化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6091-6110.DOI: 10.16085/j.issn.1000-6613.2023-1730
• 能源加工与技术 • 上一篇
生物质基催化转化制备己醇和己二醇及其衍生品
洪泽龙1( ), 周本1, 邱佳容1(
), 周本1, 邱佳容1( ), 张良清1(
), 张良清1( ), 陈剑锋1, 汪炳叔1, 曾宪海2, 林鹿2
), 陈剑锋1, 汪炳叔1, 曾宪海2, 林鹿2
- 1.福州大学先进制造学院,福建 晋江 362251
2.厦门大学能源学院,福建 厦门 361102
-
收稿日期:2023-10-07修回日期:2024-01-25出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:邱佳容,张良清 -
作者简介:洪泽龙(1999—),男,硕士研究生,研究方向为绿色催化。E-mail:1429594183@qq.com。 -
基金资助:国家自然科学基金(22108038);齐鲁工业大学(山东省科学院)生物基材料与绿色造纸国家重点实验室开放基金(GZKF202308);国家重点研发计划(2021YFC2101604);广东省重点领域研发计划(2020B0101070001);福建省自然科学基金(2019J06005)
Biomass-based catalytic transformation for producing hexanol, hexanediol, and their derivatives
HONG Zelong1( ), ZHOU Ben1, QIU Jiarong1(
), ZHOU Ben1, QIU Jiarong1( ), ZHANG Liangqing1(
), ZHANG Liangqing1( ), CHEN Jianfeng1, WANG Bingshu1, ZENG Xianhai2, LIN Lu2
), CHEN Jianfeng1, WANG Bingshu1, ZENG Xianhai2, LIN Lu2
- 1.School of Advanced Manufacturing, Fuzhou University, Jinjiang 362251, Fujian, China
2.College of Energy, Xiamen University, Xiamen 361102, Fujian, China
-
Received:2023-10-07Revised:2024-01-25Online:2024-11-15Published:2024-12-07 -
Contact:QIU Jiarong, ZHANG Liangqing
摘要:
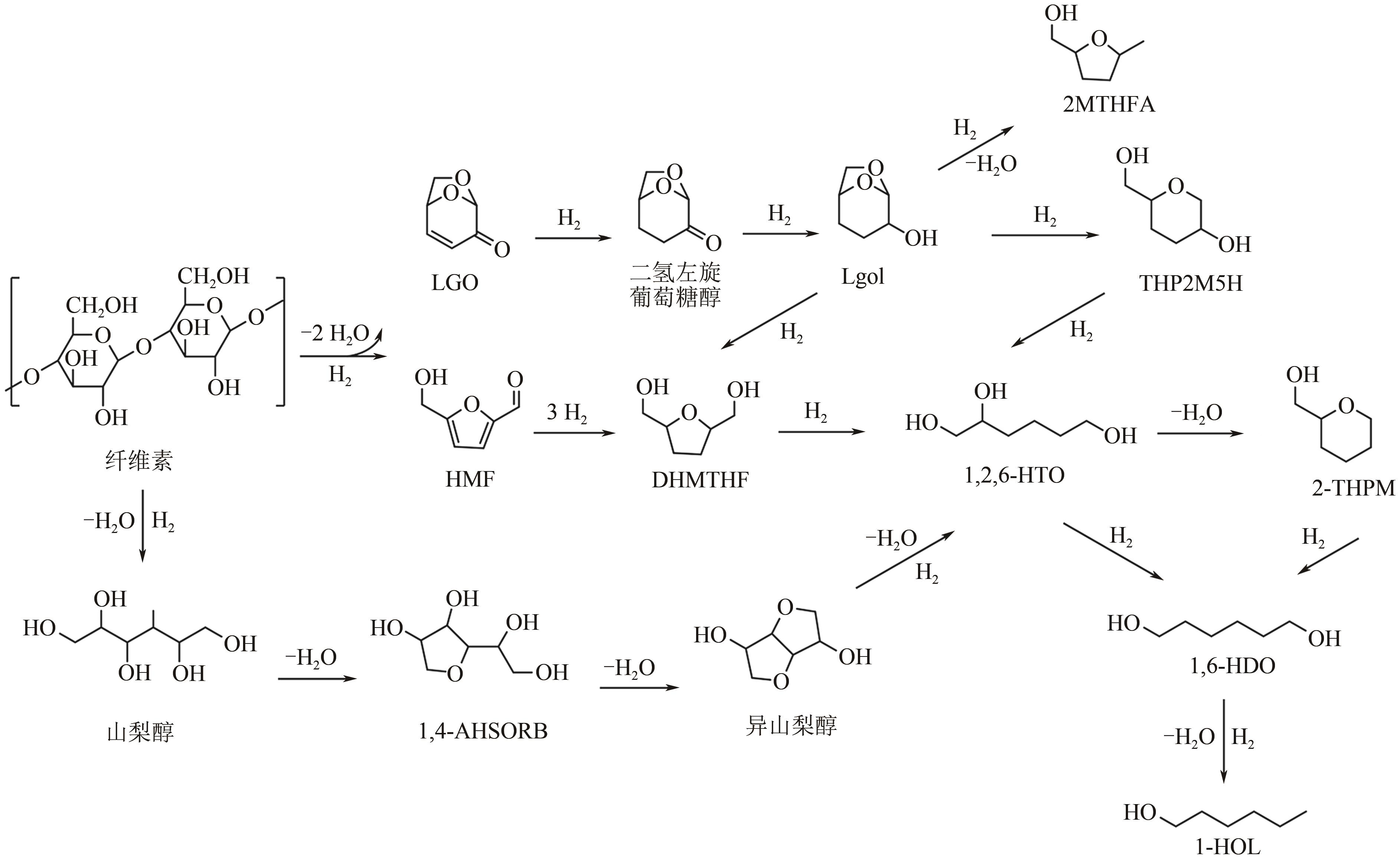
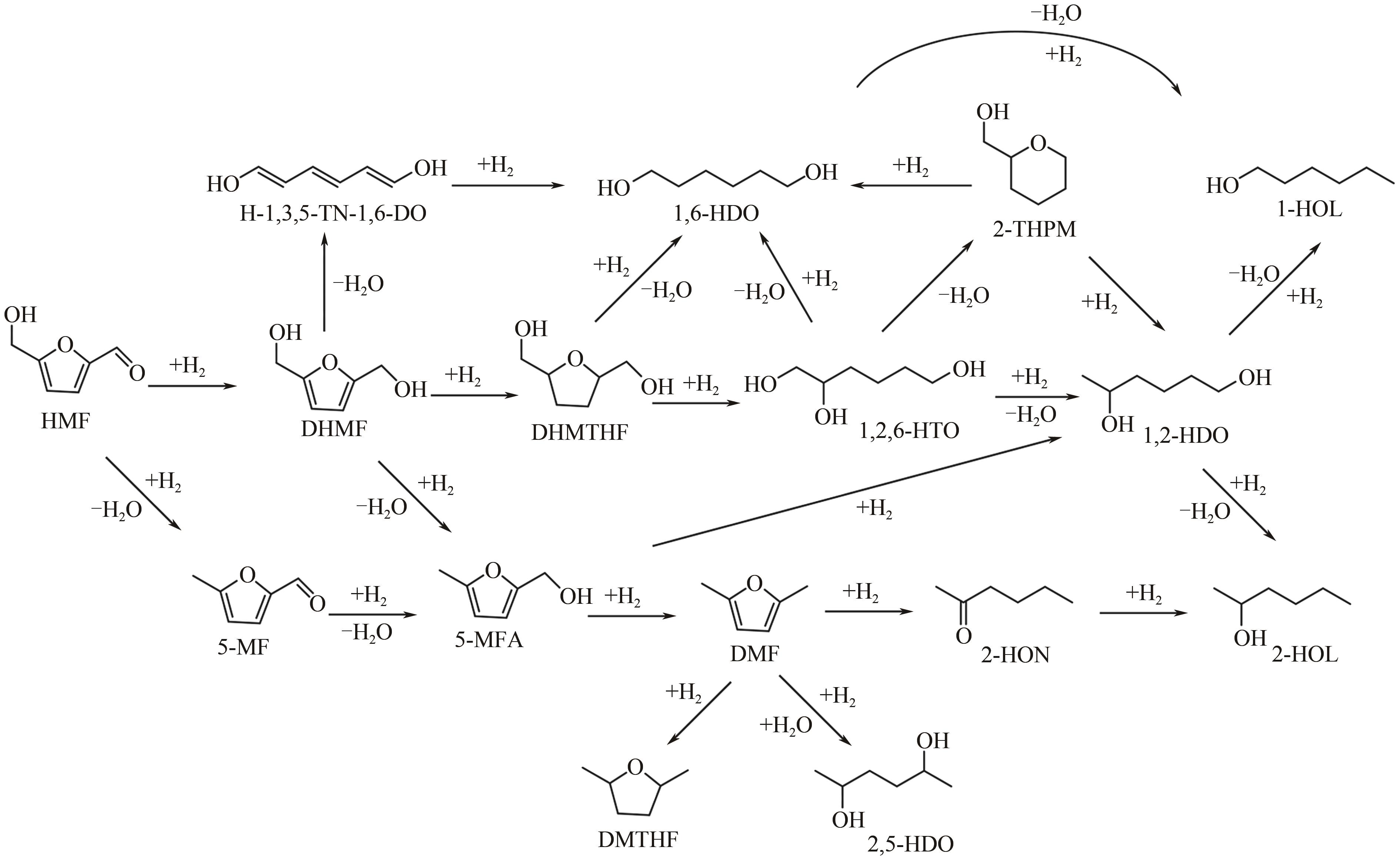
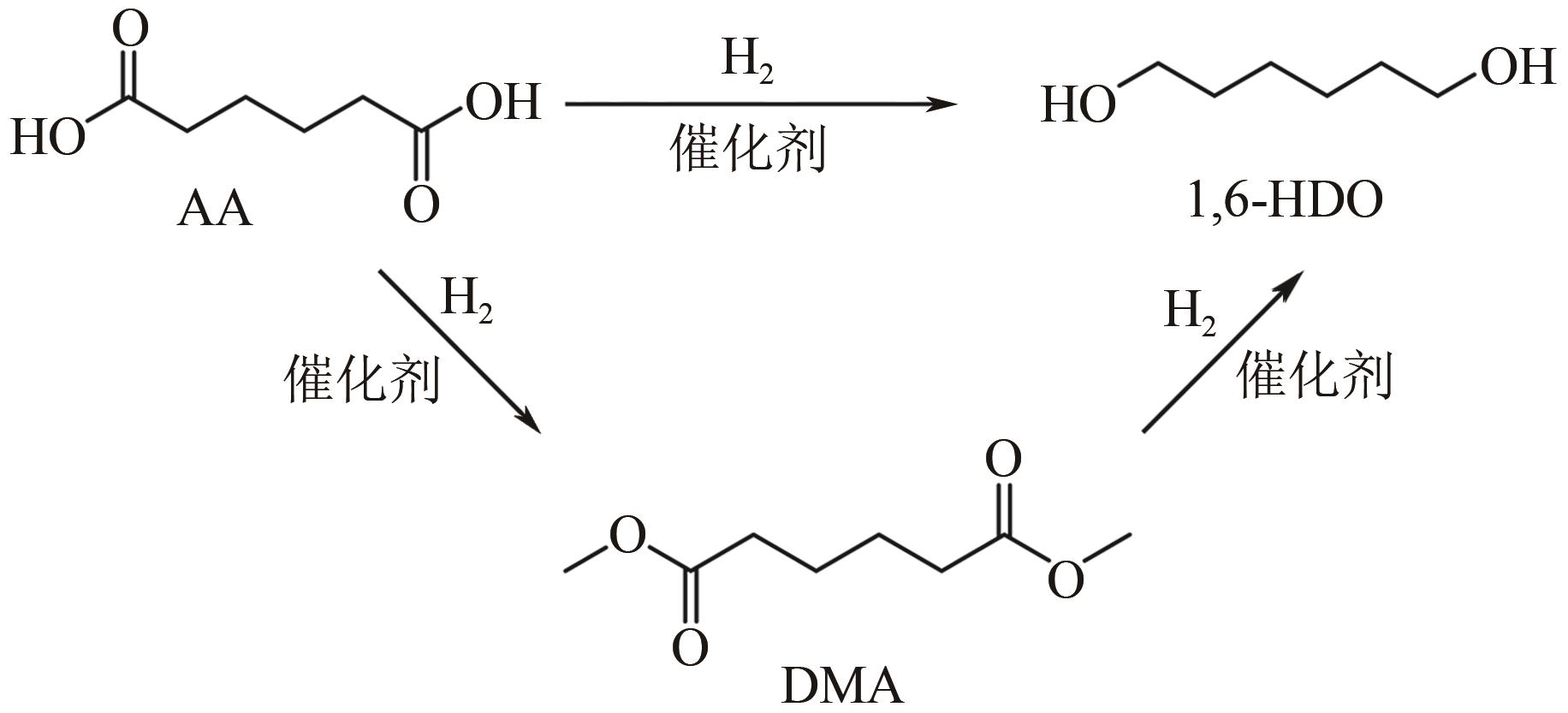
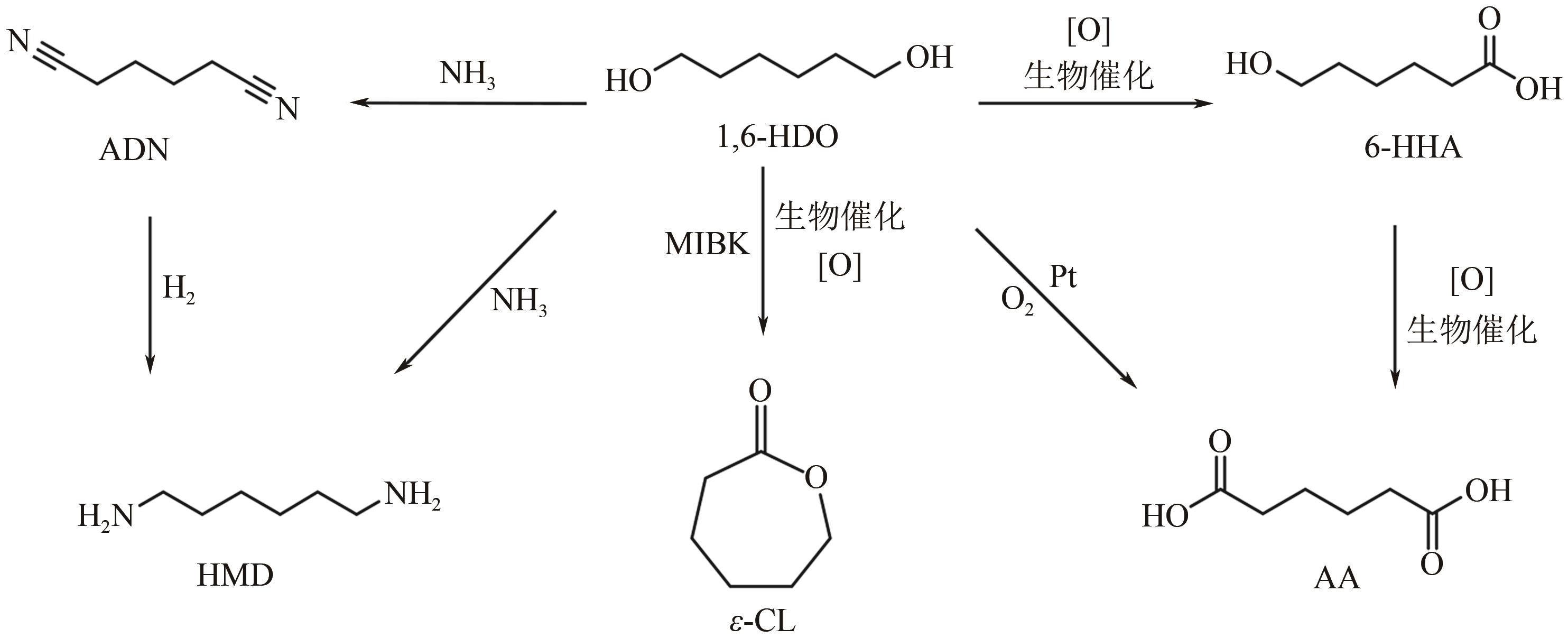
在如今能源危机以及环境污染的大背景下,生物质作为一种能够代替化石燃料的可再生能源之一,如何将生物质催化转化为有价值的己醇和己二醇在生物质转化领域受到了广泛的研究关注。己醇和己二醇被认为是一种具有高度工业价值的C6醇,其中己二醇在聚酯工业发展的领域有重要的作用,己醇则被认为是一种合适的替代燃料。本文根据原料和不同催化剂对生物质基制备己醇和己二醇的研究工作进行了归纳总结,系统阐述了近年来通过纤维素基、5-羟甲基糠醛及其衍生物、己二酸及其酯类等生物质基催化转化制备己醇和己二醇的研究进展,分析了1,6-己二醇在催化制备C6化合物(ε-己内酯、己二酸、6-羟基己酸和己二胺)方面的应用,并在此基础上对催化加氢制备己醇和己二醇的发展趋势进行了展望,为未来进一步绿色地生产可持续的C6化合物提供理论指导和有益参考。
中图分类号:
引用本文
洪泽龙, 周本, 邱佳容, 张良清, 陈剑锋, 汪炳叔, 曾宪海, 林鹿. 生物质基催化转化制备己醇和己二醇及其衍生品[J]. 化工进展, 2024, 43(11): 6091-6110.
HONG Zelong, ZHOU Ben, QIU Jiarong, ZHANG Liangqing, CHEN Jianfeng, WANG Bingshu, ZENG Xianhai, LIN Lu. Biomass-based catalytic transformation for producing hexanol, hexanediol, and their derivatives[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6091-6110.
| 序号 | 催化剂 | 底物 | 溶剂 | 反应条件 | 转化率/% | 目标产物,产率/% | 目标产物选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ir-ReO x /SiO2 | 纤维素 | 正癸烷+H2O | 24h;10MPa H2; 413K | — | HOL,60 | — | — | [ |
| 2 | Ru-MoO x /Mo2C | 山梨醇 | H2O | 6MPa H2; 523K | — | 1-HOL,28.7 | — | 72h | [ |
| 3 | Pt-WO x /SiO2 | 山梨醇 | H2O | 72h;7MPa H2; 453K | 100 | HOL,65.8 HDO,9.4 | — | 3次 | [ |
| 4 | ReO x -Pd/CeO2 | 山梨醇 | 1,4-diox | 72h;8MPa H2; 433K | >99 | HDO,85 | — | — | [ |
| 5 | Pt/C+Pt/W/SiO2 | LGO | H2O | 6h;5.5MPa H2; 433K | — | 1,6-HDO,62 | — | — | [ |
| 6 | 5%Au/ZrO2 | HMF | 1,4-diox | 12h;5MPa H2; 503K | 100 | 2-HOL,65.8 | — | 5次 | [ |
| 7 | Pd/ZrP | HMF | FA | 21h; 413K | 96.9 | 1,6-HDO,43 | — | 5次 | [ |
| 8 | Pd/SiO2+Ir-ReO x /SiO2 | HMF | H2O+THF | 7MPa H2; 373K | 100 | 1,6-HDO,57.8 1-HOL,8.2 | 1,6-HDO,57.8 1-HOL,8.2 | 24h | [ |
| 9 | Cu-Mg-Al | HMF | IA | 5h;4MPa H2; 423K | 100 | 1,2-HDO,42 | — | 4次 | [ |
| 10 | Pt/POM | DHMF | H2O | 3h;1MPa H2; 353K | 100 | 2-HOL,72.5 | — | — | [ |
表1 不同催化剂催化生物质基制备己醇和己二醇
| 序号 | 催化剂 | 底物 | 溶剂 | 反应条件 | 转化率/% | 目标产物,产率/% | 目标产物选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ir-ReO x /SiO2 | 纤维素 | 正癸烷+H2O | 24h;10MPa H2; 413K | — | HOL,60 | — | — | [ |
| 2 | Ru-MoO x /Mo2C | 山梨醇 | H2O | 6MPa H2; 523K | — | 1-HOL,28.7 | — | 72h | [ |
| 3 | Pt-WO x /SiO2 | 山梨醇 | H2O | 72h;7MPa H2; 453K | 100 | HOL,65.8 HDO,9.4 | — | 3次 | [ |
| 4 | ReO x -Pd/CeO2 | 山梨醇 | 1,4-diox | 72h;8MPa H2; 433K | >99 | HDO,85 | — | — | [ |
| 5 | Pt/C+Pt/W/SiO2 | LGO | H2O | 6h;5.5MPa H2; 433K | — | 1,6-HDO,62 | — | — | [ |
| 6 | 5%Au/ZrO2 | HMF | 1,4-diox | 12h;5MPa H2; 503K | 100 | 2-HOL,65.8 | — | 5次 | [ |
| 7 | Pd/ZrP | HMF | FA | 21h; 413K | 96.9 | 1,6-HDO,43 | — | 5次 | [ |
| 8 | Pd/SiO2+Ir-ReO x /SiO2 | HMF | H2O+THF | 7MPa H2; 373K | 100 | 1,6-HDO,57.8 1-HOL,8.2 | 1,6-HDO,57.8 1-HOL,8.2 | 24h | [ |
| 9 | Cu-Mg-Al | HMF | IA | 5h;4MPa H2; 423K | 100 | 1,2-HDO,42 | — | 4次 | [ |
| 10 | Pt/POM | DHMF | H2O | 3h;1MPa H2; 353K | 100 | 2-HOL,72.5 | — | — | [ |
| 序号 | 催化剂 | 底物 | 溶剂 | 反应条件 | 转化率/% | 目标产物,产率/% | 目标产物,选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Rh-ReO x /SiO2+ Nafion SAC-13 | BHMTHF | H2O | 20h;8MPa H2; 392K | 100 | 1,6-HDO,86 1,5-HDO,14 | — | — | [ |
| 2 | Pt-WO x /TiO2 | BHMTHF | H2O | 8h;5.5MPa H2; 433K | 100 | 1,6-HDO,70 | — | 46h | [ |
| 3 | Pt/W/TiO2 | BHMTHF | H2O | 24h;5.5MPa H2; 433K | — | 1,6-HDO,83 | — | — | [ |
| 4 | Pt-WO x /TiO2 | 1,2,6-HTO | H2O | 4h;6.9MPa H2; 453K | 89 | 1,6-HDO,62 | — | — | [ |
| 5 | Pt/ZrO2 | 1,2,6-HTO | H2O | 2.5h;4.6MPa H2; 433K | 91 | 1,6-HDO,61 | 1,6-HDO,68 | — | [ |
| 6 | Pt-Mo/SiO2 | 1,2,6-HTO | H2O | 2.5h;4.6MPa H2; 433K | 78 | 1,6-HDO,55 | 1,6-HDO,69 | — | [ |
| 7 | Pt-W/β | 1,2,6-HTO | H2O | 2.5h;4.6 MPa H2; 433 K | 100 | 1,6-HDO,65 | 1,6-HDO,65 | — | [ |
| 8 | Rh-ReO x /C | 1,2,6-HTO | H2O | 14h;3.4MPa H2; 393K | 59.3 | — | 1,6-HDO,61.9 | 120h | [ |
| 9 | Rh-MoO x /C | 2-THPM | H2O | 4h;3.4MPa H2; 393 K | 25.8 | — | 1,6-HDO,88.6 1-HOL,3.5 | — | [ |
| 10 | Rh-ReO x /C | 2-THPM | H2O | 4h;3.4 MPa H2; 393K | 27.3 | — | 1,6-HDO,97 1-HOL,3 | — | [ |
| 11 | Rh-ReO x /C | 2-THPM | H2O | 84h;8MPa H2; 373K | — | 1,6-HDO,84 | — | 4次 | [ |
表2 不同催化剂催化HMF衍生物制备己醇和己二醇
| 序号 | 催化剂 | 底物 | 溶剂 | 反应条件 | 转化率/% | 目标产物,产率/% | 目标产物,选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Rh-ReO x /SiO2+ Nafion SAC-13 | BHMTHF | H2O | 20h;8MPa H2; 392K | 100 | 1,6-HDO,86 1,5-HDO,14 | — | — | [ |
| 2 | Pt-WO x /TiO2 | BHMTHF | H2O | 8h;5.5MPa H2; 433K | 100 | 1,6-HDO,70 | — | 46h | [ |
| 3 | Pt/W/TiO2 | BHMTHF | H2O | 24h;5.5MPa H2; 433K | — | 1,6-HDO,83 | — | — | [ |
| 4 | Pt-WO x /TiO2 | 1,2,6-HTO | H2O | 4h;6.9MPa H2; 453K | 89 | 1,6-HDO,62 | — | — | [ |
| 5 | Pt/ZrO2 | 1,2,6-HTO | H2O | 2.5h;4.6MPa H2; 433K | 91 | 1,6-HDO,61 | 1,6-HDO,68 | — | [ |
| 6 | Pt-Mo/SiO2 | 1,2,6-HTO | H2O | 2.5h;4.6MPa H2; 433K | 78 | 1,6-HDO,55 | 1,6-HDO,69 | — | [ |
| 7 | Pt-W/β | 1,2,6-HTO | H2O | 2.5h;4.6 MPa H2; 433 K | 100 | 1,6-HDO,65 | 1,6-HDO,65 | — | [ |
| 8 | Rh-ReO x /C | 1,2,6-HTO | H2O | 14h;3.4MPa H2; 393K | 59.3 | — | 1,6-HDO,61.9 | 120h | [ |
| 9 | Rh-MoO x /C | 2-THPM | H2O | 4h;3.4MPa H2; 393 K | 25.8 | — | 1,6-HDO,88.6 1-HOL,3.5 | — | [ |
| 10 | Rh-ReO x /C | 2-THPM | H2O | 4h;3.4 MPa H2; 393K | 27.3 | — | 1,6-HDO,97 1-HOL,3 | — | [ |
| 11 | Rh-ReO x /C | 2-THPM | H2O | 84h;8MPa H2; 373K | — | 1,6-HDO,84 | — | 4次 | [ |
| 序号 | 催化剂 | 底物 | 反应条件;溶剂 | 转化率/% | 目标产物,产率/% | 目标产物,选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru-Sn/Al2O3 | AA | 6.5MPa H2,513K;1,4-diox | 97.3 | 1,6-HDO,89.4 | — | — | [ |
| 2 | Pt-W/SiO2 | AA | 2.5h,4.6MPa H2,393K;H2O | 100 | 1,6-HDO,88 | — | — | [ |
| 3 | Ru-MoB/γ-Al2O3-300 | AA | 5.5MPa H2,493K;H2O | 90 | 1,6-HDO,77 | 1,6-HDO,85.6 | 5次 | [ |
| 4 | Re-Pd/SiO2 | AA | 96h,8MPa H2,413K;1,4-diox | 100 | 1,6-HDO,74 | — | — | [ |
| 5 | Ir-Re/AC | AA | 16h,10MPa H2,453K;H2O | 100 | — | 1,6-HDO,59 | 4次 | [ |
| 6 | 20CuPS-red | AA | 6h,9MPa H2,513K;H2O | 100 | 1,6-HDO,96.6 | 1,6-HDO,97.4 | 3次 | [ |
| 7 | Ru-Sn/TiO2 | DMA | 16h,5MPa H2,528K;H2O | 57 | — | 1,6-HDO,70 | — | [ |
| 8 | Ru-Sn/Al2O3 | DMA | 5MPa H2,528K;H2O | 98 | — | 1,6-HDO,49 | — | [ |
| 9 | Ru-Sn-Co/AlO(OH) | DMA | 10h,5MPa H2,493K;H2O | 98 | — | 1,6-HDO,95 | — | [ |
| 10 | Cu-Zn-Al-500 | DMA | 0.5h,5MPa H2,488K;H2O | 99 | — | 1,6-HDO,99 | — | [ |
| 11 | CuO-ZnO-Al2O3 | DMA | 4h,553K | 98.8 | 1,6-HDO,73 | — | — | [ |
| 12 | 732树脂 | DMA | 5.5h,2.5MPa H2,523K;H2O | — | 1,6-HDO,>96 | — | 7次 | [ |
| 13 | DNW树脂 | DMA | 6MPa H2,473K;H2O | 99.71 | — | 1,6-HDO,97.35 | — | [ |
| 14 | CuAl5/SBA-15 | DMA | 6h,6MPa H2,513K;1,4-diox | — | 1,6-HDO,87 | — | 36h | [ |
表3 不同催化剂催化己二酸和己二酸酯类制备己二醇
| 序号 | 催化剂 | 底物 | 反应条件;溶剂 | 转化率/% | 目标产物,产率/% | 目标产物,选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru-Sn/Al2O3 | AA | 6.5MPa H2,513K;1,4-diox | 97.3 | 1,6-HDO,89.4 | — | — | [ |
| 2 | Pt-W/SiO2 | AA | 2.5h,4.6MPa H2,393K;H2O | 100 | 1,6-HDO,88 | — | — | [ |
| 3 | Ru-MoB/γ-Al2O3-300 | AA | 5.5MPa H2,493K;H2O | 90 | 1,6-HDO,77 | 1,6-HDO,85.6 | 5次 | [ |
| 4 | Re-Pd/SiO2 | AA | 96h,8MPa H2,413K;1,4-diox | 100 | 1,6-HDO,74 | — | — | [ |
| 5 | Ir-Re/AC | AA | 16h,10MPa H2,453K;H2O | 100 | — | 1,6-HDO,59 | 4次 | [ |
| 6 | 20CuPS-red | AA | 6h,9MPa H2,513K;H2O | 100 | 1,6-HDO,96.6 | 1,6-HDO,97.4 | 3次 | [ |
| 7 | Ru-Sn/TiO2 | DMA | 16h,5MPa H2,528K;H2O | 57 | — | 1,6-HDO,70 | — | [ |
| 8 | Ru-Sn/Al2O3 | DMA | 5MPa H2,528K;H2O | 98 | — | 1,6-HDO,49 | — | [ |
| 9 | Ru-Sn-Co/AlO(OH) | DMA | 10h,5MPa H2,493K;H2O | 98 | — | 1,6-HDO,95 | — | [ |
| 10 | Cu-Zn-Al-500 | DMA | 0.5h,5MPa H2,488K;H2O | 99 | — | 1,6-HDO,99 | — | [ |
| 11 | CuO-ZnO-Al2O3 | DMA | 4h,553K | 98.8 | 1,6-HDO,73 | — | — | [ |
| 12 | 732树脂 | DMA | 5.5h,2.5MPa H2,523K;H2O | — | 1,6-HDO,>96 | — | 7次 | [ |
| 13 | DNW树脂 | DMA | 6MPa H2,473K;H2O | 99.71 | — | 1,6-HDO,97.35 | — | [ |
| 14 | CuAl5/SBA-15 | DMA | 6h,6MPa H2,513K;1,4-diox | — | 1,6-HDO,87 | — | 36h | [ |
| 序号 | 催化剂 | 底物 | 反应条件 | 转化率/% | 目标产物, 产率/% | 目标产物, 选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [{Ru(cymene)Cl}2]2/DPPF | 1,6-HDO | T=70℃;溶剂: MIBK;0.5h | — | ε-CL,>99 | — | — | [ |
| 2 | RuCl2(PPh3)3 | 1,6-HDO | 20%(摩尔分数)Bu4NBr; MIBK+K2CO3 | 95 | — | ε-CL;97 | — | [ |
| 3 | [RuCl2(p-cymene)]2+dppp | 1,6-HDO | 20%(摩尔分数)Bu4NBr; MIBK+K2CO3 | 99 | — | ε-CL;98 | — | [ |
| 4 | Pt/C | 1,6-HDO | T=70℃;1MPa O2;24h | 100 | AA,86 | — | — | [ |
| 5 | Pt-Bi/C | 1,6-HDO | T=55℃;0.02MPa O2;36h | — | AA,98 | — | — | [ |
| 6 | Pt/C | 1,6-HDO | T=55℃;0.02MPa O2;36h | — | AA,43 | — | — | [ |
| 7 | Pt/ZrO2 | 1,6-HDO | T=140℃;3MPa N2;1~2h | 100 | AA;>99 | — | — | [ |
| 8 | Au-Pt/ZrO2 | 1,6-HDO | T=70℃;4MPa;48h | 100 | AA;96 | — | 6次 | [ |
| 9 | G. oxydans DSM | 1,6-HDO | 4.6mmol/L 1,6-HDO; pH=5;T=30℃;t=24h | 99.5 | 6-HHA,2.1 AA,97.8 | 6-HHA,2.14 AA,97.8 | — | [ |
| 10 | G. oxydans DSM | 1,6-HDO | 253.8mmol/L 1,6-HDO; pH=6.5~7;T=30℃;t=120h | 100 | 6-HHA,100 | 6-HHA,100 | — | [ |
| 11 | 分枝杆菌MS16 | 1,6-HDO | 42.3mmol/L 1,6-HDO; pH=7;T=30℃;t=36h | 100 | 6-HHA,100 | — | — | [ |
| 12 | Au40Pd60-DDAO/HT | 1,6-HDO | T=80℃;30% H2O2;0.5mol/L NaOH;t=8h;溶剂: H2O | 87 | 6-HHA,81 | 6-HHA,93 AA,5 | 5次 | [ |
| 13 | RANEY®-Ni | ADN | T=100℃;3.5MPa N2;t=2h;溶剂: NH3 | 100 | HMD,92 | — | — | [ |
| 14 | 吖啶基钌螯合物 | 1,6-HDO | T=155℃;溶剂: NH3;t=16h | — | HMD,88 | — | — | [ |
| 15 | ChnD + TA | 1,6-HDO | 20mmol/L 1,6-HDO;pH=8;T=25℃;t=21h | — | HMD,73 | — | — | [ |
表4 不同催化剂催化1,6-HDO制备ε-CL、AA和6-HHA
| 序号 | 催化剂 | 底物 | 反应条件 | 转化率/% | 目标产物, 产率/% | 目标产物, 选择性/% | 催化剂稳定性 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 1 | [{Ru(cymene)Cl}2]2/DPPF | 1,6-HDO | T=70℃;溶剂: MIBK;0.5h | — | ε-CL,>99 | — | — | [ |
| 2 | RuCl2(PPh3)3 | 1,6-HDO | 20%(摩尔分数)Bu4NBr; MIBK+K2CO3 | 95 | — | ε-CL;97 | — | [ |
| 3 | [RuCl2(p-cymene)]2+dppp | 1,6-HDO | 20%(摩尔分数)Bu4NBr; MIBK+K2CO3 | 99 | — | ε-CL;98 | — | [ |
| 4 | Pt/C | 1,6-HDO | T=70℃;1MPa O2;24h | 100 | AA,86 | — | — | [ |
| 5 | Pt-Bi/C | 1,6-HDO | T=55℃;0.02MPa O2;36h | — | AA,98 | — | — | [ |
| 6 | Pt/C | 1,6-HDO | T=55℃;0.02MPa O2;36h | — | AA,43 | — | — | [ |
| 7 | Pt/ZrO2 | 1,6-HDO | T=140℃;3MPa N2;1~2h | 100 | AA;>99 | — | — | [ |
| 8 | Au-Pt/ZrO2 | 1,6-HDO | T=70℃;4MPa;48h | 100 | AA;96 | — | 6次 | [ |
| 9 | G. oxydans DSM | 1,6-HDO | 4.6mmol/L 1,6-HDO; pH=5;T=30℃;t=24h | 99.5 | 6-HHA,2.1 AA,97.8 | 6-HHA,2.14 AA,97.8 | — | [ |
| 10 | G. oxydans DSM | 1,6-HDO | 253.8mmol/L 1,6-HDO; pH=6.5~7;T=30℃;t=120h | 100 | 6-HHA,100 | 6-HHA,100 | — | [ |
| 11 | 分枝杆菌MS16 | 1,6-HDO | 42.3mmol/L 1,6-HDO; pH=7;T=30℃;t=36h | 100 | 6-HHA,100 | — | — | [ |
| 12 | Au40Pd60-DDAO/HT | 1,6-HDO | T=80℃;30% H2O2;0.5mol/L NaOH;t=8h;溶剂: H2O | 87 | 6-HHA,81 | 6-HHA,93 AA,5 | 5次 | [ |
| 13 | RANEY®-Ni | ADN | T=100℃;3.5MPa N2;t=2h;溶剂: NH3 | 100 | HMD,92 | — | — | [ |
| 14 | 吖啶基钌螯合物 | 1,6-HDO | T=155℃;溶剂: NH3;t=16h | — | HMD,88 | — | — | [ |
| 15 | ChnD + TA | 1,6-HDO | 20mmol/L 1,6-HDO;pH=8;T=25℃;t=21h | — | HMD,73 | — | — | [ |
| 1 | ZHANG Liangqing, QIU Jiarong, TANG Xing, et al. Efficient synthesis of sugar alcohols over a synergistic and sustainable catalyst[J]. Chinese Journal of Chemistry, 2021, 39(9): 2467-2476. |
| 2 | ZHANG Liangqing, QIU Jiarong, DENG Jiahui, et al. Efficient transformation of hemicellulosic biomass into sugar alcohols with non-precious and stable bimetallic support catalyst[J]. Industrial Crops and Products, 2023, 194: 116378. |
| 3 | LI Fukun, FRANCE Liam John, CAI Zhenping, et al. Catalytic transfer hydrogenation of butyl levulinate to γ-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites[J]. Applied Catalysis B: Environmental, 2017, 214: 67-77. |
| 4 | FENG Yunchao, LONG Sishi, TANG Xing, et al. Earth-abundant 3d-transition-metal catalysts for lignocellulosic biomass conversion[J]. Chemical Society Reviews, 2021, 50(10): 6042-6093. |
| 5 | ZANGOEI Samane, SALEHNIA Narges, MASHHADI Mehdi Khodaparast. A comparative study on the effect of alternative and fossil energy consumption on economic growth and foreign direct investment in selected countries using SUR approach[J]. Environmental Science and Pollution Research, 2021, 28(16): 19799-19809. |
| 6 | WANG Huiqiang, LI Jiachen, ZENG Xianhai, et al. Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent[J]. Cellulose, 2020, 27(3): 1301-1314. |
| 7 | 杨启悦, 吴巧妹, 邱佳容, 等. 生物基平台化合物催化转化制备糠醇[J]. 化学进展, 2022, 34(8): 1748-1759. |
| YANG Qiyue, WU Qiaomei, QIU Jiarong, et al. Catalytic conversion of bio-based platform compounds to fufuryl alcohol[J]. Progress in Chemistry, 2022, 34(8): 1748-1759. | |
| 8 | 伊帆, 周春兵, 魏浩. 我国1,6-己二醇的产业化现状与发展建议[J]. 山西化工, 2019, 39(6): 21-22. |
| YI Fan, ZHOU Chunbing, WEI Hao. Present situation and development suggestions of 1,6-hexanediol industrialization in China[J]. Shanxi Chemical Industry, 2019, 39(6): 21-22. | |
| 9 | SONG Uhram, KIM Jieun. Assessment of the potential risk of 1,2-hexanediol using phytotoxicity and cytotoxicity testing[J]. Ecotoxicology and Environmental Safety, 2020, 201: 110796. |
| 10 | 张存胜, 刘岩, 杨莉, 等. 工业废弃合成气厌氧发酵产己醇研究进展[J]. 化工进展, 2021, 40(3): 1604-1610. |
| ZHANG Cunsheng, LIU Yan, YANG Li, et al. Research progress of hexanol production through anaerobic fermentation of wasted industrial syngas[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1604-1610. | |
| 11 | 陈新. Ru-MoO x /Mo2C选择性氢解糖醇C—O键性能的研究[D]. 广州: 广东工业大学, 2021. |
| CHEN Xin. Study on properties of Ru-MoO x /Mo2C selective hydrogenolysis of sugar alcohol C—O bond[D]. Guangzhou: Guangdong University of Technology, 2021. | |
| 12 | HU Xiaohong, LI Zhijian, WANG Haiyong, et al. Selective hydrogenolysis of 5-hydroxymethylfurfural to 2-hexanol over Au/ZrO2 catalysts[J]. ChemSusChem, 2022, 15(13): e202200092. |
| 13 | 王新龙, 王晓东, 雷小楠, 等. 1,6-己二醇制备工艺进展[J]. 石油化工, 2019, 48(5): 513-521. |
| WANG Xinlong, WANG Xiaodong, LEI Xiaonan, et al. Review on the preparation process of 1,6-hexanediol[J]. Petrochemical Technology, 2019, 48(5): 513-521. | |
| 14 | HE Jiayue, LIU Mingjie, HUANG Kefeng, et al. Production of levoglucosenone and 5-hydroxymethylfurfural from cellulose in polar aprotic solvent-water mixtures[J]. Green Chemistry, 2017, 19(15): 3642-3653. |
| 15 | 匡碧锋, 江婷, 余雅玲, 等. 生物质催化转化制备1,5-戊二醇和1,6-己二醇研究进展[J]. 精细化工, 2019, 36(5): 781-785. |
| KUANG Bifeng, JIANG Ting, YU Yaling, et al. Research progress on hydrogenolysis of biomass to 1,5-pentanediol and 1,6-hexanediol[J]. Fine Chemicals, 2019, 36(5): 781-785. | |
| 16 | ENJAMURI Nagasuresh, DARBHA Srinivas. Solid catalysts for conversion of furfural and its derivatives to alkanediols[J]. Catalysis Reviews, 2020, 62(4): 566-606. |
| 17 | 吴巧妹, 杨启悦, 曾宪海, 等. 纤维素基生物质催化转化制备二醇[J]. 化学进展, 2022, 34(10): 2173-2189. |
| WU Qiaomei, YANG Qiyue, ZENG Xianhai, et al. Catalytic conversion of cellulose-based biomass to diols[J]. Progress in Chemistry, 2022, 34(10): 2173-2189. | |
| 18 | LIU Yue, LUO Chen, LIU Haichao. Tungsten trioxide promoted selective conversion of cellulose into propylene glycol and ethylene glycol on a ruthenium catalyst[J]. Angewandte Chemie International Edition, 2012, 51(13): 3249-3253. |
| 19 | ZHANG Liangqing, HUANG Suchang, QIU Jiarong, et al. Selective transformation of biomass-derived substrates to 1,2-butanediol: A comprehensive review and new insights[J]. Industrial Crops and Products, 2023, 202: 116984. |
| 20 | ZHENG Mingyuan, PANG Jifeng, SUN Ruiyan, et al. Selectivity control for cellulose to diols: Dancing on eggs[J]. ACS Catalysis, 2017, 7(3): 1939-1954. |
| 21 | VILCOCQ Léa, CABIAC Amandine, ESPECEL Catherine, et al. New insights into the mechanism of sorbitol transformation over an original bifunctional catalytic system[J]. Journal of Catalysis, 2014, 320: 16-25. |
| 22 | KRISHNA Siddarth H, MCCLELLAND Daniel J, RASHKE Quinn A, et al. Hydrogenation of levoglucosenone to renewable chemicals[J]. Green Chemistry, 2017, 19(5): 1278-1285. |
| 23 | WANG Wei, WANG Yao, ZHAN Zixiang, et al. Heterogeneous catalysis for deoxygenation of cellulose and its derivatives to chemicals[J]. Acta Physico Chimica Sinica, 2022, 38(10): 2205032. |
| 24 | BUNTARA Teddy, NOEL Sebastien, PHUA Pim Huat, et al. Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone[J]. Angewandte Chemie International Edition, 2011, 50(31): 7083-7087. |
| 25 | YANG Zhirong, ZHANG Jing, QIAN Gang, et al. Production of biomass-derived monomers through catalytic conversion of furfural and hydroxymethylfurfural[J]. Green Chemical Engineering, 2021, 2(2): 158-173. |
| 26 | XI Jinxu, DING Daqian, SHAO Yi, et al. Production of ethylene glycol and its monoether derivative from cellulose[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(10): 2355-2362. |
| 27 | HE Jiayue, HUANG Kefeng, BARNETT Kevin J, et al. New catalytic strategies for α, ω-diols production from lignocellulosic biomass[J]. Faraday Discussions, 2017, 202: 247-267. |
| 28 | KIM Hyunwoo, LEE Shinje, LEE Jinwon, et al. Simultaneous production of 1,6-hexanediol, furfural, and high-purity lignin from white birch: Process integration and techno-economic evaluation[J]. Bioresource Technology, 2021, 331: 125009. |
| 29 | KIM Hyunwoo, LEE Shinje, WON Wangyun. System-level analyses for the production of 1,6-hexanediol from cellulose[J]. Energy, 2021, 214: 118974. |
| 30 | Nobuhiko OTA, TAMURA Masazumi, NAKAGAWA Yoshinao, et al. Hydrodeoxygenation of vicinal OH groups over heterogeneous rhenium catalyst promoted by palladium and ceria support[J]. Angewandte Chemie International Edition, 2015, 54(6): 1897-1900. |
| 31 | ALLGEIER Alan Martin, DE SILVA Wathudura Indika Namal, KOROVESSI Ekaterini, et al. Process for preparing 1,6-hexanediol: US8865940[P]. 2014-10-21. |
| 32 | OKUYAMA Yasuyo, TONISHIGE Kellchi, IMAI Akio, et al. Method for producing hexanol/pentanol: JP2016033129A[P]. 2016-03-10. |
| 33 | LIU Sibao, TAMURA Masazumi, NAKAGAWA Yoshinao, et al. One-pot conversion of cellulose into n-hexane over the Ir-ReO x /SiO2 catalyst combined with HZSM-5[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(7): 1819-1827. |
| 34 | LIU Sibao, OKUYAMA Yasuyo, TAMURA Masazumi, et al. Production of renewable hexanols from mechanocatalytically depolymerized cellulose by using Ir-ReO x /SiO2 catalyst[J]. ChemSusChem, 2015, 8(4): 628-635. |
| 35 | LI Ning, HUBER George W. Aqueous-phase hydrodeoxygenation of sorbitol with Pt/SiO2-Al2O3: Identification of reaction intermediates[J]. Journal of Catalysis, 2010, 270(1): 48-59. |
| 36 | CHEN Xin, ZHENG Yanni, ZHANG Qian, et al. Controlling transformation of sorbitol into 1-hexanol over Ru-MoO x /Mo2C catalyst via aqueous-phase hydrodeoxygenation[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(27): 9033-9044. |
| 37 | YUN Guoxia, GUAN Qingxin, LI Wei. Nondestructive construction of Lewis acid sites on the surface of supported nickel phosphide catalysts by atomic-layer deposition[J]. Journal of Catalysis, 2018, 361: 12-22. |
| 38 | LIU Lujie, CAO Ji, NAKAGAWA Yoshinao, et al. Hydrodeoxygenation of C4-C6 sugar alcohols to diols or mono-alcohols with the retention of the carbon chain over a silica-supported tungsten oxide-modified platinum catalyst[J]. Green Chemistry, 2021, 23(15): 5665-5679. |
| 39 | TUTEJA Jaya, CHOUDHARY Hemant, NISHIMURA Shun, et al. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source[J]. ChemSusChem, 2014, 7(1): 96-100. |
| 40 | XIAO Bin, ZHENG Mingyuan, LI Xinsheng, et al. Synthesis of 1,6-hexanediol from HMF over double-layered catalysts of Pd/SiO2+Ir–ReO x /SiO2 in a fixed-bed reactor[J]. Green Chemistry, 2016, 18(7): 2175-2184. |
| 41 | SHAO Yuewen, WANG Junzhe, SUN Kai, et al. Cu-based nanoparticles as catalysts for selective hydrogenation of biomass-derived 5-hydroxymethylfurfural to 1,2-hexanediol[J]. ACS Applied Nano Materials, 2022, 5(4): 5882-5894. |
| 42 | SHERBI Magdy, STUCKART Maria, ALBERT Jakob. Selective catalytic hydrogenation of biomass derived furans to secondary alcohols using Pt/polyoxometalate catalysts under mild reaction conditions[J]. Biofuels, Bioproducts and Biorefining, 2021, 15(5): 1431-1446. |
| 43 | YU Yaling, ZHANG Qian, CHEN Xin, et al. Aqueous phase hydrogenolysis of sugar alcohol to higher alcohols over Ru-Mo/CMK-3 catalyst[J]. Fuel Processing Technology, 2020, 197: 106195. |
| 44 | LI Qian, ZHANG Zhongwei, ZHAO Jing, et al. Recent advances in the sustainable production of α, ω-C6 bifunctional compounds enabled by chemo-/ biocatalysts[J]. Green Chemistry, 2022, 24(11): 4270-4303. |
| 45 | ZHANG Shunli, WU Changqing, MA Cuiluan, et al. Transformation of bread waste into 2,5-furandimethanol via an efficient chemoenzymatic approach in a benign reaction system[J]. Bioresource Technology, 2023, 371: 128579. |
| 46 | CHEN Yulong, MU Yuesong, HE Zejian, et al. New bio-based polyester with excellent spinning performance: Poly(tetrahydrofuran dimethanol-co-ethylene terephthalate)[J]. RSC Advances, 2022, 12(45): 29516-29524. |
| 47 | KATAOKA Hiroto, KOSUGE Daichi, OGURA Keiji, et al. Reductive conversion of 5-hydroxymethylfurfural to 1,2,6-hexanetriol in water solvent using supported Pt catalysts[J]. Catalysis Today, 2020, 352: 60-65. |
| 48 | BURT Samuel P, BARNETT Kevin J, MCCLELLAND Daniel J, et al. Production of 1,6-hexanediol from tetrahydropyran-2-methanol by dehydration–hydration and hydrogenation[J]. Green Chemistry, 2017, 19(5): 1390-1398. |
| 49 | HANSEN Thomas S, BARTA Katalin, ANASTAS Paul T, et al. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol[J]. Green Chemistry, 2012, 14(9): 2457-2461. |
| 50 | DE VOS Dirk E, DE WILDEMAN Stefaan, SELS Bert F, et al. Selective alkene oxidation with H2O2 and a heterogenized Mn catalyst: Epoxidation and a new entry to vicinalcis-diols[J]. Angewandte Chemie International Edition, 1999, 38(7): 980-983. |
| 51 | ALSHAMMARI Hamed, MIEDZIAK Peter J, BAWAKED Salem, et al. Solvent-free liquid-phase oxidation of 1-hexene using supported gold catalysts[J]. ChemCatChem, 2012, 4(10): 1565-1571. |
| 52 | YAO Shengxi, WANG Xicheng, JIANG Yijun, et al. One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2,6-hexanetriol over Ni-Co-Al mixed oxide catalysts under mild conditions[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(2): 173-180. |
| 53 | TANG Xing, WEI Junnan, DING Ning, et al. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels[J]. Renewable and Sustainable Energy Reviews, 2017, 77: 287-296. |
| 54 | SHAO Yuewen, WANG Junzhe, DU Huining, et al. Importance of magnesium in Cu-based catalysts for selective conversion of biomass-derived furan compounds to diols[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(13): 5217-5228. |
| 55 | FU Xiaomin, REN Xiaoqian, SHEN Jiecan, et al. Synergistic catalytic hydrogenation of furfural to 1,2-pentanediol and 1,5-pentanediol with LDO derived from CuMgAl hydrotalcite[J]. Molecular Catalysis, 2021, 499: 111298. |
| 56 | LIU Hailong, HUANG Zhiwei, ZHAO Feng, et al. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2- and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst[J]. Catalysis Science & Technology, 2016, 6(3): 668-671. |
| 57 | LIU Hailong, HUANG Zhiwei, KANG Haixiao, et al. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts[J]. Chinese Journal of Catalysis, 2016, 37(5): 700-710. |
| 58 | 丁璟, 赵俊琦, 程时标, 等. 生物基1,6-己二醇的研究进展[J]. 化工进展, 2015, 34(12): 4209-4213. |
| DING Jing, ZHAO Junqi, CHENG Shibiao, et al. Advances in production of biobased 1,6-HDO[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4209-4213. | |
| 59 | HE Jiayue, BURT Samuel P, BALL Madelyn, et al. Synthesis of 1,6-hexanediol from cellulose derived tetrahydrofuran-dimethanol with Pt-WO x /TiO2 catalysts[J]. ACS Catalysis, 2018, 8(2): 1427-1439. |
| 60 | ABILLARD Olivier, TEBBEN Gerd Dieter, PINKOS Rolf, et al. Process for preparing 1,6-hexanediol: CA2744126A1[P]. 2010-06-10. |
| 61 | STEPHENS Kyle J, ALLGEIER Alan M, BELL Alysha L, et al. A mechanistic study of polyol hydrodeoxygenation over a bifunctional Pt-WO x /TiO2 catalyst[J]. ACS Catalysis, 2020, 10(21): 12996-13007. |
| 62 | DIAS Eric L, SHOEMAKER James A W, BOUSSIE Thomas R, et al. Process for production of hexamethylenediamine from 5-hydroxymethylfurfural: EP2084848A1[P]. 2014-11-26. |
| 63 | MEI Chia, PAGÁN-TORRES Yomaira J, HIBBITTS David, et al. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts[J]. Journal of the American Chemical Society, 2011, 133(32): 12675-12689. |
| 64 | MEI Chia, O’NEILL Brandon J, ALAMILLO Ricardo, et al. Bimetallic RhRe/C catalysts for the production of biomass-derived chemicals[J]. Journal of Catalysis, 2013, 308: 226-236. |
| 65 | CHEN Kaiyou, KOSO Shuichi, KUBOTA Takeshi, et al. Chemoselective hydrogenolysis of tetrahydropyran-2-methanol to 1,6-hexanediol over rhenium-modified carbon-supported rhodium catalysts[J]. ChemCatChem, 2010, 2(5): 547-555. |
| 66 | KING David L, ZHANG Liang, XIA Gordon, et al. Aqueous phase reforming of glycerol for hydrogen production over Pt-Re supported on carbon[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 206-213. |
| 67 | HUANG Zexing, WANG Jianhua, LEI Jing, et al. Recent advances in the catalytic hydroconversion of 5-hydroxymethylfurfural to valuable diols[J]. Frontiers in Chemistry, 2022, 10: 925603. |
| 68 | MONTI Eleonora, VENTIMIGLIA Alessia, GARCIA SOTO Carolina Alejandra, et al. Effect of the colloidal preparation method for supported preformed colloidal Au nanoparticles for the liquid phase oxidation of 1,6-hexanediol to adipic acid[J]. Catalysts, 2022, 12(2): 196. |
| 69 | POSPELOVA Violetta, AUBRECHT Jaroslav, KIKHTYANIN Oleg, et al. CuZn catalysts superior to Adkins catalysts for dimethyl adipate hydrogenolysis[J]. ChemCatChem, 2019, 11(8): 2169-2178. |
| 70 | MURPHY Vincent J, DIAS Eric L, SHOEMAKER James A W. Production of 1,6-hexanediol from adipic acid: US10150719[P]. 2018-12-11. |
| 71 | MARTÍNEZ-PRIETO Luis M, PUCHE Marta, Christian CEREZO-NAVARRETE, et al. Uniform Ru nanoparticles on N-doped graphene for selective hydrogenation of fatty acids to alcohols[J]. Journal of Catalysis, 2019, 377: 429-437. |
| 72 | GONG Wei, WANG Xuyun, JI Shan, et al. Amorphous RuCoP ultrafine nanoparticles supported on carbon as efficient catalysts for hydrogenation of adipic acid to 1,6-hexanediol[J]. Materials, 2022, 15(22): 8084. |
| 73 | 巩伟. 己二酸加氢合成1,6-己二醇负载型过渡金属催化剂的制备与应用研究[D]. 青岛: 青岛科技大学, 2020. |
| GONG Wei. Study on preparation and application of supported transition metal catalyst for hydrogenation of adipic acid to 1,6-hexanediol[D]. Qingdao: Qingdao University of Science & Technology, 2020. | |
| 74 | TOYAO Takashi, S M A Hakim SIDDIKI, TOUCHY Abeda S, et al. TiO2-supported Re as a general and chemoselective heterogeneous catalyst for hydrogenation of carboxylic acids to alcohols[J]. Chemistry, 2017, 23(5): 1001-1006. |
| 75 | TAKEDA Yasuyuki, TAMURA Masazumi, NAKAGAWA Yoshinao, et al. Hydrogenation of dicarboxylic acids to diols over Re-Pd catalysts[J]. Catalysis Science & Technology, 2016, 6(14): 5668-5683. |
| 76 | LI Xiaoyue, LUO Jingjie, LIANG Changhai. Hydrogenation of adipic acid to 1,6-hexanediol by supported bimetallic Ir-Re catalyst[J]. Molecular Catalysis, 2020, 490: 110976. |
| 77 | JIANG Jiawei, TU Cheng-Chieh, CHEN Chaohuang, et al. Highly selective silica-supported copper catalysts derived from copper phyllosilicates in the hydrogenation of adipic acid to 1,6-hexanediol[J]. ChemCatChem, 2018, 10(23): 5449-5458. |
| 78 | SILVA Adriana M, MORALES Marco A, BAGGIO-SAITOVITCH Elisa M, et al. Selective hydrogenation of dimethyl adipate on titania-supported RuSn catalysts[J]. Applied Catalysis A: General, 2009, 353(1): 101-106. |
| 79 | KIKHTYANIN Oleg, AUBRECHT Jaroslav, POSPELOVA Violetta, et al. On the origin of the transesterification reaction route during dimethyl adipate hydrogenolysis[J]. Applied Catalysis A: General, 2020, 606: 117825. |
| 80 | JIANG Hongbin, JIANG Haijun, SU Ke, et al. A Ru-Sn-Co/AlO(OH) as a highly efficient catalyst for hydrogenation of dimethyl adipate to 1,6-hexanodiol in aqueous phase[J]. Applied Catalysis A: General, 2012, 447: 164-170. |
| 81 | 程光剑, 黄集钺, 石鸣彦, 等. 用于生产1,6-己二醇的加氢催化剂制备及评价[J]. 石化技术与应用, 2008, 26(2): 136-139. |
| CHENG Guangjian, HUANG Jiyue, SHI Mingyan, et al. Preparation and evaluation of hydrogenation catalyst for 1,6-hexanediol production[J]. Petrochemical Technology & Application, 2008, 26(2): 136-139. | |
| 82 | YUAN Peng, LIU Zhongyi, HU Tianjun, et al. Highly efficient Cu-Zn-Al catalyst for the hydrogenation of dimethyl adipate to 1,6-hexanediol: Influence of calcination temperature[J]. Reaction Kinetics, Mechanisms and Catalysis, 2010, 100(2): 427-439. |
| 83 | 尚开龙. 己二酸二甲酯加氢合成1,6-己二醇铜基催化剂的研究[D]. 郑州: 郑州大学, 2014. |
| SHANG Kailong. Study of copper-based catalyst for 1,6-hexanediol synthesis via dimethyl adipate hydrogenation[D]. Zhengzhou: Zhengzhou University, 2014. | |
| 84 | 荆宏健, 王俊伟, 杨丰科, 等. 1,6-己二醇制备的研究[J]. 应用化工, 2011, 40(7): 1222-1225. |
| JING Hongjian, WANG Junwei, YANG Fengke, et al. Preparation of 1,6-hexanediol[J]. Applied Chemical Industry, 2011, 40(7): 1222-1225. | |
| 85 | 魏晓霞, 霍稳周, 陈明, 等. 己二酸酯化加氢生产1,6-己二醇工艺研究[C]//中国化工学会2010年石油化工学术年会论文集. 上海: 中国化工学会, 2010: 933-935. |
| 86 | 刘书林, 杨娜, 张龙飞, 等. Al掺杂的Cu/SBA-15催化剂用于己二酸二甲酯加氢合成1,6-己二醇[J]. 化工进展, 2023, 42(1): 289-296. |
| LIU Shulin, YANG Na, ZHANG Longfei, et al. Al-doped Cu/SBA-15 catalysts for the hydrogenation of dimethyl adipate to 1,6-hexanediol[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 289-296. | |
| 87 | HE Ming, YANG Guihua, CHO Byoung-Uk, et al. Effects of addition method and fibrillation degree of cellulose nanofibrils on furnish drainability and paper properties[J]. Cellulose, 2017, 24(12): 5657-5669. |
| 88 | WANG Yu, BROWN Caroline A, CHEN Rachel. Industrial production, application, microbial biosynthesis and degradation of furanic compound, hydroxymethylfurfural (HMF)[J]. AIMS Microbiology, 2018, 4(2): 261-273. |
| 89 | Sang-Hyun PYO, PARK Ji Hoon, SREBNY Vanessa, et al. A sustainable synthetic route for biobased 6-hydroxyhexanoic acid, adipic acid and ε-caprolactone by integrating bio- and chemical catalysis[J]. Green Chemistry, 2020, 22(14): 4450-4455. |
| 90 | 张涵, 孙志强, 李帅, 等. ε-己内酯产业化开发的现状与展望[J]. 高分子材料科学与工程, 2021, 37(1): 218-222, 251. |
| ZHANG Han, SUN Zhiqiang, LI Shuai, et al. Present and prospect of ε-caprolactone industrialization[J]. Polymer Materials Science & Engineering, 2021, 37(1): 218-222, 251. | |
| 91 | 赵鑫, 常静. ε-己内酯与聚己内酯研究应用进展[J]. 煤炭与化工, 2021, 44(4): 130-134. |
| ZHAO Xin, CHANG Jing. Progress in research and application of ε-Caprolactone and polycaprolactone[J]. Coal and Chemical Industry, 2021, 44(4): 130-134. | |
| 92 | MORMUL Jaroslaw, BREITENFELD Jan, TRAPP Oliver, et al. Synthesis of adipic acid, 1,6-hexanediamine, and 1,6-hexanediol via double-n-selective hydroformylation of 1,3-butadiene[J]. ACS Catalysis, 2016, 6(5): 2802-2810. |
| 93 | GERARDUS De Vries Johannes, TEDDY, Huat Phua PIM, et al. Preparation of caprolaction, caprolactam, 2,5-tetrahydrofuran-dimethanol, 1,6-hexanediol or 1,2,6-hexanediol from 5-hydroxymethyl-2-furfuraldehyde: US9199961(B2) [P]. 2015-12-01. |
| 94 | KARA Selin, SPICKERMANN Dominik, SCHRITTWIESER Joerg H, et al. Access to lactone building blocks via horse liver alcohol dehydrogenase-catalyzed oxidative lactonization[J]. ACS Catalysis, 2013, 3(11): 2436-2439. |
| 95 | BORNADEL Amin, Rajni HATTI-KAUL, HOLLMANN Frank, et al. A Bi-enzymatic convergent cascade for ε-caprolactone synthesis employing 1,6-hexanediol as a ‘double-smart cosubstrate’[J]. ChemCatChem, 2015, 7(16): 2442-2445. |
| 96 | SCHMIDT Sandy, SCHERKUS Christian, MUSCHIOL Jan, et al. An enzyme cascade synthesis of ε-caprolactone and its oligomers[J]. Angewandte Chemie International Edition, 2015, 54(9): 2784-2787. |
| 97 | IDE Matthew S, DAVIS Robert J. Perspectives on the kinetics of diol oxidation over supported platinum catalysts in aqueous solution[J]. Journal of Catalysis, 2013, 308: 50-59. |
| 98 | XIE Jiahan, HUANG Benjamin, YIN Kehua, et al. Influence of dioxygen on the promotional effect of Bi during Pt-catalyzed oxidation of 1,6-hexanediol[J]. ACS Catalysis, 2016, 6(7): 4206-4217. |
| 99 | DIAS Eric L, MURPHY Vincent J, SHOEMAKER James A W. Process for production of adipic acid from 1,6-hexanediol: US10077226[P]. 2018-09-18. |
| 100 | Modibo MOUNGUENGUI-DIALLO, VERMERSCH François, PERRET Noémie, et al. Base free oxidation of 1,6-hexanediol to adipic acid over supported noble metal mono- and bimetallic catalysts[J]. Applied Catalysis A: General, 2018, 551: 88-97. |
| 101 | TUTEJA Jaya, NISHIMURA Shun, CHOUDHARY Hemant, et al. Selective oxidation of 1,6-hexanediol to 6-hydroxycaproic acid over reusable hydrotalcite-supported Au-Pd bimetallic catalysts[J]. ChemSusChem, 2015, 8(11): 1862-1866. |
| 102 | ZHANG Zhongwei, FANG Lin, WANG Fei, et al. Transforming inert cycloalkanes into α, ω-diamines by designed enzymatic cascade catalysis[J]. Angewandte Chemie International Edition, 2023, 62(16): e202215935. |
| 103 | GILKEY Matthew J, MIRONENKO Alexander V, VLACHOS Dionisios G, et al. Adipic acid production via metal-free selective hydrogenolysis of biomass-derived tetrahydrofuran-2,5-dicarboxylic acid[J]. ACS Catalysis, 2017, 7(10): 6619-6634. |
| 104 | CAPECE Noemi, SADIER Achraf, PALOMBO FERRAZ Camila, et al. Aerobic oxidation of 1,6-hexanediol to adipic acid over Au-based catalysts: The role of basic supports[J]. Catalysis Science & Technology, 2020, 10(8): 2644-2651. |
| 105 | SARAK Sharad, PAGAR Amol D, KHOBRAGADE Taresh P, et al. A multienzyme biocatalytic cascade as a route towards the synthesis of α, ω-diamines from corresponding cycloalkanols[J]. Green Chemistry, 2023, 25(2): 543-549. |
| [1] | 李新月, 李振京, 韩沂杭, 郭永强, 闫瑜, 哈力米热·卡热木拉提, 赵会吉, 柴永明, 刘东, 殷长龙. 油脂加氢脱氧生产绿色柴油催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 351-364. |
| [2] | 李帅哲, 聂懿宸, PHIDSAVARD Keomeesay, 顾雯, 张伟, 刘娜, 徐高翔, 刘莹, 李兴勇, 陈玉保. 非贵金属催化生物质加氢脱氧制备烃基生物燃料的研究进展[J]. 化工进展, 2024, 43(S1): 225-242. |
| [3] | 熊磊, 丁飞燕, 李聪, 王群乐, 吕起, 翟晓娜, 刘峰. 金属Pt负载型非均相催化剂研究进展[J]. 化工进展, 2024, 43(S1): 295-304. |
| [4] | 宋财城, 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 碳基载体负载加氢脱硫催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 305-314. |
| [5] | 韩洪晶, 车宇, 田宇轩, 王海英, 张亚男, 陈彦广. 木质素催化氢解催化剂及溶剂的研究进展[J]. 化工进展, 2024, 43(S1): 315-324. |
| [6] | 胡兴, 刘易, 杜泽学. 3-氯丙烯直接合成环氧氯丙烷催化剂研究进展[J]. 化工进展, 2024, 43(S1): 325-334. |
| [7] | 于梦洁, 吴语童, 罗发祥, 豆义波. 低浓度二氧化碳还原光催化剂结构设计的研究进展[J]. 化工进展, 2024, 43(S1): 335-350. |
| [8] | 何世坤, 张荣花, 李昊阳, 潘晖, 冯君锋. 脱铝分子筛固体酸催化葡萄糖制备5-羟甲基糠醛[J]. 化工进展, 2024, 43(S1): 374-381. |
| [9] | 张日东, 吕建华, 刘继东, 郭豹, 李文松. Ru-K-NaY催化草酸二甲酯脱羰基制备碳酸二甲酯[J]. 化工进展, 2024, 43(S1): 382-390. |
| [10] | 高聪志, 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平. 新戊二醇的合成工艺[J]. 化工进展, 2024, 43(S1): 469-478. |
| [11] | 李琳, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王春霞, 王俊莲, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 铝基废催化剂载体的回收与再生制备[J]. 化工进展, 2024, 43(S1): 640-649. |
| [12] | 刘振涛, 梅金林, 王春雅, 段爱军, 巩雁军, 徐春明, 王喜龙. 一步法加氢制生物航煤催化剂研究进展[J]. 化工进展, 2024, 43(9): 4909-4924. |
| [13] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| [14] | 修浩然, 王云刚, 白彦渊, 刘涛, 张兴邦, 张益嘉. H2O2低温催化氧化法脱硫脱硝中试实验特性[J]. 化工进展, 2024, 43(9): 4941-4950. |
| [15] | 付维, 宁淑英, 蔡晨, 陈佳音, 周皞, 苏亚欣. Cu改性MIL-100(Fe)催化剂的SCR-C3H6脱硝特性[J]. 化工进展, 2024, 43(9): 4951-4960. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||