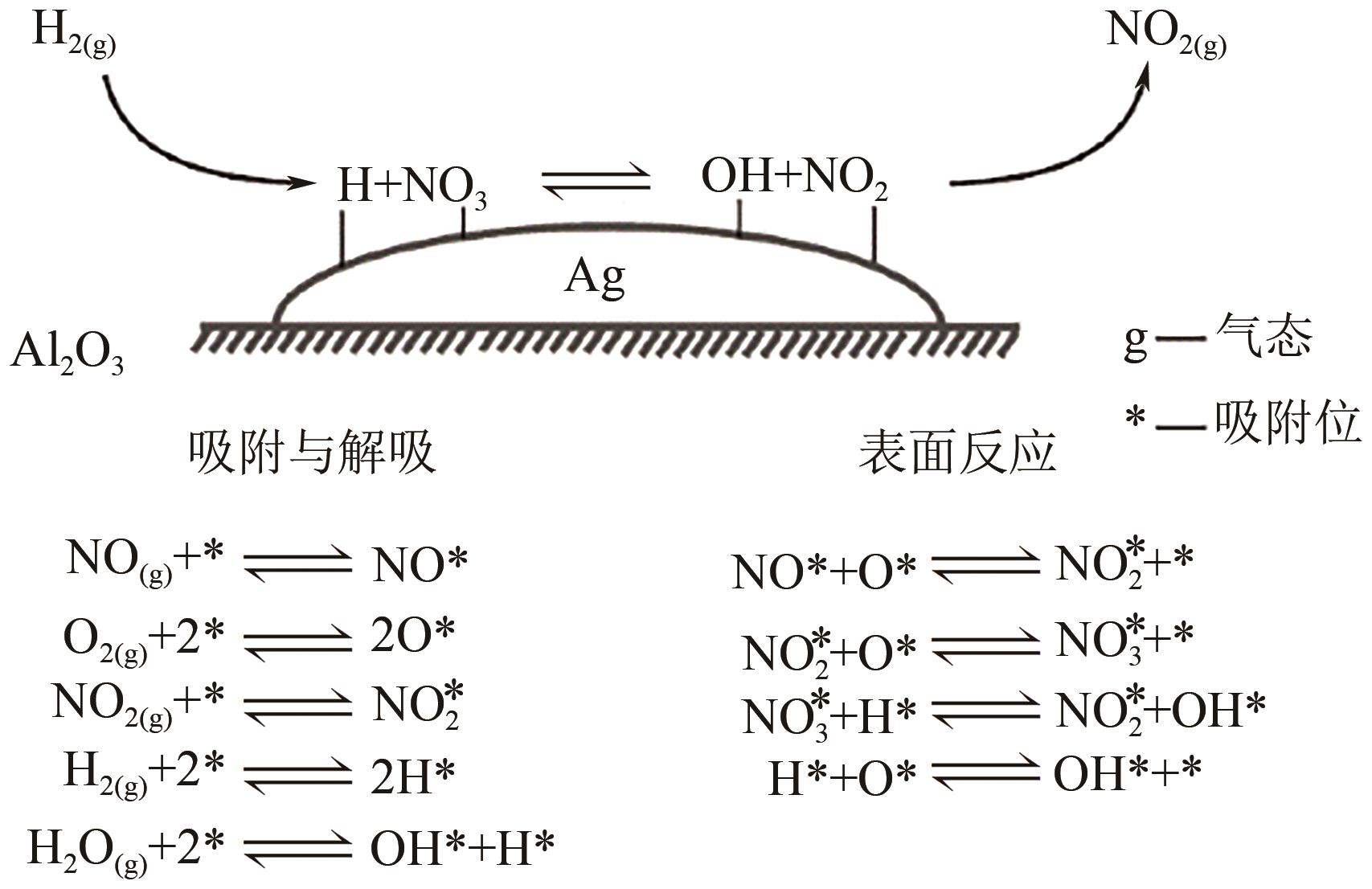
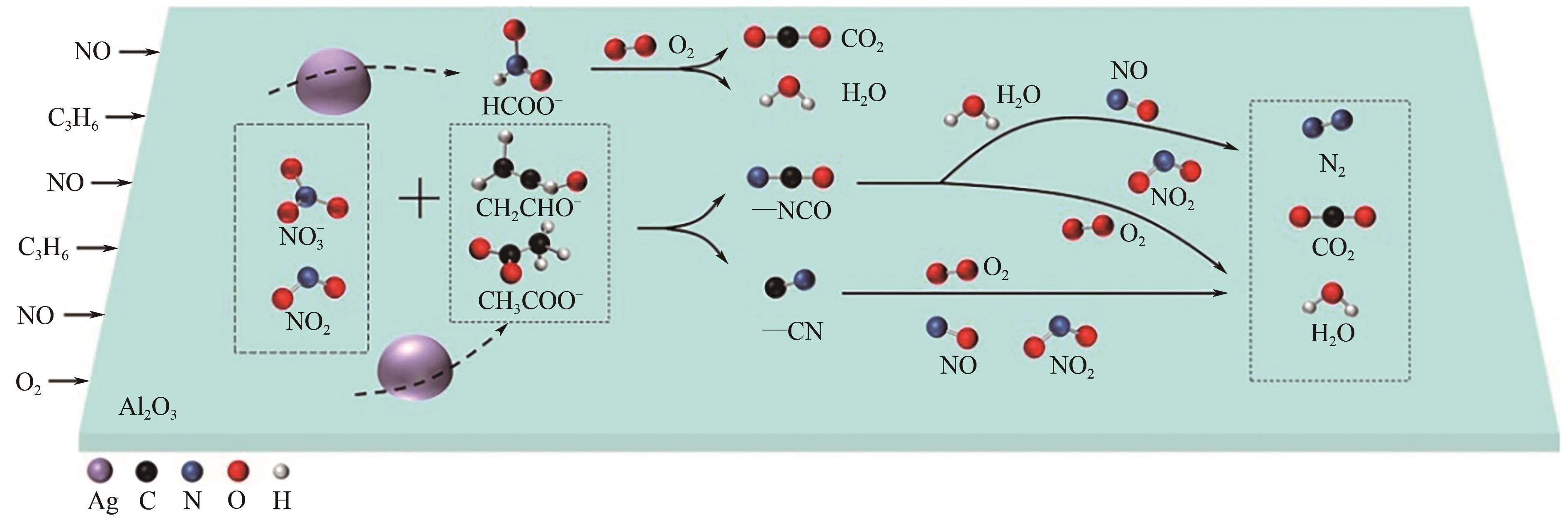
柴油发动机尾气是氮氧化物(NO x )主要来源之一,烃类化合物(HC)选择催化还原氮氧化物(HC-SCR)技术是一种常见的脱除氮氧化物方法,但是在HC-SCR催化还原NO x 中,Ag基氧化物催化剂的低温脱硝活性较差,活性温度窗口较窄。在HC-SCR反应气氛中添加少量氢气(H2)能显著提升Ag/γ-Al2O3等催化剂的低温脱硝活性,拓宽活性温度窗口。本文以贫燃柴油发动机尾气脱硝为背景,归纳了H2对活性中心Ag物种种类、烃类化合物和氧气的活化以及活性含氮中间体转化的影响,总结了H2在提升HC-SCR催化剂抗硫和抗水性能起到的作用,列举了近年来新型催化剂在H2辅助HC-SCR(H2-HC-SCR)脱硝体系中的应用。相关研究表明H2促进HC-SCR中具有催化活性的Ag物种的生成,促进O2活化为活性氧物种,加速烃类化合物转化为异氰酸盐、烯醇物种等关键中间体,并减少可毒化活性中心硝酸盐物种的含量,从而提高HC-SCR脱硝活性。H2辅助HC-SCR脱硝技术有望在机动车尾气脱硝中发挥重要作用,推动HC-SCR脱硝技术的发展。
中图分类号:
引用本文
周强, 殷成阳, 刘百军, 赵震. 氢气辅助HC-SCR脱硝性能和作用机制的研究进展[J]. 化工进展, 2024, 43(11): 6140-6154.
ZHOU Qiang, YIN Chengyang, LIU Baijun, ZHAO Zhen. Research progress on the performance and mechanism of H2-assisted HC-SCR denitration[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6140-6154.
| 发动机操作条件 | O2/% | CO/% | CO2/% | H2/% | HC/% | NO x /% | H2O①/% | 发动机尾气温度/℃ |
|---|---|---|---|---|---|---|---|---|
| 发动机负荷25% 发动机转速1500r/min 平均指示缸内压力0.3MPa | 15.8 | 0.01 | 3.3 | 0.004 | ~0.07 | 0.038 | 4.0 | 195 |
| 发动机负荷50% 发动机转速1500r/min 平均指示缸内压力0.43MPa | 13.7 | 0.01 | 5.0 | 0.008 | ~0.075 | 0.059 | 4.9 | 260 |
发动机负荷75% 发动机转速1500r/min 平均指示缸内压力0.59MPa | 11.1 | 0.01 | 6.7 | 0.007 | ~0.085 | 0.093 | 7.0 | 360 |
表1 不同柴油发动机负荷下的实际尾气物质组成(体积分数)[35]
| 发动机操作条件 | O2/% | CO/% | CO2/% | H2/% | HC/% | NO x /% | H2O①/% | 发动机尾气温度/℃ |
|---|---|---|---|---|---|---|---|---|
| 发动机负荷25% 发动机转速1500r/min 平均指示缸内压力0.3MPa | 15.8 | 0.01 | 3.3 | 0.004 | ~0.07 | 0.038 | 4.0 | 195 |
| 发动机负荷50% 发动机转速1500r/min 平均指示缸内压力0.43MPa | 13.7 | 0.01 | 5.0 | 0.008 | ~0.075 | 0.059 | 4.9 | 260 |
发动机负荷75% 发动机转速1500r/min 平均指示缸内压力0.59MPa | 11.1 | 0.01 | 6.7 | 0.007 | ~0.085 | 0.093 | 7.0 | 360 |
| 序号 | 催化剂种类 | 基本反应条件 | [H2]①/% | XNOmax②/%-TNOmax③/℃ | T50④/℃ | ∆T50⑤/℃ | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | 2%Ag/γ-Al2O3 | 0.1%NO/0.15%C3H6/10% O2/Ar | 0 | 约92-400 | 约350 | 约50 | [ |
| 0.1 | 约95-400 | 约300 | |||||
| 2 | 1%Ag/γ-Al2O3 (未水热老化) | 0.05%NO/0.1333%C3H6/8% O2/0.05%CO/5 %H2O/N2 | 0 | 约92-450 | 约410 | 约170 | [ |
| 1 | 约100-300 | 约240 | |||||
| 3 | 2%Ag/γ-Al2O3-WO x | 0.05%NO/0.0625%C3H8/0.02%CO/2% O2/N2 | 0 | 约80-450 | 约410 | 约260 | [ |
| 0.066 | 约65-170 | 约150 | |||||
| 4 | 2%Ag/CeZr0.4 | 0.07%NO/0.07%C3H6/3% O2/Ar | 0 | 约72-350 | 约225 | 约50 | [ |
| 0.5 | 约86-400 | 约175 | |||||
| 5 | 2%Ag-Ce0.4ZrO2-δ | 0.1% NO/0.1% C3H6/10% O2/He | 0 | 约70-320 | 约220 | 约40 | [ |
| 10 | 约82-500 | 约180 |
表2 H2对Ag基催化剂HC-SCR脱硝活性的影响
| 序号 | 催化剂种类 | 基本反应条件 | [H2]①/% | XNOmax②/%-TNOmax③/℃ | T50④/℃ | ∆T50⑤/℃ | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | 2%Ag/γ-Al2O3 | 0.1%NO/0.15%C3H6/10% O2/Ar | 0 | 约92-400 | 约350 | 约50 | [ |
| 0.1 | 约95-400 | 约300 | |||||
| 2 | 1%Ag/γ-Al2O3 (未水热老化) | 0.05%NO/0.1333%C3H6/8% O2/0.05%CO/5 %H2O/N2 | 0 | 约92-450 | 约410 | 约170 | [ |
| 1 | 约100-300 | 约240 | |||||
| 3 | 2%Ag/γ-Al2O3-WO x | 0.05%NO/0.0625%C3H8/0.02%CO/2% O2/N2 | 0 | 约80-450 | 约410 | 约260 | [ |
| 0.066 | 约65-170 | 约150 | |||||
| 4 | 2%Ag/CeZr0.4 | 0.07%NO/0.07%C3H6/3% O2/Ar | 0 | 约72-350 | 约225 | 约50 | [ |
| 0.5 | 约86-400 | 约175 | |||||
| 5 | 2%Ag-Ce0.4ZrO2-δ | 0.1% NO/0.1% C3H6/10% O2/He | 0 | 约70-320 | 约220 | 约40 | [ |
| 10 | 约82-500 | 约180 |
| 序号 | 催化剂种类 | 反应条件 | [H2]/% | Tmax①/℃ | (XNOxtoN2)max②/% | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 0.5%Pt/γ-Al2O3 | 0.1%NO/0.1% C3H6/2% O2/Ar | 0 | 约220 | 约25 | [ |
| 0.5 | 约110 | 约72 | ||||
| 2 | 0.5%Ir/γ-Al2O3 | 0.1%NO/0.1% C3H6/2% O2/Ar | 0 | 约325 | 约20 | [ |
| 0.5 | 约280 | 约60 | ||||
| 3 | 0.07g Au(1.6%)/γ-Al2O3+0.30g γ-Al2O3 | 0.037%NO/0.04%C3H6/8% O2/He | 0 | 400 | 约30 | [ |
| 0.21 | 400 | 约43 | ||||
| 4 | 0.5%Au/γ-Al2O3 | 0.0385%NO x (~96% NO)/0.04%C3H6/8% O2/He | 0 | 约400 | 约39 | [ |
| 0.21 | 约390 | 约57 |
表3 H2对贵金属催化剂HC-SCR脱硝活性的影响
| 序号 | 催化剂种类 | 反应条件 | [H2]/% | Tmax①/℃ | (XNOxtoN2)max②/% | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 0.5%Pt/γ-Al2O3 | 0.1%NO/0.1% C3H6/2% O2/Ar | 0 | 约220 | 约25 | [ |
| 0.5 | 约110 | 约72 | ||||
| 2 | 0.5%Ir/γ-Al2O3 | 0.1%NO/0.1% C3H6/2% O2/Ar | 0 | 约325 | 约20 | [ |
| 0.5 | 约280 | 约60 | ||||
| 3 | 0.07g Au(1.6%)/γ-Al2O3+0.30g γ-Al2O3 | 0.037%NO/0.04%C3H6/8% O2/He | 0 | 400 | 约30 | [ |
| 0.21 | 400 | 约43 | ||||
| 4 | 0.5%Au/γ-Al2O3 | 0.0385%NO x (~96% NO)/0.04%C3H6/8% O2/He | 0 | 约400 | 约39 | [ |
| 0.21 | 约390 | 约57 |
| 催化剂 | 反应条件 | 烃类化合物种类 | 温度/℃ | (XNO | 参考文献 | |
|---|---|---|---|---|---|---|
| 不添加H2 | 添加H2 | |||||
| γ-Al2O3 | R#1 | C3H8 | 500 | 15 | 35 | [ |
| 2%Ag/γ-Al2O3 | R#1 | C3H8 | 300 | 0 | 36 | [ |
| 2%Ag/TiO2 | R#1 | C3H8 | 500 | 3 | 3 | [ |
| 2%Ag/Ga2O3 | R#1 | C3H8 | 500 | 34 | 12 | [ |
| 2%Co/γ-Al2O3 | R#1 | C3H8 | 350 | 12 | 10 | [ |
| 0.5%Pt/γ-Al2O3(Engelhard公司生产) | R#1 | C3H8 | 325 | 8 | 6 | [ |
| 0.5%Pt/γ-Al2O3(初湿浸渍法) | R#2 | C3H6 | 约110 | 约0 | 约72 | [ |
| 0.5%Pd/γ-Al2O3 | R#2 | C3H6 | 200 | 约12 | 约16 | [ |
| 0.5%Ir/γ-Al2O3 | R#2 | C3H6 | 约280 | 约0 | 约61 | [ |
表4 H2-HC-SCR催化剂的活性对比
| 催化剂 | 反应条件 | 烃类化合物种类 | 温度/℃ | (XNO | 参考文献 | |
|---|---|---|---|---|---|---|
| 不添加H2 | 添加H2 | |||||
| γ-Al2O3 | R#1 | C3H8 | 500 | 15 | 35 | [ |
| 2%Ag/γ-Al2O3 | R#1 | C3H8 | 300 | 0 | 36 | [ |
| 2%Ag/TiO2 | R#1 | C3H8 | 500 | 3 | 3 | [ |
| 2%Ag/Ga2O3 | R#1 | C3H8 | 500 | 34 | 12 | [ |
| 2%Co/γ-Al2O3 | R#1 | C3H8 | 350 | 12 | 10 | [ |
| 0.5%Pt/γ-Al2O3(Engelhard公司生产) | R#1 | C3H8 | 325 | 8 | 6 | [ |
| 0.5%Pt/γ-Al2O3(初湿浸渍法) | R#2 | C3H6 | 约110 | 约0 | 约72 | [ |
| 0.5%Pd/γ-Al2O3 | R#2 | C3H6 | 200 | 约12 | 约16 | [ |
| 0.5%Ir/γ-Al2O3 | R#2 | C3H6 | 约280 | 约0 | 约61 | [ |
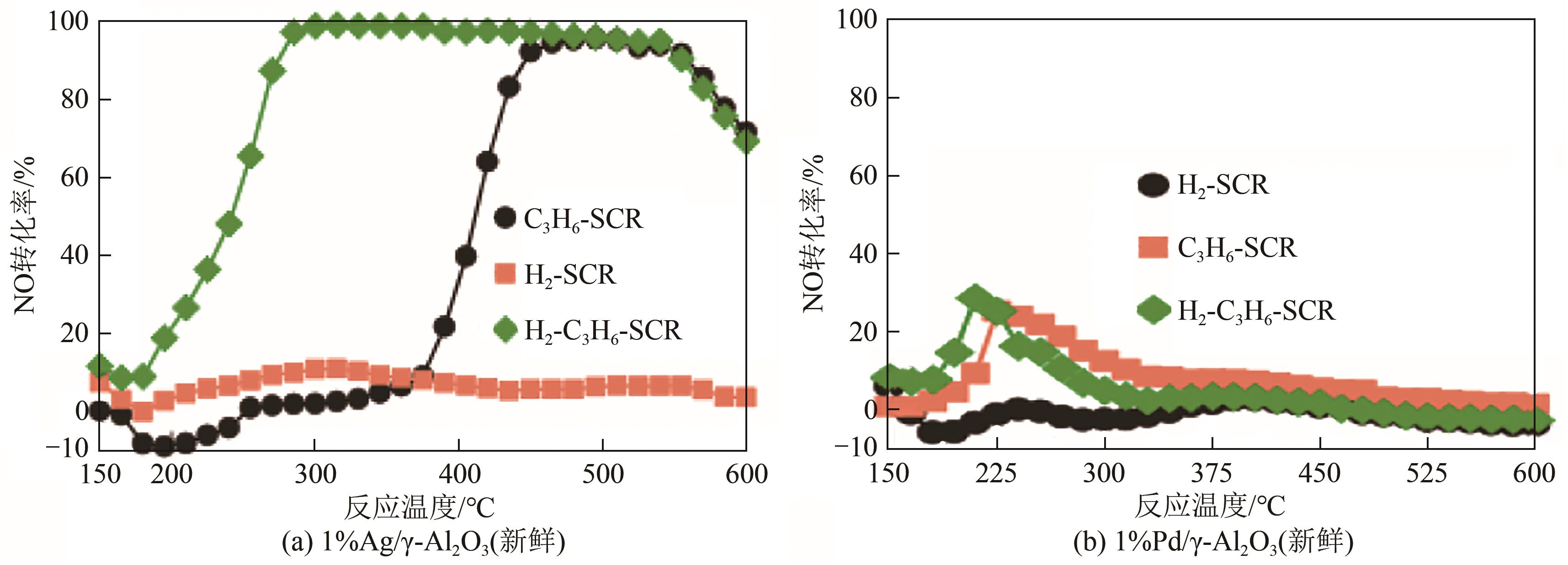
图10 Ag/γ-Al2O3和Pd/γ-Al2O3催化剂在不同还原气氛下的脱硝活性[16](基本反应条件:[NO]=0.05%,[O2]=8%,[CO]=0.05%,[H2O]=5%;H2–SCR:[H2]=1%;C3H6–SCR:[C3H6]=0.1333%;H2-C3H6–SCR:[H2]=1%,[C3H6]=0.1333%)
| 26 | REITZ R D, RUTLAND C J. Development and testing of diesel engine CFD models[J]. Progress in Energy and Combustion Science, 1995, 21(2): 173-196. |
| 27 | ROY Sounak, HEGDE M S, MADRAS Giridhar. Catalysis for NO x abatement[J]. Applied Energy, 2009, 86(11): 2283-2297. |
| 28 | PRASAD Ram, SINGH Pratichi. Applications and preparation methods of copper chromite catalysts: a review[J]. Bulletin of Chemical Reaction Engineering & Catalysis, 2011, 6(2): 63-113. |
| 29 | REŞITOĞLU İbrahim Aslan, Kemal ALTINIŞIK, KESKIN Ali. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems[J]. Clean Technologies and Environmental Policy, 2015, 17(1): 15-27. |
| 30 | QI Gongshin, YANG Ralph T, RINALDI Fabrizio C. Selective catalytic reduction of nitric oxide with hydrogen over Pd-based catalysts[J]. Journal of Catalysis, 2006, 237(2): 381-392. |
| 31 | GARIN François. Mechanism of NO x decomposition[J]. Applied Catalysis A: General, 2001, 222(1/2): 183-219. |
| 32 | MACLEOD Norman, CROPLEY Rachael, KEEL James M, et al. Exploiting the synergy of titania and alumina in lean NO x reduction: In situ ammonia generation during the Pd/TiO2/Al2O3-catalysed H2/CO/NO/O2 reaction[J]. Journal of Catalysis, 2004, 221(1): 20-31. |
| 33 | QI Gongshin, YANG Ralph T, THOMPSON Levi T. Catalytic reduction of nitric oxide with hydrogen and carbon monoxide in the presence of excess oxygen by Pd supported on pillared clays[J]. Applied Catalysis A: General, 2004, 259(2): 261-267. |
| 34 | MACLEOD Norman, LAMBERT Richard M. Lean NO x reduction with CO+H2 mixtures over Pt/Al2O3 and Pd/Al2O3 catalysts[J]. Applied Catalysis B: Environmental, 2002, 35(4): 269-279. |
| 35 | ABU-JRAI A, TSOLAKIS A. The effect of H2 and CO on the selective catalytic reduction of NO x under real diesel engine exhaust conditions over Pt/Al2O3 [J]. International Journal of Hydrogen Energy, 2007, 32(12): 2073-2080. |
| 36 | 苏庆运, 解亮, 冯廷智, 等. 柴油机LNT再生过程铂催化CO还原NO x 反应机理[J]. 内燃机学报, 2016, 34(4): 339-345. |
| SU Qingyun, XIE Liang, FENG Tingzhi, et al. Detailed mechanism of NO x reduction by CO over Pt catalyst during regeneration period of diesel LNT[J]. Transactions of CSICE, 2016, 34(4): 339-345. | |
| 1 | GHOLAMI Fatemeh, TOMAS Martin, GHOLAMI Zahra, et al. Technologies for the nitrogen oxides reduction from flue gas: A review[J]. Science of the Total Environment, 2020, 714: 136712. |
| 2 | WU Peng, YU Qing, YAN Jingjing, et al. Progress in selective catalytic reduction of NO x by hydrogen in excess oxygen[J]. Chinese Journal of Catalysis, 2010, 31(8): 912-918. |
| 3 | 汤常金, 孙敬方, 董林. 超低温(<150℃)SCR脱硝技术研究进展[J]. 化工学报, 2020, 71(11): 4873-4884. |
| TANG Changjin, SUN Jingfang, DONG Lin. Recent progress on elimination of NO x from flue gas via SCR technology under ultra-low temperatures (<150℃)[J]. CIESC Journal, 2020, 71(11): 4873-4884. | |
| 4 | 单玉龙, 彭悦, 楚碧武, 等. 我国重点行业氮氧化物管控现状及减排策略[J]. 环境科学研究, 2023, 36(3): 431-438. |
| SHAN Yulong, PENG Yue, CHU Biwu, et al. Control status and emission reduction strategies of nitrogen oxides in key industries in China[J]. Research of Environmental Sciences, 2023, 36(3): 431-438. | |
| 5 | 中华人民共和国生态环境部. 重型柴油车污染物排放限值及测量方法(中国第六阶段): [S]. 北京: 中国环境科学出版社, 2018. |
| Ministry of Ecology and Environment of the People’s Republic of China. Limits and measurement methods for emissions from diesel fuelled heavy-duty vehicles (): GB 17691—2018[S]. Beijing: China Environment Science Press, 2018. | |
| 6 | TOFAN C, KLVANA D, KIRCHNEROVA J. Decomposition of nitric oxide over perovskite oxide catalysts: Effect of CO2, H2O and CH4 [J]. Applied Catalysis B: Environmental, 2002, 36(4): 311-323. |
| 7 | GOULA M A, CHARISIOU N D, PAPAGERIDIS K N, et al. A comparative study of the H2-assisted selective catalytic reduction of nitric oxide by propene over noble metal (Pt, Pd, Ir)/γ-Al2O3 catalysts[J]. Journal of Environmental Chemical Engineering, 2016, 4(2): 1629-1641. |
| 8 | DUAN Jun, ZHAO Ling, GAO Shengjun, et al. New aspects on a low-medium temperature mechanism of H2-assisted C3H6-SCR over xAg-CeZr catalyst[J]. Fuel, 2021, 305: 121574. |
| 9 | 宁淑英, 苏亚欣, 杨洪海, 等. 用于HC-SCR还原NO x 的Cu基分子筛催化剂研究进展[J]. 化工进展, 2023, 42(3): 1308-1320. |
| NING Shuying, SU Yaxin, YANG Honghai, et al. Research progress on supported Cu-based zeolite catalysts for the selective catalytic reduction of NO x with hydrocarbons[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1308-1320. | |
| 10 | LEE Kyungseok, CHOI Byungchul, LEE Chunbeom, et al. Effects of SiO2/Al2O3 ratio, reaction atmosphere and metal additive on de-NO x performance of HC-SCR over Cu-based ZSM-5[J]. Journal of Industrial and Engineering Chemistry, 2020, 90: 132-144. |
| 11 | BREEN J P, BURCH R. A review of the effect of the addition of hydrogen in the selective catalytic reduction of NO x with hydrocarbons on silver catalysts[J]. Topics in Catalysis, 2006, 39(1/2): 53-58. |
| 12 | SATOKAWA Shigeo. Enhancing the NO/C3H8/O2 reaction by using H2 over Ag/Al2O3 catalysts under lean-exhaust conditions[J]. Chemistry Letters, 2000, 29(3): 294-295. |
| 13 | SITTICHOMPOO Sak, THEINNOI Kampanart, SAWATMONGKHON Boonlue, et al. Promotion effect of hydrogen addition in selective catalytic reduction of nitrogen oxide emissions from diesel engines fuelled with diesel-biodiesel-ethanol blends[J]. Alexandria Engineering Journal, 2022, 61(7): 5383-5395. |
| 14 | HERNÁNDEZ-TERÁN María E, LÓPEZ CURIEL Julio C, FUENTES Gustavo A. Study of the reversibility of the H2 effect over Ag/γ-Al2O3 catalyst during selective catalytic reduction (SCR) of NO x by propane[J]. Topics in Catalysis, 2022, 65(13/14/15/16): 1505-1515. |
| 15 | WANG Jia, YOU Rui, QIAN Kun, et al. Effect of the modification of alumina supports with chloride on the structure and catalytic performance of Ag/Al2O3 catalysts for the selective catalytic reduction of NO x with propene and H2/propene[J]. Chinese Journal of Catalysis, 2021, 42(12): 2242-2253. |
| 16 | LEE Kyungseok, CHOI Byungchul. HC-SCR system combining Ag/Al2O3 and Pd/Al2O3 catalysts with resistance to hydrothermal aging for simultaneous removal of NO, HC, and CO[J]. Journal of Industrial and Engineering Chemistry, 2021, 102: 51-68. |
| 17 | KHATRI Prateek, BHATIA Divesh. Effect of gas composition on the NO x adsorption and reduction activity of a dual-function Ag/MgO/γ- Al2O3 catalyst[J]. Applied Catalysis A: General, 2021, 618: 118114. |
| 18 | YAN Tao, ZHAO Kaiwen, GAO Zhaojun, et al. Investigation of the effect of bimetallic Zn-Mg modified Al2O3 carriers modulating Ag species on the performance of H2-assisted C3H6-SCR and the mechanism[J]. Applied Surface Science, 2023, 615: 156323. |
| 19 | AZIS Muhammad Mufti. Experimental and kinetic studies of H2 effect on lean exhaust aftertreatment processes: HC-SCR and DOC[D]. Sweden: Chalmers Tekniska Hogskola, 2015. |
| 20 | XU Guangyan, WANG Honghong, YU Yunbo, et al. Role of silver species in H2-NH3-SCR of NO x over Ag/Al2O3 catalysts: Operando spectroscopy and DFT calculations[J]. Journal of Catalysis, 2021, 395: 1-9. |
| 37 | GUAN Yu, LIU Yinhe, LV Qiang, et al. Review on the selective catalytic reduction of NO x with H2 by using novel catalysts[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106770. |
| 38 | CHOI Byungchul, LEE Kyungseok, Geonseog SON. Review of recent after-treatment technologies for de-NO x process in diesel engines[J]. International Journal of Automotive Technology, 2020, 21(6): 1597-1618. |
| 39 | HELD Wolfgang, Axel KÖNIG, RICHTER Thomas, et al. Catalytic NO x reduction in net oxidizing exhaust gas[C]//SAE Technical Paper Series, 400 Commonwealth Drive. United States: SAE International, 1990: 209-216. |
| 40 | CHENG Xingxing, BI Xiaotao T. A review of recent advances in selective catalytic NO x reduction reactor technologies[J]. Particuology, 2014, 16: 1-18. |
| 41 | LIU Zhiming, Seong IHL WOO. Recent advances in catalytic DeNO x science and technology[J]. Catalysis Reviews, 2006, 48(1): 43-89. |
| 42 | MEUNIER F C, ZUZANIUK V, BREEN J P, et al. Mechanistic differences in the selective reduction of NO by propene over cobalt- and silver-promoted alumina catalysts: Kinetic and in situ DRIFTS study[J]. Catalysis Today, 2000, 59(3): 287-304. |
| 43 | CHENG Xingxing, BI Xiaotao T. Reaction kinetics of selective catalytic reduction of NO x by propylene over Fe/ZSM-5[J]. Chemical Engineering Journal, 2012, 211/212: 453-462. |
| 44 | MEUNIER F C, BREEN J P, ZUZANIUK V, et al. Mechanistic aspects of the selective reduction of NO by propene over alumina and silver-alumina catalysts[J]. Journal of Catalysis, 1999, 187(2): 493-505. |
| 45 | CHANSAI Sarayute, BURCH Robbie, HARDACRE Christopher, et al. Investigating the promotional effect of methanol on the low temperature SCR reaction on Ag/Al2O3 [J]. Applied Catalysis B: Environmental, 2014, 160/161: 356-364. |
| 46 | DENG Hua, YU Yunbo, HE Hong. Water effect on preparation of Ag/Al2O3 catalyst for reduction of NO x by ethanol[J]. The Journal of Physical Chemistry C, 2016, 120(42): 24294-24301. |
| 47 | XU Guangyan, MA Jinzhu, WANG Lian, et al. Insight into the origin of sulfur tolerance of Ag/Al2O3 in the H2-C3H6-SCR of NO x [J]. Applied Catalysis B: Environmental, 2019, 244: 909-918. |
| 48 | KRUTZSCH Bernd, GOERIGK Christian, KURZE Stefan, et al. Process and apparatus for reducing nitrogen oxides in engine emissions: US5921076[P]. 1999-07-13. |
| 21 | XU Guangyan, MA Jinzhu, WANG Lian, et al. Mechanism of the H2 effect on NH3-selective catalytic reduction over Ag/Al2O3: Kinetic and diffuse reflectance infrared Fourier transform spectroscopy studies[J]. ACS Catalysis, 2019, 9(11): 10489-10498. |
| 22 | Linda STRÖM, CARLSSON Per-Anders, SKOGLUNDH Magnus, et al. Surface species and metal oxidation state during H2-assisted NH3-SCR of NO x over alumina-supported silver and indium[J]. Catalysts, 2018, 8(1): 38. |
| 23 | WANG Xiuyun, JIANG Lilong, WANG Jinyun, et al. Ag/bauxite catalysts: improved low-temperature activity and SO2 tolerance for H2-promoted NH3-SCR of NO x [J]. Applied Catalysis B: Environmental, 2015, 165: 700-705. |
| 24 | DORONKIN Dmitry E, FOGEL Sebastian, Pär GABRIELSSON, et al. Ti and Si doping as a way to increase low temperature activity of sulfated Ag/Al2O3 in H2-assisted NH3-SCR of NO x [J]. Applied Catalysis B: Environmental, 2014, 148/149: 62-69. |
| 25 | YASUMURA Shunsaku, KATO Taisetsu, TOYAO Takashi, et al. An automated reaction route mapping for the reaction of NO and active species on Ag4 clusters in zeolites[J]. Physical Chemistry Chemical Physics, 2023, 25(12): 8524-8531. |
| 49 | THOMAS Cyril. On an additional promoting role of hydrogen in the H2-assisted C3H6-SCR of NO x on Ag/Al2O3: A lowering of the temperature of formation-decomposition of the organo-NO x intermediates?[J]. Applied Catalysis B: Environmental, 2015, 162: 454-462. |
| 50 | SADOKHINA N A, PROKHOROVA A F, KVON R I, et al. Dependence of the catalytic activity of Ag/Al2O3 on the silver concentration in the selective reduction of NO x with n-hexane in the presence of H2 [J]. Kinetics and Catalysis, 2012, 53(1): 107-116. |
| 51 | BABA Toshihide, AKINAKA Noboru, NOMURA Mamoru, et al. Reversible redox behaviour between silver cations and silver metal particles in hydrated Ag-Y zeolites[J]. Journal of the Chemical Society, Faraday Transactions, 1993, 89(3): 595-599. |
| 52 | SATSUMA Atsushi, SHIBATA Junji, SHIMIZU Ken-ichi, et al. Ag clusters as active species for HC-SCR over Ag-zeolites[J]. Catalysis Surveys from Asia, 2005, 9(2): 75-85. |
| 53 | SHIBATA Junji, TAKADA Yuu, SHICHI Akira, et al. Ag cluster as active species for SCR of NO by propane in the presence of hydrogen over Ag-MFI[J]. Journal of Catalysis, 2004, 222(2): 368-376. |
| 54 | KIM Pyung Soon, KIM Mun Kyu, CHO Byong K, et al. Effect of H2 on deNO x performance of HC-SCR over Ag/Al2O3: Morphological, chemical, and kinetic changes[J]. Journal of Catalysis, 2013, 301: 65-76. |
| 55 | Kari ERÄNEN, KLINGSTEDT Fredrik, ARVE Kalle, et al. On the mechanism of the selective catalytic reduction of NO with higher hydrocarbons over a silver/alumina catalyst[J]. Journal of Catalysis, 2004, 227(2): 328-343. |
| 56 | BREEN J P, BURCH R, HARDACRE C, et al. A fast transient kinetic study of the effect of H2 on the selective catalytic reduction of NO x with octane using isotopically labelled 15NO[J]. Journal of Catalysis, 2007, 246(1): 1-9. |
| 57 | SHIMIZU Kenichi, SAWABE Kyoichi, SATSUMA Atsushi. Unique catalytic features of Ag nanoclusters for selective NO x reduction and green chemical reactions[J]. Catalysis Science & Technology, 2011, 1(3): 331-341. |
| 58 | SHIMIZU Ken-ichi, SHIBATA Junji, SATSUMA Atsushi. Kinetic and in situ infrared studies on SCR of NO with propane by silver-alumina catalyst: Role of H2 on O2 activation and retardation of nitrate poisoning[J]. Journal of Catalysis, 2006, 239(2): 402-409. |
| 59 | RICHTER M. The effect of hydrogen on the selective catalytic reduction of NO in excess oxygen over Ag/Al2O3 [J]. Applied Catalysis B: Environmental, 2004, 51(4): 261-274. |
| 60 | SHIMIZU Ken-ichi, SUGINO Kenji, KATO Kazuo, et al. Reaction mechanism of H2-promoted selective catalytic reduction of NO with C3H8 over Ag-MFI zeolite[J]. The Journal of Physical Chemistry C, 2007, 111(17): 6481-6487. |
| 61 | ZHANG Xiuli, HE Hong, MA Zichuan. Hydrogen promotes the selective catalytic reduction of NO by ethanol over Ag/Al2O3 [J]. Catalysis Communications, 2007, 8(2): 187-192. |
| 62 | SATOKAWA Shigeo, SHIBATA Junji, SHIMIZU Ken-ichi, et al. Promotion effect of H2 on the low temperature activity of the selective reduction of NO by light hydrocarbons over Ag/Al2O3 [J]. Applied Catalysis B: Environmental, 2003, 42(2): 179-186. |
| 63 | ARVE Kalle, BACKMAN Henrik, KLINGSTEDT Fredrik, et al. Hydrogen as a remedy for the detrimental effect of aromatic and cyclic compounds on the HC-SCR over Ag/alumina[J]. Applied Catalysis B: Environmental, 2007, 70(1/2/3/4): 65-72. |
| 64 | YU Yunbo, LI Yi, ZHANG Xiuli, et al. Promotion effect of H2 on ethanol oxidation and NO x reduction with ethanol over Ag/Al2O3 catalyst[J]. Environmental Science & Technology, 2015, 49(1): 481-488. |
| 65 | YU Yunbo, HE Hong, ZHANG Xiuli, et al. A common feature of H2-assisted HC-SCR over Ag/Al2O3 [J]. Catalysis Science & Technology, 2014, 4(5): 1239-1245. |
| 66 | KANNISTO Hannes, INGELSTEN Hanna Härelind, SKOGLUNDH Magnus. Aspects of the role of hydrogen in H2-assisted HC-SCR over Ag-Al2O3 [J]. Topics in Catalysis, 2009, 52(13/14/15/16/17/18/19/20): 1817-1820. |
| 67 | BURCH R. Knowledge and know-how in emission control for mobile applications[J]. Catalysis Reviews, 2004, 46(3/4): 271-334. |
| 68 | JOHNSON William L, FISHER Galen B, TOOPS Todd J. Mechanistic investigation of ethanol SCR of NO x over Ag/Al2O3 [J]. Catalysis Today, 2012, 184(1): 166-177. |
| 69 | BURCH R, BREEN J P, HILL C J, et al. Exceptional activity for NO x reduction at low temperatures using combinations of hydrogen and higher hydrocarbons on Ag/Al2O3 catalysts[J]. Topics in Catalysis, 2004, 30/31(1/2/3/4): 19-25. |
| 70 | BENTRUP Ursula, RICHTER Manfred, FRICKE Rolf. Effect of H2 admixture on the adsorption of NO, NO2 and propane at Ag/Al2O3 catalyst as examined by in situ FTIR[J]. Applied Catalysis B: Environmental, 2005, 55(3): 213-220. |
| 71 | AZIS Muhammad Mufti, Hanna HÄRELIND, CREASER Derek. On the role of H2 to modify surface NO x species over Ag-Al2O3 as lean NO x reduction catalyst: TPD and DRIFTS studies[J]. Catalysis Science & Technology, 2015, 5(1): 296-309. |
| 72 | SHIMIZU Ken-ichi, TSUZUKI Masao, SATSUMA Atsushi. Effects of hydrogen and oxygenated hydrocarbons on the activity and SO2-tolerance of Ag/Al2O3 for selective reduction of NO[J]. Applied Catalysis B: Environmental, 2007, 71(1/2): 80-84. |
| 73 | SHIMIZU Ken-ichi, HIGASHIMATA Takaaki, TSUZUKI Masao, et al. Effect of hydrogen addition on SO2 tolerance of silver-alumina for SCR of NO with propane[J]. Journal of Catalysis, 2006, 239(1): 117-124. |
| 74 | WANG Zhijian, XU Guangyan, LIU Xin, et al. Investigation of water and sulfur tolerance of precipitable silver compound Ag/Al2O3 catalysts in H2-assisted C3H6-SCR of NO x [J]. ACS Omega, 2020, 5(45): 29593-29600. |
| 75 | XU Guangyan, YU Yunbo, HE Hong. Silver valence state determines the water tolerance of Ag/Al2O3 for the H2-C3H6-SCR of NO x [J]. The Journal of Physical Chemistry C, 2018, 122(1): 670-680. |
| 76 | XU Guangyan, MA Jinzhu, HE Guangzhi, et al. An alumina-supported silver catalyst with high water tolerance for H2 assisted C3H6-SCR of NO x [J]. Applied Catalysis B: Environmental, 2017, 207: 60-71. |
| 77 | SAZAMA P, ČAPEK L, DROBNÁ H, et al. Enhancement of decane-SCR-NO over Ag/alumina by hydrogen. Reaction kinetics and in situ FTIR and UV-vis study[J]. Journal of Catalysis, 2005, 232(2): 302-317. |
| 78 | BACKMAN Henrik, ARVE Kalle, KLINGSTEDT Fredrik, et al. Kinetic considerations of H2 assisted hydrocarbon selective catalytic reduction of NO over Ag/Al2O3: II. Kinetic modelling[J]. Applied Catalysis A: General, 2006, 304: 86-92. |
| 79 | 张秀丽, 贺泓, 余运波. 氢气对Ag/Al2O3和Cu/Al2O3催化剂选择性催化C3H6还原NO x 反应的影响[J]. 催化学报, 2007, 28(2): 117-123. |
| ZHANG Xiuli, HE Hong, YU Yunbo. Effect of H2 on selective catalytic reduction of NO x by C3H6 over Ag/Al2O3 and Cu/Al2O3 catalysts[J]. Chinese Journal of Catalysis, 2007, 28(2): 117-123. | |
| 80 | DUAN Jun, ZHAO Ling, GAO Shengjun, et al. Reaction mechanism of H2-assisted C3H6-SCR over Ag-Ce x Zr catalyst as investigated by in situ FTIR[J]. Chemical Research in Chinese Universities, 2020, 36(5): 885-893. |
| 81 | POPOVYCH Nataliia O, KYRIIENKO Pavlo I, SOLOVIEV Sergiy O, et al. Influence of partial dealumination of BEA zeolites on physicochemical and catalytic properties of AgAlSiBEA in H2-promoted SCR of NO with ethanol[J]. Microporous and Mesoporous Materials, 2016, 226: 10-18. |
| 82 | ZHANG Xiuli, HE Hong, GAO Hongwei, et al. Experimental and theoretical studies of surface nitrate species on Ag/Al2O3 using DRIFTS and DFT[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2008, 71(4): 1446-1451. |
| 83 | Marika MÄNNIKKÖ, SKOGLUNDH Magnus, INGELSTEN Hanna Härelind. Selective catalytic reduction of NO x with methanol over supported silver catalysts[J]. Applied Catalysis B: Environmental, 2012, 119/120: 256-266. |
| 84 | GONZÁLEZ HERNÁNDEZ Naomi N, LUIS Contreras José, MARCOS Pinto, et al. Improved NO x reduction using C3H8 and H2 with Ag/Al2O3 catalysts promoted with Pt and WO x [J]. Catalysts, 2020, 10(10): 1212. |
| 85 | KALAMARAS Christos M, OLYMPIOU George G, PÂRVULESCU Vasile I, et al. Selective catalytic reduction of NO by H2/C3H6 over Pt/Ce1- x Zr x O2- δ : The synergy effect studied by transient techniques[J]. Applied Catalysis B: Environmental, 2017, 206: 308-318. |
| 86 | CHAIEB Tesnim, DELANNOY Laurent, CASALE Sandra, et al. Evidence for an H2 promoting effect in the selective catalytic reduction of NO x by propene on Au/Al2O3 [J]. Chemical Communications, 2015, 51(4): 796-799. |
| 87 | CHAIEB Tesnim, THOMAS Cyril, CASALE Sandra, et al. Selective catalytic reduction of NO x over Au/Al2O3: Influence of the gold loading on the promoting effect of H2 in H2-assisted C3H6-SCR of NO x [J]. Catalysis Letters, 2018, 148(2): 539-546. |
| 88 | YADAV Deepak, KAVAIYA Ashish R, MOHAN Devendra, et al. Low temperature selective catalytic reduction (SCR) of NO x emissions by Mn-doped Cu/Al2O3 catalysts[J]. Bulletin of Chemical Reaction Engineering & Catalysis, 2017, 12(3): 415-429. |
| 89 | KESKIN Zeycan, Tayfun ÖZGÜR, Himmet ÖZARSLAN, et al. Effects of hydrogen addition into liquefied petroleum gas reductant on the activity of Ag-Ti-Cu/cordierite catalyst for selective catalytic reduction system[J]. International Journal of Hydrogen Energy, 2021, 46(10): 7634-7641. |
| 90 | YADAV Deepak, SINGH Pratichi, PRASAD Ram. MnCo2O4 spinel catalysts synthesized by nanocasting method followed by different calcination routes for low-temperature reduction of NO x using various reductants[J]. International Journal of Hydrogen Energy, 2018, 43(10): 5346-5357. |
| [1] | 李新月, 李振京, 韩沂杭, 郭永强, 闫瑜, 哈力米热·卡热木拉提, 赵会吉, 柴永明, 刘东, 殷长龙. 油脂加氢脱氧生产绿色柴油催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 351-364. |
| [2] | 李帅哲, 聂懿宸, PHIDSAVARD Keomeesay, 顾雯, 张伟, 刘娜, 徐高翔, 刘莹, 李兴勇, 陈玉保. 非贵金属催化生物质加氢脱氧制备烃基生物燃料的研究进展[J]. 化工进展, 2024, 43(S1): 225-242. |
| [3] | 郝静远, 齐宝金, 魏进家. 不同压力下榆神地区富油煤原位热解产物特性[J]. 化工进展, 2024, 43(S1): 268-281. |
| [4] | 熊磊, 丁飞燕, 李聪, 王群乐, 吕起, 翟晓娜, 刘峰. 金属Pt负载型非均相催化剂研究进展[J]. 化工进展, 2024, 43(S1): 295-304. |
| [5] | 宋财城, 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 碳基载体负载加氢脱硫催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 305-314. |
| [6] | 韩洪晶, 车宇, 田宇轩, 王海英, 张亚男, 陈彦广. 木质素催化氢解催化剂及溶剂的研究进展[J]. 化工进展, 2024, 43(S1): 315-324. |
| [7] | 胡兴, 刘易, 杜泽学. 3-氯丙烯直接合成环氧氯丙烷催化剂研究进展[J]. 化工进展, 2024, 43(S1): 325-334. |
| [8] | 于梦洁, 吴语童, 罗发祥, 豆义波. 低浓度二氧化碳还原光催化剂结构设计的研究进展[J]. 化工进展, 2024, 43(S1): 335-350. |
| [9] | 何世坤, 张荣花, 李昊阳, 潘晖, 冯君锋. 脱铝分子筛固体酸催化葡萄糖制备5-羟甲基糠醛[J]. 化工进展, 2024, 43(S1): 374-381. |
| [10] | 张日东, 吕建华, 刘继东, 郭豹, 李文松. Ru-K-NaY催化草酸二甲酯脱羰基制备碳酸二甲酯[J]. 化工进展, 2024, 43(S1): 382-390. |
| [11] | 谢钰麟, 饶瑞晔, 黄建, 蒿佳怡, 王友益, 黄琦. 连续ZIF-8膜制备及在氢气分离中的研究进展[J]. 化工进展, 2024, 43(S1): 403-418. |
| [12] | 高聪志, 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平. 新戊二醇的合成工艺[J]. 化工进展, 2024, 43(S1): 469-478. |
| [13] | 李琳, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王春霞, 王俊莲, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 铝基废催化剂载体的回收与再生制备[J]. 化工进展, 2024, 43(S1): 640-649. |
| [14] | 刘振涛, 梅金林, 王春雅, 段爱军, 巩雁军, 徐春明, 王喜龙. 一步法加氢制生物航煤催化剂研究进展[J]. 化工进展, 2024, 43(9): 4909-4924. |
| [15] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||