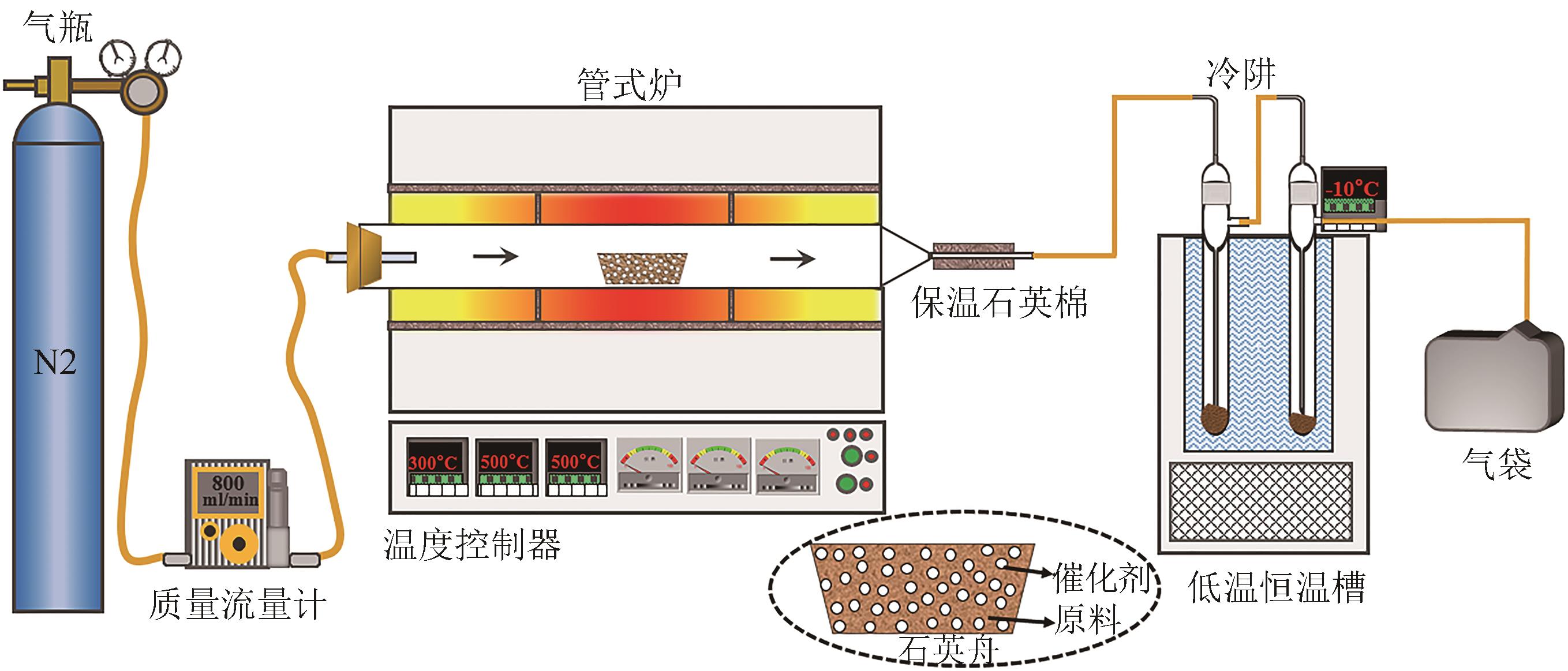
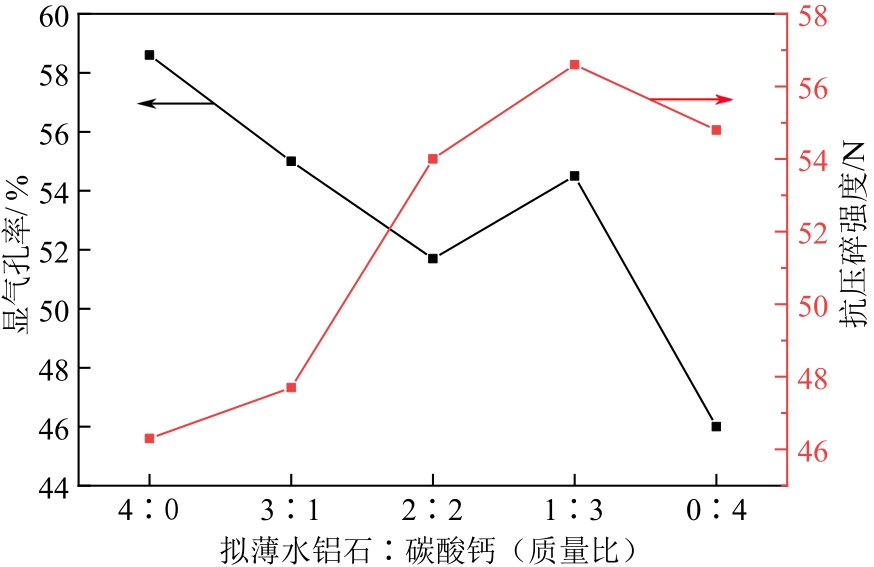
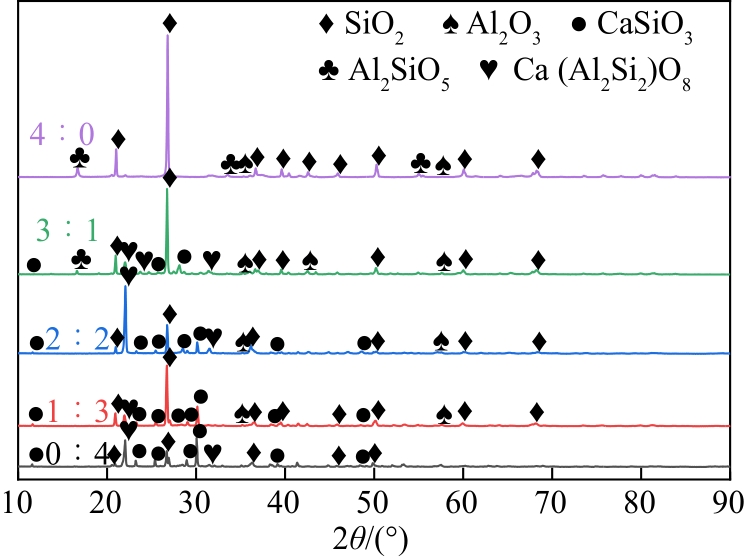
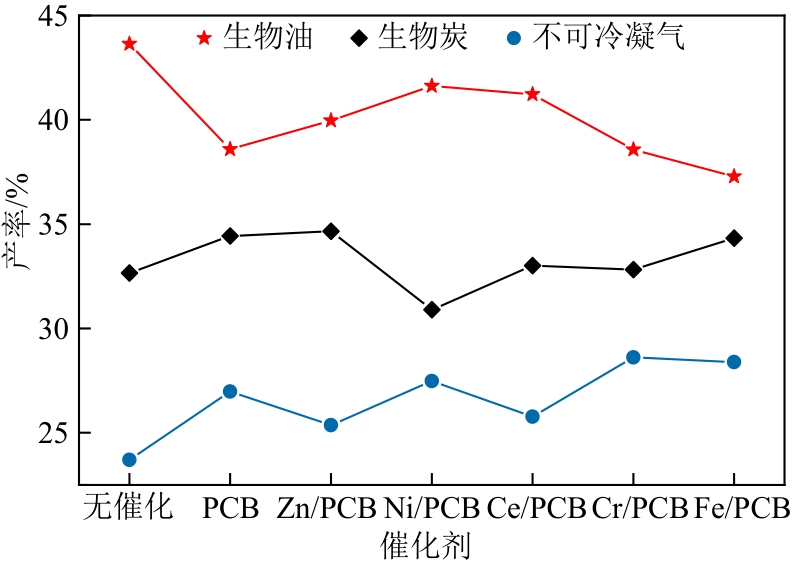
化工进展 ›› 2022, Vol. 41 ›› Issue (7): 3597-3607.DOI: 10.16085/j.issn.1000-6613.2021-1833
催化型多孔陶瓷球制备及催化玉米秸秆热解
李玉峰1,2( ), 王绍庆1,2, 张安东1,2, 毕冬梅1,2, 李志合1,2(
), 王绍庆1,2, 张安东1,2, 毕冬梅1,2, 李志合1,2( ), 高亮1,2, 万震1,2
), 高亮1,2, 万震1,2
- 1.山东理工大学农业工程与食品科学学院,山东 淄博 255000
2.山东省清洁能源工程技术研究中心,山东 淄博 255000
-
收稿日期:2021-08-26修回日期:2021-09-08出版日期:2022-07-25发布日期:2022-07-23 -
通讯作者:李志合 -
作者简介:李玉峰(1995—),男,硕士研究生,研究方向为生物质能源与材料。E-mail:lyf1067176193@163.com 。 -
基金资助:国家重点研发计划(2019YFD1100600);国家自然科学基金(52176192);山东省自然科学基金(ZR2020ME184);周村区校城融合发展计划(2020ZCXCZH09)
Preparation of catalytic porous ceramic balls and catalytic pyrolysis of corn stover
LI Yufeng1,2( ), WANG Shaoqing1,2, ZHANG Andong1,2, BI Dongmei1,2, LI Zhihe1,2(
), WANG Shaoqing1,2, ZHANG Andong1,2, BI Dongmei1,2, LI Zhihe1,2( ), GAO Liang1,2, WAN Zhen1,2
), GAO Liang1,2, WAN Zhen1,2
- 1.School of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo 255000, Shandong, China
2.Shandong Research Center of Engineering & Technology for Clean Energy, Zibo 255000, Shandong, China
-
Received:2021-08-26Revised:2021-09-08Online:2022-07-25Published:2022-07-23 -
Contact:LI Zhihe
摘要:
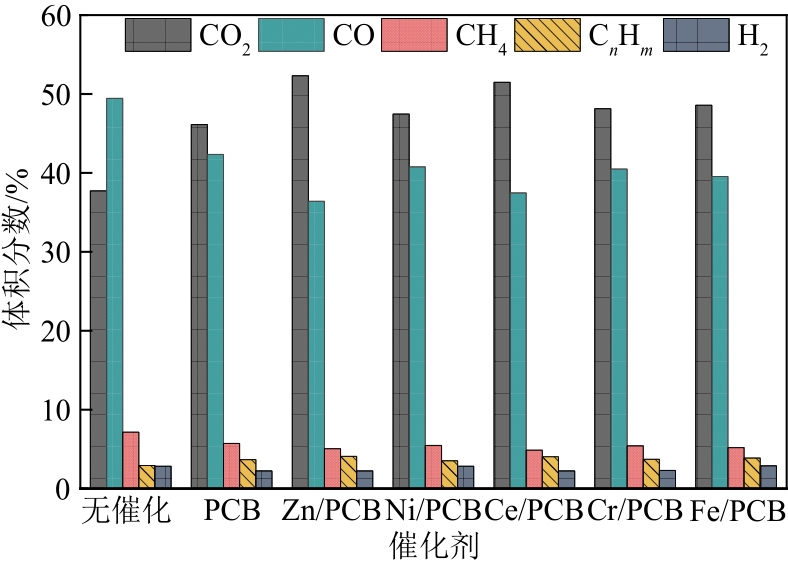
针对生物质热解液化过程中生物油品质差的问题,本文以陶瓷球作热载体为研究基础,制备了5种负载金属氧化物(ZnO、NiO、CeO2、Cr2O3、Fe2O3)的多孔陶瓷球,在固定床反应器上研究多孔陶瓷球催化剂对玉米秸秆热解过程的催化效果。结果表明:多孔陶瓷球基体在热解过程中有一定催化活性,经浸渍改性处理后,都能促进生物油产率的提高,其中Ni基多孔陶瓷球热解的产率高达41.62%。多孔陶瓷球负载的5种金属氧化物可促使生物油中酚类、呋喃类物质的含量明显增加,酸类物质的含量明显降低,且种类减少,其中CeO2降酸效果显著,降低幅度为37.15%。此外,催化型多孔陶瓷球的引入促进了不可冷凝气中C n H m (n≥2)的生成,烯烃类中乙烯的增长幅度最大,为50.53%,同时生物炭的理化特性在一定程度上得到改善和提高。
中图分类号:
引用本文
李玉峰, 王绍庆, 张安东, 毕冬梅, 李志合, 高亮, 万震. 催化型多孔陶瓷球制备及催化玉米秸秆热解[J]. 化工进展, 2022, 41(7): 3597-3607.
LI Yufeng, WANG Shaoqing, ZHANG Andong, BI Dongmei, LI Zhihe, GAO Liang, WAN Zhen. Preparation of catalytic porous ceramic balls and catalytic pyrolysis of corn stover[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3597-3607.
| 元素分析 wd/% | 工业分析 wd/% | 组分分析 wd/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O* | 灰分 | 挥发分 | 固定碳 | 纤维素 | 半纤维素 | 木质素 | ||
| 0.63 | 43.87 | 5.57 | 40.11 | 9.82 | 71.31 | 18.87 | 31.98 | 29.23 | 2.84 | ||
表1 玉米秸秆的元素分析、工业分析和组分分析(质量分数)
| 元素分析 wd/% | 工业分析 wd/% | 组分分析 wd/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O* | 灰分 | 挥发分 | 固定碳 | 纤维素 | 半纤维素 | 木质素 | ||
| 0.63 | 43.87 | 5.57 | 40.11 | 9.82 | 71.31 | 18.87 | 31.98 | 29.23 | 2.84 | ||
| 生物油组分 | 相对峰面积/% | ||||||
|---|---|---|---|---|---|---|---|
| 无催化 | PCB | Zn/PCB | Ni/PCB | Ce/PCB | Cr/PCB | Fe/PCB | |
| 醛类 | |||||||
| 甲醛 | 1.27 | 0.71 | 0.25 | 0.36 | 0.32 | 0.22 | 0.28 |
| 甲基乙二醛 | 0.60 | 0.77 | 1.22 | 1.62 | 1.40 | 2.50 | 1.80 |
| 2-甲基十一醛 | — | — | 1.53 | 1.33 | 1.81 | 1.36 | 1.40 |
| 3-羟基苯甲醛 | 0.88 | 0.81 | 1.44 | 1.52 | 1.67 | 1.51 | 1.37 |
| 醇类 | |||||||
| 甲醇 | 4.91 | 4.26 | 2.48 | 2.02 | 1.53 | 2.33 | 2.18 |
| 1,7,7-三甲基-双环[2,2,1]庚烷-2,3-二醇 | 1.25 | 1.59 | — | — | — | — | — |
| 酮类 | |||||||
| 2,3-丁二酮 | 1.78 | 1.65 | — | — | — | — | — |
| 1-羟基-2-丙酮 | 6.29 | 6.42 | 5.06 | 5.26 | 4.42 | 4.35 | 5.18 |
| 1-乙酰氧基-2-丁酮 | 1.87 | 1.66 | 1.50 | 1.38 | 1.29 | 1.12 | 1.28 |
| 1-乙酰氧基-2-丙酮 | 1.70 | 1.52 | 1.21 | 1.26 | 1.23 | 1.07 | 1.34 |
| 2-甲基-1-戊烯-1酮 | 0.88 | 0.93 | 1.60 | 1.60 | 1.95 | 1.42 | 1.01 |
| 3-甲基-1,2-环戊二酮 | — | — | 1.45 | 1.57 | 1.67 | 1.20 | 1.52 |
| 酸类 | |||||||
| 甲酸 | 1.27 | 0.54 | — | — | — | — | — |
| 乙酸 | 22.9 | 23.05 | 19.39 | 20.37 | 17.54 | 20.09 | 24.82 |
| 丙酸 | 2.62 | 2.57 | 1.33 | 1.60 | 1.41 | 1.20 | 1.68 |
| 亚甲基环丙烷羧酸 | 1.95 | 2.03 | — | — | — | — | — |
| 酯类 | |||||||
| 2-氧代丙酸甲酯 | 1.11 | 0.91 | 0.61 | 0.52 | 0.53 | 0.41 | 0.52 |
| 丁内酯 | 0.97 | 1.22 | 1.18 | 1.41 | 1.36 | 1.32 | 1.47 |
| 酚类 | |||||||
| 苯酚 | 3.48 | 4.01 | 3.81 | 4.08 | 3.69 | 3.73 | 4.07 |
| 2-甲基苯酚 | 1.39 | 1.56 | 1.12 | 1.10 | 1.03 | 1.06 | 1.11 |
| 对甲酚 | 1.85 | 2.14 | 2.43 | 2.23 | 2.25 | 2.23 | 2.45 |
| 4-乙基苯酚 | 1.91 | 2.55 | 2.47 | 2.58 | 2.32 | 2.17 | 2.23 |
| 邻苯二酚 | — | — | 1.96 | 1.67 | 1.94 | 0.43 | 0.72 |
| 对苯二酚 | — | — | 1.33 | 1.33 | 1.38 | 1.39 | 1.30 |
| 呋喃类 | |||||||
| 糠醛 | 6.02 | 6.25 | 3.08 | 3.22 | 3.23 | 3.64 | 4.22 |
| 2-呋喃甲醇 | — | — | 1.43 | 1.51 | 1.39 | 1.41 | 1.44 |
| 3-甲基-2,5-呋喃二酮 | 0.94 | 0.89 | 1.79 | 1.38 | 1.55 | 2.02 | 1.79 |
| 2,3-二氢苯并呋喃 | 4.06 | 4.92 | 7.04 | 6.81 | 6.94 | 7.71 | 7.00 |
| 5-羟甲基糠醛 | 1.10 | 0.94 | 1.87 | 1.65 | 1.89 | 1.94 | 1.90 |
| 糖类 | |||||||
| 1,4:3,6-二脱水-α-D-吡喃葡萄糖 | — | — | 1.67 | 1.56 | 1.70 | 1.70 | 1.78 |
| 1,6-脱水-β-D-吡喃葡萄糖 | 1.57 | 1.44 | 2.61 | 2.59 | 3.22 | 3.65 | 2.14 |
表2 生物油主要组分及相对含量
| 生物油组分 | 相对峰面积/% | ||||||
|---|---|---|---|---|---|---|---|
| 无催化 | PCB | Zn/PCB | Ni/PCB | Ce/PCB | Cr/PCB | Fe/PCB | |
| 醛类 | |||||||
| 甲醛 | 1.27 | 0.71 | 0.25 | 0.36 | 0.32 | 0.22 | 0.28 |
| 甲基乙二醛 | 0.60 | 0.77 | 1.22 | 1.62 | 1.40 | 2.50 | 1.80 |
| 2-甲基十一醛 | — | — | 1.53 | 1.33 | 1.81 | 1.36 | 1.40 |
| 3-羟基苯甲醛 | 0.88 | 0.81 | 1.44 | 1.52 | 1.67 | 1.51 | 1.37 |
| 醇类 | |||||||
| 甲醇 | 4.91 | 4.26 | 2.48 | 2.02 | 1.53 | 2.33 | 2.18 |
| 1,7,7-三甲基-双环[2,2,1]庚烷-2,3-二醇 | 1.25 | 1.59 | — | — | — | — | — |
| 酮类 | |||||||
| 2,3-丁二酮 | 1.78 | 1.65 | — | — | — | — | — |
| 1-羟基-2-丙酮 | 6.29 | 6.42 | 5.06 | 5.26 | 4.42 | 4.35 | 5.18 |
| 1-乙酰氧基-2-丁酮 | 1.87 | 1.66 | 1.50 | 1.38 | 1.29 | 1.12 | 1.28 |
| 1-乙酰氧基-2-丙酮 | 1.70 | 1.52 | 1.21 | 1.26 | 1.23 | 1.07 | 1.34 |
| 2-甲基-1-戊烯-1酮 | 0.88 | 0.93 | 1.60 | 1.60 | 1.95 | 1.42 | 1.01 |
| 3-甲基-1,2-环戊二酮 | — | — | 1.45 | 1.57 | 1.67 | 1.20 | 1.52 |
| 酸类 | |||||||
| 甲酸 | 1.27 | 0.54 | — | — | — | — | — |
| 乙酸 | 22.9 | 23.05 | 19.39 | 20.37 | 17.54 | 20.09 | 24.82 |
| 丙酸 | 2.62 | 2.57 | 1.33 | 1.60 | 1.41 | 1.20 | 1.68 |
| 亚甲基环丙烷羧酸 | 1.95 | 2.03 | — | — | — | — | — |
| 酯类 | |||||||
| 2-氧代丙酸甲酯 | 1.11 | 0.91 | 0.61 | 0.52 | 0.53 | 0.41 | 0.52 |
| 丁内酯 | 0.97 | 1.22 | 1.18 | 1.41 | 1.36 | 1.32 | 1.47 |
| 酚类 | |||||||
| 苯酚 | 3.48 | 4.01 | 3.81 | 4.08 | 3.69 | 3.73 | 4.07 |
| 2-甲基苯酚 | 1.39 | 1.56 | 1.12 | 1.10 | 1.03 | 1.06 | 1.11 |
| 对甲酚 | 1.85 | 2.14 | 2.43 | 2.23 | 2.25 | 2.23 | 2.45 |
| 4-乙基苯酚 | 1.91 | 2.55 | 2.47 | 2.58 | 2.32 | 2.17 | 2.23 |
| 邻苯二酚 | — | — | 1.96 | 1.67 | 1.94 | 0.43 | 0.72 |
| 对苯二酚 | — | — | 1.33 | 1.33 | 1.38 | 1.39 | 1.30 |
| 呋喃类 | |||||||
| 糠醛 | 6.02 | 6.25 | 3.08 | 3.22 | 3.23 | 3.64 | 4.22 |
| 2-呋喃甲醇 | — | — | 1.43 | 1.51 | 1.39 | 1.41 | 1.44 |
| 3-甲基-2,5-呋喃二酮 | 0.94 | 0.89 | 1.79 | 1.38 | 1.55 | 2.02 | 1.79 |
| 2,3-二氢苯并呋喃 | 4.06 | 4.92 | 7.04 | 6.81 | 6.94 | 7.71 | 7.00 |
| 5-羟甲基糠醛 | 1.10 | 0.94 | 1.87 | 1.65 | 1.89 | 1.94 | 1.90 |
| 糖类 | |||||||
| 1,4:3,6-二脱水-α-D-吡喃葡萄糖 | — | — | 1.67 | 1.56 | 1.70 | 1.70 | 1.78 |
| 1,6-脱水-β-D-吡喃葡萄糖 | 1.57 | 1.44 | 2.61 | 2.59 | 3.22 | 3.65 | 2.14 |
| 催化剂 | 比表面积/m2·g-1 | 孔容/10-3cm3·g-1 | 孔径/nm |
|---|---|---|---|
| 无 | 3.78 | 6.75 | 13.54 |
| PCB | 4.44 | 6.89 | 11.53 |
| Zn/PCB | 4.96 | 7.71 | 7.93 |
| Ni/PCB | 5.43 | 7.77 | 7.12 |
| Ce/PCB | 5.11 | 9.52 | 7.79 |
| Cr/PCB | 8.28 | 14.99 | 7.45 |
| Fe/PCB | 9.37 | 15.91 | 7.03 |
表3 生物炭的孔隙特征
| 催化剂 | 比表面积/m2·g-1 | 孔容/10-3cm3·g-1 | 孔径/nm |
|---|---|---|---|
| 无 | 3.78 | 6.75 | 13.54 |
| PCB | 4.44 | 6.89 | 11.53 |
| Zn/PCB | 4.96 | 7.71 | 7.93 |
| Ni/PCB | 5.43 | 7.77 | 7.12 |
| Ce/PCB | 5.11 | 9.52 | 7.79 |
| Cr/PCB | 8.28 | 14.99 | 7.45 |
| Fe/PCB | 9.37 | 15.91 | 7.03 |
| 1 | WU Q H, WANG Y P, PENG Y J, et al. Microwave-assisted pyrolysis of waste cooking oil for hydrocarbon bio-oil over metal oxides and HZSM-5 catalysts[J]. Energy Conversion and Management, 2020, 220: 113124. |
| 2 | DAI L L, WANG Y P, LIU Y H, et al. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: a state-of-the-art review[J]. Renewable and Sustainable Energy Reviews, 2019, 107: 20-36. |
| 3 | 仉俐, 姚宗路, 赵立欣, 等. 生物质热解制备高品质生物油研究进展[J]. 化工进展, 2021, 40(1): 139-150. |
| ZHANG Li, YAO Zonglu, ZHAO Lixin, et al. Researd progress on preparation of high quality bio-oil by pyrolysis of biomass[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 139-150. | |
| 4 | 方书起, 王毓谦, 李攀, 等. 生物质热解利用中主要催化剂的研究进展[J]. 化工进展, 2021, 40(9): 5195-5203. |
| FANG Shuqi, WANG Yuqian, LI Pan, et al. Researd progress of main catalyst in biomass pyrolysis and utilization[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5195-5203. | |
| 5 | LIU C, WANG H, KARIM A M, et al. Catalytic fast pyrolysis of lignocellulosic biomass[J]. Chemical Society Reviews, 2014, 43(22): 7594-7623. |
| 6 | CHEN X, CHE Q F, LI S J, et al. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield[J]. Fuel Processing Technology, 2019, 196: 106180. |
| 7 | ZHANG C T, ZHANG L J, LI Q Y, et al. Catalytic pyrolysis of poplar wood over transition metal oxides: correlation of catalytic behaviors with physiochemical properties of the oxides[J]. Biomass and Bioenergy, 2019, 124: 125-141. |
| 8 | 方书起, 石崇, 李攀, 等. Fe-Zn共改性ZSM-5催化作用下生物质快速热解特性研究[J]. 化工学报, 2020, 71(4): 1637-1645. |
| FANG Shuqi, SHI Chong, LI Pan, et al. Study on rapid pyrolysis characteristics of biomass catalyzed by Fe-Zn co-modified ZSM-5[J]. CIESC Journal, 2020, 71(4): 1637-1645. | |
| 9 | MANTE O D, RODRIGUEZ J A, SENANAYAKE S D, et al. Catalytic conversion of biomass pyrolysis vapors into hydrocarbon fuel precursors[J]. Green Chemistry, 2015, 17(4): 2362-2368. |
| 10 | LI Z H, LI N, YI W M, et al. Design and operation of a down-tube reactor demonstration plant for biomass fast pyrolysis[J]. Fuel Processing Technology, 2017, 161: 182-192. |
| 11 | FU P, YI W M, LI Z H, et al. Comparative study on fast pyrolysis of agricultural straw residues based on heat carrier circulation heating[J]. Bioresource Technology, 2019, 271: 136-142. |
| 12 | FU P, BAI X Y, LI Z H, et al. Fast pyrolysis of corn stovers with ceramic ball heat carriers in a novel dual concentric rotary cylinder reactor[J]. Bioresource Technology, 2018, 263: 467-474. |
| 13 | 毕冬梅, 张凯真, 易维明, 等. 白云石基多孔陶瓷负载Al2O3催化生物质热解试验[J]. 农业机械学报, 2019, 50(10): 315-322. |
| BI Dongmei, ZHANG Kaizhen, YI Weiming, et al. Catalytic pyrolysis of biomass with porous ceramic loading aluminum oxide[J]. Transactions of the Chinese Society for Agricultural Machinery, 2019, 50(10): 315-322. | |
| 14 | 李润东, 张杨, 李秉硕, 等. 玉米秸秆催化液化制备生物油实验研究[J]. 燃料化学学报, 2016, 44(1): 69-75. |
| LI Rundong, ZHANG Yang, LI Bingshuo, et al. Hydrothermal catalytic liquefaction of corn stalk for preparation of bio-oil[J]. Journal of Fuel Chemistry and Technology, 2016, 44(1): 69-75. | |
| 15 | 王绍庆, 李志合, 易维明, 等. 活化赤泥催化热解玉米芯木质素制备高值单酚[J]. 农业工程学报, 2020, 36(13): 203-211. |
| WANG Shaoqing, LI Zhihe, YI Weiming, et al. Catalytic pyrolysis of maize cob lignin over activated red mud catalyst for value-added mono-phenol production[J]. Transactions of the Chinese Society of Agricultural Engineering, 2020, 36(13): 203-211. | |
| 16 | ZOU A C, LIANG H X, JIAO C, et al. Fabrication and properties of CaSiO3/ Sr3(PO4)2 composite scaffold based on extrusion deposition[J]. Ceramics International, 2021, 47(4): 4783-4792. |
| 17 | HU X, GHOLIZADEH M. Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage[J]. Journal of Energy Chemistry, 2019, 39: 109-143. |
| 18 | 王亚珂, 朱保顺, 力国民, 等. 利用固废煤矸石制备Fe/C/Mullite-基陶粒复合型吸波材料[J]. 燃料化学学报, 2021, 49(2): 238-246. |
| WANG Yake, ZHU Baoshun, LI Guomin, et al. Preparation of Fe/C/Mullite-based ceramsite composite absorbing materials by recycling solid waste coal gangue[J]. Journal of Fuel Chemistry and Technology, 2021, 49(2): 238-246. | |
| 19 | YU Y X, KONG J, WANG M J, et al. Structure and oxidation reactivity of char: effects of pyrolysis heating rate and pressure[J]. Journal of Fuel Chemistry and Technology, 2018, 46(9): 1025-1035. |
| 20 | 胡二峰, 赵立欣, 吴娟, 等. 生物质热解影响因素及技术研究进展[J]. 农业工程学报, 2018, 34(14): 212-220. |
| HU Erfeng, ZHAO Lixin, WU Juan, et al. Research advance on influence factors and technologies of biomass pyrolysis[J]. Transactions of the Chinese Society of Agricultural Engineering, 2018, 34(14): 212-220. | |
| 21 | JAHIRUL M, RASUL M, CHOWDHURY A, et al. Biofuels production through biomass pyrolysis—A technological review[J]. Energies, 2012, 5(12): 4952-5001. |
| 22 | CHEN S, WOJCIESZAK R, DUMEIGNIL F, et al. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural[J]. Chemical Reviews, 2018, 118(22): 11023-11117. |
| 23 | WANG J Z, LIU Q, ZHOU J, et al. Production of high-value chemicals by biomass pyrolysis with metal oxides and zeolites[J]. Waste and Biomass Valorization, 2021, 12(6): 3049-3057. |
| 24 | 丁爽, 葛庆峰, 祝新利. 金属氧化物催化生物质衍生羧酸酮基化研究进展[J]. 化学学报, 2017, 75(5): 439-447. |
| DING Shuang, GE Qingfeng, ZHU Xinli. Research progress in ketonization of biomass-derived carboxylic acids over metal oxides[J]. Acta Chimica Sinica, 2017, 75(5): 439-447. | |
| 25 | CHEN X, LI S J, LIU Z H, et al. Pyrolysis characteristics of lignocellulosic biomass components in the presence of CaO[J]. Bioresource Technology, 2019, 287: 121493. |
| 26 | FU P, YI W M, LI Z H, et al. Evolution of char structural features during fast pyrolysis of corn straw with solid heat carriers in a novel V-shaped down tube reactor[J]. Energy Conversion and Management, 2017, 149: 570-578. |
| 27 | ZHANG A D, LIANG C M, WANG L H, et al. Study on the trigger mechanism and carbon behavior of catalytic reforming of wood vinegar[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14669-14678. |
| 28 | ZHANG A D, LI Z H, YI W M, et al. Study the reaction mechanism of catalytic reforming of acetic acid through the instantaneous gas production[J]. International Journal of Hydrogen Energy, 2019, 44(39): 21279-21289. |
| 29 | GAO L, LI Z H, YI W M, et al. Impacts of pyrolysis temperature on lead adsorption by cotton stalk-derived biochar and related mechanisms[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105602. |
| [1] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [2] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [3] | 郑梦启, 王成业, 汪炎, 王伟, 袁守军, 胡真虎, 何春华, 王杰, 梅红. 菌藻共生技术在工业废水零排放中的应用与展望[J]. 化工进展, 2023, 42(8): 4424-4431. |
| [4] | 关红玲, 杨辉, 井红权, 刘玉琼, 谷守玉, 王好斌, 侯翠红. 木质素基控释材料及其在药物输送和肥料控释中的应用[J]. 化工进展, 2023, 42(7): 3695-3707. |
| [5] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [6] | 于丁一, 李圆圆, 王晨钰, 纪永升. pH响应性木质素水凝胶的制备及药物控释[J]. 化工进展, 2023, 42(6): 3138-3146. |
| [7] | 朱薇, 齐鹏刚, 苏银海, 张书平, 熊源泉. 生物油分级多孔碳超级电容器电极材料的制备及性能[J]. 化工进展, 2023, 42(6): 3077-3086. |
| [8] | 王雪, 徐期勇, 张超. 木质纤维素类生物质水热炭化机理及水热炭应用进展[J]. 化工进展, 2023, 42(5): 2536-2545. |
| [9] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| [10] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| [11] | 万茂华, 张小红, 安兴业, 龙垠荧, 刘利琴, 管敏, 程正柏, 曹海兵, 刘洪斌. MXene在生物质基储能纳米材料领域中的应用研究进展[J]. 化工进展, 2023, 42(4): 1944-1960. |
| [12] | 杨自强, 李风海, 郭卫杰, 马名杰, 赵薇. 市政污泥热处理过程中磷迁移转化的研究进展[J]. 化工进展, 2023, 42(4): 2081-2090. |
| [13] | 邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539. |
| [14] | 郑云武, 裴涛, 李冬华, 王继大, 李继容, 郑志锋. 金属氧化物活化P/HZSM-5催化生物质热解气重整制备富烃生物油[J]. 化工进展, 2023, 42(3): 1353-1364. |
| [15] | 宋叶, 陈玉卓, 宋云彩, 冯杰. 有机固废合成气原位净化催化剂设计及反应器分析[J]. 化工进展, 2023, 42(3): 1383-1396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||