化工进展 ›› 2021, Vol. 40 ›› Issue (2): 1114-1121.DOI: 10.16085/j.issn.1000-6613.2020-0735
以三氯乙烯为供氢剂将四氯化碳转化为氯仿
- 1.北京化工大学化学工程学院,北京 100029
2.生态环境部对外合作与交流中心,北京 100035
-
收稿日期:2020-05-06出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:王倩,李春喜 -
作者简介:姜亚光(1994—),女,硕士研究生,研究方向为绿色化工。E-mail:jyg928@163.com 。 -
基金资助:生态环境部对外经济合作办公室(H2014048)
Converting carbon tetrachloride to chloroform by using trichloroethene as hydrogen donor
Yaguang JIANG1( ), Ruikang WANG1, Qian WANG2(
), Ruikang WANG1, Qian WANG2( ), Chunxi LI1(
), Chunxi LI1( )
)
- 1.College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2.Foreign Environmental Cooperation Center, Ministry of Ecology and Environment, Beijing 100029, China
-
Received:2020-05-06Online:2021-02-05Published:2021-02-09 -
Contact:Qian WANG,Chunxi LI
摘要:
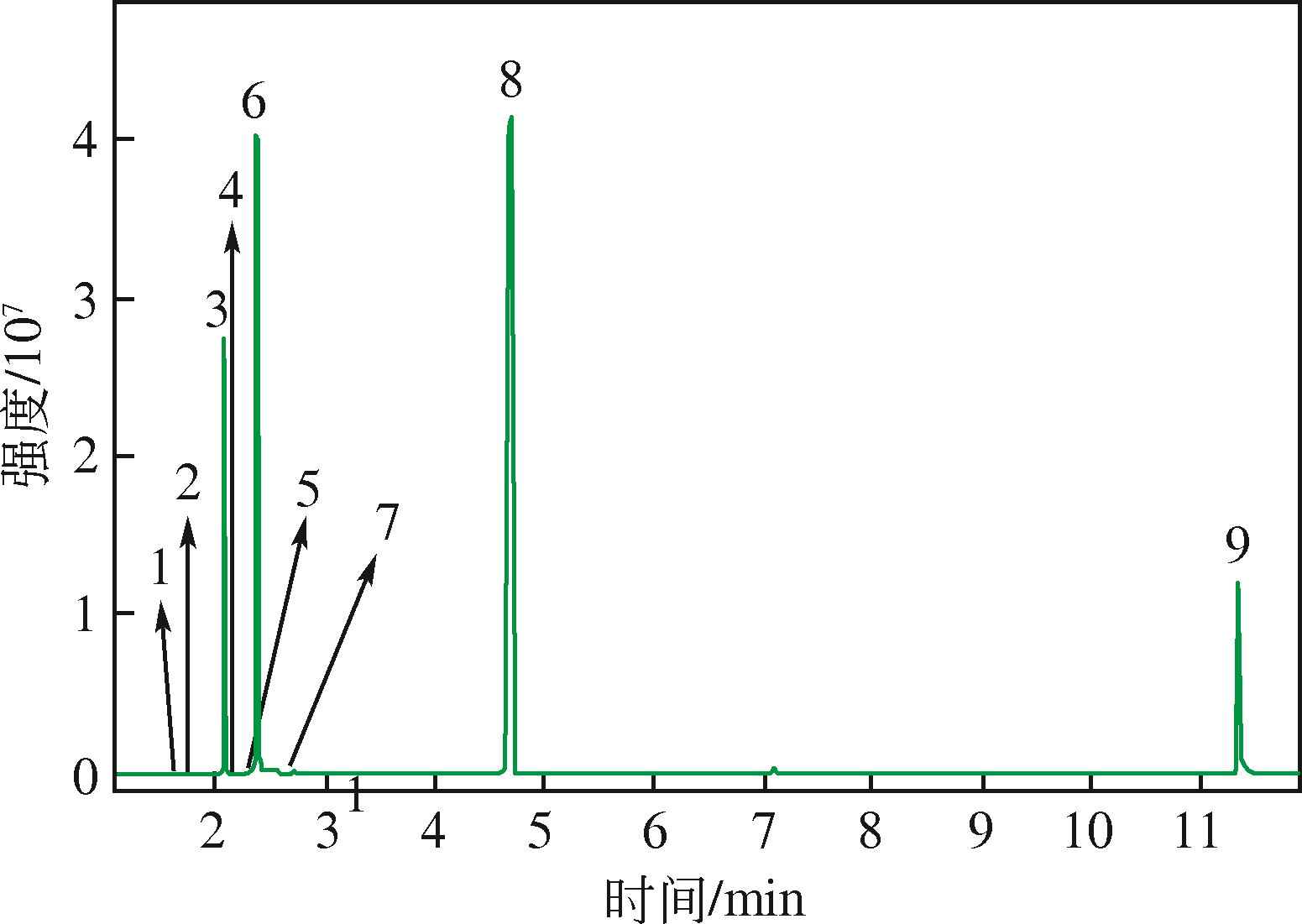
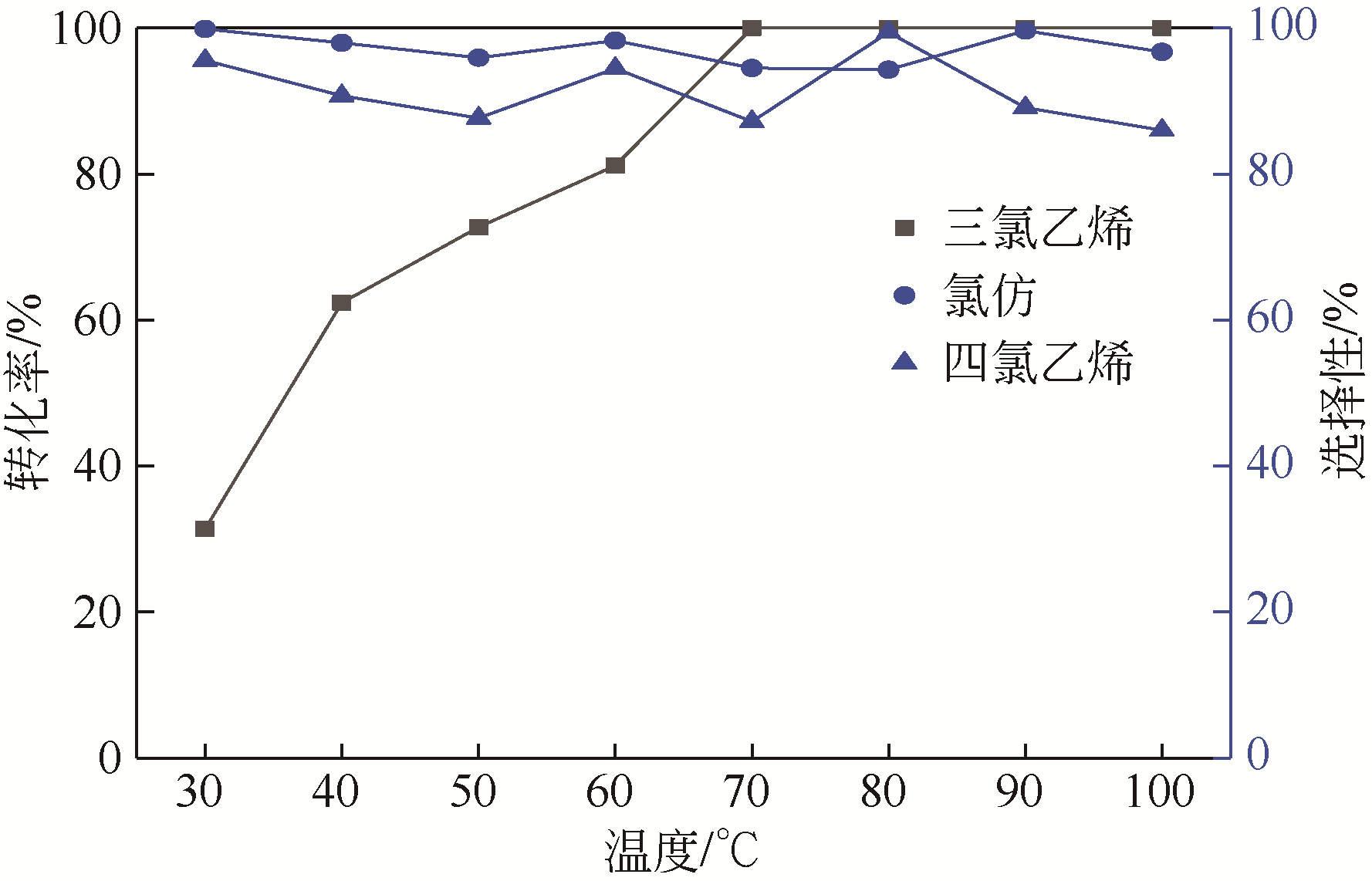
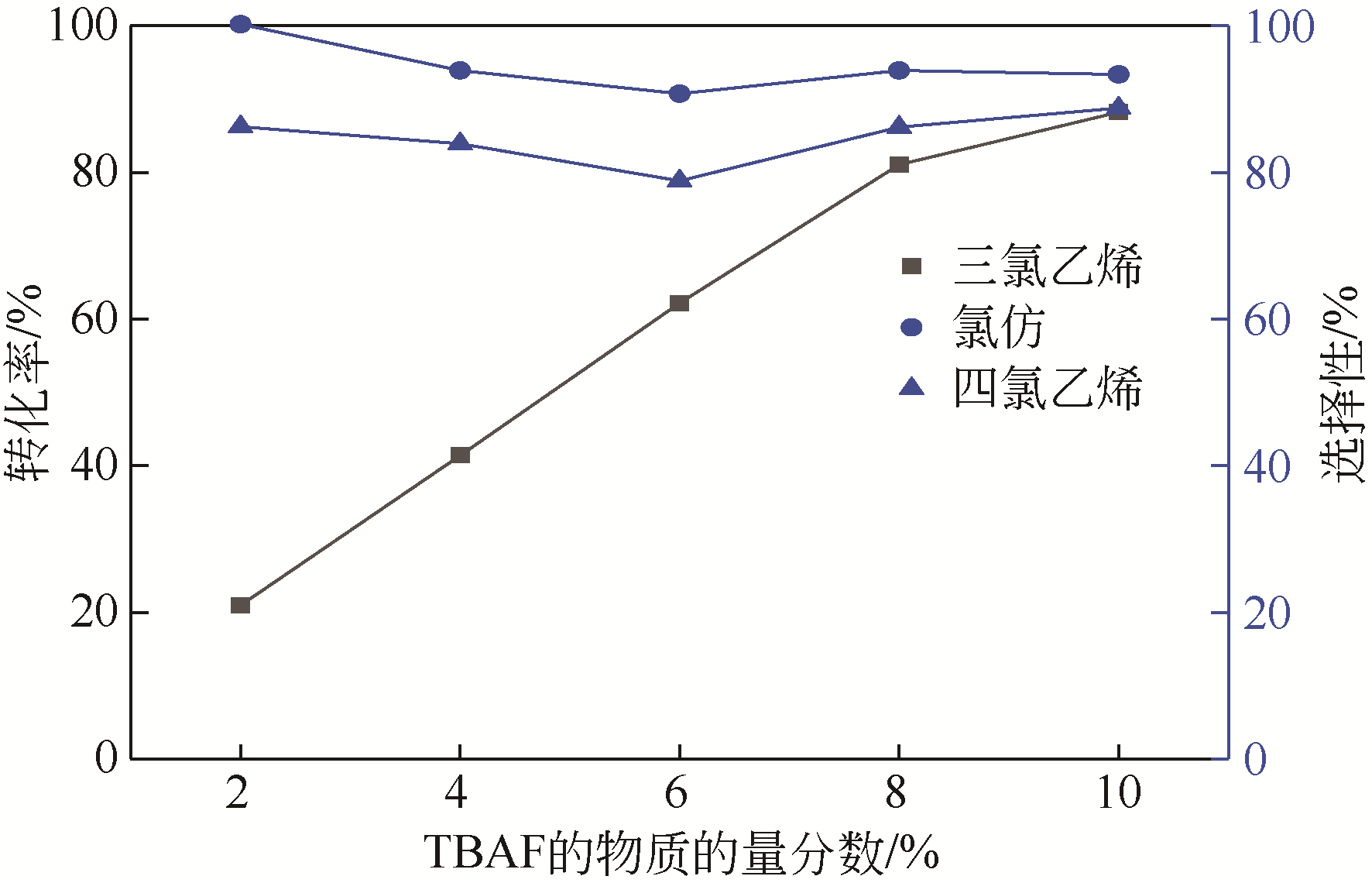
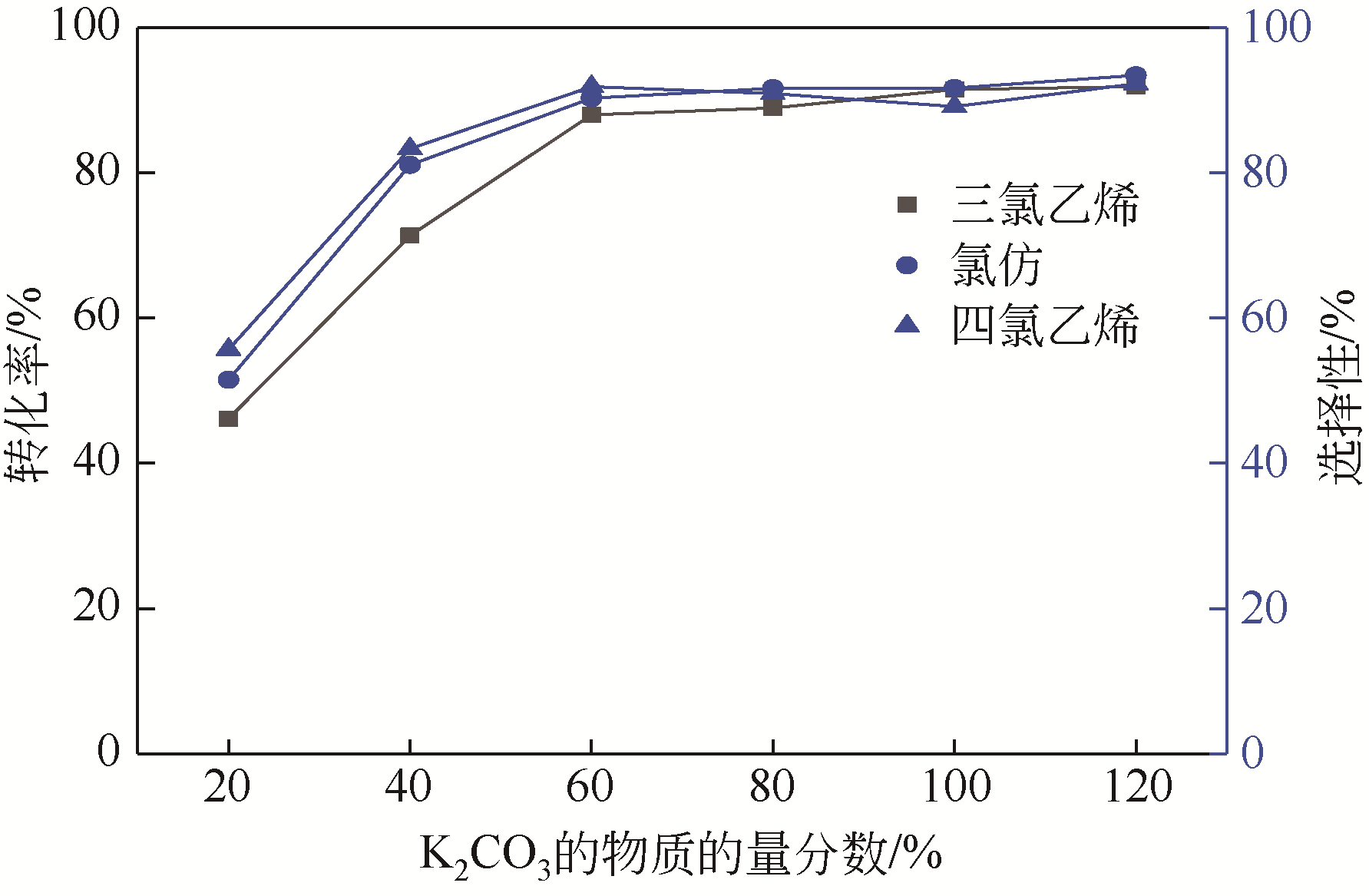
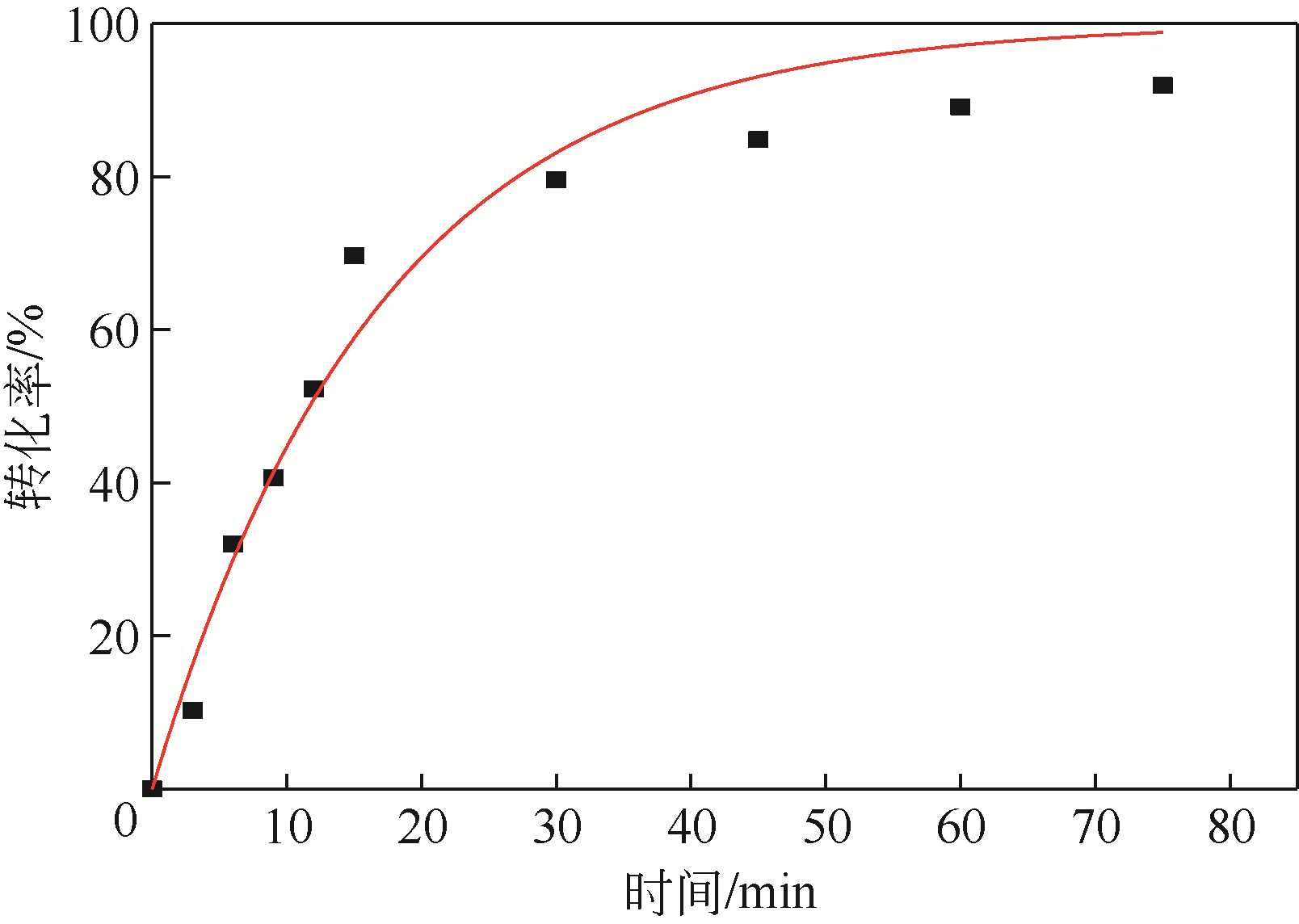
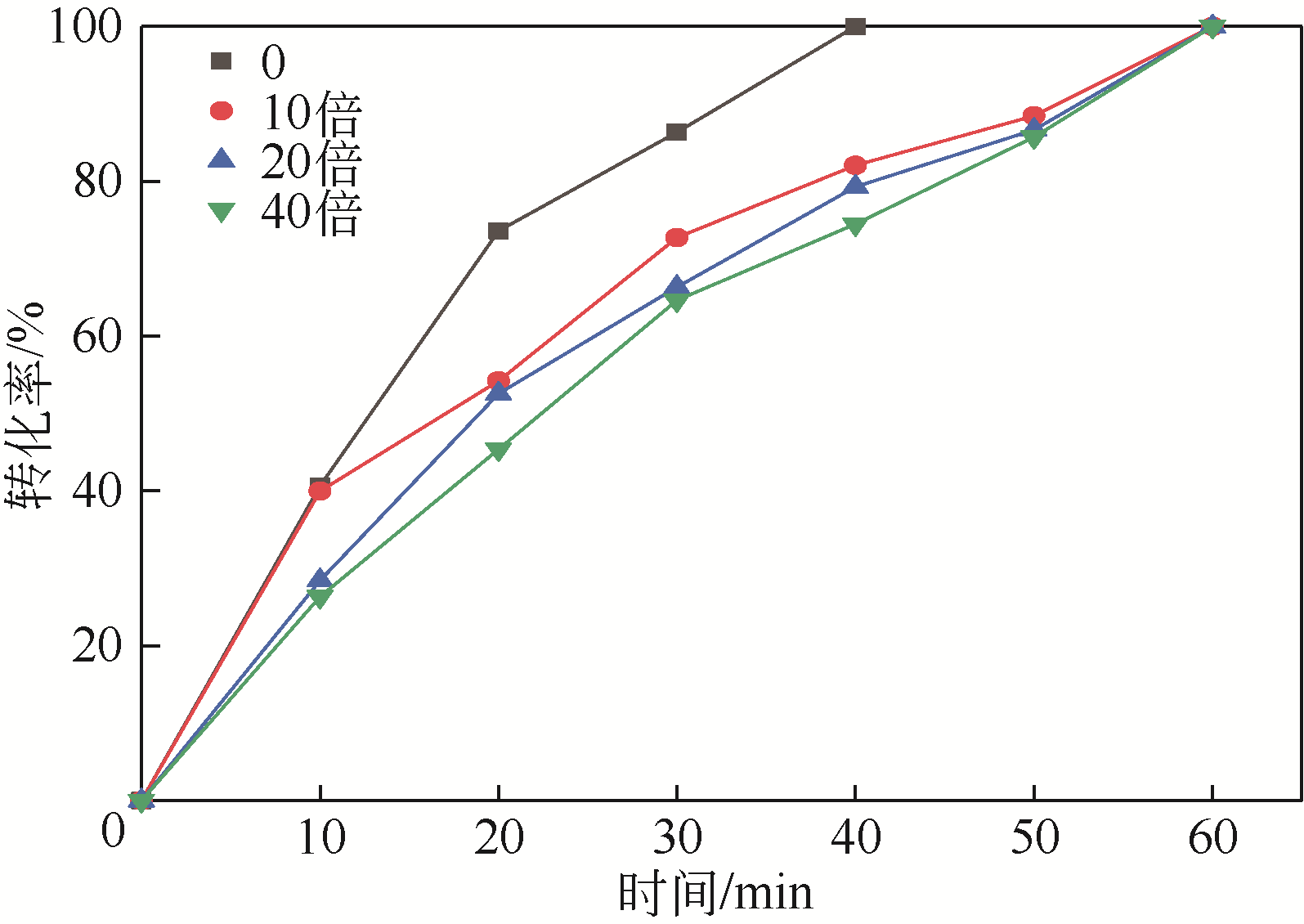
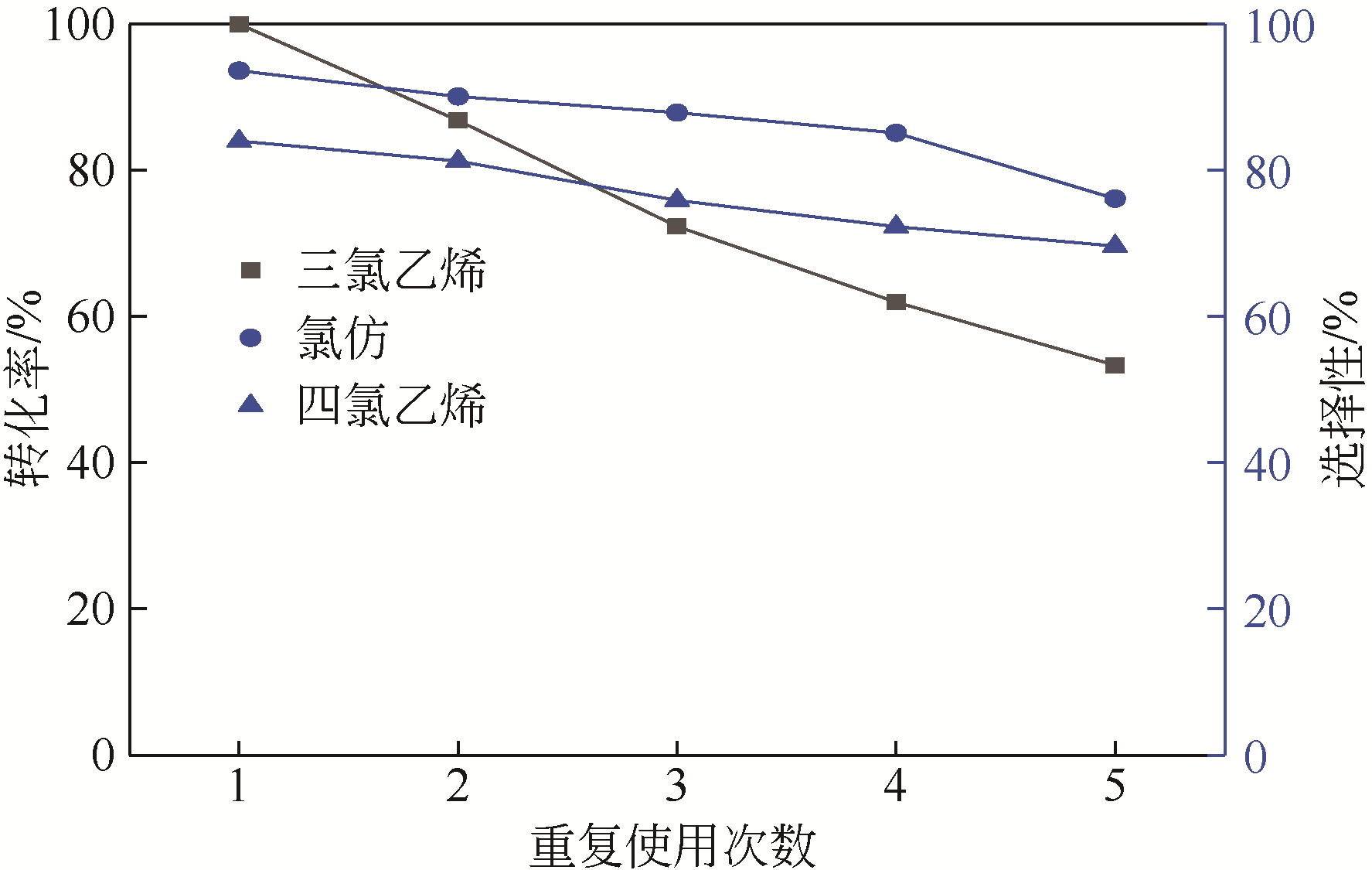
四氯化碳(CTC)是甲烷氯化物生产的副产品,也是一种消耗臭氧层物质(ODS),将其转化为氯仿,可同时实现ODS的生产淘汰和资源化循环利用。本文开发了一种以四丁基氟化铵(TBAF)为相转移催化剂、K2CO3为助催化剂、三氯乙烯为供氢剂将CTC转化为氯仿的新方法,研究了温度、催化剂种类和用量对反应的影响,考察了反应动力学特征及反应放大效应。结果表明:在碱性条件下,三氯乙烯的酸性H原子可与四氯化碳发生取代反应,生成四氯乙烯和氯仿;反应速率随相转移催化剂用量的增加以及助催化剂碱性的增强而提高;循环使用过程中催化剂活性的降低与TBAF中氟离子的取代反应消耗有关;表观反应速率符合拟一级反应动力学模型,过程放大效应不明显。在优化反应条件下(30~60℃,9g CTC,10mmol 三氯乙烯,1mmol TBAF,10mmol K2CO3,0.5h),三氯乙烯被完全转化,氯仿选择性大于95%。
中图分类号:
引用本文
姜亚光, 王瑞康, 王倩, 李春喜. 以三氯乙烯为供氢剂将四氯化碳转化为氯仿[J]. 化工进展, 2021, 40(2): 1114-1121.
Yaguang JIANG, Ruikang WANG, Qian WANG, Chunxi LI. Converting carbon tetrachloride to chloroform by using trichloroethene as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1114-1121.
| 碱性物质 | pH(c=0.1mol·L-1) | 反应时间 | 转化率/% |
|---|---|---|---|
| KOH | 12.63 | 5min | 100 |
| NaAlO2 | 12.17 | 30min | 100 |
| K3PO4 | 11.92 | 2h | 100 |
| K2CO3 | 11.14 | 40min | 100 |
| K2HPO4 | 9.08 | 12h | 17.0 |
| KHCO3 | 8.34 | 12h | 13.0 |
| K2SO3 | 7.28 | 12h | 0 |
| 二氧六环 | 12h | 0 | |
| 三乙胺(Et3N) | 12h | 0 | |
| N-甲基咪唑(MIM) | 12h | 0 | |
| AlF3-KOH | 12h | 0 | |
| CsF-KOH | 12h | 0 | |
| 无 | 12h | 0 |
表1 碱性物质的碱性及其助催化效果
| 碱性物质 | pH(c=0.1mol·L-1) | 反应时间 | 转化率/% |
|---|---|---|---|
| KOH | 12.63 | 5min | 100 |
| NaAlO2 | 12.17 | 30min | 100 |
| K3PO4 | 11.92 | 2h | 100 |
| K2CO3 | 11.14 | 40min | 100 |
| K2HPO4 | 9.08 | 12h | 17.0 |
| KHCO3 | 8.34 | 12h | 13.0 |
| K2SO3 | 7.28 | 12h | 0 |
| 二氧六环 | 12h | 0 | |
| 三乙胺(Et3N) | 12h | 0 | |
| N-甲基咪唑(MIM) | 12h | 0 | |
| AlF3-KOH | 12h | 0 | |
| CsF-KOH | 12h | 0 | |
| 无 | 12h | 0 |
| 1 | 陆颖舟, 杨礼荣, 王开祥, 等. 四氯化碳淘汰背景下氯化聚丙烯的生产技术选择[J]. 化工进展, 2011, 30(7): 1537-1541. |
| LU Yingzhou,YANG Lirong, WANG Kaixiang, et al. Production technology selection of chlorinated polypropylene under the background of carbon tetrachloride phase-out[J]. Chemical Industry and Engineering Progress, 2011, 30(7): 1537-1541. | |
| 2 | 冯拥军, 李春喜, 徐咏蓝. 四氯化碳作为反应原料的研究进展[J]. 化工进展, 2002, 21(3): 183-185. |
| FENG Yongjun,LI Chunxi,XU Yonglan. Research progress of carbon tetrachloride as a reaction raw material[J]. Chemical Industry and Engineering Progress, 2002, 21(3): 183-185. | |
| 3 | 杜静, 吴克安, 张建君. 实验室和分析用途四氯化碳的管理现状[J]. 有机氟工业, 2018(3): 32-37. |
| DU Jing, WU Kean, ZHANG Jianjun. Management status of CTC in laboratory and analytical uses[J]. Organo-Fluorine Industry, 2018(3):32-37. | |
| 4 | 国家环保总局办公厅关于严格控制新(扩)建四氯化碳生产项目的通知(环办[2003]28号). . |
| Notice of the general office of the State Environmental Protection Administration on strictly controlling of the new construction(expansion) of the carbon tetrachloride production project([2003]28). . | |
| 5 | 常州大学技术转移中心. 四氯化碳加氢转化制备氯仿或四氯乙烯[J]. 乙醛醋酸化工, 2017(10): 50. |
| University of Changzhou Center for Technology Tramfer. Hydrogenation of carbon tetrachloride to produce chloroform or tetrachloroethylene[J]. Acetaldehyde Acetic Acid Chemical, 2017(10): 50. | |
| 6 | 张明慧, 肖光, 康健. 四氯化碳生产四氯乙烯应用介绍及优化[J]. 价值工程, 2013, 32(27): 311-312. |
| ZHANG Minghui, XIAO Guang, KANG Jian. Application introduction and optimization of tetrachloroethylene production by carbon tetrachloride [J] .Value Engineering, 2013, 32(27): 311-312. | |
| 7 | 尚建壮, 李春喜, 王子镐. 四氯化碳转化为一氯甲烷的气相反应研究[J]. 化工进展, 2005, 24(4): 408-410. |
| SHANG Jianzhuang, LI Chunxi, WANG Zihao. Study on the gas-phase reaction of carbon tetrachloride converted to monochloromethane [J]. Chemical Industry and Engineering Progress, 2005, 24(4): 408-410. | |
| 8 | 陈彦, 杨理想. 催化分解四氯化碳与甲醇生成一氯甲烷技术[J]. 化工生产与技术, 2014, 21(1): 34-36, 41. |
| CHEN Yan, YANG Lixiang. Technology of methane chloride produced by catalytic decomposition carbon tetrachloride and methanol[J]. Chemical Production and Technology, 2014, 21(1): 34-36, 41. | |
| 9 | 陈刚, 祁芳菲, 谢文健, 等. 一种四氯化碳气相加氢脱氯制氯仿的催化剂: CN107876046A[P]. 2018-04-06. |
| CHEN Gang, QI Fangfei, XIE Wenjian, et al. A catalyst for the preparation of chloroform from carbon tetrachloride by gas phase hydrochlorination: CN107876046A[P]. 2018-04-06. | |
| 10 | 张霓, 卢贵武, 李春喜, 等. 四氯化碳催化加氢制氯仿的实验研究[J]. 北京化工大学学报, 2004, 31(2): 18-20. |
| ZHANG Ni, LU Guiwu, LI Chunxi, et al. Experimental study on the catalytic hydrogenation of carbon tetrachloride to chloroform[J]. Journal of Beijing University of Chemical Technology, 2004, 31(2):18-20. | |
| 11 | 林涛, 万克柔, 程杰, 等. 反应条件对四氯化碳选择性脱氯用 PtMn/C催化剂性能的影响[J]. 山东化工, 2018, 47(12): 11-12, 15. |
| LIN Tao, WAN Kerou, CHENG Jie, et al. Effect of Mn on dechlorination of carbon tetrachloride by PtMn/C catalyst[J]. Shandong Chemical Industry, 2018, 47(12): 11-12, 15. | |
| 12 | 朱一明, 张豪, 张军, 等. 四氯化碳液相催化加氢制氯仿多元金属催化剂的制备及其催化性能[J]. 应用化学, 2017, 34(2): 242-244. |
| ZHU Yiming, ZHANG Hao, ZHANG Jun, et al. Preparation and catalytic performance evaluation of multi-metal catalysts for conversion of tetrachloromethane to trichloromethane by liquid phase hydrogenation [J]. Chinese Journal of Applied Chemistry, 2017, 34(2): 242-244. | |
| 13 | 成春春, 王保华, 张东宝, 等. 四氯化碳催化加氢反应在液相和气相中的比较[J]. 无机盐工业, 2014, 46(6): 24-27, 55. |
| CHENG Chunchun, WANG Baohua, ZHANG Dongbao, et al. Comparative study on catalytic hydrodechlorination of carbon tetrachloride in liquid-phase and gas-phase[J]. Inorganic Chemicals Industry, 2014,46(6): 24-27, 55. | |
| 14 | 高扬, 项斌, 何敏焕, 等.以苯乙烯和四氯化碳为原料合成肉桂酸[J]. 浙江化工, 2010(3): 22-23. |
| GAO Yang, XIANG Bin, HE Minhuan, et al. Synthesis of cinnamic acid from styrene and carbon tetrachloride[J]. Zhejiang Chemical Industry, 2010(3): 22-23. | |
| 15 | 苏刚, 许响生. 四氯化碳法合成二苯甲酮研究[J]. 化工生产与技术, 2013, 20(2): 19-21. |
| SU Gang, XU Xiangsheng. Synthesis of benzophenon from carbon tetrachloride [J]. Chemical Production and Technology, 2013, 20(2): 19-21. | |
| 16 | 禹兴利. 一种清洁生产二苯甲酮的应用实践[J]. 上海化工, 2019, 44(12): 11-15. |
| YU Xingli. Application of a clean production of benzophenone[J].Shanghai Chemical Industry, 2019, 44 (12): 11-15. | |
| 17 | 丁元胜, 张建君, 吴江平, 等. 1,1,1,3-四氯丙烷合成的反应动力学[J]. 化工进展, 2008, 27(4): 550-552. |
| DING Yuansheng, ZHNAG Jianjun, WU Jiangping, et al. Reaction kinetics of 1,1,1,3-tetrachloropropane synthesis[J]. Chemical Industry and Engineering Progress, 2008, 27(4): 550-552. | |
| 18 | 李家才, 王亮, 何邦友. 一种制备1,1,1,3-四氯丙烷的装置: CN 209885775 U[P]. 2020-01-03. |
| LI Jiacai, WANG Liang, HE Bangyou. A device for preparing1,1,1,3-tetrachloropane: CN 209885775 U[P]. 2020-01-03. | |
| 19 | 许晓涛, 龙辉, 张未星. 铁粉催化合成1,1,1,3,3-五氯丙烷[J]. 化工进展, 2013, 32(11): 2723-2726. |
| XU Xiaotao, LONG Hui, ZHANG Weixing. Catalytic synthesis of 1,1,1,3,3-pentachloropropane with iron powder[J]. Chemical Industry and Engineering Progress, 2013, 32(11): 2723-2726. | |
| 20 | 许晓涛, 张未星. 1,1,1,3,3-五氯丙烷合成的反应动力学[J]. 化工学报, 2014, 65(1): 176-181. |
| XU Xiaotao, ZHANG Weixing. Reaction kinetics of 1,1,1,3,3-pentachloropropane synthesis[J]. CIESC Journal, 2014, 65(1): 176-181. | |
| 21 | 吕杨, 李宏峰, 蒋琪, 等. 一种2,3,3,3-四氟丙烯的连续制备方法: CN 109796300A[P]. 2019-05-24. |
| Yang LYU, LI Hongfeng, JIANG Qi, et al. A continuous preparation method of2,3,3,3-tetrafluoropropylene: CN 109796300A[P]. 2019-05-24. | |
| 22 | 王金明, 吕杨. 一种1,1,2,3-四氯丙烯的制备方法: CN 110511112A[P]. 2019-11-29. |
| WANG Jinming, LYU Yang. A preparation method of 1,1,2,3-tetrachloropropene: CN 110511112A[P]. 2019-11-29. | |
| 23 | 吕剑, 曾纪珺, 韩升. 一种合成2-氯-1,1,1,4,4,4-六氟-2-丁烯的方法: CN 110283042A[P]. 2019-09-27. |
| Jian LYU, ZENG Jijun, HAN Sheng. A method for the synthesis of2-chloro-1,1,1,4,4,4-hexafluoro-2-butene: CN110283042A[P].2019-09-27. | |
| 24 | 梁初, 潘良斌, 曾称福, 等. 一种低温合成二硫化碳的方法: CN110510612 A[P]. 2019-11-29. |
| LIANG Chu, PAN Liangbin, ZENG Chengfu, et al. A method of synthesizing carbon disulfide at low temperature: CN110510612 A[P]. 2019-11-29. | |
| 25 | 姜兴茂, 张可琢, 周雅倩, 等. 一种交联聚苯乙烯吸附剂及其制备方法和应用: CN 109701505A[P]. 2019-05-03. |
| JIANG Xingmao, ZHANG Kezuo, ZHOU Yaqian, et al. A crosslinked polystyrene adsorbent and its preparation and application: CN 109701505A[P]. 2019-05-03. | |
| 26 | 刘金章, 李岩, 孙麓, 等. 镁热还原四氯化碳法制备石墨烯: CN 106241792A[P].2016-12-21. |
| LIU Jinzhang, LI Yan, SUN Lu, et al. Preparation of graphene by magnesium thermal reduction of carbon tetrachloride: CN 106241792A[P]. 2016-12-21. | |
| 27 | 肖自胜, 尹笃林, 兰支利, 等. 一种2,4,4,4-四氯丁腈的合成法:CN109535029A[P]. 2019-03-29. |
| XIAO Zisheng, YIN Dulin, LAN Zhili, et al. Synthesis of2,4,4,4-tetrachloobutyronitrile: CN109535029A[P]. 2019-03-29. | |
| 28 | 张天宇, 严宁宁, 李效军. 3,5-二叔丁基-4-羟基苯甲酸的合成[J]. 石油化工, 2019, 48(11): 1105-1109. |
| ZHANG Tianyu, YAN Ningning, LI Xiaojun. Synthesis of 3,5-di-tert-butyl-4-hydroxybenzoic acid[J]. Petrochemical Technology, 2019, 48(11): 1105-1109. | |
| 29 | 张宇. 高温法合成甲基二氯化磷[J]. 安徽化工, 2019, 45(2): 72-74. |
| ZHANG Yu. Synthesis of methyl phosphorus dichloride by high temperature method[J]. Anhui Chemical Industry, 2019, 45(2): 72-74. | |
| 30 | 张东宝, 朱建军, 单玉华, 等. 四氯化碳催化加氢研究进展[J]. 化工进展, 2007, 26(4): 477-480. |
| ZHNAG Dongbao, ZHU Jianjun, SHAN Yuhua, et al. Advances in hydrodechlorination of carbon tetrachloride[J]. Chemical Industry and Engineering Progress, 2007, 26(4): 477-480. | |
| 31 | WEISS A H, GAMBHIR B S, LEONR R B. Hydrodechlorination of carbon tetrachloride [J]. Journal of Catalysis, 1971, 22(2): 240-245. |
| 32 | ZHANG Z C, BEARD B C. Genesis of durable catalyst for selective hydrodechlorination of CCl4 to CHCl3 [J]. Applied Catalysis A: General, 1998, 174: 33-39. |
| 33 | GOMEZ-SAINERO L M, CORTES A, SEOANE X L, et al. Hydrodechlorination of carbon tetrachloride to chloroform in the liquid-phase with metal supported catalysts. Effect of the catalyst components [J]. Industrial Engineering Chemistry Research,2000, 39:2849. |
| 34 | MICHELE R, CARLO R, ANTONIO P, et al. Catalysts for hydrodechlorination of carbon tetrachloride to chloroform: US 6627777[P]. 2003-09-30. |
| 35 | Santo V DAL, DOSSI C, RECCHIA S, et al. Carbon tetrachloride hydrodechlorination with organometallics-based platinum and palladium catalysts on MgO[J]. Journal of Molecular Catalysis A: Chemical, 2002,182/183:157-166. |
| 36 | GOLUBINA E V, LOKTEVA E S, LUNIN V V, et al. Modification of the supported palladium catalysts surface during hydrodechlorination of carbon tetrachloride [J]. Applied Catalysis A: General, 2003, 241 (1/2):123-132. |
| 37 | IMRE B, HANNUS I, KONYA Z, et al. Hydrodechlorination of carbon tetrachloride on Pt-containing zeolites[J]. Studies in Surface Science and Catalysis, 2004, 154:2536-2542. |
| 38 | ADRIANA G F S, Manoel P B AYR, ALEXANDRE C C R, et al. Hydrodechlorination of carbon tetrachloride over Pt/NaX zeolite: deactivation studies[J]. Catalysis Today, 2005, 107/108: 493-499. |
| 39 | GARETTO T F, VIGNATTI C I, BORGNA A, et al. Deactivation and regeneration of Pt/Al2O3 catalysts during the hydrodechlorination of carbon tetrachloride[J]. Applied Catalysis B: Environmental, 2009,87(3/4): 211-219. |
| 40 | 鲁墨弘, 周秀莲, 朱劼, 等. 甲醇为氢源四氯化碳原位加氢脱氯反应研究[J]. 常州大学学报(自然科学版), 2016, 28(3): 31-35. |
| LU Mohong, ZHOU Xiulian, ZHU Jie, et al. Catalytic hydrodechlorination of carbon tetrachloride with methanol as H-donor over Ag/C catalyst[J], Journal of Changzhou University(Natural Sci. Ed.), 2016, 28(3): 31-35. | |
| 41 | 周秀莲, 鲁墨弘, 朱劼, 等. Pd和甲醛对Ag/C催化四氯化碳原位液相加氢脱氯性能的影响[J]. 石油学报(石油加工), 2013, 29(2):269-276. |
| ZHOU Xiulian, LU Mohong, ZHU Jie, et al. Effect of Pd and formaldehyde on in-situ liquid phase catalytic hydrodechlorination of CCl4 over Ag/C catalysts[J].Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(2): 269-276 | |
| 42 | YOEL S S, OWEN W W. Quaternary ammonium fluoride catalysed halogenation of carbon acids by polyhaloalkanes[J]. Journal of the Chemical Society, Chemical Communications, 1992, 17: 1200-1201. |
| 43 | 郑结斌. 中国三氯乙烯行业现状及发展展望[J]. 中国氯碱, 2013(12): 21-22. |
| ZHENG Jiebin. Present situation and prospect of China trichloroethylene industry[J]. China Chlor-Alkali, 2013(12): 21-22. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [14] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [15] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||