化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6620-6630.DOI: 10.16085/j.issn.1000-6613.2023-0090
• 资源与环境化工 • 上一篇
液化残渣基CO2吸附剂的制备与性能优化
崔倩1( ), 王岸楠1, 陈再明2, 孙峤昳1, 王保登1, 王永胜3, 孙楠楠4, 胡剑2, 李井峰5, 熊日华1(
), 王岸楠1, 陈再明2, 孙峤昳1, 王保登1, 王永胜3, 孙楠楠4, 胡剑2, 李井峰5, 熊日华1( )
)
- 1.国家能源集团北京低碳清洁能源研究院,北京 102209
2.国能浙江宁海发电有限公司,浙江 宁波 315612
3.中国神华煤制油化工有限公司鄂尔多斯煤制油分公司,内蒙古 鄂尔多斯 017209
4.中国科学院上海高等 研究院低碳转化科学与工程重点实验室,上海 201210
5.国家能源投资集团有限责任公司,北京 100011
-
收稿日期:2023-01-19修回日期:2023-03-20出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:熊日华 -
作者简介:崔倩(1991—),女,硕士,工程师,研究方向为CCUS、新能源。E-mail:qian.cui.c@chnenergy.com.cn。
Preparation and performance optimization of liquefied residue-based CO2 adsorbents
CUI Qian1( ), WANG Annan1, CHEN Zaiming2, SUN Qiaoyi1, WANG Baodeng1, WANG Yongsheng3, SUN Nannan4, HU Jian2, LI Jingfeng5, XIONG Rihua1(
), WANG Annan1, CHEN Zaiming2, SUN Qiaoyi1, WANG Baodeng1, WANG Yongsheng3, SUN Nannan4, HU Jian2, LI Jingfeng5, XIONG Rihua1( )
)
- 1.National Institute of Clean and Low Carbon Energy, Beijing 102209, China
2.Guoneng Zhejiang Ninghai Power Generation Co. , Ltd. , Ningbo 315612, Zhejiang, China
3.Ordos Coal-to-Oil Sub-Company, China Shenhua Coal-to-Oil Chemical Co. , Ltd. , Ordos 017209, Inner Mongolia, China
4.CAS Key Lab of Low-Carbon Conversion Science and Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
5.CHN Energy Investment Group Co. , Ltd. , Beijing 100011, China
-
Received:2023-01-19Revised:2023-03-20Online:2023-12-25Published:2024-01-08 -
Contact:XIONG Rihua
摘要:
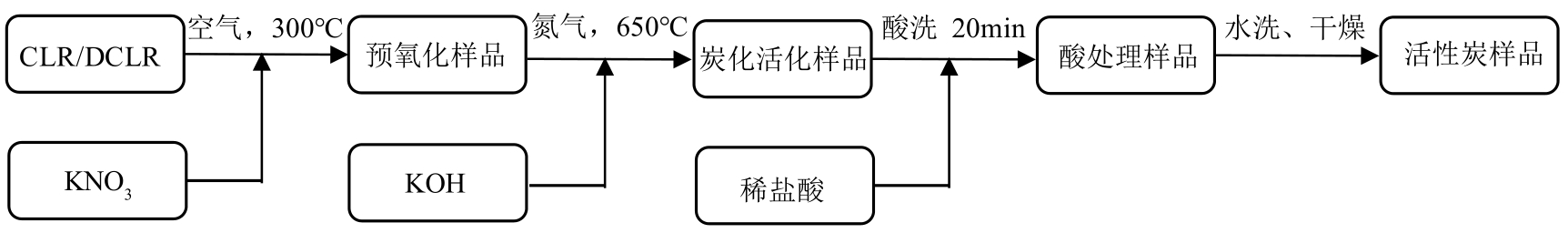
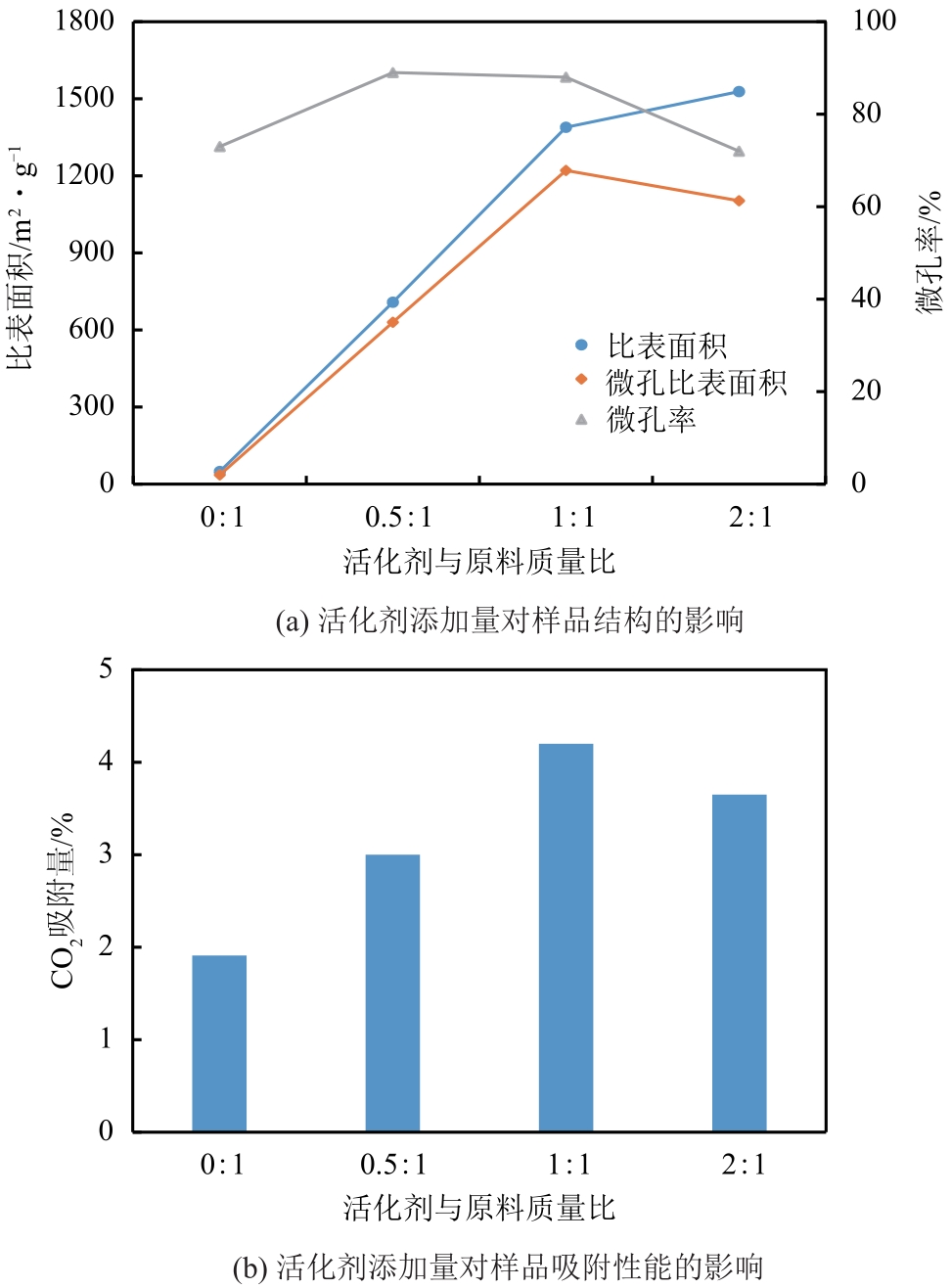
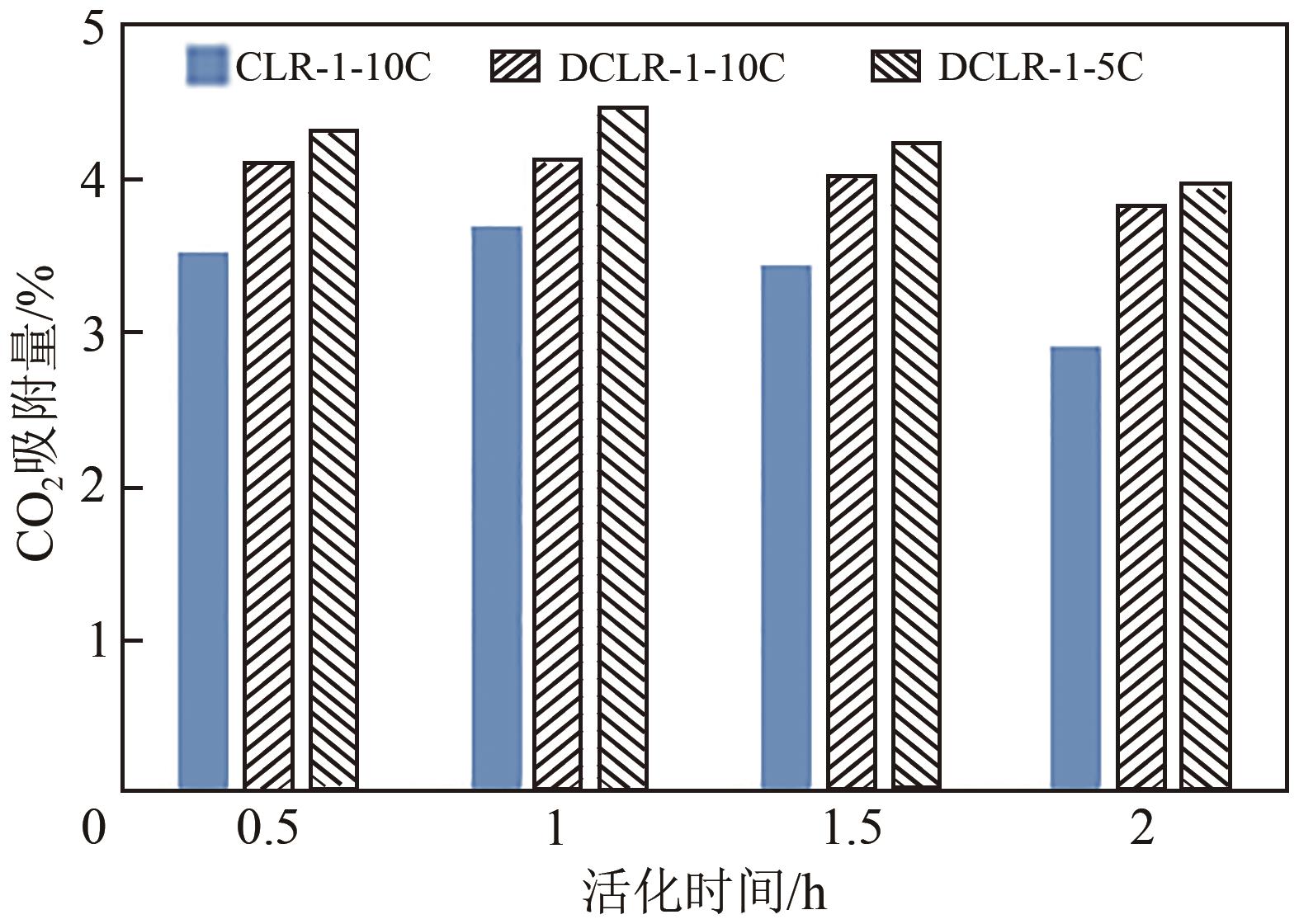
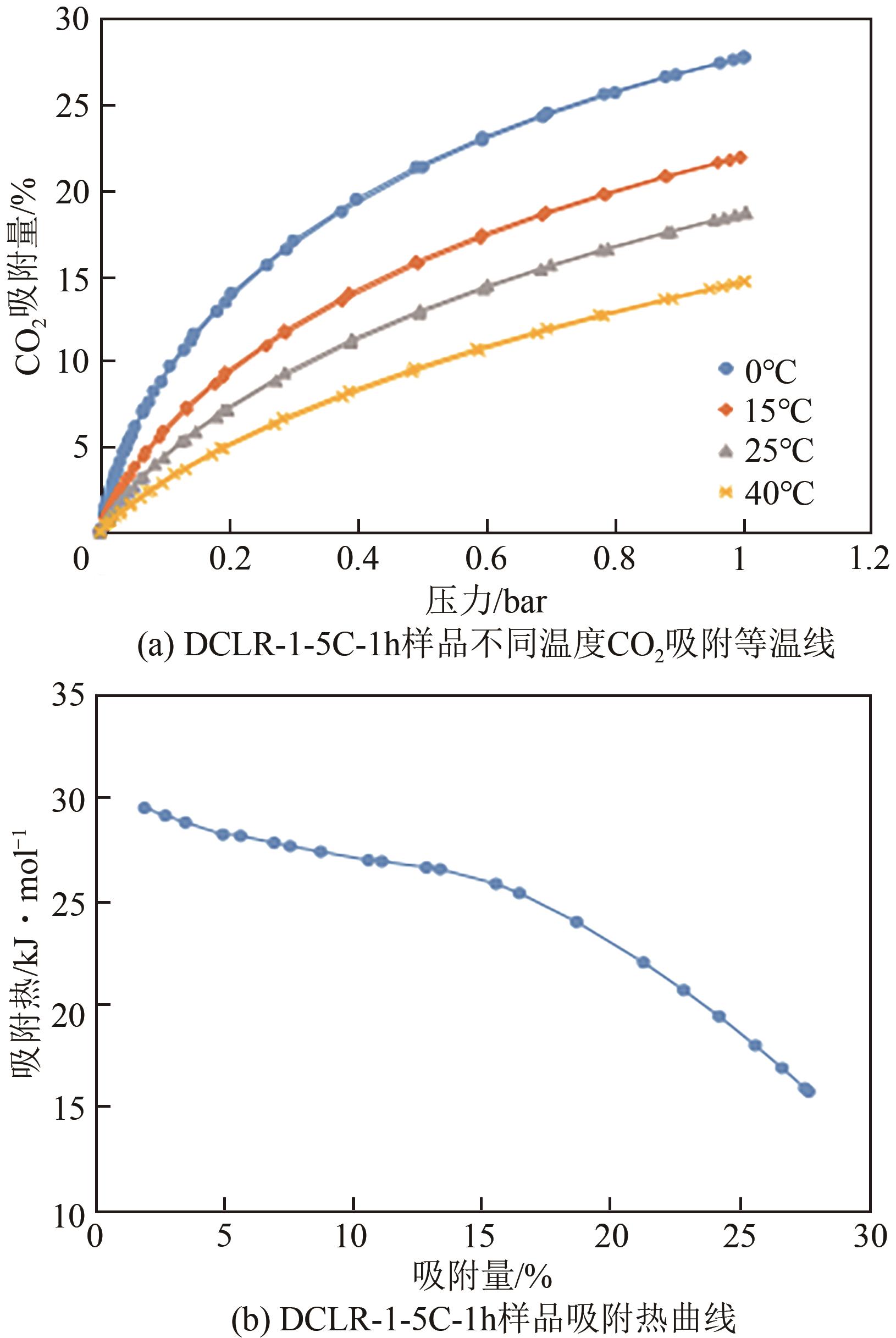
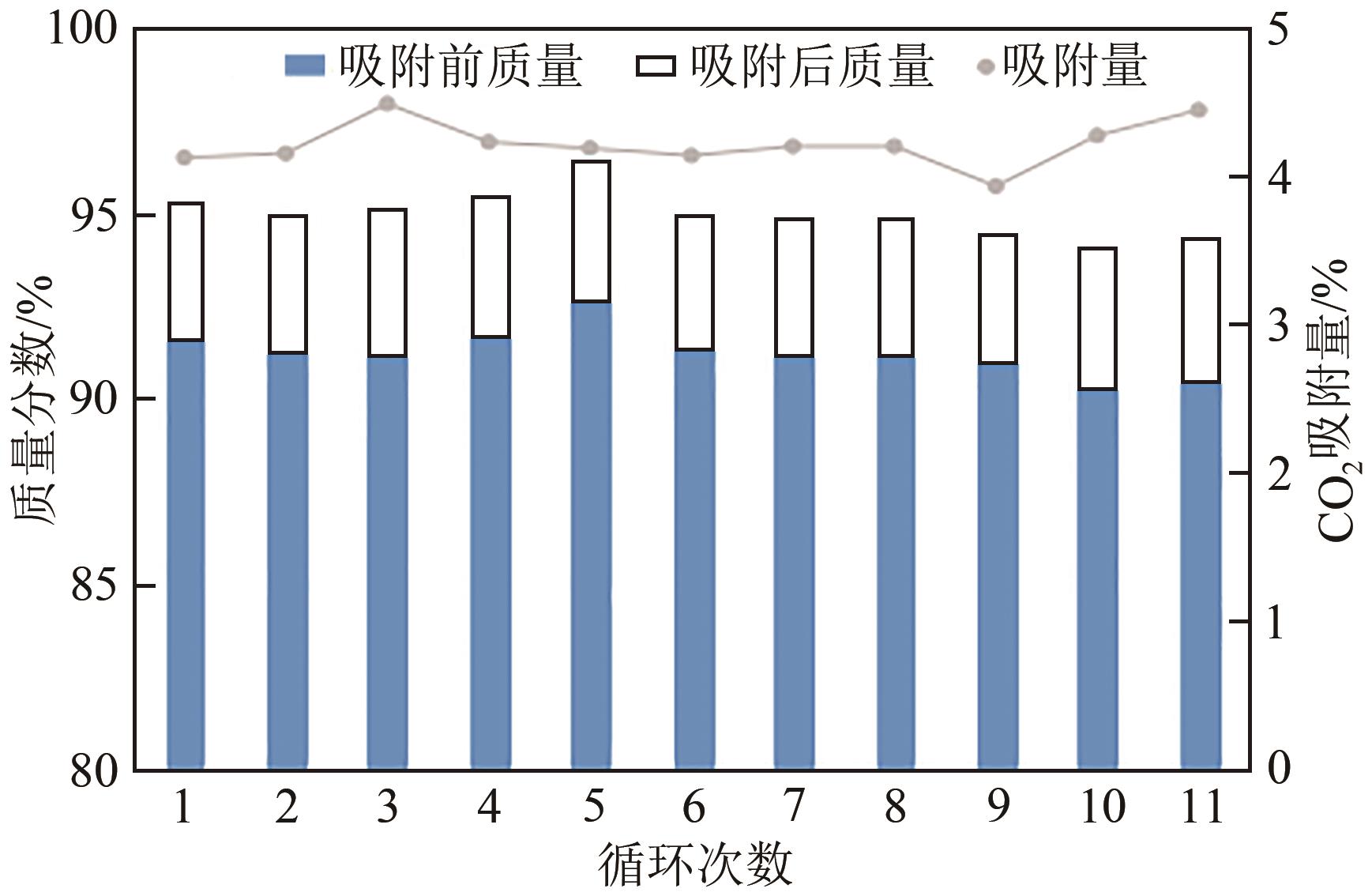
以煤液化残渣(CLR)为原料制备CO2吸附剂,在实现碳减排的同时,可有效提高煤直接液化工艺的经济性和环保价值。本文采用神华煤直接液化残渣为碳源,通过预氧化、炭化活化等工艺,制备出了吸附性能较优的CO2吸附剂。利用热重分析仪(TGA)、低温氮吸附仪(BET)、扫描电镜(SEM)以及透射电子显微镜(TEM)等分析手段对吸附材料的微观形貌、孔径结构以及吸附性能进行了表征测试。结果显示,低灰分液化残渣所制备的吸附材料具有更高的比表面积和更多的微孔结构,更有利于CO2的吸附。以低灰分液化残渣为原料,在较优活化条件下(活化剂/原料质量比为1∶1、升温速率5℃/min、活化时间1h)制备的CO2吸附剂表现出良好的吸附性能,在40℃、15%(体积分数)CO2模拟烟气条件下的CO2吸附容量为4.47%(质量分数),在0℃、1bar(1bar=105Pa)条件下的CO2吸附容量可高达27.70%(质量分数),低温吸附性能优异,吸附速率快且具有良好的循环稳定性。
中图分类号:
引用本文
崔倩, 王岸楠, 陈再明, 孙峤昳, 王保登, 王永胜, 孙楠楠, 胡剑, 李井峰, 熊日华. 液化残渣基CO2吸附剂的制备与性能优化[J]. 化工进展, 2023, 42(12): 6620-6630.
CUI Qian, WANG Annan, CHEN Zaiming, SUN Qiaoyi, WANG Baodeng, WANG Yongsheng, SUN Nannan, HU Jian, LI Jingfeng, XIONG Rihua. Preparation and performance optimization of liquefied residue-based CO2 adsorbents[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6620-6630.
| 样品 | 工业分析 | 元素分析 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | FCd | C | H | N | S | ||
| CLR | 0.31 | 26.28 | 33.56 | 40.17 | 67.60 | 4.26 | 0.53 | 4.99 | |
| DCLR | 0.26 | 0.35 | 51.91 | 47.75 | 91.20 | 6.41 | 1.14 | 0.21 | |
表1 煤液化残渣工业分析和元素分析(质量分数,%)
| 样品 | 工业分析 | 元素分析 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vd | FCd | C | H | N | S | ||
| CLR | 0.31 | 26.28 | 33.56 | 40.17 | 67.60 | 4.26 | 0.53 | 4.99 | |
| DCLR | 0.26 | 0.35 | 51.91 | 47.75 | 91.20 | 6.41 | 1.14 | 0.21 | |
| 样品 | 成分 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CaO | SO3 | SiO2 | Al2O3 | TiO2 | MgO | Na2O | 其他 | |
| CLR | 53.48 | 12.39 | 12.19 | 11.79 | 5.77 | 1.16 | 1.05 | 1.02 | 1.15 |
| DCLR | 45.88 | 11.92 | 22.33 | 7.13 | 4.70 | 1.08 | 1.57 | 3.99 | 1.40 |
表2 煤液化残渣的灰分分析(质量分数,%)
| 样品 | 成分 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe2O3 | CaO | SO3 | SiO2 | Al2O3 | TiO2 | MgO | Na2O | 其他 | |
| CLR | 53.48 | 12.39 | 12.19 | 11.79 | 5.77 | 1.16 | 1.05 | 1.02 | 1.15 |
| DCLR | 45.88 | 11.92 | 22.33 | 7.13 | 4.70 | 1.08 | 1.57 | 3.99 | 1.40 |
| 样品 | SBET/m2·g-1 | Smic /m2·g-1 | Vtol /cm3·g-1 | Vmic /cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-1-10C-0.5h | 1308 | 1103 | 0.61 | 0.46 | 4.73 |
| CLR-1-10C-1h | 1259 | 1121 | 0.59 | 0.47 | 2.98 |
| CLR-1-10C-1.5h | 1251 | 1038 | 0.57 | 0.43 | 4.65 |
| CLR-1-10C-2h | 1328 | 996 | 0.78 | 0.39 | 4.84 |
表3 样品结构性质数据
| 样品 | SBET/m2·g-1 | Smic /m2·g-1 | Vtol /cm3·g-1 | Vmic /cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-1-10C-0.5h | 1308 | 1103 | 0.61 | 0.46 | 4.73 |
| CLR-1-10C-1h | 1259 | 1121 | 0.59 | 0.47 | 2.98 |
| CLR-1-10C-1.5h | 1251 | 1038 | 0.57 | 0.43 | 4.65 |
| CLR-1-10C-2h | 1328 | 996 | 0.78 | 0.39 | 4.84 |
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-1-3C-1h | 1311 | 1244 | 0.60 | 0.53 | 1.83 |
| CLR-1-5C-1h | 1389 | 1221 | 0.63 | 0.52 | 2.22 |
| CLR-1-10C-1h | 1259 | 1121 | 0.59 | 0.47 | 2.98 |
表4 样品结构性质数据
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-1-3C-1h | 1311 | 1244 | 0.60 | 0.53 | 1.83 |
| CLR-1-5C-1h | 1389 | 1221 | 0.63 | 0.52 | 2.22 |
| CLR-1-10C-1h | 1259 | 1121 | 0.59 | 0.47 | 2.98 |
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-0-5C-1h | 49 | 36 | 0.03 | 0.01 | 3.98 |
| CLR-0.5-5C-1h | 708 | 630 | 0.37 | 0.24 | 2.10 |
| CLR-1-5C-1h | 1389 | 1221 | 0.63 | 0.52 | 2.22 |
| CLR-2-5C-1h | 1528 | 1103 | 0.95 | 0.50 | 2.87 |
表5 样品结构性质数据
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-0-5C-1h | 49 | 36 | 0.03 | 0.01 | 3.98 |
| CLR-0.5-5C-1h | 708 | 630 | 0.37 | 0.24 | 2.10 |
| CLR-1-5C-1h | 1389 | 1221 | 0.63 | 0.52 | 2.22 |
| CLR-2-5C-1h | 1528 | 1103 | 0.95 | 0.50 | 2.87 |
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
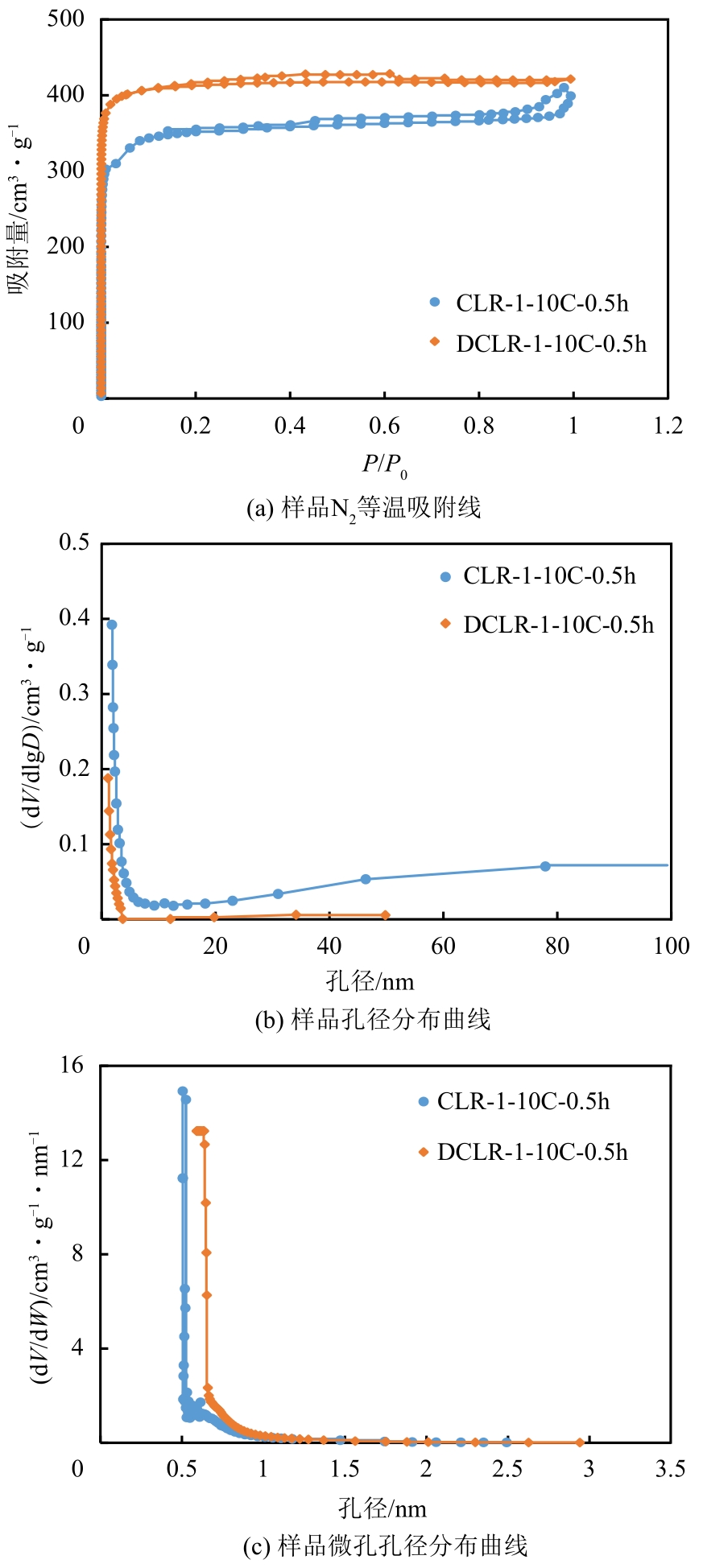
| CLR-1-10C-0.5h | 1308 | 1103 | 0.61 | 0.46 | 4.73 |
| DCLR-1-10C-0.5h | 1673 | 1534 | 0.63 | 0.58 | 1.66 |
表6 样品结构性质数据
| 样品 | SBET/m2·g-1 | Smic/m2·g-1 | Vtol/cm3·g-1 | Vmic/cm3·g-1 | Da/nm |
|---|---|---|---|---|---|
| CLR-1-10C-0.5h | 1308 | 1103 | 0.61 | 0.46 | 4.73 |
| DCLR-1-10C-0.5h | 1673 | 1534 | 0.63 | 0.58 | 1.66 |
| 样品 | 灰分 | Fe2O3 | CaO | SO3 | SiO2 | Al2O3 | TiO2 | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLR-1-10C-0.5h | 2.54 | 36.37 | 1.31 | 0.63 | 50.14 | 3.62 | 2.44 | 0.47 | 2.81 | 2.00 | 0.21 |
| DCLR-1-10C-0.5h | 1.21 | 23.83 | 0.80 | 0.70 | 63.49 | 2.87 | 0.42 | 0.25 | 3.11 | 4.24 | 0.29 |
表7 样品灰分含量及成分(质量分数,%)
| 样品 | 灰分 | Fe2O3 | CaO | SO3 | SiO2 | Al2O3 | TiO2 | MgO | Na2O | K2O | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLR-1-10C-0.5h | 2.54 | 36.37 | 1.31 | 0.63 | 50.14 | 3.62 | 2.44 | 0.47 | 2.81 | 2.00 | 0.21 |
| DCLR-1-10C-0.5h | 1.21 | 23.83 | 0.80 | 0.70 | 63.49 | 2.87 | 0.42 | 0.25 | 3.11 | 4.24 | 0.29 |
| 项目 | 前体 | 活化剂 | 吸附温度/℃ | 吸附压力/bar | CO2吸附量/% |
|---|---|---|---|---|---|
| DCLR-1-5C-1h | 液化残渣 | KOH | 40 | 0.15 | 4.47 |
| 0 | 1 | 27.70 | |||
| 文献[ | 淀粉/纤维素/木屑 | KOH | 0 | 1 | 25.52 |
| 文献[ | 莴笋叶 | KOH | 0 | 1 | 26.40 |
| 文献[ | 亚烟煤 | K2CO3+ CO2 | 0 | 1 | 19.18 |
| 文献[ | 榛子壳 | NaNH2 | 0 | 1 | 26.00 |
| 文献[ | 无烟煤 | H2O | 0 | 1 | 12.89 |
| 文献[ | 咖啡渣 | CO2 | 0 | 1 | 14.08 |
| 市售活性炭1 | 煤质 | — | 40 | 0.15 | 2.57 |
| 市售活性炭2 | 椰壳 | — | 40 | 0.15 | 3.48 |
| 市售活性炭3 | 椰壳 | — | 40 | 0.15 | 2.91 |
表8 不同原料活性炭样品CO2吸附性能对照表
| 项目 | 前体 | 活化剂 | 吸附温度/℃ | 吸附压力/bar | CO2吸附量/% |
|---|---|---|---|---|---|
| DCLR-1-5C-1h | 液化残渣 | KOH | 40 | 0.15 | 4.47 |
| 0 | 1 | 27.70 | |||
| 文献[ | 淀粉/纤维素/木屑 | KOH | 0 | 1 | 25.52 |
| 文献[ | 莴笋叶 | KOH | 0 | 1 | 26.40 |
| 文献[ | 亚烟煤 | K2CO3+ CO2 | 0 | 1 | 19.18 |
| 文献[ | 榛子壳 | NaNH2 | 0 | 1 | 26.00 |
| 文献[ | 无烟煤 | H2O | 0 | 1 | 12.89 |
| 文献[ | 咖啡渣 | CO2 | 0 | 1 | 14.08 |
| 市售活性炭1 | 煤质 | — | 40 | 0.15 | 2.57 |
| 市售活性炭2 | 椰壳 | — | 40 | 0.15 | 3.48 |
| 市售活性炭3 | 椰壳 | — | 40 | 0.15 | 2.91 |
| 1 | 蔡博峰, 李琦, 张贤, 等. 中国二氧化碳捕集、利用与封存 (CCUS) 年度报告: 中国 CCUS 路径研究[R]. 生态环境部环境规划院, 中国科学院武汉岩土力学研究所, 中国 21 世纪议程管理中心, 2021. |
| CAI Bofeng, LI Qi, ZHANG Xian, et al. China carbon dioxide capture, utilization and storage (CCUS) annual report: China CCUS pathway study[R]. Institute of Environmental Planning, Ministry of Ecology and Environment, Wuhan Institute of Rock and Soil Mechanics, Chinese Academy of Sciences, China Management Center for Agenda 21, 2021. | |
| 2 | 宋亚楠. CCUS技术的减排作用与应用前景[J]. 金融纵横, 2021(9): 35-43. |
| SONG Yanan. Emission reduction effects and application prospects of CCUS[J]. Financial Perspectives Journal, 2021(9): 35-43. | |
| 3 | 李禾. 任重道远 CCUS技术体系尚待完善[N]. 科技日报, 2022-09-27(5). |
| LI He. The CCUS technology system still needs to be improved[N]. Science and Technology Daily, 2022-09-27(5). | |
| 4 | 何亮, 王延斌, 张景阳. 厚积薄发 CCUS重点工程加速推进[N]. 科技日报, 2022-09-27(5). |
| HE Liang, WANG Yanbing, ZHANG Jingyang. Accelerate the progress of CCUS key projects through thick accumulation and thin development[N]. Science and Technology Daily, 2022-09-27(5). | |
| 5 | 何亮. 变害为宝 CCUS助力“双碳”目标实现[N]. 科技日报, 2022-09-27(5). |
| HE Liang. Turning harm into treasure CCUS helps achieve the “double carbon” goal[N]. Science and Technology Daily, 2022-09-27(5). | |
| 6 | WANG Baodeng, CUI Qian, ZHANG Guoping, et al. Post-combustion slipstream CO2-capture test facility at Jiangyou Power Plant, Sichuan, China: Facility design and validation using 30% wt monoethanolamine (MEA) testing[J]. Clean Energy, 2020, 4(2): 107-119. |
| 7 | CUI Qian, WANG Baodeng, ZHAO Xinglei, et al. Post-combustion slipstream CO2-capture test facility at Jiangyou Power Plant, Sichuan, China: Performance of a membrane separation module under dynamic power-plant operations[J]. Clean Energy, 2021, 5(4): 742-755. |
| 8 | CAO Shicheng, ZHAO Hongyu, HU Deng, et al. Preparation of potassium intercalated carbons by in situ activation and speciation for CO2 capture from flue gas[J]. Journal of CO2 Utilization, 2020, 35: 59-66. |
| 9 | WANG Baodeng, ZHANG Zhongzheng, ZHU Chenming, et al. Enhancing low pressure CO2 adsorption of solvent-free derived mesoporous carbon by highly dispersed potassium species[J]. RSC Advances, 2016, 6(40): 33580-33588. |
| 10 | BALSAMO M, BUDINOVA T, ERTO A, et al. CO2 adsorption onto synthetic activated carbon: Kinetic, thermodynamic and regeneration studies[J]. Separation and Purification Technology, 2013, 116: 214-221. |
| 11 | 刘之琳. 氨基功能化MCM-41材料制备及其二氧化碳吸附性能研究[D]. 北京: 华北电力大学(北京), 2016. |
| LIU Zhilin. Preparation and CO2 adsorption performance of amine-functionalized MCM-41[D]. Beijing: North China Electric Power University(Beijing), 2016. | |
| 12 | SJOSTROM Sharon, SENIOR Constance. Pilot testing of CO2 capture from a coal-fired power plant—Part 2: Results from 1-MWe pilot tests[J]. Clean Energy, 2020, 4(1): 12-25. |
| 13 | 何利梅, 姜伟丽, 李继聪, 等. CO2吸附材料的研究进展[J]. 石油化工, 2022, 51(1): 83-91. |
| HE Limei, JIANG Weili, LI Jicong, et al. Research progress in the adsorption materials of CO2 [J]. Petrochemical Technology, 2022, 51(1): 83-91. | |
| 14 | MESFER Mohammed K AL. Synthesis and characterization of high-performance activated carbon from walnut shell biomass for CO2 capture[J]. Environmental Science and Pollution Research, 2020, 27(13): 15020-15028. |
| 15 | ZENG Ganning, LOU Sa, YING Huijuan, et al. Preparation of microporous carbon from Sargassum horneri by hydrothermal carbonization and KOH activation for CO2 capture[J]. Journal of Chemistry, 2018, 2018: 1-11. |
| 16 | KHUONG Duy Anh, NGUYEN Hong Nam, TSUBOTA Toshiki. Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents[J]. Biomass and Bioenergy, 2021, 148: 106039. |
| 17 | 张建波. 煤直接液化残渣基炭材料的制备及应用[D]. 大连: 大连理工大学, 2013. |
| ZHANG Jianbo. Preparation and applications of carbon materials from direct coal liquefaction residue[D]. Dalian: Dalian University of Technology, 2013. | |
| 18 | 段林娥. 液化残渣在CO2气氛下热转化特性热重研究[D]. 西安: 西北大学, 2015. |
| DUAN Lin'e. Thermogravimetric study on thermal conversion characteristics of liquefied residue in CO2 atmosphere.[D]. Xi'an: Northwest University, 2015. | |
| 19 | 舒成, 李克健, 章序文, 等. 原料预处理对氢氧化钾活化法制备活性炭的影响[J]. 炭素技术, 2014, 33(2): 5-8. |
| SHU Cheng, LI Kejian, ZHANG Xuwen, et al. Effects of raw material pretreatment on activated carbons prepared with KOH activation[J]. Carbon Techniques, 2014, 33(2): 5-8. | |
| 20 | 辛凡文, 李克健, 舒歌平, 等. 预氧化对煤液化沥青制备超级活性炭的影响研究[J]. 煤炭工程, 2017, 49(8): 141-144. |
| XIN Fanwen, LI Kejian, SHU Geping, et al. Influence of pre-oxidation to super activated carbon prepared from coal liquefaction asphaltene[J]. Coal Engineering, 2017, 49(8): 141-144. | |
| 21 | 罗化峰, 乔元栋, 李通达, 等. 煤液化残渣基炭材料吸附瓦斯实验研究[J]. 煤炭技术, 2020, 39(1): 89-92. |
| LUO Huafeng, QIAO Yuandong, LI Tongda, et al. Experimental study on adsorption of gas by coal liquefaction residue based carbon material[J]. Coal Technology, 2020, 39(1): 89-92. | |
| 22 | 徐春霞. 煤直接液化残渣半焦CO2气化特性及动力学研究[J]. 煤炭加工与综合利用, 2021(7): 62-67. |
| XU Chunxia. Study on characteristics anddynamics of direct liquefaction residue semi-coke gasification with CO2 [J]. Coal Processing & Comprehensive Utilization, 2021(7): 62-67. | |
| 23 | 李肖, 李昱琳, 田晓冬, 等. 煤液化残渣的性质及应用现状[J]. 化学研究, 2020, 31(1): 9-16, 95. |
| LI Xiao, LI Yulin, TIAN Xiaodong, et al. Properties and application of coal liquefaction residues[J]. Chemical Research, 2020, 31(1): 9-16, 95. | |
| 24 | LEE Seul-Yi, PARK Soo-Jin. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers[J]. Journal of Colloid and Interface Science, 2013, 389(1): 230-235. |
| 25 | JANG Eunji, CHOI Seung Wan, HONG Seok-Min, et al. Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation[J]. Applied Surface Science, 2018, 429: 62-71. |
| 26 | KIM Min-Jeong, CHOI Seung Wan, KIM Hyunwook, et al. Simple synthesis of spent coffee ground-based microporous carbons using K2CO3 as an activation agent and their application to CO2 capture[J]. Chemical Engineering Journal, 2020, 397: 125404. |
| 27 | SEVILLA Marta, FUERTES Antonio B. Sustainable porous carbons with a superior performance for CO2 capture[J]. Energy & Environmental Science, 2011, 4(5): 1765-1771. |
| 28 | WANG Jiacheng, HEERWIG Andreas, LOHE Martin R, et al. Fungi-based porous carbons for CO2 adsorption and separation[J]. Journal of Materials Chemistry, 2012, 22(28): 13911-13913. |
| 29 | WANG Lijie, SUN Fei, HAO Fei, et al. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors[J]. Chemical Engineering Journal, 2020, 383: 123205. |
| 30 | LIU Shenfang, MA Rui, HU Xin, et al. CO2 adsorption on hazelnut-shell-derived nitrogen-doped porous carbons synthesized by single-step sodium amide activation[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 7046-7053. |
| 31 | 张双全, 马蓉, 朱文魁, 等. 用于吸附分离CO2的活性炭研究[J]. 中国矿业大学学报, 2004, 33(6): 683-686. |
| ZHANG Shuangquan, MA Rong, ZHU Wenkui, et al. Research on activated carbon for separating CO2 by adsorption[J]. Journal of China University of Mining & Technology, 2004, 33(6): 683-686. | |
| 32 | PLAZA M G, GONZÁLEZ A S, PEVIDA C, et al. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications[J]. Applied Energy, 2012, 99: 272-279. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [7] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [13] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [14] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [15] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||