化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6319-6337.DOI: 10.16085/j.issn.1000-6613.2022-0442
氨分解制氢镍基催化剂研究进展
关静莹( ), 张欢欢, 苏子恺, 史大昕(
), 张欢欢, 苏子恺, 史大昕( ), 吴芹, 陈康成, 张耀远, 黎汉生(
), 吴芹, 陈康成, 张耀远, 黎汉生( )
)
- 北京理工大学化学与化工学院,北京 102401
-
收稿日期:2022-03-21修回日期:2022-05-24出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:史大昕,黎汉生 -
作者简介:关静莹(1998—),女,硕士研究生,研究方向为氨分解制氢。E-mail:guanjingying11@163.com。 -
基金资助:国家自然科学基金(22108013);中国石油科技创新基金(2020D-5007-0402)
Recent progress in nickel-based catalysts for ammonia decomposition to hydrogen
GUAN Jingying( ), ZHANG Huanhuan, SU Zikai, SHI Daxin(
), ZHANG Huanhuan, SU Zikai, SHI Daxin( ), WU Qin, CHEN Kangcheng, ZHANG Yaoyuan, LI Hansheng(
), WU Qin, CHEN Kangcheng, ZHANG Yaoyuan, LI Hansheng( )
)
- School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102401, China
-
Received:2022-03-21Revised:2022-05-24Online:2022-12-20Published:2022-12-29 -
Contact:SHI Daxin, LI Hansheng
摘要:
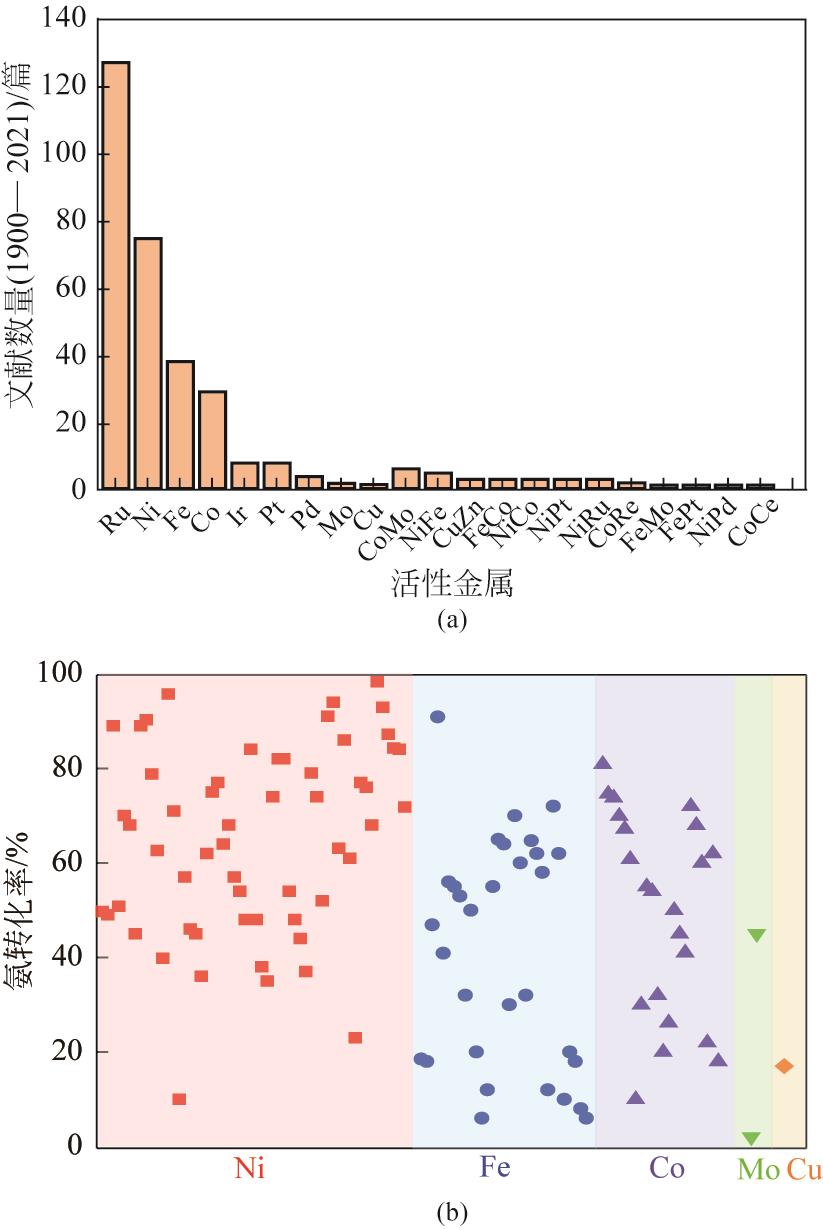
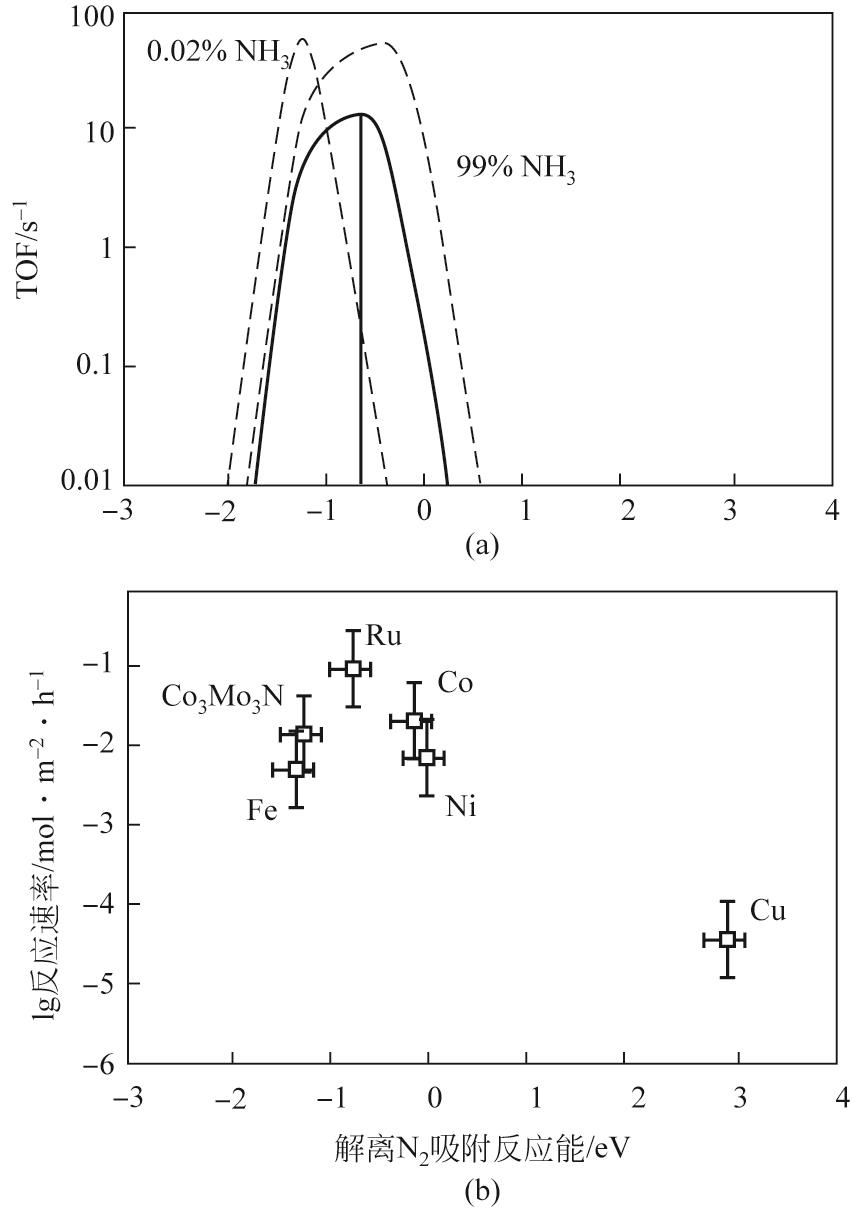
氨分解制氢清洁高效,易于工业化使用,是一种极具前景的便携式制氢方法。镍作为氨分解非贵金属催化剂中性能最好、应用最广的催化剂,但仍存在低温活性低、易烧结等问题亟需改进。本文概括了氨分解反应的反应机理、动力学和热力学,综述了近年来国内外氨分解镍基催化剂的研究现状。研究者从镍金属活性中心调控出发进行研究,发现调节镍粒子尺寸、加入第二金属(Fe、Co、Mo等)、载体(Al2O3、SiO2、分子筛等)、助剂(碱土金属、稀土金属等)以及设计核壳结构进行调控,可提高镍金属的分散性和抗烧结能力。本文在以上基础上提出了镍基催化剂的改进措施和未来发展方向,以期为进一步设计出低温高活性镍基催化剂提供依据。
中图分类号:
引用本文
关静莹, 张欢欢, 苏子恺, 史大昕, 吴芹, 陈康成, 张耀远, 黎汉生. 氨分解制氢镍基催化剂研究进展[J]. 化工进展, 2022, 41(12): 6319-6337.
GUAN Jingying, ZHANG Huanhuan, SU Zikai, SHI Daxin, WU Qin, CHEN Kangcheng, ZHANG Yaoyuan, LI Hansheng. Recent progress in nickel-based catalysts for ammonia decomposition to hydrogen[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6319-6337.
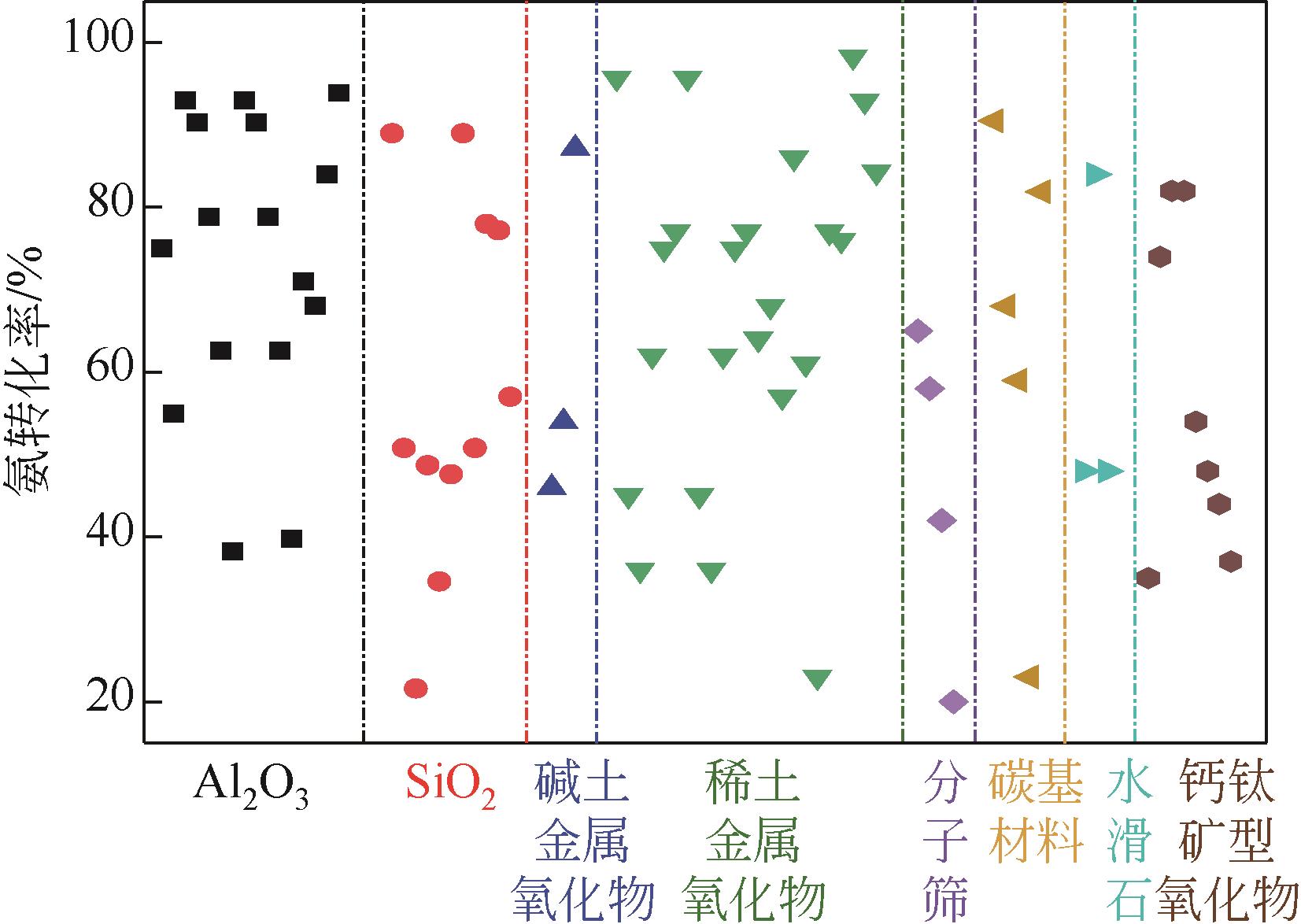
| 催化剂 | 制备方法 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | TOF/s-1 | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ni1Co9/Ce0.6Zr0.3Y0.1O2 | 湿浸渍(WI) | 10 | 550 | 6000 | 100 | 0.68① | 6.13 | [ |
| Ni-Co/α-Al2O3 | 浸渍(IM) | 10 | 500 | W/F=2.45kg·s/L | 73 | — | — | [ |
| Ni5Co5/SiO2 | IM | 10 | 550 | 30000 | 76.8 | — | 25.71 | [ |
| Ni5Co5/SiO2-K | IM | 10 | 550 | 30000 | 78.1 | — | — | [ |
| Fe-Ni/γ-Al2O3 | WI | 10 | 650 | 28500 | — | — | 31.7mL·min/g | [ |
| Ni-Ir/γ-Al2O3 | WI | 10.7 | 400 | 9500 | 43.55 | — | — | [ |
| Ni-Pt/Al2O3 | — | — | 600 | W/F=3.4g·h/mol | 78.1 | — | — | [ |
| Ni-Ru/CeO2 | WI | 12 | 450 | 13800 | 90 | — | — | [ |
| 10Ni2Ru/CeO2 | WI | 12 | 450 | 15000 | 84.5 | 0.88 | — | [ |
| 5Ni1Ru/CeO2 | WI | 6 | 450 | 15000 | 86.1 | 1.01 | — | [ |
| 2.5Ni0.5Ru/CeO2 | WI | 3 | 450 | 15000 | 88.7 | 2 | — | [ |
| 2Ni0.4Ru/CeO2 | WI | 2.4 | 450 | 15000 | 81.2 | 1.57 | — | [ |
| 1.5Ni0.5Ru/CeO2 | WI | 2 | 450 | 15000 | 86.3 | 2.44 | — | [ |
| 2.5Ni0.3Ru/CeO2 | WI | 2.8 | 450 | 15000 | 73.4 | 1.11 | — | [ |
表1 双金属镍基催化剂的氨分解催化活性
| 催化剂 | 制备方法 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | TOF/s-1 | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Ni1Co9/Ce0.6Zr0.3Y0.1O2 | 湿浸渍(WI) | 10 | 550 | 6000 | 100 | 0.68① | 6.13 | [ |
| Ni-Co/α-Al2O3 | 浸渍(IM) | 10 | 500 | W/F=2.45kg·s/L | 73 | — | — | [ |
| Ni5Co5/SiO2 | IM | 10 | 550 | 30000 | 76.8 | — | 25.71 | [ |
| Ni5Co5/SiO2-K | IM | 10 | 550 | 30000 | 78.1 | — | — | [ |
| Fe-Ni/γ-Al2O3 | WI | 10 | 650 | 28500 | — | — | 31.7mL·min/g | [ |
| Ni-Ir/γ-Al2O3 | WI | 10.7 | 400 | 9500 | 43.55 | — | — | [ |
| Ni-Pt/Al2O3 | — | — | 600 | W/F=3.4g·h/mol | 78.1 | — | — | [ |
| Ni-Ru/CeO2 | WI | 12 | 450 | 13800 | 90 | — | — | [ |
| 10Ni2Ru/CeO2 | WI | 12 | 450 | 15000 | 84.5 | 0.88 | — | [ |
| 5Ni1Ru/CeO2 | WI | 6 | 450 | 15000 | 86.1 | 1.01 | — | [ |
| 2.5Ni0.5Ru/CeO2 | WI | 3 | 450 | 15000 | 88.7 | 2 | — | [ |
| 2Ni0.4Ru/CeO2 | WI | 2.4 | 450 | 15000 | 81.2 | 1.57 | — | [ |
| 1.5Ni0.5Ru/CeO2 | WI | 2 | 450 | 15000 | 86.3 | 2.44 | — | [ |
| 2.5Ni0.3Ru/CeO2 | WI | 2.8 | 450 | 15000 | 73.4 | 1.11 | — | [ |
| 催化剂 | 制备方法 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Ni/SBA-15 | 沉积-沉淀(DP) | 23.4 | 550 | 30000 | 89.0 | 29.8 | [ |
| Ni/SBA-15 | WI | 10 | 550 | 30000 | 50.8 | 17.0 | [ |
| Ni/SiO2 | WI | 10 | 600 | 30000h-1 | 78.0 | — | [ |
| Ni/ATP | 均相沉淀(HP) | 15.7 | 650 | 30000 | 77.2 | 25.8 | [ |
| Ni/Al2O3 | 共沉淀(CP) | 49.9 | 500 | 30000 | 38.2 | — | [ |
| Ni/Al2O3 | CP | 90 | 600 | 36000 | 93.0 | — | [ |
| Ni/Mg-Al-O | — | 20 | 550 | 6000 | 90.3 | — | [ |
| Ni/Sr-Al-O | — | 20 | 550 | 6000 | 78.8 | — | [ |
| Ni/Ca-Al-O | — | 20 | 550 | 6000 | 62.6 | — | [ |
| Ni/Ba-Al-O | — | 20 | 550 | 6000 | 39.8 | — | [ |
| Ni/Ce0.8Zr0.2O2 | IM | 13.2 | 550 | 15mL/min | 95.7 | — | [ |
| Ni/Al2O3 | WI | 10 | 550 | — | 71.0 | — | [ |
| Ni/ZrO2 | WI | 10 | 550 | — | 10.0 | — | [ |
| Ni/SiO2 | WI | 10 | 550 | — | 57.0 | — | [ |
| Ni/MgO | WI | 10 | 550 | — | 46.0 | — | [ |
| Ni/CeO2 | WI | 10 | 550 | — | 45.0 | — | [ |
| Ni/TiO2 | WI | 10 | 550 | — | 36.0 | — | [ |
| Ni/La2O3 | WI | 10 | 550 | — | 62.0 | — | [ |
| Ni/La2O3 | WI | 26.5 | 550 | 6000 | 75.0 | 5.0 | [ |
| Ni/La2O3 | 柠檬酸络合(CAC) | 26.5 | 550 | 6000 | 77.0 | 5.1 | [ |
| Ni/La2O3 | NH3-CP | 26.5 | 550 | 6000 | 64.0 | 4.4 | [ |
| Ni/La2O3 | NaOH-CP | 26.5 | 550 | 6000 | 68.0 | 4.6 | [ |
| Ni/La2O3 | 热解(PR) | 26.5 | 550 | 6000 | 57.0 | 4.0 | [ |
| Ni/MgO-La2O3 | — | 5 | 550 | 30000 | 54.0 | — | [ |
| Ni/ZSM-5 | WI | 5 | 650 | 30000 | 50.1 | 16.8 | [ |
| Ni/ZSM-5 | DP | 5 | 650 | 30000 | 81.3 | 27.2 | [ |
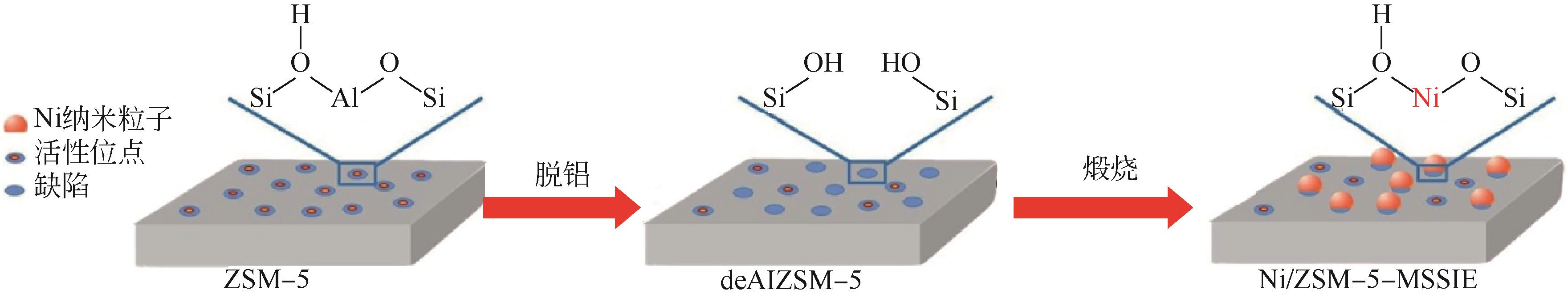
| Ni/ZSM-5 | 固态离子(SSIE) | 5 | 650 | 30000 | 92.9 | 31.1 | [ |
| Ni/ZSM-5 | 改性固态离子交换(MSSIE) | 5 | 650 | 30000 | 97.6 | 32.7 | [ |
| Ni0.6(Mg0.29Al0.57O n ) | CP | 40.1 | 600 | 30000 | 99.3 | 33.3 | [ |
| Ni x /LDHs | IM | 23.6 | 550 | 10000 | 48.0 | — | [ |
| Ni x /LDHs | 镍诱导(ST) | 23.6 | 550 | 10000 | 84.0 | — | [ |
| Ni-MgAl(6∶1) | CP | 15 | 550 | 30000 | 48.0 | — | [ |
| Ni-KNbO3 | IM | 40 | 550 | — | 35.0 | — | [ |
| Ni-LaAlO3 | IM | 40 | 550 | — | 74.0 | — | [ |
| Ni-SmAlO3 | IM | 40 | 550 | — | 82.0 | — | [ |
| Ni-GdAlO3 | IM | 40 | 550 | — | 82.0 | — | [ |
| Ni-CaMnO3 | IM | 40 | 550 | — | 54.0 | — | [ |
| Ni-SrMnO3 | IM | 40 | 550 | — | 48.0 | — | [ |
| Ni-BaMnO3 | IM | 40 | 550 | — | 44.0 | — | [ |
| Ni-CaTiO3 | IM | 40 | 550 | — | 37.0 | — | [ |
| Ni-SrTiO3 | IM | 40 | 550 | — | 79.0 | — | [ |
| Ni-BaTiO3 | IM | 40 | 550 | — | 74.0 | — | [ |
| Ni-CaZrO3 | IM | 40 | 550 | — | 52.0 | — | [ |
| Ni-SrZrO3 | IM | 40 | 550 | — | 91.0 | — | [ |
| Ni-BaZrO3 | IM | 40 | 550 | — | 94.0 | — | [ |
| Ni/海泡石 | WI | 5.2 | 600 | 15500 | 63.1 | 10.0 | [ |
| Ni/Y2O3 | IM | 10 | 550 | — | 86.0 | — | [ |
| Ni/La2O3 | IM | 10 | 550 | — | 61.0 | — | [ |
| Ni/CeO2 | IM | 10 | 550 | — | 23.0 | — | [ |
| Ni/Sm2O3 | IM | 10 | 550 | — | 77.0 | — | [ |
| Ni/Gd2O3 | IM | 10 | 550 | — | 76.0 | — | [ |
| Ni/Al2O3 | IM | 10 | 550 | — | 68.0 | — | [ |
| Ni/Y2O3 | CP | 30 | 550 | — | 98.3 | — | [ |
| Ni/CeO2 | CP | 30 | 550 | — | 92.9 | — | [ |
| Ni/MgO | CP | 30 | 550 | — | 87.2 | — | [ |
| Ni/La2O3 | CP | 30 | 550 | — | 84.3 | — | [ |
| Ni/Al2O3 | CP | 30 | 550 | — | 84.0 | — | [ |
| Ni/ZrO2 | CP | 30 | 550 | — | 71.8 | — | [ |
| Ni/云母 | WI | 15 | 650 | 30000 | 97.2 | 32.5 | [ |
| Ni/MRM | HP | 15 | 700 | 50mL/min | 97.9 | 32.8 | [ |
| Ni/MRM | IM | 12 | 700 | 50mL/min | 95.5 | 32.0 | [ |
| Ni-CNFs | — | 2 | 650 | — | 68.0 | 23.0 | [ |
| Ni/MWCNTs | — | 5 | 500 | 6000h-1 | 59.0 | — | [ |
| Ni/AC | — | 5 | 500 | 6000h-1 | 23.0 | — | [ |
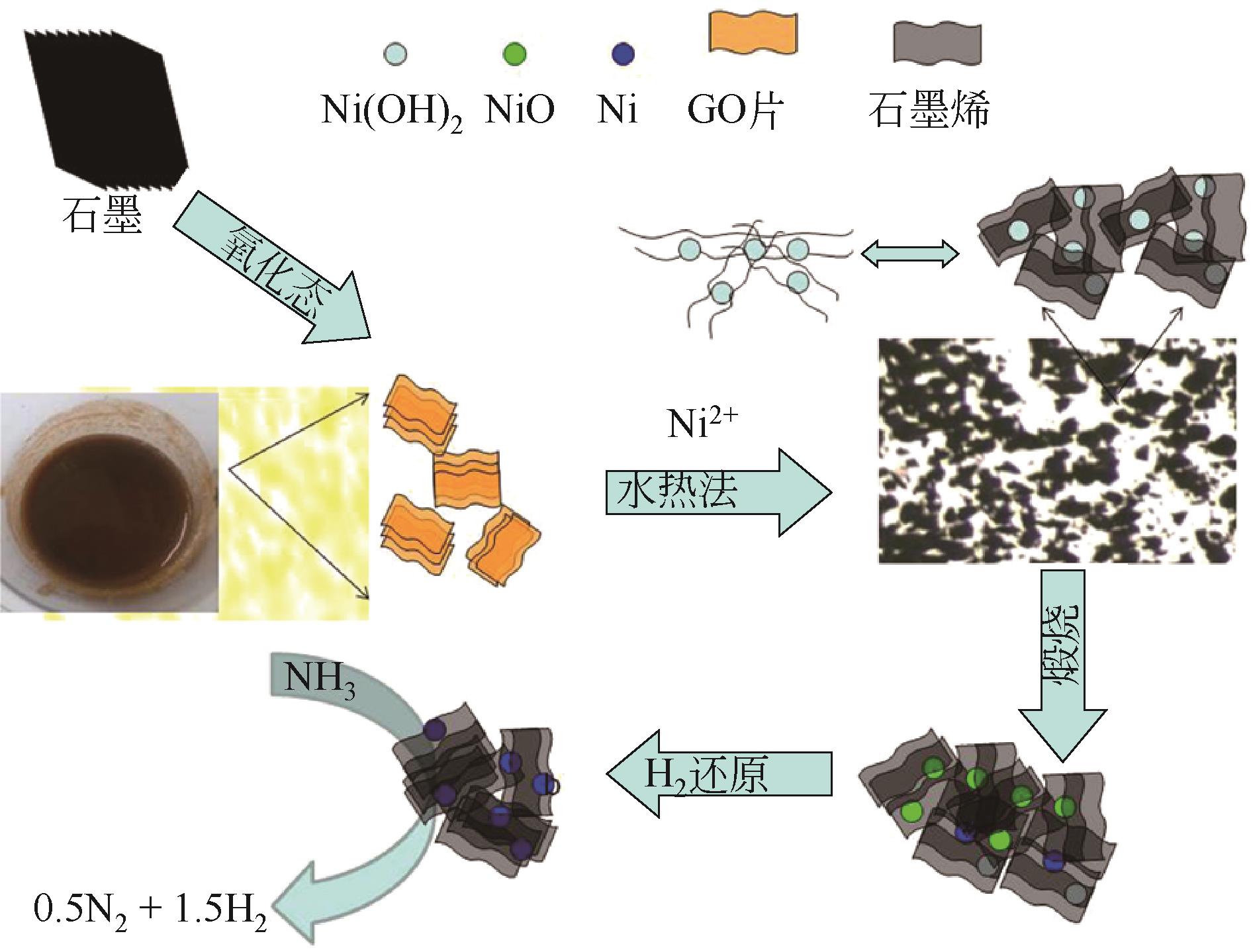
| Ni-rGO | — | 10 | 700 | 50mL/min | 81.9 | 27.4 | [ |
| Ni@Al2O3 | — | — | 600 | 24000 | 93.9 | — | [ |
表2 不同载体负载镍基催化剂的氨分解催化活性
| 催化剂 | 制备方法 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Ni/SBA-15 | 沉积-沉淀(DP) | 23.4 | 550 | 30000 | 89.0 | 29.8 | [ |
| Ni/SBA-15 | WI | 10 | 550 | 30000 | 50.8 | 17.0 | [ |
| Ni/SiO2 | WI | 10 | 600 | 30000h-1 | 78.0 | — | [ |
| Ni/ATP | 均相沉淀(HP) | 15.7 | 650 | 30000 | 77.2 | 25.8 | [ |
| Ni/Al2O3 | 共沉淀(CP) | 49.9 | 500 | 30000 | 38.2 | — | [ |
| Ni/Al2O3 | CP | 90 | 600 | 36000 | 93.0 | — | [ |
| Ni/Mg-Al-O | — | 20 | 550 | 6000 | 90.3 | — | [ |
| Ni/Sr-Al-O | — | 20 | 550 | 6000 | 78.8 | — | [ |
| Ni/Ca-Al-O | — | 20 | 550 | 6000 | 62.6 | — | [ |
| Ni/Ba-Al-O | — | 20 | 550 | 6000 | 39.8 | — | [ |
| Ni/Ce0.8Zr0.2O2 | IM | 13.2 | 550 | 15mL/min | 95.7 | — | [ |
| Ni/Al2O3 | WI | 10 | 550 | — | 71.0 | — | [ |
| Ni/ZrO2 | WI | 10 | 550 | — | 10.0 | — | [ |
| Ni/SiO2 | WI | 10 | 550 | — | 57.0 | — | [ |
| Ni/MgO | WI | 10 | 550 | — | 46.0 | — | [ |
| Ni/CeO2 | WI | 10 | 550 | — | 45.0 | — | [ |
| Ni/TiO2 | WI | 10 | 550 | — | 36.0 | — | [ |
| Ni/La2O3 | WI | 10 | 550 | — | 62.0 | — | [ |
| Ni/La2O3 | WI | 26.5 | 550 | 6000 | 75.0 | 5.0 | [ |
| Ni/La2O3 | 柠檬酸络合(CAC) | 26.5 | 550 | 6000 | 77.0 | 5.1 | [ |
| Ni/La2O3 | NH3-CP | 26.5 | 550 | 6000 | 64.0 | 4.4 | [ |
| Ni/La2O3 | NaOH-CP | 26.5 | 550 | 6000 | 68.0 | 4.6 | [ |
| Ni/La2O3 | 热解(PR) | 26.5 | 550 | 6000 | 57.0 | 4.0 | [ |
| Ni/MgO-La2O3 | — | 5 | 550 | 30000 | 54.0 | — | [ |
| Ni/ZSM-5 | WI | 5 | 650 | 30000 | 50.1 | 16.8 | [ |
| Ni/ZSM-5 | DP | 5 | 650 | 30000 | 81.3 | 27.2 | [ |
| Ni/ZSM-5 | 固态离子(SSIE) | 5 | 650 | 30000 | 92.9 | 31.1 | [ |
| Ni/ZSM-5 | 改性固态离子交换(MSSIE) | 5 | 650 | 30000 | 97.6 | 32.7 | [ |
| Ni0.6(Mg0.29Al0.57O n ) | CP | 40.1 | 600 | 30000 | 99.3 | 33.3 | [ |
| Ni x /LDHs | IM | 23.6 | 550 | 10000 | 48.0 | — | [ |
| Ni x /LDHs | 镍诱导(ST) | 23.6 | 550 | 10000 | 84.0 | — | [ |
| Ni-MgAl(6∶1) | CP | 15 | 550 | 30000 | 48.0 | — | [ |
| Ni-KNbO3 | IM | 40 | 550 | — | 35.0 | — | [ |
| Ni-LaAlO3 | IM | 40 | 550 | — | 74.0 | — | [ |
| Ni-SmAlO3 | IM | 40 | 550 | — | 82.0 | — | [ |
| Ni-GdAlO3 | IM | 40 | 550 | — | 82.0 | — | [ |
| Ni-CaMnO3 | IM | 40 | 550 | — | 54.0 | — | [ |
| Ni-SrMnO3 | IM | 40 | 550 | — | 48.0 | — | [ |
| Ni-BaMnO3 | IM | 40 | 550 | — | 44.0 | — | [ |
| Ni-CaTiO3 | IM | 40 | 550 | — | 37.0 | — | [ |
| Ni-SrTiO3 | IM | 40 | 550 | — | 79.0 | — | [ |
| Ni-BaTiO3 | IM | 40 | 550 | — | 74.0 | — | [ |
| Ni-CaZrO3 | IM | 40 | 550 | — | 52.0 | — | [ |
| Ni-SrZrO3 | IM | 40 | 550 | — | 91.0 | — | [ |
| Ni-BaZrO3 | IM | 40 | 550 | — | 94.0 | — | [ |
| Ni/海泡石 | WI | 5.2 | 600 | 15500 | 63.1 | 10.0 | [ |
| Ni/Y2O3 | IM | 10 | 550 | — | 86.0 | — | [ |
| Ni/La2O3 | IM | 10 | 550 | — | 61.0 | — | [ |
| Ni/CeO2 | IM | 10 | 550 | — | 23.0 | — | [ |
| Ni/Sm2O3 | IM | 10 | 550 | — | 77.0 | — | [ |
| Ni/Gd2O3 | IM | 10 | 550 | — | 76.0 | — | [ |
| Ni/Al2O3 | IM | 10 | 550 | — | 68.0 | — | [ |
| Ni/Y2O3 | CP | 30 | 550 | — | 98.3 | — | [ |
| Ni/CeO2 | CP | 30 | 550 | — | 92.9 | — | [ |
| Ni/MgO | CP | 30 | 550 | — | 87.2 | — | [ |
| Ni/La2O3 | CP | 30 | 550 | — | 84.3 | — | [ |
| Ni/Al2O3 | CP | 30 | 550 | — | 84.0 | — | [ |
| Ni/ZrO2 | CP | 30 | 550 | — | 71.8 | — | [ |
| Ni/云母 | WI | 15 | 650 | 30000 | 97.2 | 32.5 | [ |
| Ni/MRM | HP | 15 | 700 | 50mL/min | 97.9 | 32.8 | [ |
| Ni/MRM | IM | 12 | 700 | 50mL/min | 95.5 | 32.0 | [ |
| Ni-CNFs | — | 2 | 650 | — | 68.0 | 23.0 | [ |
| Ni/MWCNTs | — | 5 | 500 | 6000h-1 | 59.0 | — | [ |
| Ni/AC | — | 5 | 500 | 6000h-1 | 23.0 | — | [ |
| Ni-rGO | — | 10 | 700 | 50mL/min | 81.9 | 27.4 | [ |
| Ni@Al2O3 | — | — | 600 | 24000 | 93.9 | — | [ |
| 催化剂 | 制备方法 | 镍分散度 /% | 镍质量分数 /% | 反应温度 /℃ | 空速 /mL·g-1·h-1 | 氨转化率 /% | TOF /s-1 | 氢气生成速率 /mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Ce-Ni/SBA-15 | DP | 29.5 | — | 550 | — | 88.0 | — | — | [ |
| La-Ni/SBA-15 | DP | 24.3 | — | 550 | — | 85.0 | — | — | [ |
| Ni/La–Al2O3 | CP | 25.5 | 37.9 | 500 | — | 63.9 | 21.4 | — | [ |
| Ni/Ce-掺杂Al2O3 | CP | 3.15 | — | 550 | 30000 | 87.9 | — | — | [ |
| Ni-Ce/Al2O3 | CP | 21 | 43.4 | 500 | 30000 | 71.9 | 24.1 | — | [ |
| Ni/Ce-掺杂Al2O3 | — | 4 | — | 550 | — | 89.9 | — | — | [ |
| Ni/Zr-掺杂Al2O3 | — | 3.3 | — | 550 | — | 87.0 | — | — | [ |
| Ni/Sr-掺杂Al2O3 | — | 3.4 | — | 550 | — | 84.4 | — | — | [ |
| Ni/Y-掺杂Al2O3 | — | 2 | — | 550 | — | 79.2 | — | — | [ |
| Y-Ni/Al2O3 | WI | 3.39 | 9 | 450 | 6000 | 18.8 | 0.38 | 1.3 | [ |
| La-Ni/Al2O3 | WI | 3.57 | 9 | 450 | 6000 | 20.2 | 0.38 | 1.4 | [ |
| Ce-Ni/Al2O3 | WI | 3.43 | 9 | 450 | 6000 | 15.4 | 0.29 | 1.0 | [ |
| Pr-Ni/Al2O3 | WI | 2.98 | 9 | 450 | 6000 | 19.7 | 0.43 | 1.3 | [ |
| Nd-Ni/Al2O3 | WI | 3.44 | 9 | 450 | 6000 | 19.7 | 0.37 | 1.3 | [ |
| Sm-Ni/Al2O3 | WI | 3.06 | 9 | 450 | 6000 | 18.2 | 0.38 | 1.2 | [ |
| Eu-Ni/Al2O3 | WI | 3.48 | 9 | 450 | 6000 | 15.9 | 0.31 | 1.1 | [ |
| Gd-Ni/Al2O3 | WI | 3.45 | 9 | 450 | 6000 | 15.8 | 0.31 | 1.1 | [ |
| Ni/Zr-doped Al2O3 | WI | 3.3 | 20 | 550 | 7500 | 79.8 | — | 5.5 | [ |
| Ni/Al-Ce0.8Zr0.2 | CP | — | 10 | 550 | 9000h-1 | 92.0 | 0.48 | — | [ |
| MgO–Ni/Y2O3 | — | — | 40 | 500 | — | 60.0 | — | — | [ |
| CaO–Ni/Y2O3 | — | — | 40 | 500 | — | 42.0 | — | — | [ |
| SrO–Ni/Y2O3 | — | — | 40 | 500 | — | 80.0 | — | — | [ |
| BaO–Ni/Y2O3 | — | — | 40 | 500 | — | 76.0 | — | — | [ |
表3 改性镍基催化剂氨分解催化活性
| 催化剂 | 制备方法 | 镍分散度 /% | 镍质量分数 /% | 反应温度 /℃ | 空速 /mL·g-1·h-1 | 氨转化率 /% | TOF /s-1 | 氢气生成速率 /mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Ce-Ni/SBA-15 | DP | 29.5 | — | 550 | — | 88.0 | — | — | [ |
| La-Ni/SBA-15 | DP | 24.3 | — | 550 | — | 85.0 | — | — | [ |
| Ni/La–Al2O3 | CP | 25.5 | 37.9 | 500 | — | 63.9 | 21.4 | — | [ |
| Ni/Ce-掺杂Al2O3 | CP | 3.15 | — | 550 | 30000 | 87.9 | — | — | [ |
| Ni-Ce/Al2O3 | CP | 21 | 43.4 | 500 | 30000 | 71.9 | 24.1 | — | [ |
| Ni/Ce-掺杂Al2O3 | — | 4 | — | 550 | — | 89.9 | — | — | [ |
| Ni/Zr-掺杂Al2O3 | — | 3.3 | — | 550 | — | 87.0 | — | — | [ |
| Ni/Sr-掺杂Al2O3 | — | 3.4 | — | 550 | — | 84.4 | — | — | [ |
| Ni/Y-掺杂Al2O3 | — | 2 | — | 550 | — | 79.2 | — | — | [ |
| Y-Ni/Al2O3 | WI | 3.39 | 9 | 450 | 6000 | 18.8 | 0.38 | 1.3 | [ |
| La-Ni/Al2O3 | WI | 3.57 | 9 | 450 | 6000 | 20.2 | 0.38 | 1.4 | [ |
| Ce-Ni/Al2O3 | WI | 3.43 | 9 | 450 | 6000 | 15.4 | 0.29 | 1.0 | [ |
| Pr-Ni/Al2O3 | WI | 2.98 | 9 | 450 | 6000 | 19.7 | 0.43 | 1.3 | [ |
| Nd-Ni/Al2O3 | WI | 3.44 | 9 | 450 | 6000 | 19.7 | 0.37 | 1.3 | [ |
| Sm-Ni/Al2O3 | WI | 3.06 | 9 | 450 | 6000 | 18.2 | 0.38 | 1.2 | [ |
| Eu-Ni/Al2O3 | WI | 3.48 | 9 | 450 | 6000 | 15.9 | 0.31 | 1.1 | [ |
| Gd-Ni/Al2O3 | WI | 3.45 | 9 | 450 | 6000 | 15.8 | 0.31 | 1.1 | [ |
| Ni/Zr-doped Al2O3 | WI | 3.3 | 20 | 550 | 7500 | 79.8 | — | 5.5 | [ |
| Ni/Al-Ce0.8Zr0.2 | CP | — | 10 | 550 | 9000h-1 | 92.0 | 0.48 | — | [ |
| MgO–Ni/Y2O3 | — | — | 40 | 500 | — | 60.0 | — | — | [ |
| CaO–Ni/Y2O3 | — | — | 40 | 500 | — | 42.0 | — | — | [ |
| SrO–Ni/Y2O3 | — | — | 40 | 500 | — | 80.0 | — | — | [ |
| BaO–Ni/Y2O3 | — | — | 40 | 500 | — | 76.0 | — | — | [ |
| 催化剂 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | TOF/s-1 | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Ni/ATP@SiO2 | 8.7 | 650 | 30000 | 73.4 | — | 24.6 | [ |
| Ce-Ni@SiO2 | — | 600 | 50mL/min | 86.9 | — | 29.1 | [ |
| Ni@SiO2 | — | 600 | 30000 | 78.9 | 96.9mmol/(h·mol) | 26.4 | [ |
| Fe@SiO2 | Si/Fe=0.2 | 600 | 30000 | 81.8 | — | 27.9 | [ |
| Ru@SiO2 | Si/Ru=0.2 | 600 | 30000 | 99.8 | — | 33.5 | [ |
| Co@SiO2 | Si/Co=0.2 | 600 | 30000 | 52.3 | — | — | [ |
| Ni@SiO2 | Si/Ni=0.2 | 600 | 30000 | 81.7 | — | 27.6 | [ |
| Ni@MgO | — | 600 | 30000 | 69.8 | — | — | [ |
| Ni@Al2O3 | — | 600 | 30000 | 60.4 | — | — | [ |
| Ni@SiO2 | Si/Ni=0.2 | 600 | 30000 | 89.5 | 15.3 | 27.6 | [ |
| Ni@SiO2 | Si/Ni=0.4 | 600 | 30000 | 78.5 | — | 25.6 | [ |
| La-Ni@SiO2 | — | 600 | 30000 | 36.6 | — | 32.2 | [ |
表4 核壳型镍基催化剂氨分解催化活性
| 催化剂 | 镍质量分数/% | 反应温度/℃ | 空速/mL·g-1·h-1 | 氨转化率/% | TOF/s-1 | 氢气生成速率/mmol·min-1·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| Ni/ATP@SiO2 | 8.7 | 650 | 30000 | 73.4 | — | 24.6 | [ |
| Ce-Ni@SiO2 | — | 600 | 50mL/min | 86.9 | — | 29.1 | [ |
| Ni@SiO2 | — | 600 | 30000 | 78.9 | 96.9mmol/(h·mol) | 26.4 | [ |
| Fe@SiO2 | Si/Fe=0.2 | 600 | 30000 | 81.8 | — | 27.9 | [ |
| Ru@SiO2 | Si/Ru=0.2 | 600 | 30000 | 99.8 | — | 33.5 | [ |
| Co@SiO2 | Si/Co=0.2 | 600 | 30000 | 52.3 | — | — | [ |
| Ni@SiO2 | Si/Ni=0.2 | 600 | 30000 | 81.7 | — | 27.6 | [ |
| Ni@MgO | — | 600 | 30000 | 69.8 | — | — | [ |
| Ni@Al2O3 | — | 600 | 30000 | 60.4 | — | — | [ |
| Ni@SiO2 | Si/Ni=0.2 | 600 | 30000 | 89.5 | 15.3 | 27.6 | [ |
| Ni@SiO2 | Si/Ni=0.4 | 600 | 30000 | 78.5 | — | 25.6 | [ |
| La-Ni@SiO2 | — | 600 | 30000 | 36.6 | — | 32.2 | [ |
| 69 | HENPRASERTTAE S, CHAROJROCHKUL S, LAWTRAKUL L, et al. Ni-based catalysts for hydrogen production from ammonia decomposition: effect of dopants and urine application[J]. ChemistrySelect, 2018, 3(42): 11842-11850. |
| 70 | ATSUMI R, NODA R, TAKAGI H, et al. Effects of steam on Ni/Al2O3 catalysts for ammonia decomposition[J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17849-17853. |
| 71 | LI Xiukai, JI Weijie, ZHAO Jing, et al. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15[J]. Journal of Catalysis, 2005, 236(2): 181-189. |
| 72 | MUROYAMA H, MATSUI T, EGUCHI K. Production and utilization of hydrogen carriers by using supported nickel catalysts[J]. Journal of the Japan Petroleum Institute, 2021, 64(3): 123-131. |
| 73 | SIMA Dewen, WU Haojin, TIAN Koukou, et al. Enhanced low temperature catalytic activity of Ni/Al-Ce0.8Zr0.2O2 for hydrogen production from ammonia decomposition[J]. International Journal of Hydrogen Energy, 2020, 45(16): 9342-9352. |
| 74 | ZHU Zengzan, LU Guanzhong, ZHANG Zhigang, et al. Highly active and stable Co3O4/ZSM-5 catalyst for propane oxidation: effect of the preparation method[J]. ACS Catalysis, 2013, 3(6): 1154-1164. |
| 75 | WAN Zhijian, TAO Youkun, YOU Hengzhi, et al. Na-ZSM-5 zeolite nanocrystals supported nickel nanoparticles for efficient hydrogen production from ammonia decomposition[J]. ChemCatChem, 2021,13(13): 3027-3036. |
| 76 | WAN Zhijian, TAO Youkun, YOU Hengzhi, et al. Facile synthesis of a sintering-resistant zeolite confined Ni catalyst for efficient CO x -free hydrogen generation from ammonia decomposition[J]. Sustainable Energy & Fuels, 2021, 5(12): 3182-3190. |
| 77 | INOKAWA H, ICHIKAWA T, MIYAOKA H. Catalysis of nickel nanoparticles with high thermal stability for ammonia decomposition[J]. Applied Catalysis A: General, 2015, 491: 184-188. |
| 78 | LUO Dan, ZHANG Xuqiang. The effect of oxygen-containing functional groups on the H2 adsorption of graphene-based nanomaterials: experiment and theory[J]. International Journal of Hydrogen Energy, 2018, 43(11): 5668-5679. |
| 79 | MIAO Meng, SHA Maolin, MENG Qiangqiang. The rule of N in N-doped graphene supported Pd catalyst[J]. Chemical Physics Letters, 2021, 763: 138155-138159. |
| 80 | MA Qingmin, XIE Zun, WANG Jing, et al. Structures, stabilities and magnetic properties of small Co clusters[J]. Physics Letters A, 2006, 358(4): 289-296. |
| 81 | MIAO Meng, SHI Hui, WANG Qi, al et, et al. The Ti4 cluster activates water dissociation on defective graphene[J]. Physical Chemistry Chemical Physics, 2014, 16(12): 5634-5639. |
| 1 | ACAR C, DINCER I. Review and evaluation of hydrogen production options for better environment[J]. Journal of Cleaner Production, 2019, 218: 835-849. |
| 2 | 苏玉蕾, 王少波, 宋刚祥, 等. 氨分解制氢催化剂研究进展[J]. 舰船科学技术, 2010, 32(4): 138-143. |
| SU Yulei, WANG Shaobo, SONG Gangxiang, et al. Development in catalysts for hydrogen production by ammonia decomposition[J]. Ship Science and Technology, 2010, 32(4): 138-143. | |
| 3 | OKURA K, OKANISHI T, MUROYAMA H, et al. Ammonia decomposition over nickel catalysts supported on rare-earth oxides for the on-site generation of hydrogen[J]. ChemCatChem, 2016, 8(18): 2988-2995. |
| 4 | LIU Jun, ZHENG Shuiying, ZHANG Zhixin, et al. Numerical study on the fast filling of on-bus gaseous hydrogen storage cylinder[J]. International Journal of Hydrogen Energy, 2020, 45(15): 9241-9251. |
| 5 | WIJAYANTA A T, ODA T, PURNOMO C W, et al. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: comparison review[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15026-15044. |
| 6 | KOJIMA Y. Hydrogen storage materials for hydrogen and energy carriers[J]. International Journal of Hydrogen Energy, 2019, 44(33): 18179-18192. |
| 7 | ATASHROUZ S, RAHMANI M. Predicting hydrogen storage capacity of metal-organic frameworks using group method of data handling[J]. Neural Computing and Applications, 2020, 32(18): 14851-14864. |
| 8 | LANG Chengguang, JIA Yi, YAO Xiangdong. Recent advances in liquid-phase chemical hydrogen storage[J]. Energy Storage Materials, 2020, 26: 290-312. |
| 9 | 刘红梅, 徐向亚, 张蓝溪, 等. 储氢材料的研究进展[J]. 石油化工, 2021, 50(10): 1101-1107. |
| LIU Hongmei, XU Xiangya, ZHANG Lanxi, et al. Research progress of hydrogen storage materials[J]. Petrochemical Technology, 2021, 50(10): 1101-1107. | |
| 10 | 王中华, 郑淞生, 姚育栋, 等. 电催化分解氨制氢研究进展[J]. 化工学报, 2022, 73(3): 1008-1021. |
| WANG Zhonghua, ZHENG Songsheng, YAO Yudong, et al. Research progress on electrocatalytic decomposition of ammonia for hydrogen production[J]. CIESC Journal, 2022, 73(3): 1008-1021. | |
| 11 | 蒋红华, 王亚运, 王帅, 等. 氨分解制氢镍基催化剂的制备和应用[J]. 化工生产与技术, 2020, 26(1): 5-7. |
| JIANG Honghua, WANG Yayun, WANG Shuai, et al. Preparation and application of nickel-based catalyst for ammonia decomposition to hydrogen[J]. Chemical Production and Technology, 2020, 26(1): 5-7. | |
| 12 | 邱书伟, 程群淑, 任铁真. 纳米氧化镍的制备及其氨分解制氢性能[J]. 石油化工, 2016, 45(10): 1180-1185. |
| QIU Shuwei, CHENG Qunshu, REN Tiezhen. Preparation and characterization of nano NiO catalysts for ammonia decomposition to hydrogen[J]. Petrochemical Technology, 2016, 45(10): 1180-1185. | |
| 13 | GU Yingqiu, JIN Zhao, ZHANG Hu, et al. Transition metal nanoparticles dispersed in an alumina matrix as active and stable catalysts for CO x -free hydrogen production from ammonia[J]. Journal of Materials Chemistry A, 2015, 3(33): 17172-17180. |
| 14 | 范清帅, 唐浩东, 韩文锋, 等. 氨分解制氢催化剂研究进展[J]. 工业催化, 2016, 24(8): 20-28. |
| FAN Qingshuai, TANG Haodong, HAN Wenfeng, et al. Advances in the catalysts for hydrogen production from ammonia decomposition[J]. Industrial Catalysis, 2016, 24(8): 20-28. | |
| 15 | HUANG Chuanqing, YU Yingzhi, TANG Xiaoyue, et al. Hydrogen generation by ammonia decomposition over Co/CeO2 catalyst: influence of support morphologies[J]. Applied Surface Science, 2020, 532: 147335-147343. |
| 16 | LE Thien An, KIM Youngmin, KIM Hyun Woo, et al. Ru-supported lanthania-ceria composite as an efficient catalyst for CO x -free H2 production from ammonia decomposition[J]. Applied Catalysis B: Environmental, 2021, 285: 119831-119841. |
| 17 | PINZÓN M, ROMERO A, DE LUCAS CONSUEGRA A, et al. Hydrogen production by ammonia decomposition over ruthenium supported on SiC catalyst[J]. Journal of Industrial and Engineering Chemistry, 2021, 94: 326-335. |
| 18 | LUCENTINI I, GARCÍA COLLI G, LUZI C D, et al. Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: effect of metal loading and kinetic analysis[J]. Applied Catalysis B: Environmental, 2021, 286: 119896. |
| 19 | 邱书伟, 任铁真, 李珺. 氨分解制氢催化剂改性研究进展[J]. 化工进展, 2018, 37(3):1001-1007. |
| QIU Shuwei, REN Tiezhen, LI Jun. The latest advances in the modified catalysts for hydrogen production from ammonia decomposition[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1001-1007. | |
| 20 | 常秋连, 李培霖, 陈松清, 等. 金属元素改性的超高温氨分解催化剂Ni/Mg-Al的结构及性能[J]. 石油炼制与化工, 2020, 51(2): 25-30. |
| Chang Qiulian, LI Peilin, Chen Songqing, et al. Structure and properties of Ni/Mg-Al catalyst modified by different metal elements[J]. Petroleum Processing and Petrochemicals, 2020, 51(2): 25-30. | |
| 21 | OJELADE O A, ZAMAN S F. Ammonia decomposition for hydrogen production: a thermodynamic study[J]. Chemical Papers, 2021, 75(1): 57-65. |
| 22 | YIN Shuangfeng, ZHANG Qinhui, XU Boqing, et al. Investigation on the catalysis of CO x -free hydrogen generation from ammonia[J]. Journal of Catalysis, 2004, 224(2): 384-396. |
| 23 | DUAN Xuezhi, QIAN Gang, FAN Chen, et al. First-principles calculations of ammonia decomposition on Ni(110) surface[J]. Surface Science, 2012, 606(3/4): 549-553. |
| 24 | TSAI W, WEINBERG W H. Steady-state decomposition of ammonia on the ruthenium(001) surface[J]. The Journal of Physical Chemistry, 1987, 91(20): 5302-5307. |
| 25 | TAKAHASHI A, FUJITANI T. Kinetic analysis of decomposition of ammonia over nickel and ruthenium catalysts[J]. Journal of Chemical Engineering of Japan, 2016, 49(1): 22-28. |
| 26 | STOLBOV S, RAHMAN T S. First-principles study of some factors controlling the rate of ammonia decomposition on Ni and Pd surfaces[J]. The Journal of Chemical Physics, 2005, 123(20): 204716. |
| 27 | PLANA C, ARMENISE S, MONZÓN A, et al. Process optimisation of in situ H2 generation from ammonia using Ni on alumina coated cordierite monoliths[J]. Topics in Catalysis, 2011, 54(13/14/15): 914-921. |
| 28 | OKURA K, OKANISHI T, MUROYAMA H, et al. Promotion effect of rare-earth elements on the catalytic decomposition of ammonia over Ni/Al2O3 catalyst[J]. Applied Catalysis A: General, 2015, 505: 77-85. |
| 29 | CHELLAPPA A S, FISCHER C M, THOMSON W J. Ammonia decomposition kinetics over Ni-Pt/Al2O3 for PEM fuel cell applications[J]. Applied Catalysis A: General, 2002, 227(1/2): 231-240. |
| 82 | MIAO Meng, GONG Xiaojing, LEI Shulai, et al. The graphene-supported non-noble metal catalysts activate ammonia decomposition: a DFT study[J]. Chemical Physics, 2021, 548: 111249-11254. |
| 83 | LIU Hongchao, WANG Hua, SHEN Jianghan, et al. Promotion effect of cerium and lanthanum oxides on Ni/SBA-15 catalyst for ammonia decomposition[J]. Catalysis Today, 2008, 131(1/2/3/4): 444-449. |
| 84 | VACHARAPONG P, ARAYAWATE S, HENPRASERTTAE S, et al. Effect of magnetic inducement in preparation of Ni/Ce-doped Al2O3 for ammonia decomposition[J]. ChemistrySelect, 2019, 4(40): 11913-11919. |
| 85 | ZHENG Weiqing, ZHANG Jian, GE Qingjie, et al. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen[J]. Applied Catalysis B: Environmental, 2008, 80(1/2): 98-105. |
| 86 | HENPRASERTTAE S, CHAROJROCHKUL S, KLYSUBUN W, et al. Reduced temperature ammonia decomposition using Ni/Zr-doped Al2O3 catalyst[J]. Catalysis Letters, 2018, 148(6): 1775-1783. |
| 87 | OKURA Kaname, OKANISHI Takeou, MUROYAMA Hiroki, et al. Additive effect of alkaline earth metals on ammonia decomposition reaction over Ni/Y2O3 catalysts[J]. RSC Advances, 2016, 6(88): 85142-85148. |
| 88 | LI Lei, WU Jun, SHAO Jingling, et al. Impacts of SiO2 shell structure of Ni@SiO2 nanocatalysts on their performance for catalytic decomposition of ammonia[J]. Catalysis Letters, 2017, 147(1): 141-149. |
| 89 | YAO L H, LI Y X, ZHAO J, et al. Core-shell structured nanoparticles (M@SiO2, Al2O3, MgO; M=Fe, Co, Ni, Ru) and their application in CO x -free H2 production via NH3 decomposition[J]. Catalysis Today, 2010, 158(3/4): 401-408. |
| 90 | ZHANG Lingfeng, LI Min, REN Tiezhen, et al. Ce-modified Ni nanoparticles encapsulated in SiO2 for CO x -free hydrogen production via ammonia decomposition[J]. International Journal of Hydrogen Energy, 2015, 40(6): 2648-2656. |
| 91 | YAO Lianghong, SHI Tianbao, LI Yanxing, et al. Core–shell structured nickel and ruthenium nanoparticles: very active and stable catalysts for the generation of CO x -free hydrogen via ammonia decomposition[J]. Catalysis Today, 2011, 164(1): 112-118. |
| 30 | ZHANG Jian, XU Hengyong, LI Wenzhao. Kinetic study of NH3 decomposition over Ni nanoparticles: the role of La promoter, structure sensitivity and compensation effect[J]. Applied Catalysis A: General, 2005, 296(2): 257-267. |
| 31 | CHEN Shuangjing, CHEN Xin, ZHANG Hui. Nanoscale size effect of octahedral nickel catalyst towards ammonia decomposition reaction[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17122-17128. |
| 32 | LI Yanping, WEN Jie, Arshid M ALI, et al. Size structure-catalytic performance correlation of supported Ni/MCF-17 catalysts for CO x -free hydrogen production[J]. Chemical communications, 2018, 54(49): 6364-6367. |
| 33 | ZHANG Jian, XU Hengyong, JIN Xianglan, et al. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La-Al2O3 catalysts for NH3 decomposition[J]. Applied Catalysis A: General, 2005, 290(1/2): 87-96. |
| 34 | SIMONSEN S B, CHAKRABORTY D, CHORKENDORFF I, et al. Alloyed Ni-Fe nanoparticles as catalysts for NH3 decomposition[J]. Applied Catalysis A: General, 2012, 447/448: 22-31. |
| 35 | HUANG Chuanqing, LI Huaxi, YANG Jinmei, et al. Ce0.6Zr0.3Y0.1O2 solid solutions-supported NiCo bimetal nanocatalysts for NH3 decomposition[J]. Applied Surface Science, 2019, 478: 708-716. |
| 36 | SHIMODA N, YOSHIMURA R, NUKUI T, et al. Alloying effect of nickel-cobalt based binary metal catalysts supported on α-alumina for ammonia decomposition[J]. Journal of Chemical Engineering of Japan, 2019, 52(5): 413-422. |
| 37 | WU Zewei, LI Xin, QIN Yuanhang, et al. Ammonia decomposition over SiO2-supported Ni-Co bimetallic catalyst for CO x -free hydrogen generation[J]. International Journal of Hydrogen Energy, 2020, 45(30): 15263-15269. |
| 38 | SILVA H, NIELSEN M G, FIORDALISO E M, et al. Synthesis and characterization of Fe-Ni/γ-Al2O3 egg-shell catalyst for H2 generation by ammonia decomposition[J]. Applied Catalysis A: General, 2015, 505: 548-556. |
| 39 | HAN Xue, CHU Wei, NI Ping, et al. Promoting effects of iridium on nickel based catalyst in ammonia decomposition[J]. Journal of Fuel Chemistry and Technology, 2007, 35(6): 691-695. |
| 40 | CHEN Xin, ZHOU Junwei, CHEN Shuangjing, et al. Catalytic performance of M@Ni (M = Fe, Ru, Ir) core-shell nanoparticles towards ammonia decomposition for CO x -free hydrogen production[J]. Journal of Nanoparticle Research, 2018, 20(6): 1-9. |
| 41 | YI Yanhui, WANG Li, GUO Yanjun, et al. Plasma-assisted ammonia decomposition over Fe-Ni alloy catalysts for CO x -free hydrogen[J]. AIChE Journal, 2019,65(2): 691-701. |
| 42 | 孙帅其, 易颜辉, 王丽, 等. 负载型双金属催化剂的制备及其等离子体催化氨分解制氢性能[J]. 物理化学学报, 2017, 33(6): 1123-1129. |
| SUN Shuaiqi, YI Yanhui, WANG Li, et al. Preparation and performance of supported bimetallic catalysts for hydrogen production from ammonia decomposition by plasma catalysis[J]. Acta Physico-Chimica Sinica, 2017, 33(6): 1123-1129. | |
| 43 | LUCENTINI I, CASANOVAS A, LLORCA J. Catalytic ammonia decomposition for hydrogen production on Ni, Ru and NiRu supported on CeO2 [J]. International Journal of Hydrogen Energy, 2019, 44(25): 12693-12707. |
| 44 | HANSGEN D A, VLACHOS D G, CHEN Jingguang. Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction[J]. Nature Chemistry, 2010, 2(6): 484-489. |
| 45 | XIE Pengfei, YAO Yonggang, HUANG Zhennan, et al. Highly efficient decomposition of ammonia using high-entropy alloy catalysts[J]. Nature Communications, 2019, 10(1): 4011-4022. |
| 46 | 王晓光, 韦永德, 张建, 等. 高效镍基氨分解催化体系中载体作用的研究[J]. 石油学报(石油加工), 2006, 22(5): 33-38. |
| WANG Xiaoguang, WEI Yongde, ZHANG Jian, et al. Investigation on the role of support in the high-performance nickel-based catalytic system for ammonia decomposition[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2006, 22(5): 33-38. | |
| 47 | LIU Hongchao, WANG Hua, SHEN Jianghan, et al. Preparation, characterization and activities of the nano-sized Ni/SBA-15 catalyst for producing CO x -free hydrogen from ammonia[J]. Applied Catalysis A: General, 2008, 337(2): 138-147. |
| 48 | ATSUMI R, NODA R, TAKAGI H, et al. Ammonia decomposition activity over Ni/SiO2 catalysts with different pore diameters[J]. International Journal of Hydrogen Energy, 2014, 39(26): 13954-13961. |
| 49 | LI Lei, CHEN Feng, SHAO Jingling,, et al. Attapulgite clay supported Ni nanoparticles encapsulated by porous silica: thermally stable catalysts for ammonia decomposition to CO x free hydrogen[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21157-21165. |
| 50 | IM Y, MUROYAMA H, MATSUI T, et al. Ammonia decomposition over nickel catalysts supported on alkaline earth metal aluminate for H2 production[J]. International Journal of Hydrogen Energy, 2020, 45(51): 26979-26988. |
| 51 | DENG Qingfang, ZHANG Hui, HOU Xiaoxu, et al. High-surface-area Ce0.8Zr0.2O2 solid solutions supported Ni catalysts for ammonia decomposition to hydrogen[J]. International Journal of Hydrogen Energy, 2012, 37(21): 15901-15907. |
| 52 | MUROYAMA H, SABURI C, MATSUI T, et al. Ammonia decomposition over Ni/La2O3 catalyst for on-site generation of hydrogen[J]. Applied Catalysis A: General, 2012, 443/444: 119-124. |
| 53 | YU Yingzhi, GAN Yumeng, HUANG Chuanqing, et al. Ni/La2O3 and Ni/MgO-La2O3 catalysts for the decomposition of NH3 into hydrogen[J]. International Journal of Hydrogen Energy, 2020, 45(33): 16528-16539. |
| 54 | HU Zhongpan, WENG Chenchen, CHEN Chong, et al. Catalytic decomposition of ammonia to CO x -free hydrogen over Ni/ZSM-5 catalysts: a comparative study of the preparation methods[J]. Applied Catalysis A: General, 2018, 562: 49-57. |
| 55 | SU Qin, GU Lingli, YAO Yao, et al. Layered double hydroxides derived Ni x (Mg y Al z O n ) catalysts: enhanced ammonia decomposition by hydrogen spillover effect[J]. Applied Catalysis B: Environmental, 2017, 201: 451-460. |
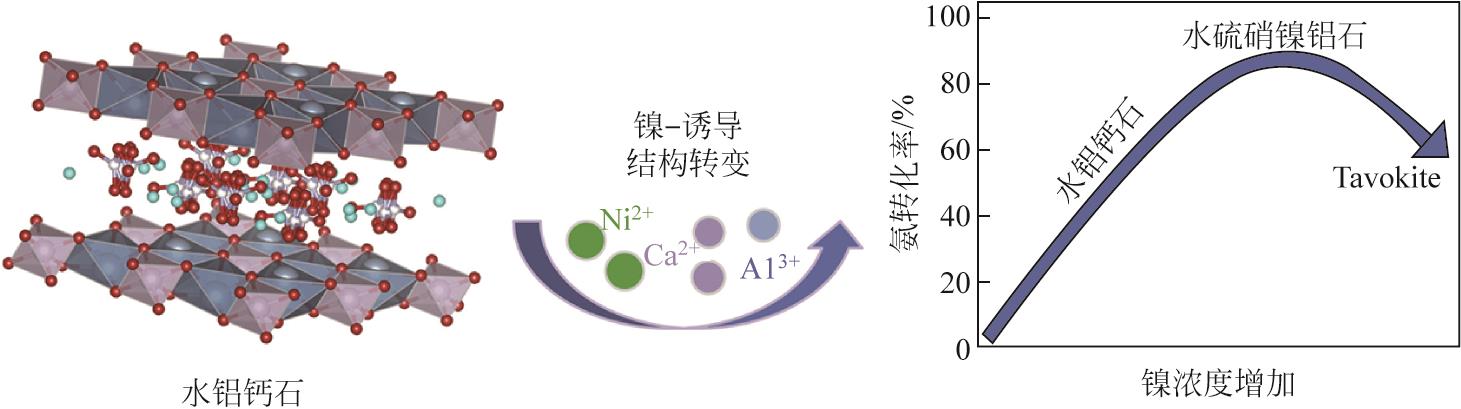
| 56 | ZHAO Jiawen, DENG Lidan, ZHENG Wei, et al. Nickel-induced structure transformation in hydrocalumite for enhanced ammonia decomposition[J]. International Journal of Hydrogen Energy, 2020, 45(22): 12244-12255. |
| 57 | SATO K, ABE N, KAWAGOE T, et al. Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition[J]. International Journal of Hydrogen Energy, 2017, 42(10): 6610-6617. |
| 58 | OKURA K, MIYAZAKI K, MUROYAMA H, et al. Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen[J]. RSC Advances, 2018, 8(56): 32102-32110. |
| 59 | KURTOĞLU S F, SARP S, YıLMAZ AKKAYA C, et al. CO x -free hydrogen production from ammonia decomposition over sepiolite-supported nickel catalysts[J]. International Journal of Hydrogen Energy, 2018, 43(21): 9954-9968. |
| 60 | NAKAMURA I, FUJITANI T. Role of metal oxide supports in NH3 decomposition over Ni catalysts[J]. Applied Catalysis A: General, 2016, 524: 45-49. |
| 61 | HU Zhongpan, WENG Chenchen, YUAN Gege, et al. Ni nanoparticles supported on mica for efficient decomposition of ammonia to CO x -free hydrogen[J]. International Journal of Hydrogen Energy, 2018, 43(20): 9663-9676. |
| 62 | CAO Jianliang, YAN Zhaoli, DENG Qingfang, et al. Homogeneous precipitation method preparation of modified red mud supported Ni mesoporous catalysts for ammonia decomposition[J]. Catalysis Science & Technology, 2014, 4(2): 361-368. |
| 63 | CAO Jianliang, YAN Zhaoli, DENG Qingfang, et al. Mesoporous modified-red-mud supported Ni catalysts for ammonia decomposition to hydrogen[J]. International Journal of Hydrogen Energy, 2014, 39(11): 5747-5755. |
| 64 | JI Jian, DUAN Xuezhi, QIAN Gang, et al. In situ production of Ni catalysts at the tips of carbon nanofibers and application in catalytic ammonia decomposition[J]. Industrial & Engineering Chemistry Research, 2013, 52(5): 1854-1858. |
| 65 | JI Jian, PHAM Thanh Hai, DUAN Xuezhi, et al. Morphology dependence of catalytic properties of Ni nanoparticles at the tips of carbon nanofibers for ammonia decomposition to generate hydrogen[J]. International Journal of Hydrogen Energy, 2014, 39(35): 20722-20730. |
| 66 | ZHANG Hui, ALHAMED Y A, KOJIMA Y, et al. Structure and catalytic properties of Ni/MWCNTs and Ni/AC catalysts for hydrogen production via ammonia decomposition[J]. International Journal of Hydrogen Energy, 2014, 39(1): 277-287. |
| 67 | MENG Tao, XU Qianqian, LI Yintao, et al. Nickle nanoparticles highly dispersed on reduced graphene oxide for ammonia decomposition to hydrogen[J]. Journal of Industrial and Engineering Chemistry, 2015, 32: 373-379. |
| 68 | GU Yingqiu, MA Yinglan, LONG Zhouyang, et al. One-pot synthesis of supported Ni@Al2O3 catalysts with uniform small-sized Ni for hydrogen generation via ammonia decomposition[J]. International Journal of Hydrogen Energy, 2021, 46(5): 4045-4054. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [13] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [14] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [15] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||