化工进展 ›› 2023, Vol. 42 ›› Issue (11): 5756-5763.DOI: 10.16085/j.issn.1000-6613.2023-0020
调节剂对UiO-66在糠醛转移加氢制糠醇反应中催化性能的影响
谢有为1( ), 陈静1, 于峰1, 史秀锋2, 范彬彬1(
), 陈静1, 于峰1, 史秀锋2, 范彬彬1( ), 李瑞丰1
), 李瑞丰1
- 1.太原理工大学化学工程与技术学院,山西 太原 030024
2.太原理工大学化学学院,山西 太原 030024
-
收稿日期:2023-01-06修回日期:2023-02-11出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:范彬彬 -
作者简介:谢有为(1997—),男,硕士研究生,研究方向为MOFs合成及催化性能。E-mail:1039258083@qq.com。 -
基金资助:国家自然科学基金(22178244);山西省自然科学基金(20210302123118)
Effect of regulators on the catalytic performance of UiO-66 in furfural transfer hydrogenation to furfuryl alcohol
XIE Youwei1( ), CHEN Jing1, YU Feng1, SHI Xiufeng2, FAN Binbin1(
), CHEN Jing1, YU Feng1, SHI Xiufeng2, FAN Binbin1( ), LI Ruifeng1
), LI Ruifeng1
- 1.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.College of Chemistry, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2023-01-06Revised:2023-02-11Online:2023-11-20Published:2023-12-15 -
Contact:FAN Binbin
摘要:
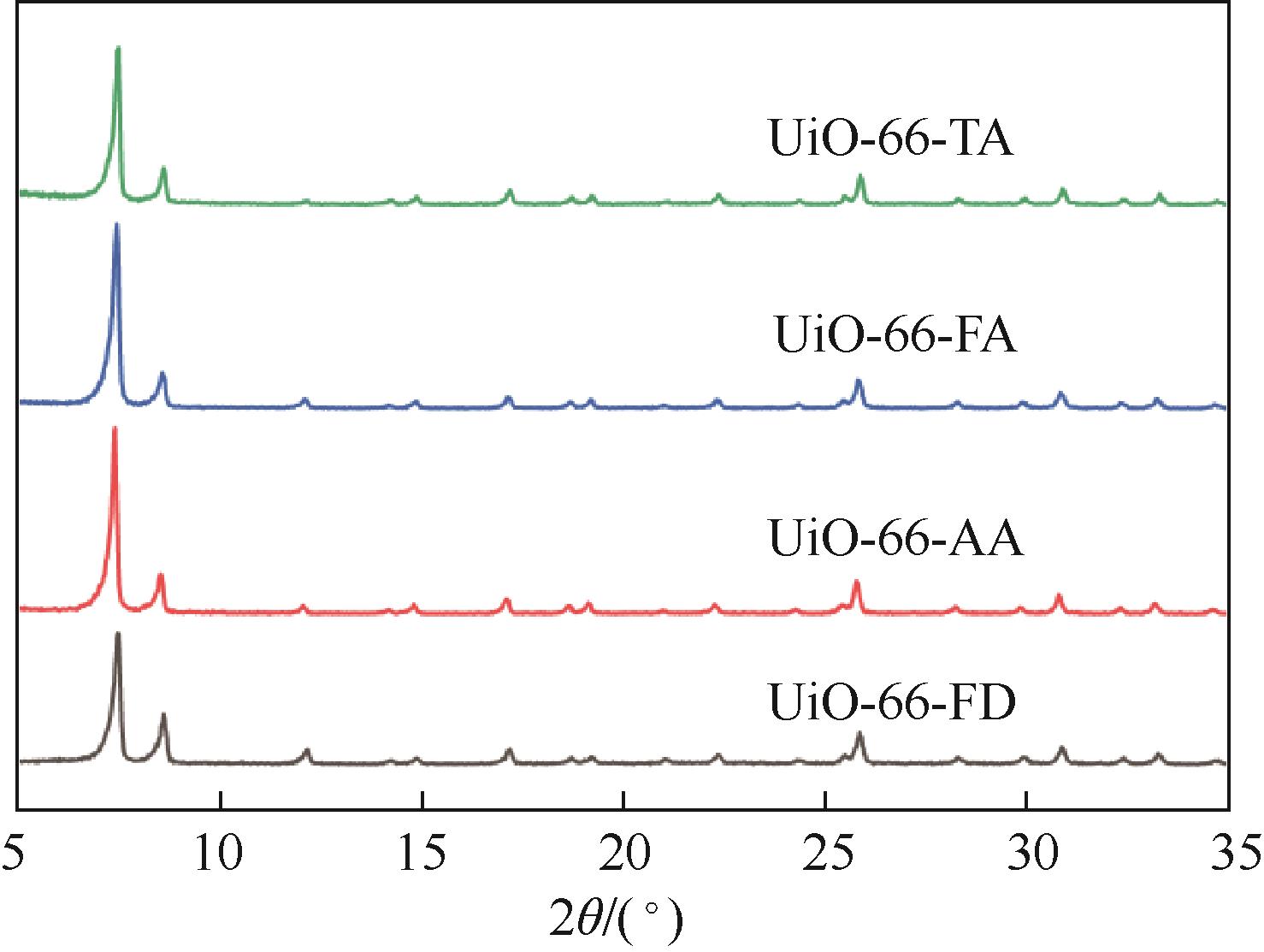
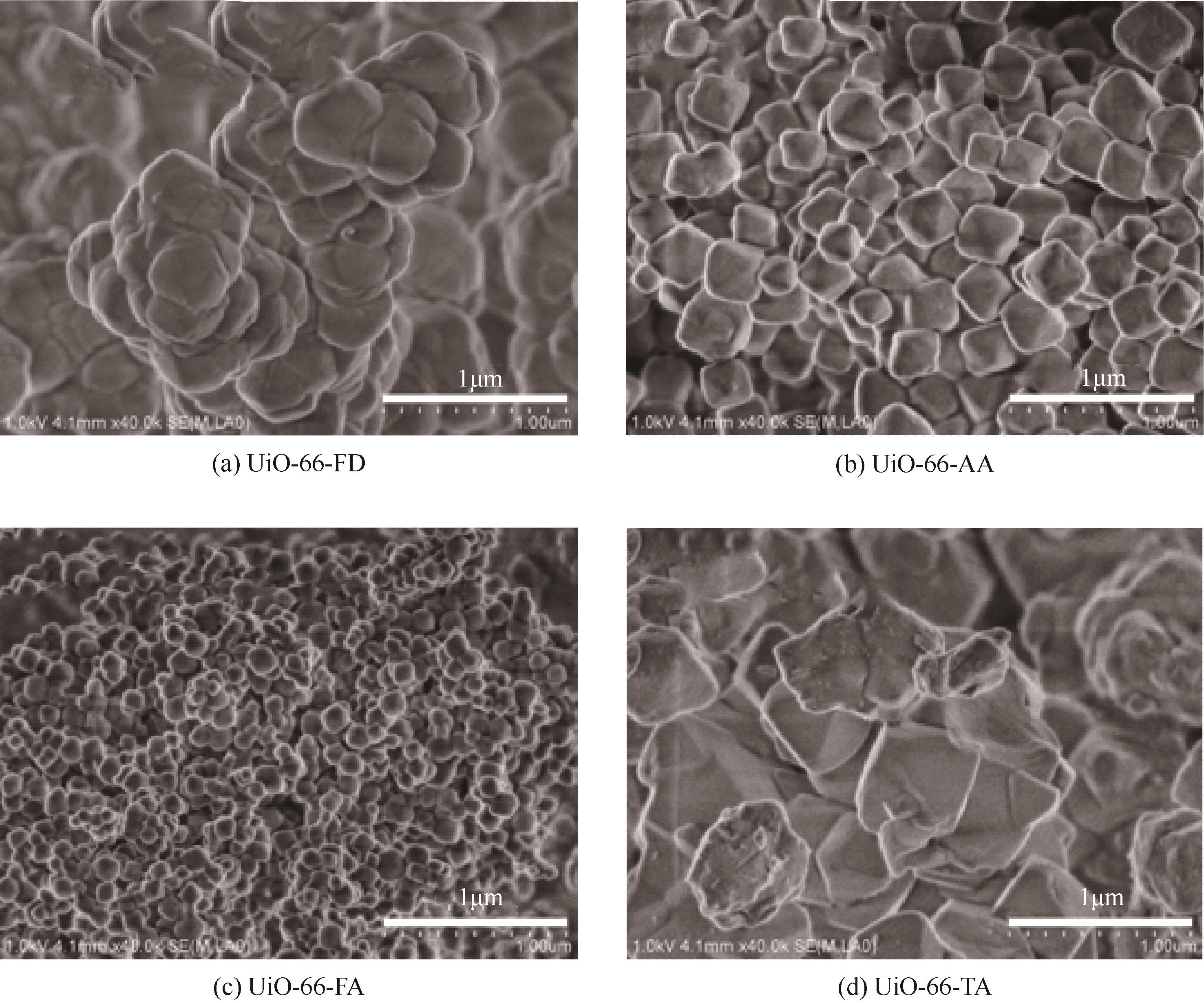
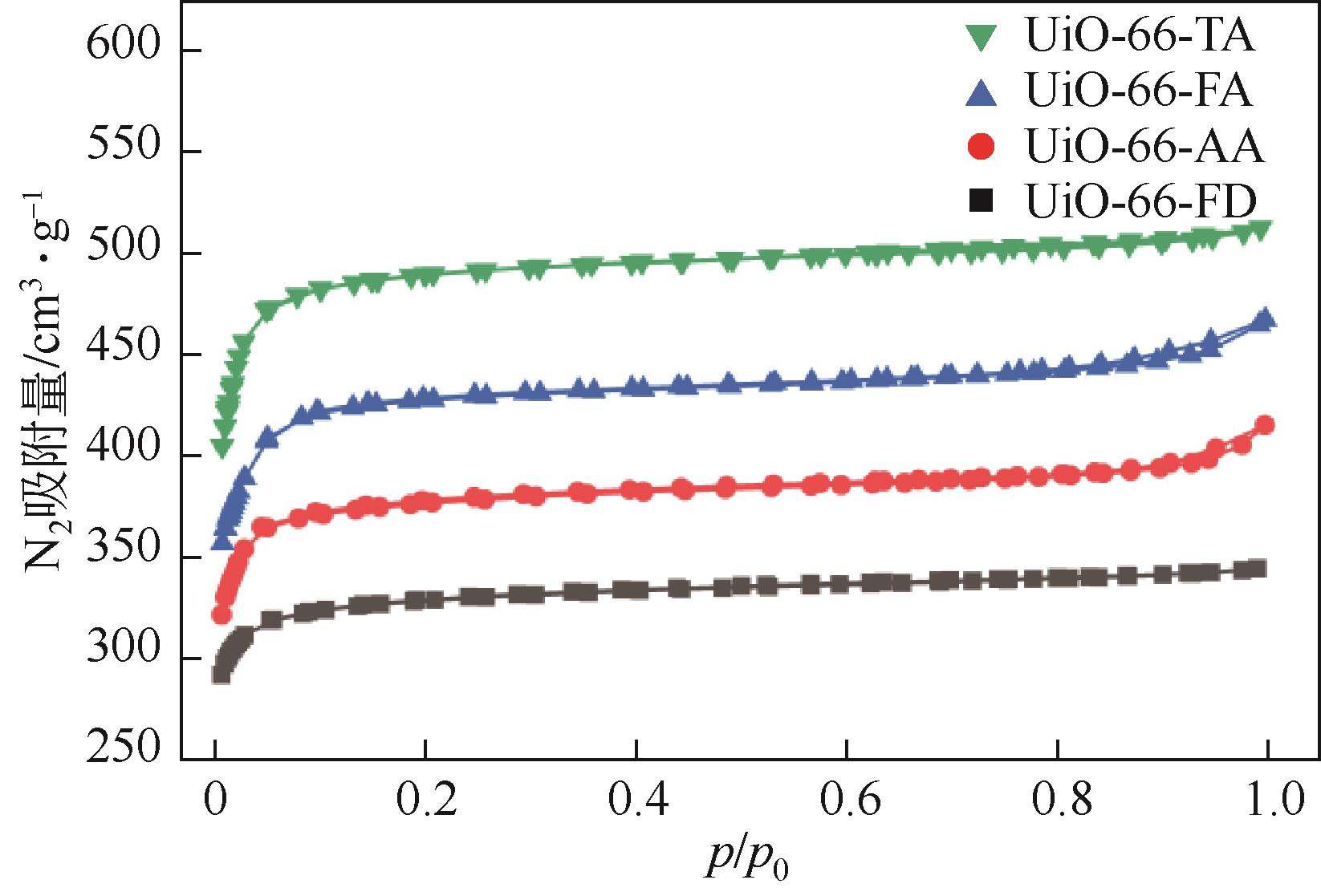
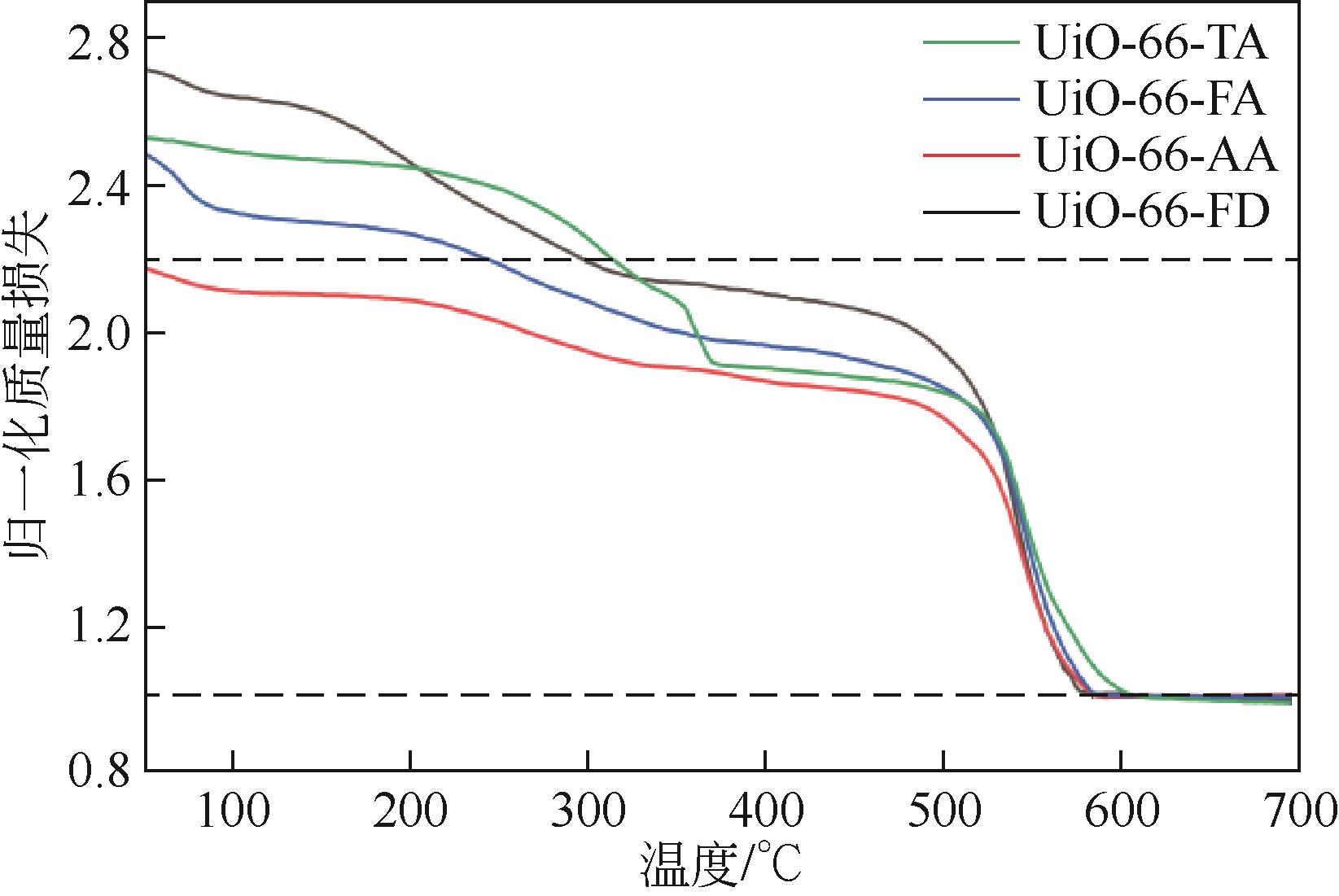
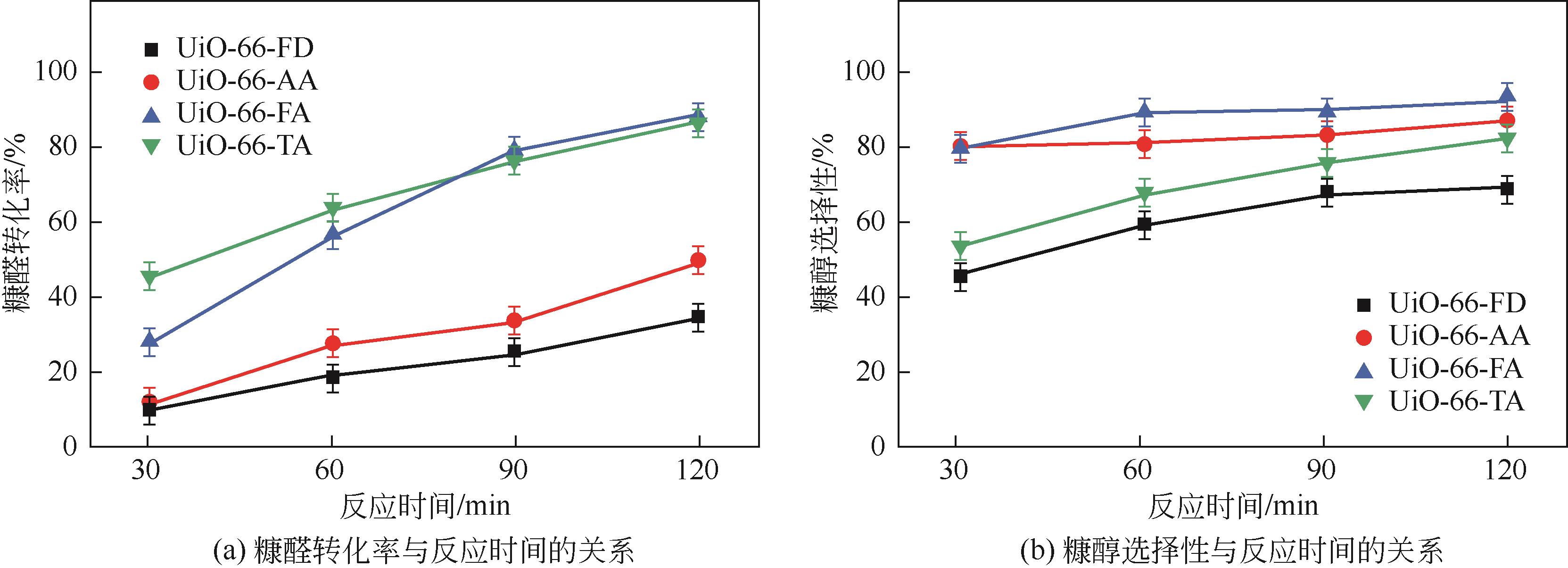
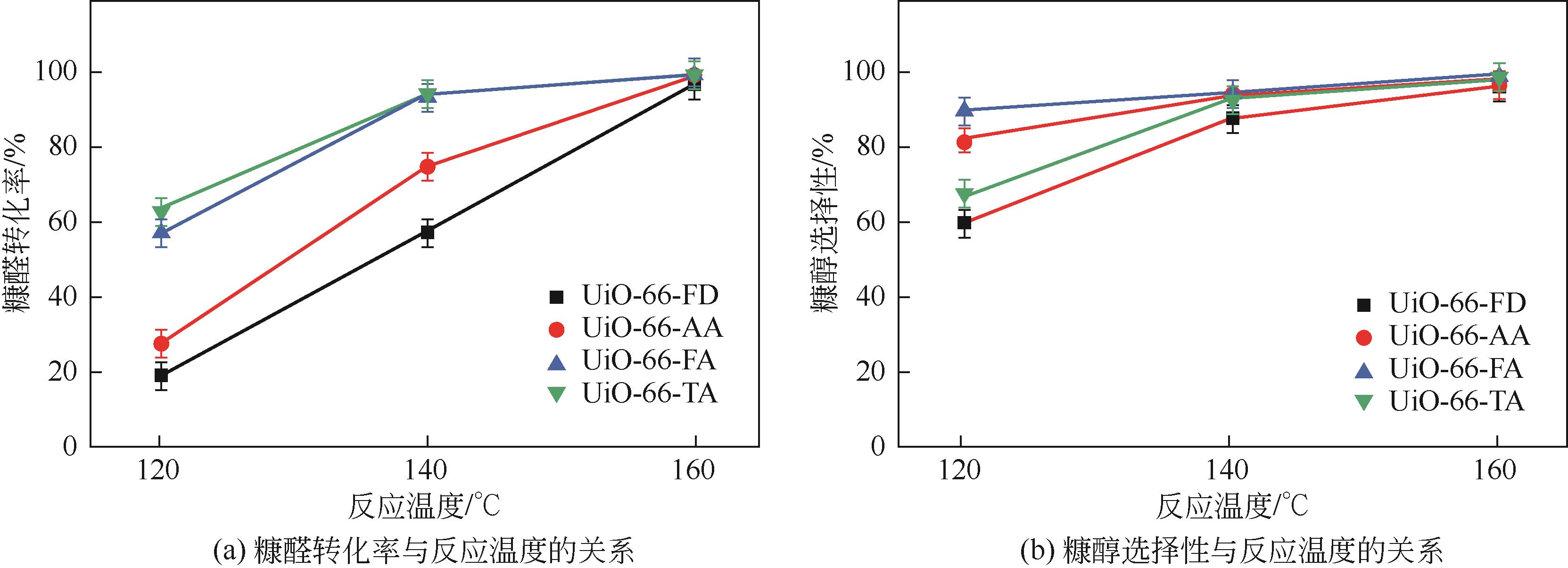
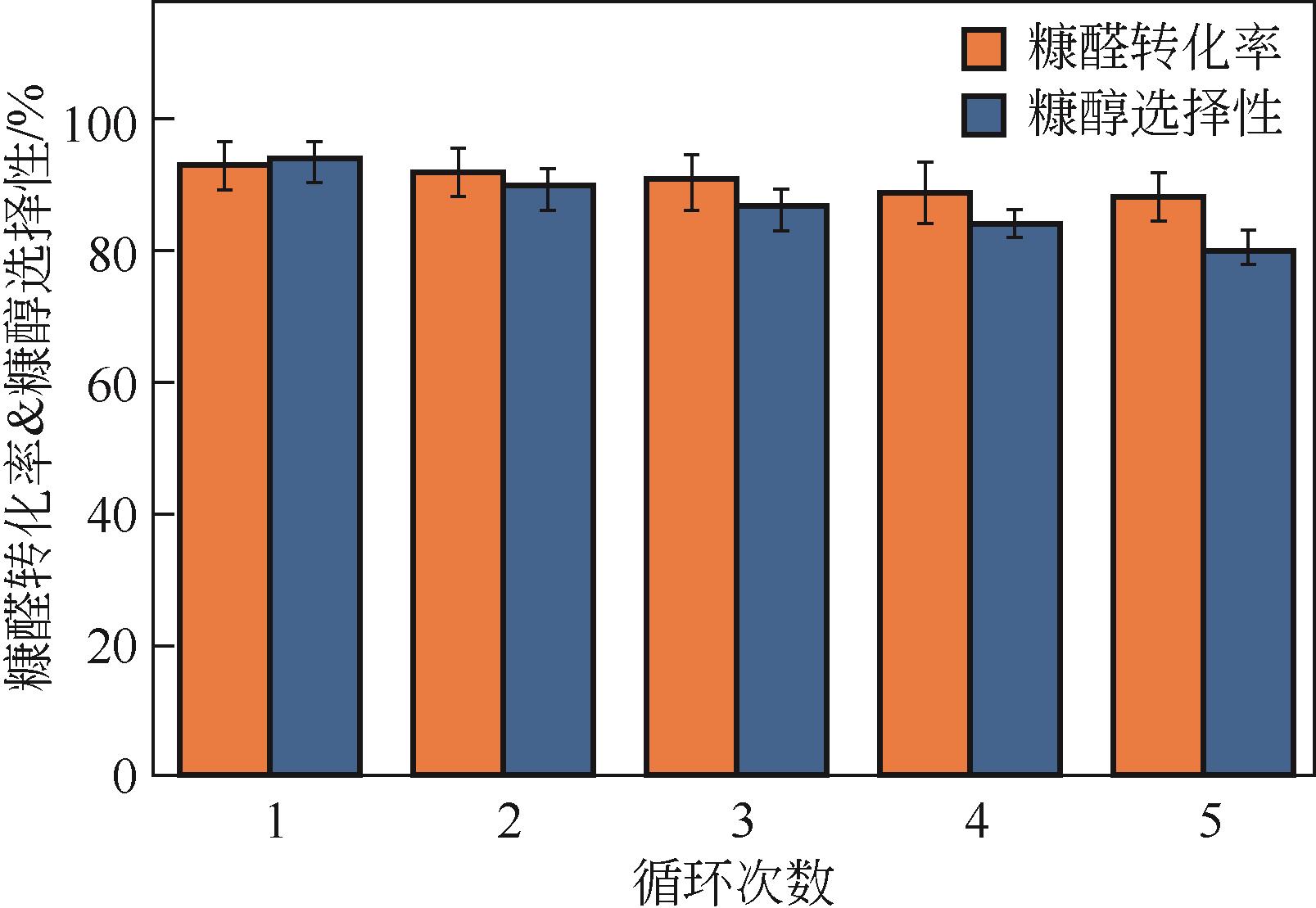
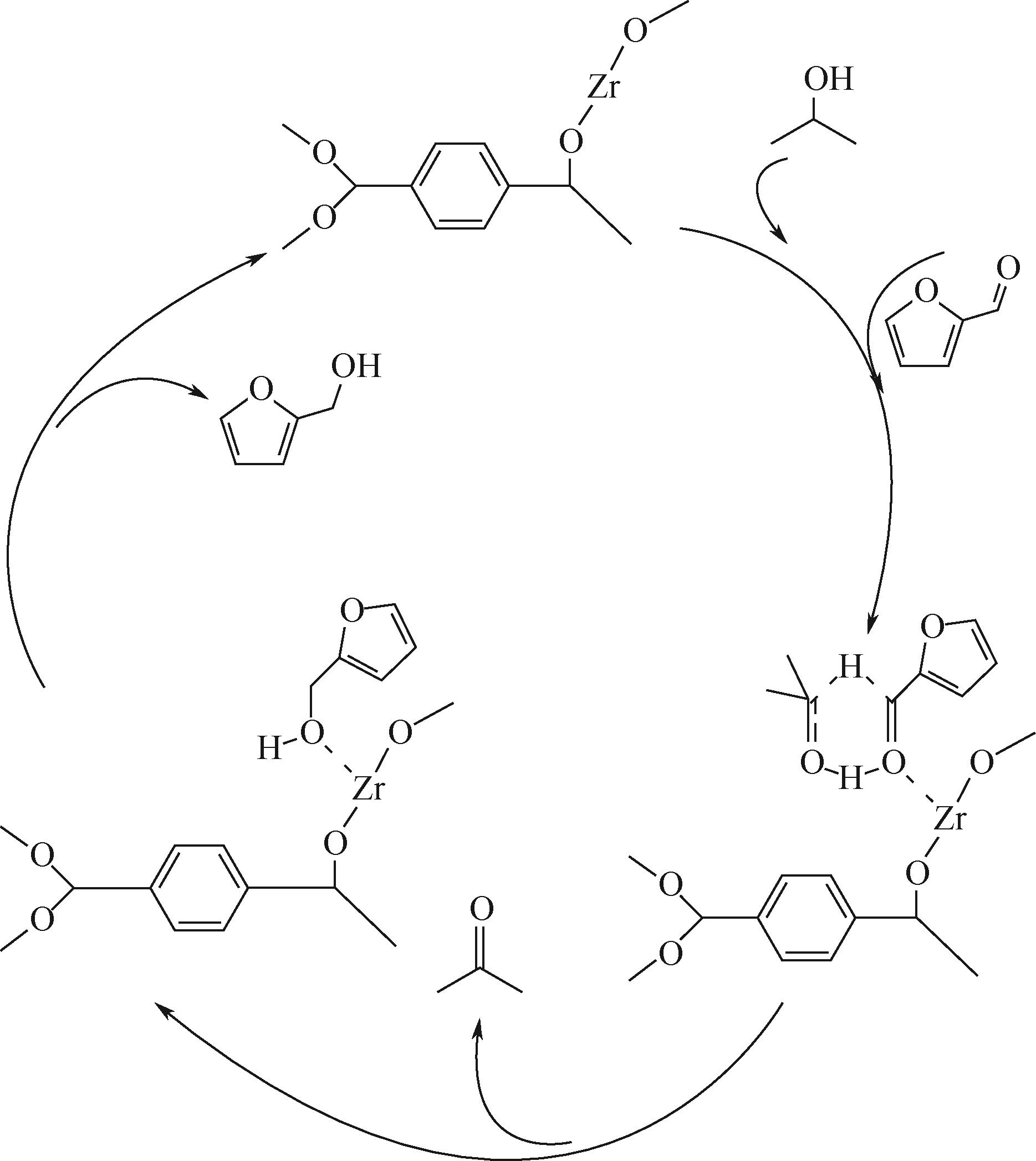
糠醛高选择性催化转移加氢制糠醇是生物质高值化利用的重要途径之一,本文以甲酸、乙酸及三氟乙酸为调节剂制备了具有不同缺陷位点的UiO-66,利用XRD、SEM、NH3-TPD、TG和N2吸附脱附等手段进行详细表征,并以异丙醇为溶剂和氢供体,考察了UiO-66催化剂缺陷位、酸性和织构性质对催化糠醛转移加氢反应的影响。结果表明,UiO-66催化剂酸性的增强有利于糠醛转移加氢反应中糠醇选择性的提高,以甲酸为调节剂制备的UiO-66在160℃的反应温度下,反应1h后糠醛的转化率和对糠醇的选择性分别达到99.4%和98.9%。
中图分类号:
引用本文
谢有为, 陈静, 于峰, 史秀锋, 范彬彬, 李瑞丰. 调节剂对UiO-66在糠醛转移加氢制糠醇反应中催化性能的影响[J]. 化工进展, 2023, 42(11): 5756-5763.
XIE Youwei, CHEN Jing, YU Feng, SHI Xiufeng, FAN Binbin, LI Ruifeng. Effect of regulators on the catalytic performance of UiO-66 in furfural transfer hydrogenation to furfuryl alcohol[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5756-5763.
| 样品 | BET比表面积/m2·g-1 | 微孔面积/m2·g-1 | 外表面积/m2·g-1 | 总孔体积/cm2·g-1 | 微孔体积/cm2·g-1 | 介孔体积/cm2·g-1 |
|---|---|---|---|---|---|---|
| UiO-66-FD | 942 | 882 | 60 | 0.39 | 0.34 | 0.05 |
| UiO-66-AA | 862 | 794 | 68 | 0.39 | 0.30 | 0.09 |
| UiO-66-FA | 938 | 876 | 62 | 0.42 | 0.34 | 0.09 |
| UiO-66-TA | 1034 | 957 | 77 | 0.42 | 0.36 | 0.07 |
表1 不同UiO-66的表面积和孔体积参数
| 样品 | BET比表面积/m2·g-1 | 微孔面积/m2·g-1 | 外表面积/m2·g-1 | 总孔体积/cm2·g-1 | 微孔体积/cm2·g-1 | 介孔体积/cm2·g-1 |
|---|---|---|---|---|---|---|
| UiO-66-FD | 942 | 882 | 60 | 0.39 | 0.34 | 0.05 |
| UiO-66-AA | 862 | 794 | 68 | 0.39 | 0.30 | 0.09 |
| UiO-66-FA | 938 | 876 | 62 | 0.42 | 0.34 | 0.09 |
| UiO-66-TA | 1034 | 957 | 77 | 0.42 | 0.36 | 0.07 |
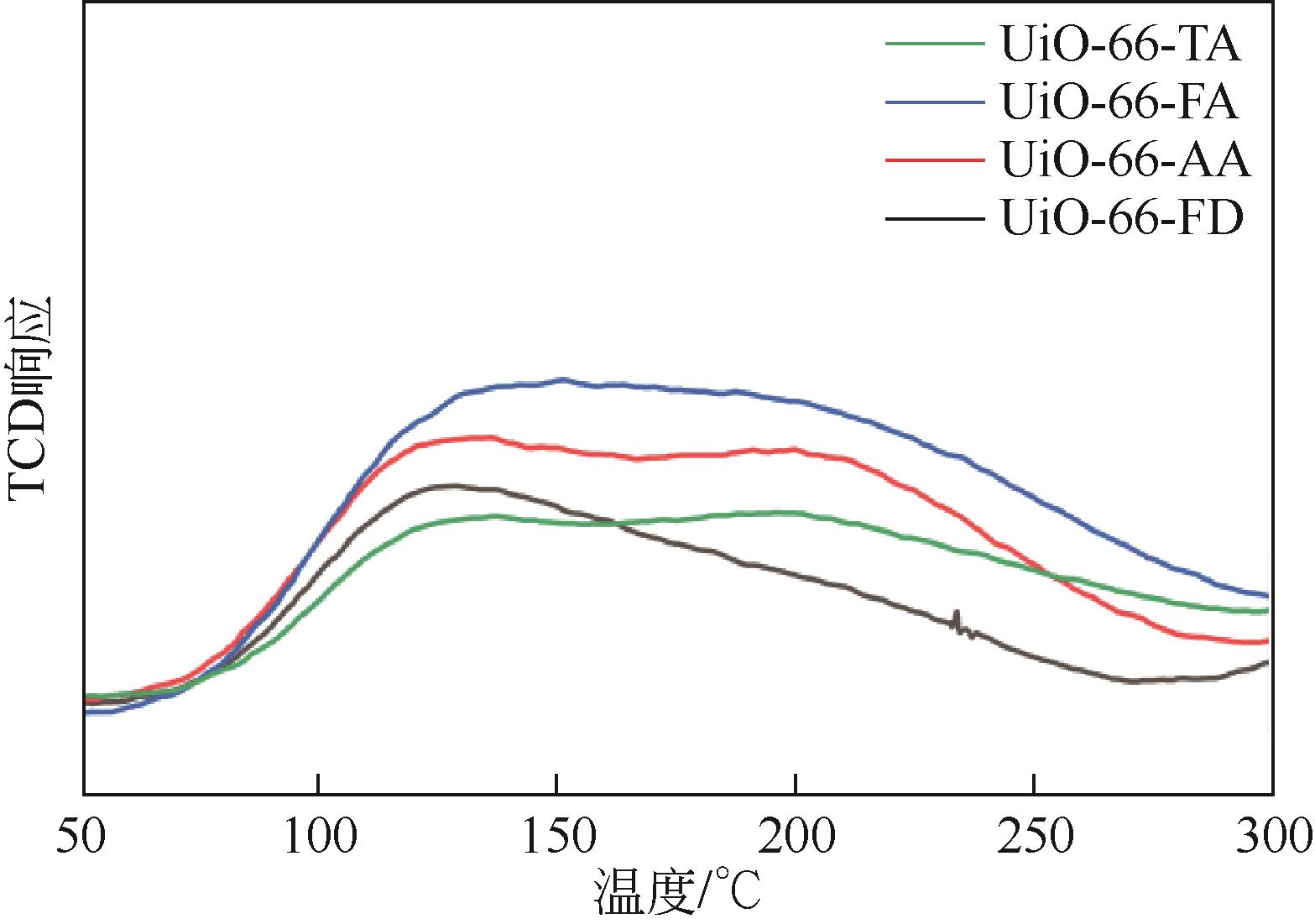
| 样品 | 脱附峰温度/℃ | NH3-TPD峰面积 | 总酸量 |
|---|---|---|---|
| UiO-66-FD | 124 | 4.3(45.7) | 9.4 |
| 177 | 5.1(54.3) | ||
| UiO-66-AA | 123 | 6.1(35.5) | 17.2 |
| 195 | 11.1(64.5) | ||
| UiO-66-FA | 129 | 7.7(37.9) | 20.3 |
| 198 | 12.6(62.1) | ||
| UiO-66-TA | 123 | 2.8(26.2) | 10.7 |
| 190 | 7.9(73.8) |
表2 不同UiO-66样品酸性质
| 样品 | 脱附峰温度/℃ | NH3-TPD峰面积 | 总酸量 |
|---|---|---|---|
| UiO-66-FD | 124 | 4.3(45.7) | 9.4 |
| 177 | 5.1(54.3) | ||
| UiO-66-AA | 123 | 6.1(35.5) | 17.2 |
| 195 | 11.1(64.5) | ||
| UiO-66-FA | 129 | 7.7(37.9) | 20.3 |
| 198 | 12.6(62.1) | ||
| UiO-66-TA | 123 | 2.8(26.2) | 10.7 |
| 190 | 7.9(73.8) |
| 催化剂 | 溶剂 | 氢供体与糠醛摩尔比 | 反应温度/K | 氢气压力/MPa | 反应时间/h | 糠醛转化率/% | 糠醛转化率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 质量分数5% Pd/Al2O3 | 异丙醇 | 25.1 | 453 | — | 5 | 78 | 63 | [ |
| 质量分数0.3% Pt-4 | 异丙醇 | 26 | 393 | — | 7.5 | 98.8 | 79.5 | [ |
| AuRu/La-ZrO2 | 异丙醇 | 43.5 | 423 | — | 5 | 83 | 84 | [ |
| Cu/AC-SO3H | 异丙醇 | 65.3 | 378 | 0.4 | 2 | 99.9 | 99.9 | [ |
| Ni/AC-SO3H | 异丙醇 | 81.6 | 333 | 4 | 8 | 99.9 | 99.9 | [ |
| Fe3O4-12 | 异丙醇 | 389.9 | 433 | — | 5 | 97.5 | 92.5 | [ |
| UiO-66-FA | 异丙醇 | 43.5 | 433 | — | 1 | 99.4 | 98.9 | 本工作 |
表3 不同催化剂在糠醛转移加氢反应中的催化性能
| 催化剂 | 溶剂 | 氢供体与糠醛摩尔比 | 反应温度/K | 氢气压力/MPa | 反应时间/h | 糠醛转化率/% | 糠醛转化率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 质量分数5% Pd/Al2O3 | 异丙醇 | 25.1 | 453 | — | 5 | 78 | 63 | [ |
| 质量分数0.3% Pt-4 | 异丙醇 | 26 | 393 | — | 7.5 | 98.8 | 79.5 | [ |
| AuRu/La-ZrO2 | 异丙醇 | 43.5 | 423 | — | 5 | 83 | 84 | [ |
| Cu/AC-SO3H | 异丙醇 | 65.3 | 378 | 0.4 | 2 | 99.9 | 99.9 | [ |
| Ni/AC-SO3H | 异丙醇 | 81.6 | 333 | 4 | 8 | 99.9 | 99.9 | [ |
| Fe3O4-12 | 异丙醇 | 389.9 | 433 | — | 5 | 97.5 | 92.5 | [ |
| UiO-66-FA | 异丙醇 | 43.5 | 433 | — | 1 | 99.4 | 98.9 | 本工作 |
| 1 | CHO E J, TRINH L T P, SONG Y H, et al. Bioconversion of biomass waste into high value chemicals[J]. Bioresource Technology, 2020, 298: 122386. |
| 2 | 王久臣, 戴林, 田宜水, 等. 中国生物质能产业发展现状及趋势分析[J]. 农业工程学报, 2007, 23(9): 276-282. |
| WANG Jiuchen, DAI Lin, TIAN Yishui, et al. Analysis of the development status and trends of biomass energy industry in China[J]. Transactions of the Chinese Society of Agricultural Engineering, 2007, 23(9): 276-282. | |
| 3 | 杜海凤, 闫超. 生物质转化利用技术的研究进展[J]. 能源化工, 2016, 37(2): 41-46. |
| DU Haifeng, YAN Chao. Study progress on technologies of biomass conversion and utilization[J]. Energy Chemical Industry, 2016, 37(2): 41-46. | |
| 4 | MORAVVEJ Z, TABRIZI F F, RAHIMPOUR M R, et al. Exploiting the potential of cobalt molybdenum catalyst in elevated hydrodeoxygenation of furfural to 2-methyl furan[J]. Fuel, 2023, 332: 126193. |
| 5 | 海雪清, 谭静静, 何静, 等. CuCo双金属催化剂催化糠醛加氢制备1,5-戊二醇的研究[J]. 燃料化学学报, 2023, 51(7): 959-969. |
| Xueqing HAI, TAN Jingjing, HE Jing, et al. Hydrogenation of furfural to 1,5-pentanediol over CuCo bimetallic catalysts[J]. Journal of Fuel Chemistry and Technology, 2023, 51(7): 959-969. | |
| 6 | 李玉成, 朱礼玉, 赵静养, 等. 新型NaNi/C催化剂合成及对糠醛选择性加氢的研究[J]. 北京林业大学学报, 2023, 45(1): 140-147. |
| LI Yucheng, ZHU Liyu, ZHAO Jingyang, et al. Synthesis of novel NaNi/C catalyst and selective hydrogenation study of furfural[J]. Journal of Beijing Forestry University, 2023, 45(1): 140-147. | |
| 7 | 萧垚鑫, 张军, 胡升, 等. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352. |
| XIAO Yaoxin, ZHANG Jun, HU Sheng, et al. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352. | |
| 8 | WANG Zhuangqing, WANG Xinchao, ZHANG Chao, et al. Selective hydrogenation of furfural to furfuryl alcohol over Pd/TiH2 catalyst[J]. Molecular Catalysis, 2021, 508: 111599. |
| 9 | CHEN Junjie, SUN Weixiao, WANG Yongxing, et al. Performant Au hydrogenation catalyst cooperated with Cu-doped Al2O3 for selective conversion of furfural to furfuryl alcohol at ambient pressure[J]. Green Energy & Environment, 2021, 6(4): 546-556. |
| 10 | 刘思乐, 卜义夫, 吴静, 等. 非均相Pd/mpg-C3N4催化糠醛选择性加氢制糠醇[J]. 化学工程, 2022, 50(12): 22-26. |
| LIU Sile, BU Yifu, WU Jing, et al. Selective hydrogenation of furfural to furfuryl alcohol catalyzed by heterogeneous Pd/mpg-C3N4 [J]. Chemical Engineering (China), 2022, 50(12): 22-26. | |
| 11 | HE J, LI H, RIISAGER A, et al. Catalytic transfer hydrogenation of furfural to furfuryl alcohol with recyclable Al-Zr@Fe mixed oxides[J]. ChemCatChem, 2018, 10(2): 430-438. |
| 12 | HE J, SCHILL L, YANG S, et al. Catalytic transfer hydrogenation of bio-based furfural with NiO nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 17220-17229. |
| 13 | Chinh NGUYEN-HUY, KIM Ji Sun, YOON Sinmyung, et al. Supported Pd nanoparticle catalysts with high activities and selectivities in liquid-phase furfural hydrogenation[J]. Fuel, 2018, 226: 607-617. |
| 14 | ALIBEGOVIC K, MORGAN D G, LOSOVYJ Y, et al. Efficient furfuryl alcohol synthesis from furfural over magnetically recoverable catalysts: Does the catalyst stabilizing medium matter?[J]. ChemistrySelect, 2017, 2(20): 5485-5491. |
| 15 | MORANDI S, MANZOLI M, CHAN-THAW C E, et al. Unraveling the effect of ZrO2 modifiers on the nature of active sites on AuRu/ZrO2 catalysts for furfural hydrogenation[J]. Sustainable Energy & Fuels, 2020, 4(3): 1469-1480. |
| 16 | GONG Wanbing, CHEN Chun, ZHANG Yong, et al. Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(3): 2172-2180. |
| 17 | GONG Wanbing, CHEN Chun, WANG Haojie, et al. Sulfonate group modified Ni catalyst for highly efficient liquid-phase selective hydrogenation of bio-derived furfural[J]. Chinese Chemical Letters, 2018, 29(11): 1617-1620. |
| 18 | MA Mingwei, HOU Pan, ZHANG Peng, et al. Magnetic Fe3O4 nanoparticles as easily separable catalysts for efficient catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol[J]. Applied Catalysis A: General, 2020, 602: 117709. |
| 19 | CHEN S, WOJCIESZAK R, DUMEIGNIL F, et al. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural[J]. Chemical Reviews, 2018, 118(22): 11023-11117. |
| 20 | GAO Xing, TIAN Suyang, JIN Yunyun, et al. Bimetallic PtFe-catalyzed selective hydrogenation of furfural to furfuryl alcohol: Solvent effect of isopropanol and hydrogen activation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(33): 12722-12730. |
| 21 | WENG M W, ZHANG Z H, OKEJIRI F, et al. Encapsulation of CuO nanoparticles within silicalite-1 as a regenerative catalyst for transfer hydrogenation of furfural[J]. iScience, 2021, 24(8): 102884. |
| 22 | JIANG Shanshan, HUANG Jin, WANG Yue, et al. Metal-organic frameworks derived magnetic Fe3O4/C for catalytic transfer hydrogenation of furfural to furfuryl alcohol[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(3): 639-649. |
| 23 | AN Zhidong, LI Jiang. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts[J]. Green Chemistry, 2022, 24(5): 1780-1808. |
| 24 | YANG Dong, GATES Bruce C. Catalysis by metal organic frameworks: Perspective and suggestions for future research[J]. ACS Catalysis, 2019, 9(3): 1779-1798. |
| 25 | NGUYEN H G T, MAO L, PETERS A W, et al. Comparative study of titanium-functionalized UiO-66: Support effect on the oxidation of cyclohexene using hydrogen peroxide[J]. Catalysis Science & Technology, 2015, 5(9): 4444-4451. |
| 26 | QIU Mo, GUO Tianmeng, XI Ran, et al. Highly efficient catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol using UiO-66 without metal catalysts[J]. Applied Catalysis A: General, 2020, 602: 117719. |
| 27 | 李佩, 秦品典, 汪艳艳, 等. 缺陷改性UiO-66糠醛催化转移加氢研究[J]. 能源化工, 2022, 43(4): 1-5. |
| LI Pei, QIN Pindian, WANG Yanyan, et al. Study on catalytic transfer hydrogenation of furfural by defects modified UiO-66[J]. Energy Chemical Industry, 2022, 43(4): 1-5. | |
| 28 | LIANG W B, COGHLAN C J, RAGON F, et al. Defect engineering of UiO-66 for CO2 and H2O uptake—A combined experimental and simulation study[J]. Dalton Transactions, 2016, 45(11): 4496-4500. |
| 29 | 杜峰, 李鹂. UiO-66(Zr)系列MOFs催化材料的制备及在乳酸乙酯合成中的应用[J]. 化工进展, 2015, 34(11): 3938-3943. |
| DU Feng, LI Li. Preparation of UiO-66(Zr)MOFs and their application as catalysts for the synthesis of ethyl lactate[J]. Chemical Industry and Engineering Progress, 2015, 34(11): 3938-3943. | |
| 30 | SHEARER G C, CHAVAN S, ETHIRAJ J, et al. Tuned to perfection: Ironing out the defects in metal-organic framework UiO-66[J]. Chemistry of Materials, 2014, 26(14): 4068-4071. |
| 31 | SHEARER G C, CHAVAN S, BORDIGA S, et al. Defect engineering: Tuning the porosity and composition of the metal-organic framework UiO-66 via modulated synthesis[J]. Chemistry of Materials, 2016, 28(11): 3749-3761. |
| 32 | KLET R C, LIU Y Y, WANG T C, et al. Evaluation of Brønsted acidity and proton topology in Zr- and Hf-based metal-organic frameworks using potentiometric acid-base titration[J]. Journal of Materials Chemistry A, 2016, 4(4): 1479-1485. |
| 33 | LING S L, SLATER B. Dynamic acidity in defective UiO-66[J]. Chemical Science, 2016, 7(7): 4706-4712. |
| 34 | VANDICHEL M, HAJEK J, VERMOORTELE F, et al. Active site engineering in UiO-66 type metal-organic frameworks by intentional creation of defects: A theoretical rationalization[J]. CrystEngComm, 2015, 17(2): 395-406. |
| 35 | VERMOORTELE F, BUEKEN B, LE BARS G, et al. Synthesis modulation as a tool to increase the catalytic activity of metal-organic frameworks: The unique case of UiO-66(Zr)[J]. Journal of the American Chemical Society, 2013, 135(31): 11465-11468. |
| 36 | XUAN Keng, PU Yanfeng, LI Feng, et al. Direct synthesis of dimethyl carbonate from CO2 and methanol over trifluoroacetic acid modulated UiO-66[J]. Journal of CO2 Utilization, 2018, 27: 272-282. |
| 37 | GONELL F, BORONAT M, CORMA A. Structure-reactivity relationship in isolated Zr sites present in Zr-zeolite and ZrO2 for the Meerwein-Ponndorf-Verley reaction[J]. Catalysis Science & Technology, 2017, 7(13): 2865-2873. |
| 38 | WU Jingcheng, LIANG Dong, SONG Xiangbo, et al. Sulfonic groups functionalized Zr-metal organic framework for highly catalytic transfer hydrogenation of furfural to furfuryl alcohol[J]. Journal of Energy Chemistry, 2022, 71: 411-417. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [10] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [11] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [12] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [13] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [14] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [15] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||