化工进展 ›› 2021, Vol. 40 ›› Issue (2): 1121-1129.DOI: 10.16085/j.issn.1000-6613.2020-0753
镧改性核桃壳生物炭制备及吸附水体磷酸盐性能
罗元1,2,3( ), 谢坤1,3, 冯弋洋1,2,3, 何秋平1,3, 张克强1,3, 沈仕洲1,3, 王风1,3(
), 谢坤1,3, 冯弋洋1,2,3, 何秋平1,3, 张克强1,3, 沈仕洲1,3, 王风1,3( )
)
- 1.农业农村部环境保护科研监测所,天津 300191
2.云南农业大学资源与环境学院,云南 昆明 650201
3.农业农村部大理农业环境科学观测实验站,云南 大理 671004
-
收稿日期:2020-05-04修回日期:2020-06-24出版日期:2021-02-05发布日期:2021-02-09 -
通讯作者:王风 -
作者简介:罗元(1995-),男,硕士研究生,研究方向为农业面源污染防治。E-mail:ly234132@163.com 。 -
基金资助:国家重点研发计划(2017YFD0800403);中央级公益性科研院所基本科研业务费专项(C2112060302008086)
Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions
Yuan LUO1,2,3( ), Kun XIE1,3, Yiyang FENG1,2,3, Qiuping HE1,3, Keqiang ZHANG1,3, Shizhou SHEN1,3, Feng WANG1,3(
), Kun XIE1,3, Yiyang FENG1,2,3, Qiuping HE1,3, Keqiang ZHANG1,3, Shizhou SHEN1,3, Feng WANG1,3( )
)
- 1.Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin 300191, China
2.College of Resources and Environment, Yunnan Agricultural University, Kunming 650201, Yunnan, China
3.Dali Agro-Environmental Science Station, Ministry of Agriculture and Rural Affairs, Dali 671004, Yunnan, China
-
Received:2020-05-04Revised:2020-06-24Online:2021-02-05Published:2021-02-09 -
Contact:Feng WANG
摘要:
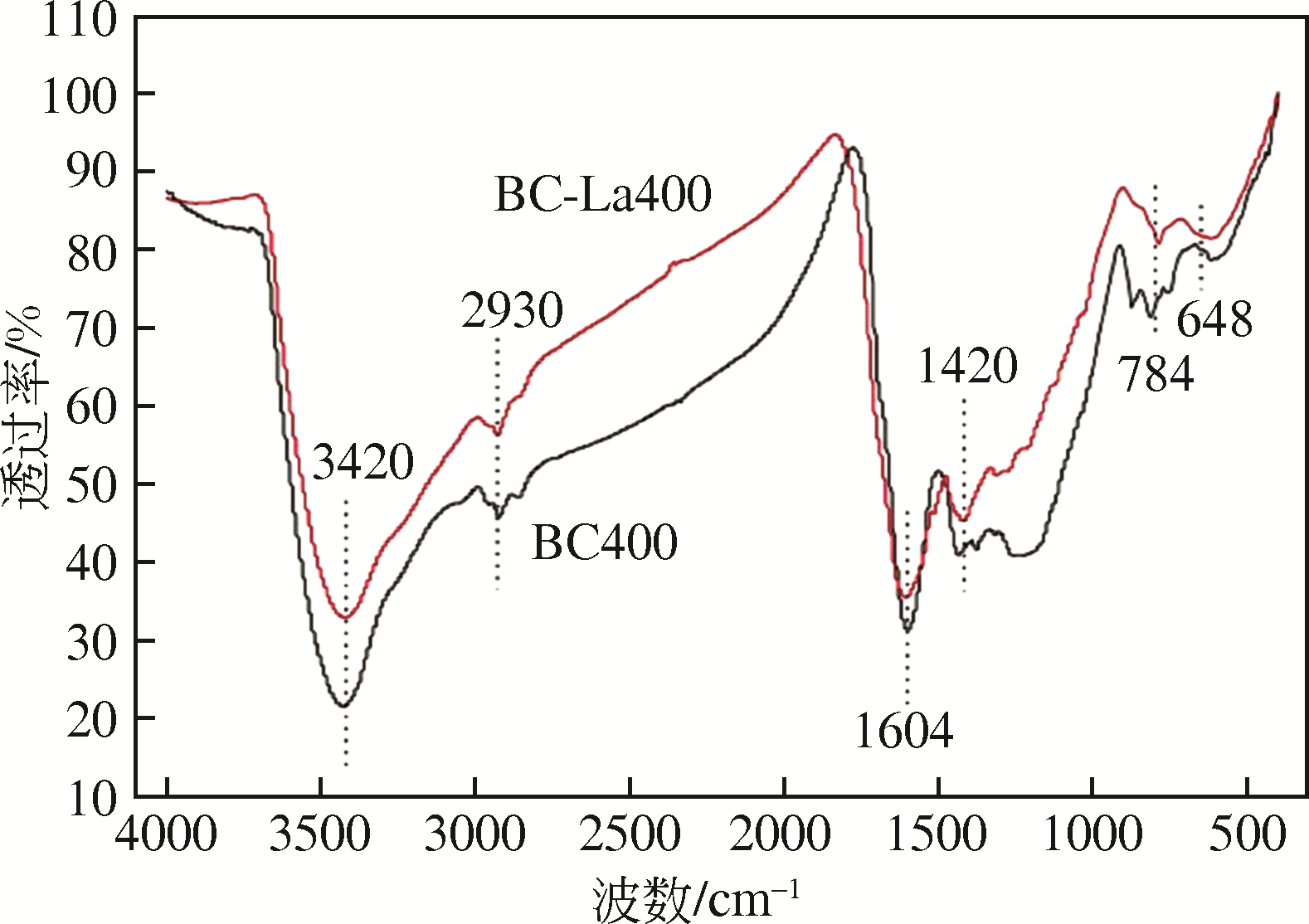
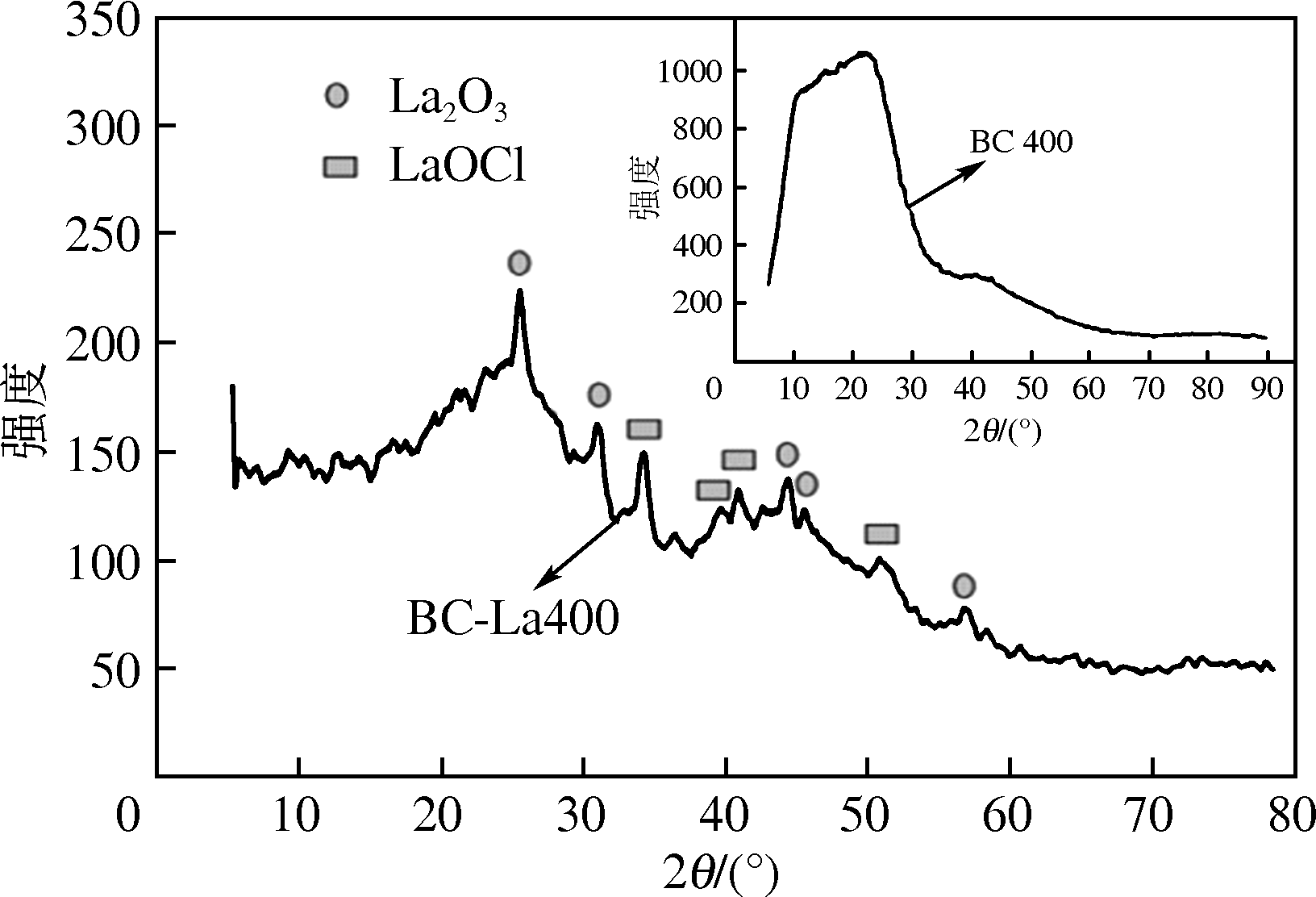
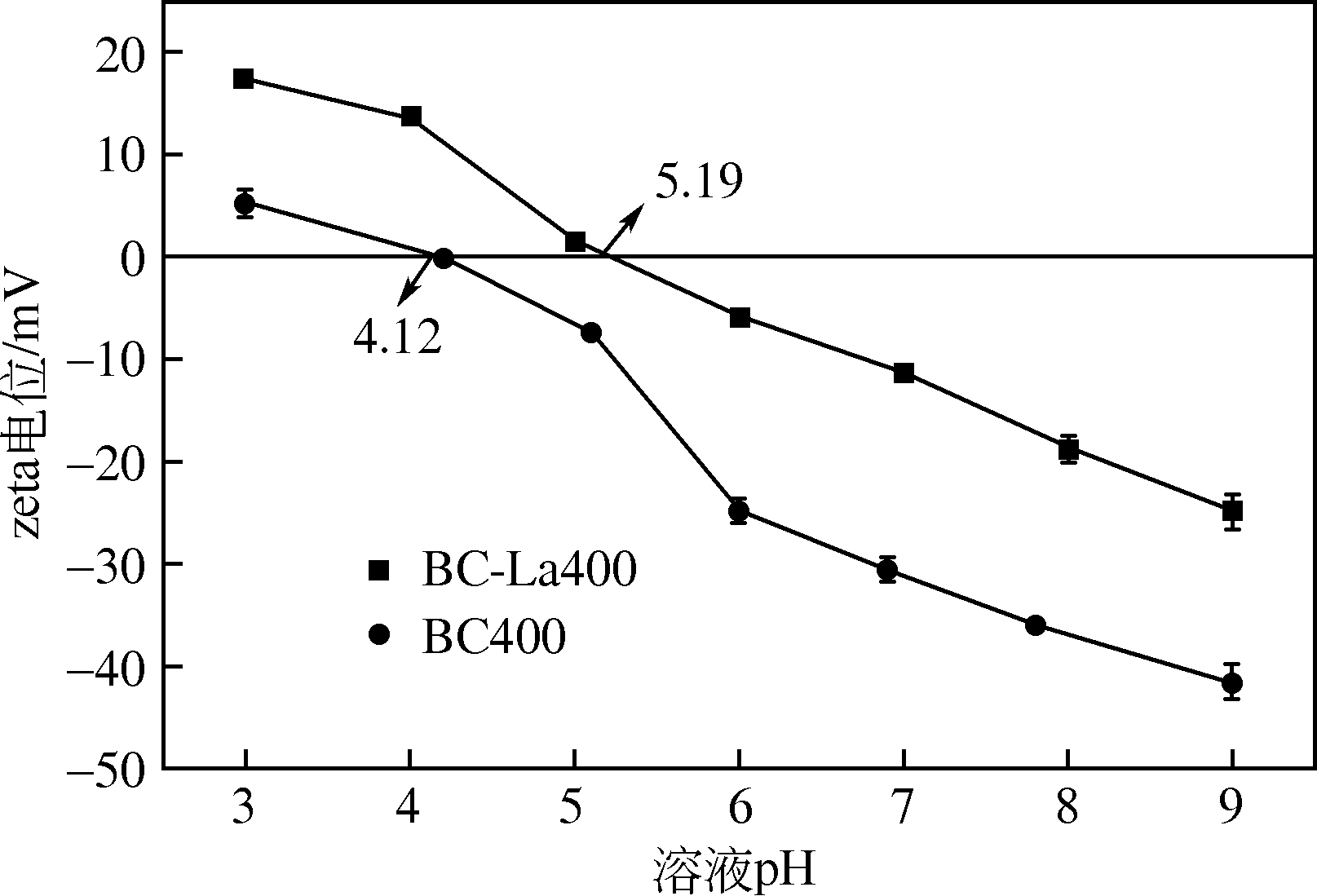
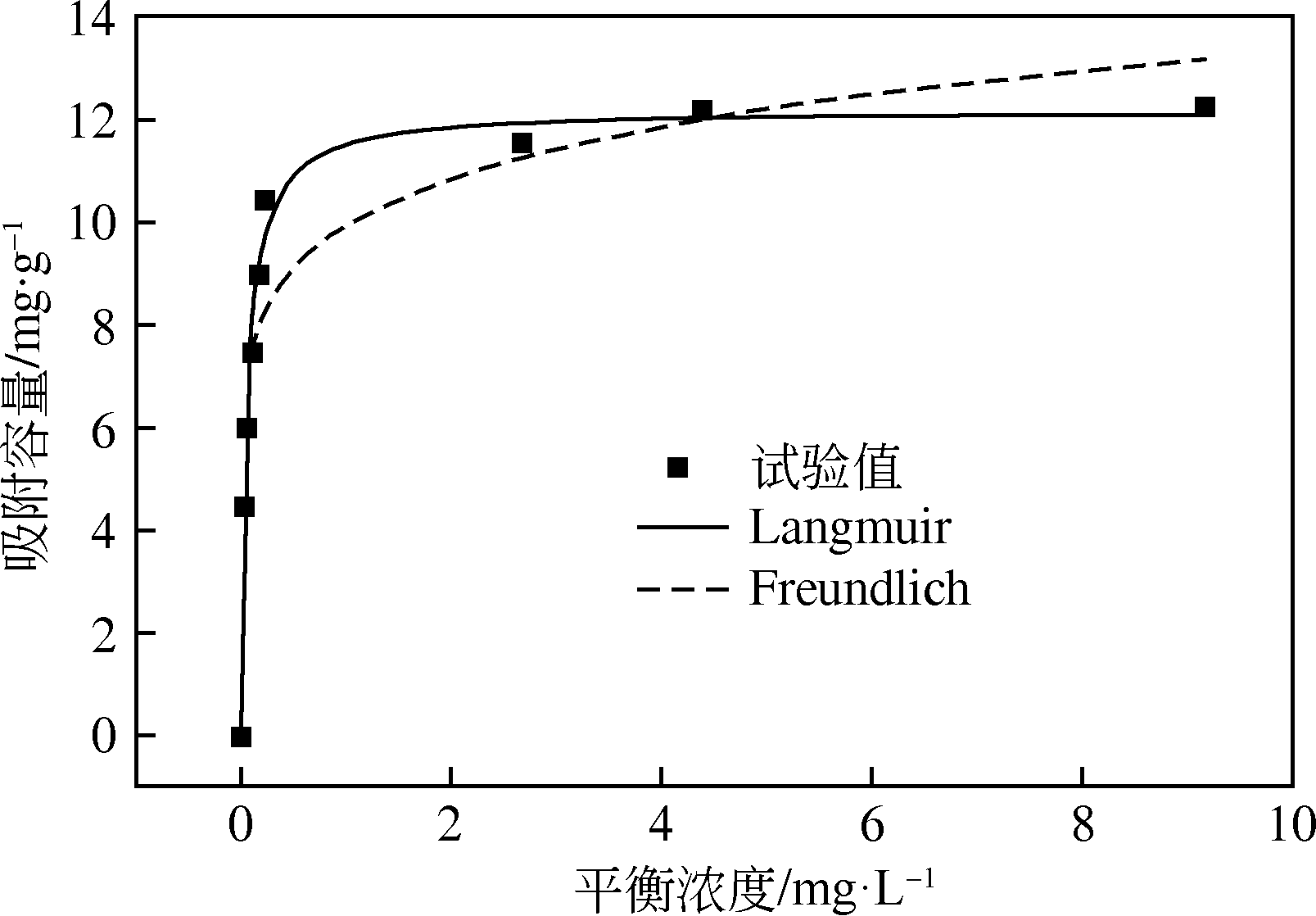
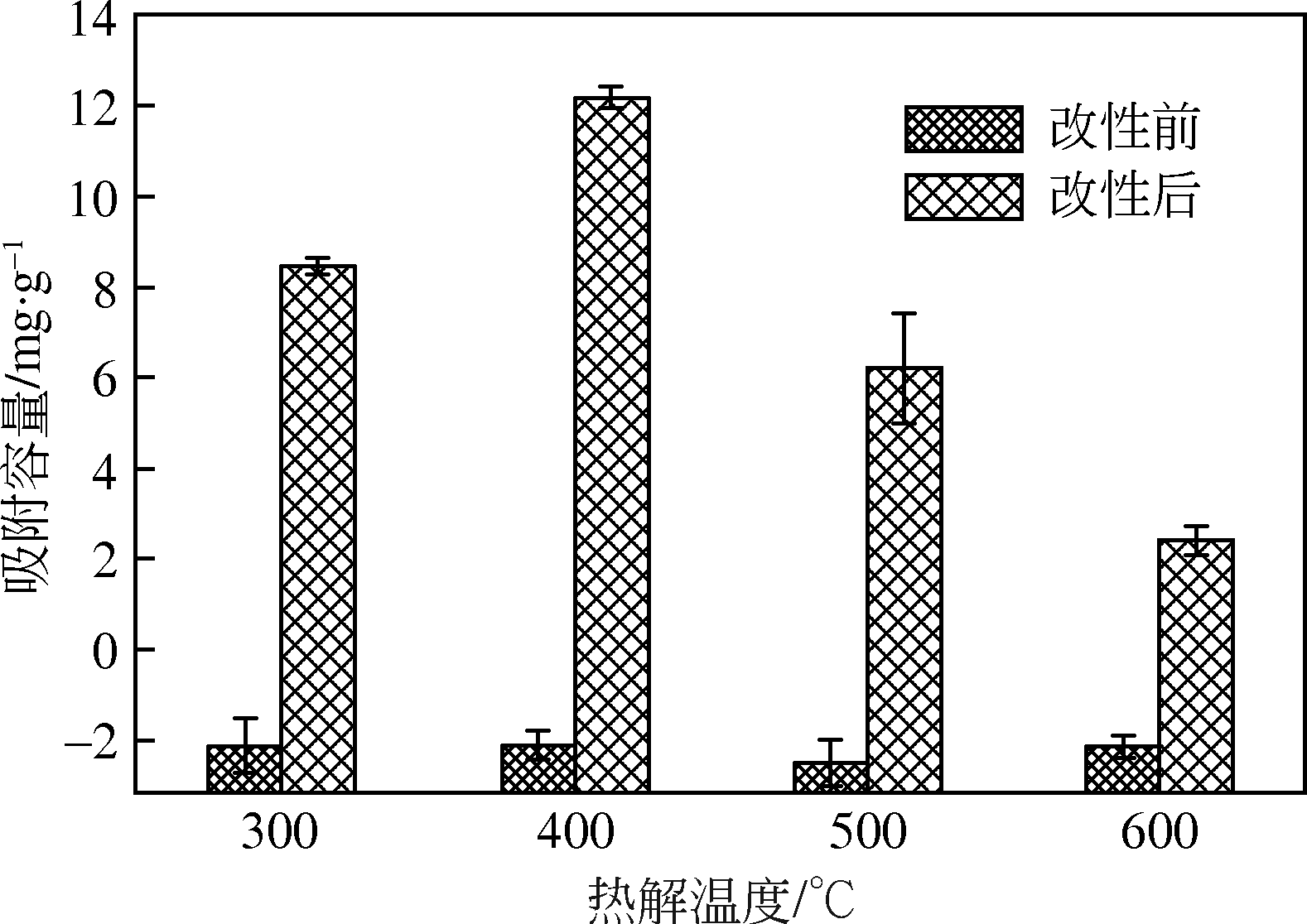
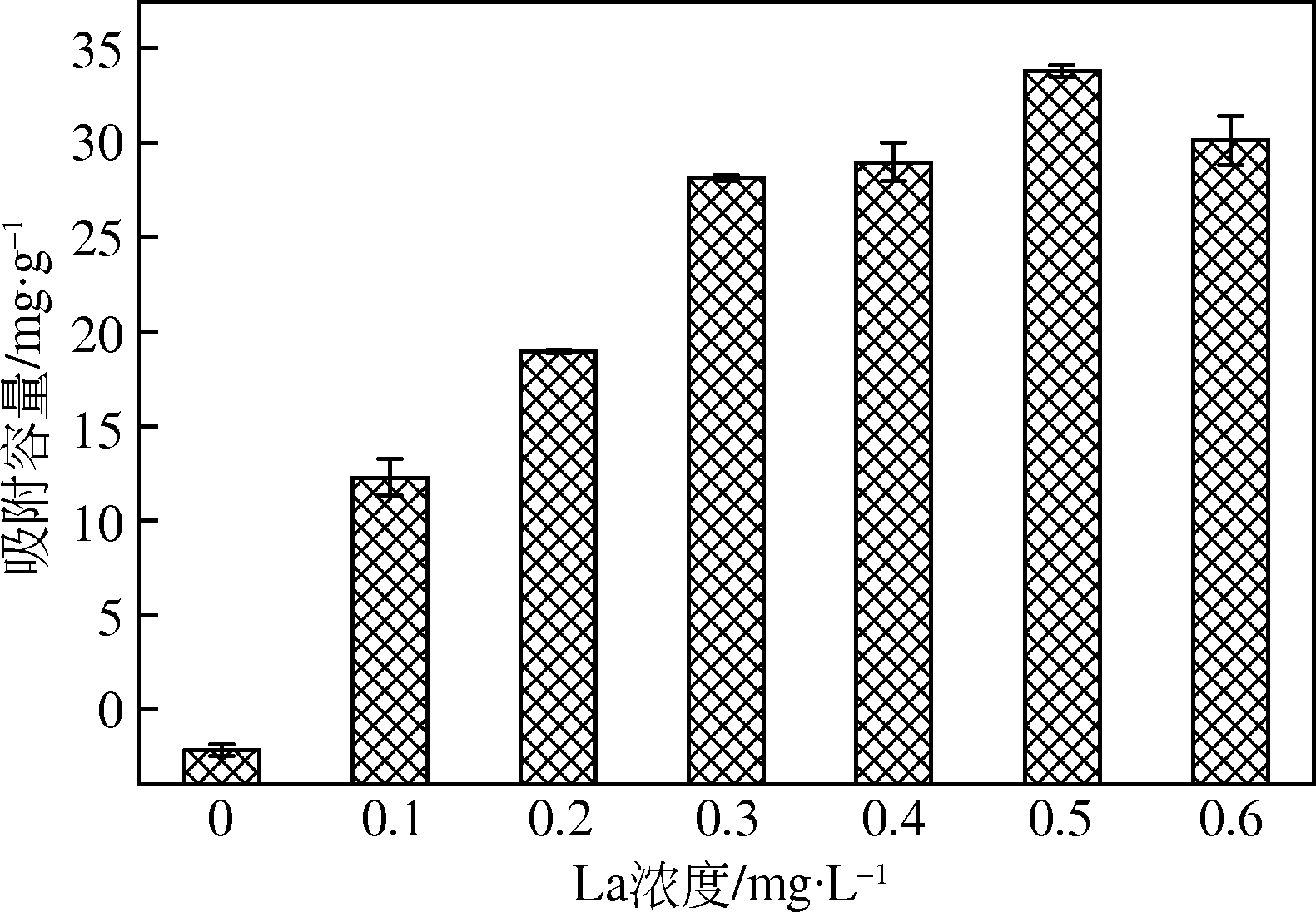
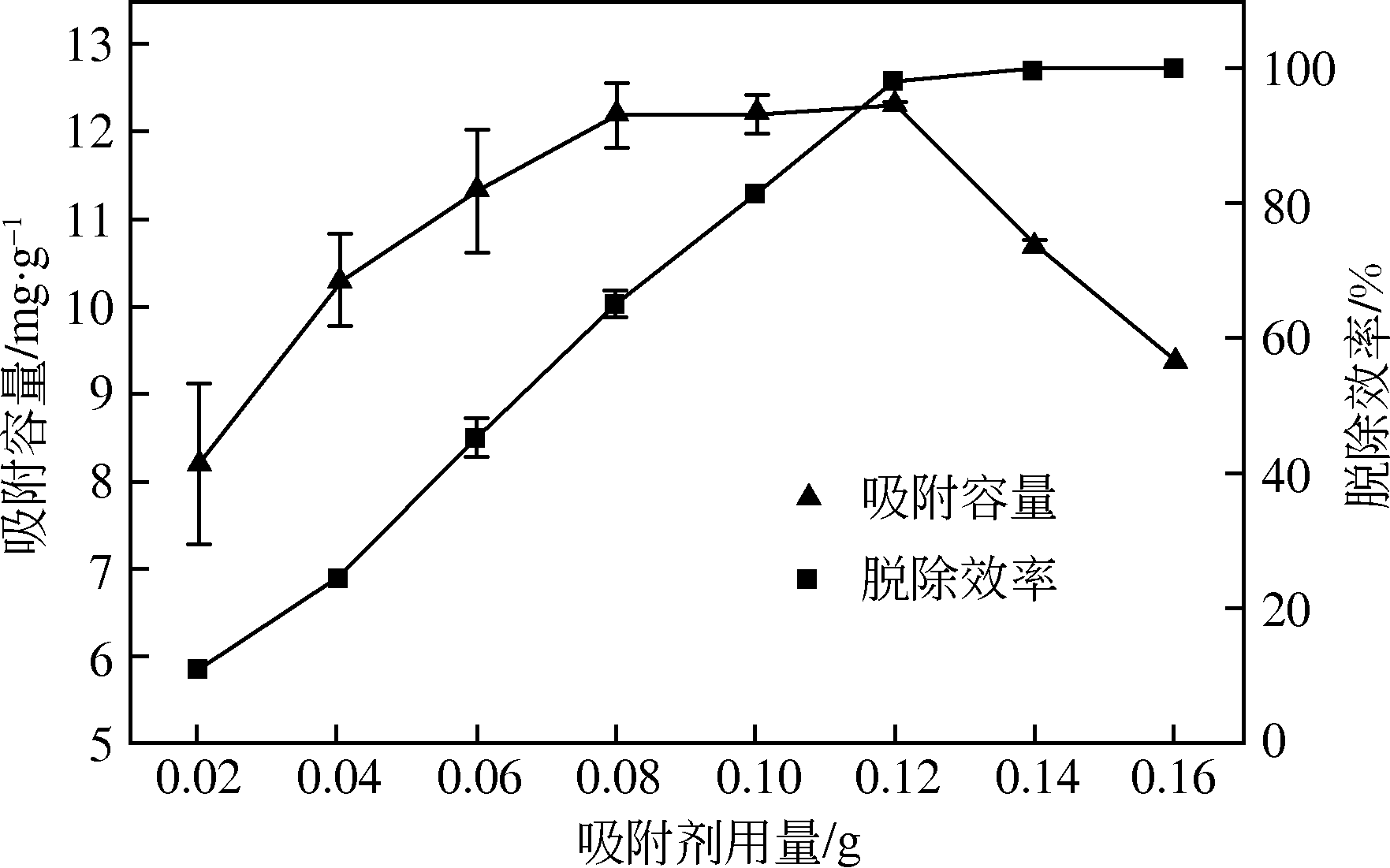
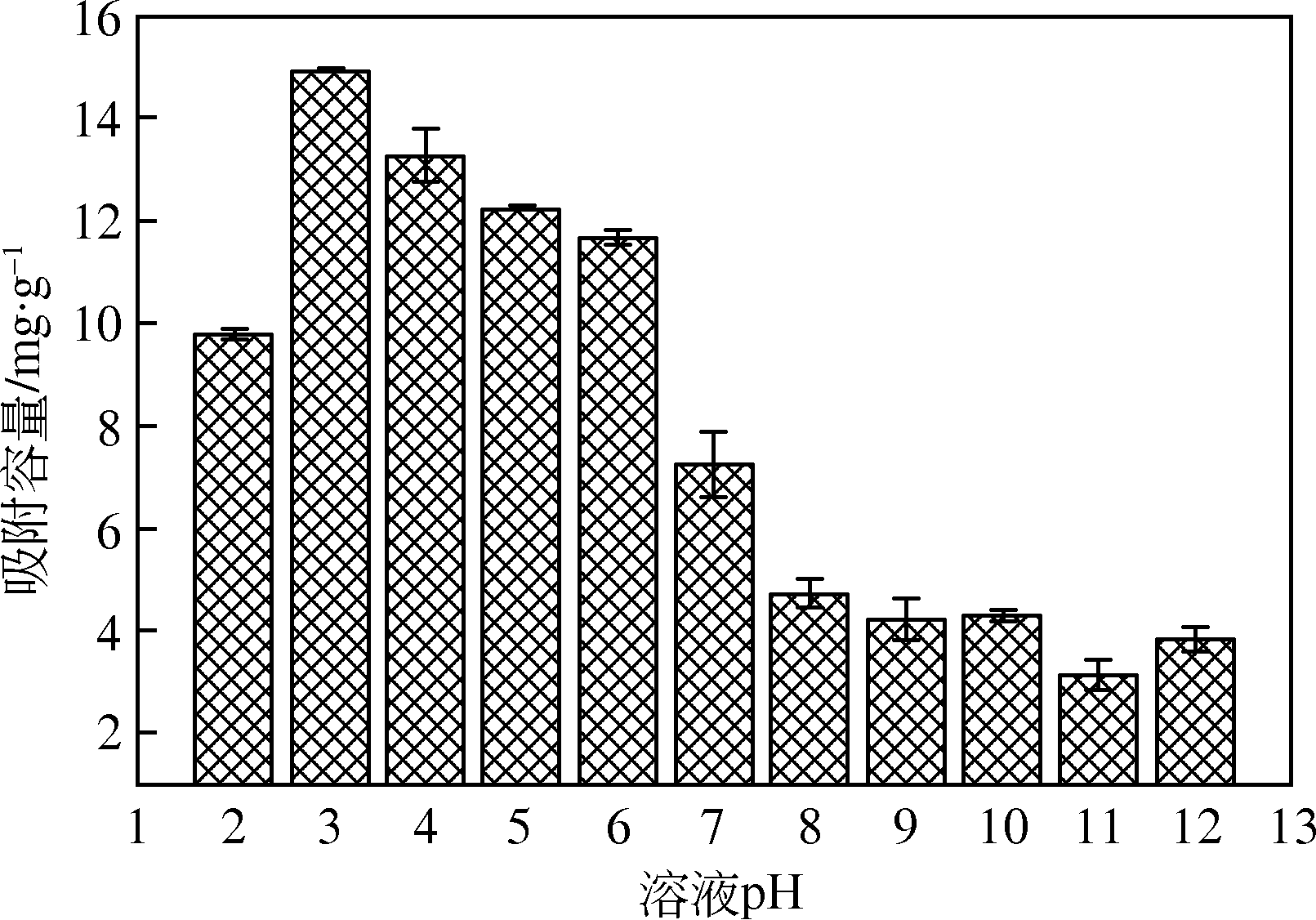
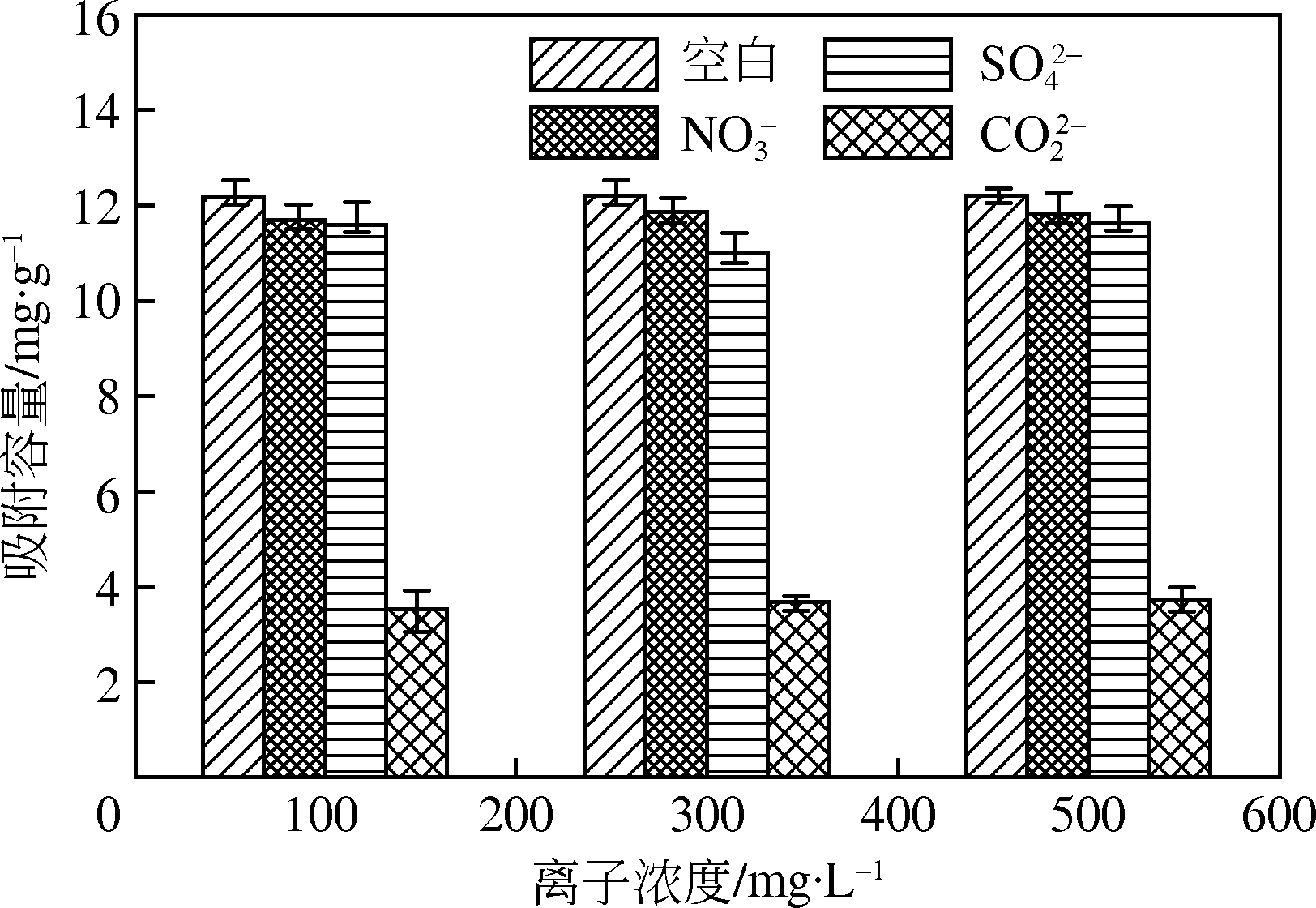
为研发低成本的磷酸盐吸附剂,以核桃壳为原料,LaCl3为改性试剂热解制备核桃壳生物炭。通过SEM-EDS、ICP-OES、FTIR和XRD对生物炭进行表征,采用吸附等温模型和动力学模型拟合生物炭的吸磷特征,并研究热解温度、La改性浓度、添加量、初始溶液pH和共存离子对生物炭吸附磷的影响。结果表明:La改性后,生物炭表面由于负载了La2O3和LaOCl,其吸附能力明显提高。热解温度为400℃、La浸渍浓度为0.1mol/L时获得的生物炭(BC-La400),其Langmuir最大磷吸附容量为12.18mg/g,吸附过程主要受化学吸附和颗粒内扩散控制。热解温度和La改性浓度过高均不利于磷的吸附。磷初始浓度为50mg/L时,BC-La400添加量为2.7g/L可获得较理想的吸附能力,但当添加量超过4.0g/L时,磷脱除率可超过98%。BC-La400吸磷时最佳初始pH为3,CO
中图分类号:
引用本文
罗元, 谢坤, 冯弋洋, 何秋平, 张克强, 沈仕洲, 王风. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能[J]. 化工进展, 2021, 40(2): 1121-1129.
Yuan LUO, Kun XIE, Yiyang FENG, Qiuping HE, Keqiang ZHANG, Shizhou SHEN, Feng WANG. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129.
| 元素 | 质量分数/% | 原子分数/% |
|---|---|---|
| C | 56.47 | 78.21 |
| O | 16.78 | 17.44 |
| Cl | 3.28 | 1.54 |
| La | 23.47 | 2.81 |
表1 BC-La400表面主要元素含量
| 元素 | 质量分数/% | 原子分数/% |
|---|---|---|
| C | 56.47 | 78.21 |
| O | 16.78 | 17.44 |
| Cl | 3.28 | 1.54 |
| La | 23.47 | 2.81 |
| 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|
| k1/h-1 | qe/mg·g-1 | R2 | k2/g·mg-1·h-1 | qe/mg·g-1 | R2 | |
| 0.4450 | 11.04 | 0.8676 | 0.0567 | 11.94 | 0.9422 | |
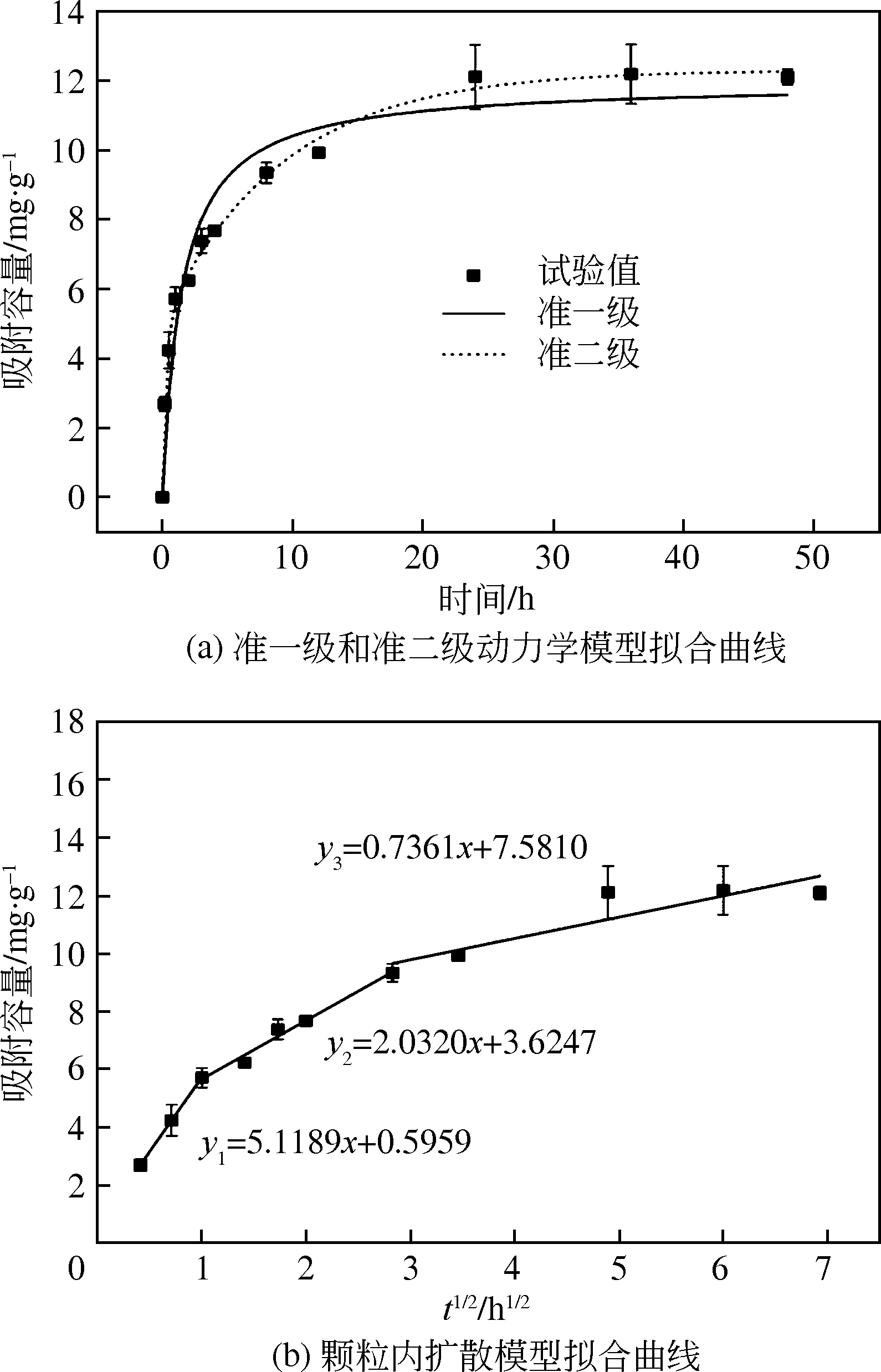
表2 BC-La400吸附磷的准一级和准二级动力学模型拟合参数
| 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|
| k1/h-1 | qe/mg·g-1 | R2 | k2/g·mg-1·h-1 | qe/mg·g-1 | R2 | |
| 0.4450 | 11.04 | 0.8676 | 0.0567 | 11.94 | 0.9422 | |
| 准一级动力学模型 | 准二级动力学模型 | 准三级动力学模型 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kd1/mg·g-1·h-1/2 | c1 | R2 | kd2/mg·g-1·h-1/2 | c2 | R2 | kd3/mg·g-1·h-1/2 | c3 | R2 | ||
| 5.1189 | 0.5959 | 0.9999 | 2.0320 | 3.6247 | 0.9792 | 0.7361 | 7.5810 | 0.7618 | ||
表3 BC-La400吸附磷的颗粒内扩散模型拟合参数
| 准一级动力学模型 | 准二级动力学模型 | 准三级动力学模型 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| kd1/mg·g-1·h-1/2 | c1 | R2 | kd2/mg·g-1·h-1/2 | c2 | R2 | kd3/mg·g-1·h-1/2 | c3 | R2 | ||
| 5.1189 | 0.5959 | 0.9999 | 2.0320 | 3.6247 | 0.9792 | 0.7361 | 7.5810 | 0.7618 | ||
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL/L·mg-1 | R2 | n | KF/mg·g-1·L1/n·mg-1/n | R2 | |
| 12.18 | 16.69 | 0.9875 | 7.8095 | 9.9218 | 0.9061 | |
表4 BC-La400吸附磷的等温方程拟合参数
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL/L·mg-1 | R2 | n | KF/mg·g-1·L1/n·mg-1/n | R2 | |
| 12.18 | 16.69 | 0.9875 | 7.8095 | 9.9218 | 0.9061 | |
| 吸附剂 | 热解温度 /℃ | La与原料 质量比 | 理论吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| La改性稻壳生物炭 | 800 | 0.20 | 45.62 | [ |
| La改性橡木生物炭 | 500 | 0.35 | 46.53 | [ |
| La/Fe改性菠萝皮生物炭 | 300 | 1.39 | 101.16 | [ |
| BC-La400 | 400 | 0.07 | 12.18 | 本研究 |
| La改性膨润土 | Nd | Nd | 10.19 | [ |
| La/Al改性蒙脱石 | Nd | Nd | 13.02 | [ |
| La改性刺柏纤维素 | Nd | 0.83 | 10.89 | [ |
| La改性符山石 | Nd | 0.16 | 6.70 | [ |
表5 La改性吸附材料对磷的吸附容量
| 吸附剂 | 热解温度 /℃ | La与原料 质量比 | 理论吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| La改性稻壳生物炭 | 800 | 0.20 | 45.62 | [ |
| La改性橡木生物炭 | 500 | 0.35 | 46.53 | [ |
| La/Fe改性菠萝皮生物炭 | 300 | 1.39 | 101.16 | [ |
| BC-La400 | 400 | 0.07 | 12.18 | 本研究 |
| La改性膨润土 | Nd | Nd | 10.19 | [ |
| La/Al改性蒙脱石 | Nd | Nd | 13.02 | [ |
| La改性刺柏纤维素 | Nd | 0.83 | 10.89 | [ |
| La改性符山石 | Nd | 0.16 | 6.70 | [ |
| 41 | 王章鸿. 稀土添加制备生物炭及炭对氮、磷吸附性能的研究[D]. 成都: 四川农业大学, 2015. |
| WANG Zhanghong. Production of rare earth/biochar composite and its N&P adsorption performances[D]. Chengdu: Sichuan Agriculture University, 2015. | |
| 42 | KOILRAI P, SASAKI K. Selective removal of phosphate using La-porous carbon composites from aqueous solutions: batch and column studies[J]. Chemical Engineering Journal, 2017, 317: 1059-1068. |
| 43 | CHEN Q, QIN J, SUN P, et al. Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil-crop system[J]. Journal of Cleaner Production, 2018, 172: 2009-2018. |
| 44 | ZHOU A, ZHU C, CHEN W, et al. Phosphorus recovery from water by lanthanum hydroxide embedded interpenetrating network poly(vinyl alcohol)/sodium alginate hydrogel beads[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 554: 237-244. |
| 45 | WU Y, LI X, YANG Q, et al. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents[J]. Journal of Environmental Management, 2019, 231: 370-379. |
| 46 | DAI L, WU B, TAN F, et al. Engineered hydrochar composites for phosphorus removal/recovery: Lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw[J]. Bioresource Technology, 2014, 161:327-332. |
| 47 | FU H, YANG Y, ZHU R, et al. Superior adsorption of phosphate by ferrihydrite-coated and lanthanum-decorated magnetite[J]. Journal of Colloid and Interface Science, 2018, 530: 704-713. |
| 1 | KUMAR P S, KORVING L, LOOSDRECHT M C M VAN, et al. Adsorption as a technology to achieve ultra-low concentrations of phosphate: research gaps and economic analysis[J]. Water Research X, 2019, 4: 100029. |
| 2 | 王荣, 贺峰, 徐栋, 等. 人工湿地基质除磷机理及影响因素研究[J]. 环境科学与技术, 2010, 33(S1): 12-18. |
| WANG Rong, HE Feng, XU Dong, et al. Studies on the mechanisms and influencing factors of substrates in constructed wetlands removing phosphorus [J]. Environmental Science & Technology, 2010, 33(S1): 12-18. | |
| 3 | 王彤彤, 崔庆亮, 王丽丽, 等. Al改性柠条生物炭对P的吸附特性及其机制[J]. 中国环境科学, 2018, 38(6): 2210-2222. |
| 48 | RASHID M, PRICE N T, GRACIA P M A, et al. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles[J]. Water Research, 2017, 123: 353-360. |
| 3 | WANG Tongtong, CUI Qingliang, WANG Lili, et al. Adsorption characteristics and mechanism of phosphate from aqueous solutions on Al modification biochar produced from Caragana Korshinskii[J]. China Environmental Science, 2018, 38(6): 2210-2222. |
| 4 | YAGHOOBI-RAHNI S, REZAEI B, MIRGHAFFARI N. Bentonite surface modification and characterization for high selective phosphate adsorption from aqueous media and its application for wastewater treatments[J]. Journal of Water Reuse and Desalination, 2017, 7(2): 175-186. |
| 5 | YIN Q, ZHANG B, WANG R, et al. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: a review[J]. Environmental Science and Pollution Research, 2017, 24(34): 26297-26309. |
| 6 | CHEN W, MENG J, HAN X, et al. Past, present, and future of biochar[J]. Biochar, 2019, 1(1): 75-87. |
| 7 | YAO Y, GAO B, INYANG M, et al. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings[J]. Journal of Hazardous Materials, 2011, 190(1/3): 501-507. |
| 8 | TAN X, LIU Y, ZENG G, et al. Application of biochar for the removal of pollutants from aqueous solutions[J]. Chemosphere, 2015, 125: 70-85. |
| 9 | NOVAIS S V, ZENERO M D O, TRONYO J, et al. Poultry manure and sugarcane straw biochars modified with MgCl2 for phosphorus adsorption[J]. Journal of Environmental Management, 2018, 214: 36-44. |
| 10 | YAO Y, GAO B, ZHANG M, et al. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil[J]. Chemosphere, 2012, 89(11): 1467-1471. |
| 11 | PINTO M D C E, SILVA D D DA, GOMES A L A, et al. Biochar from carrot residues chemically modified with magnesium for removing phosphorus from aqueous solution[J]. Journal of Cleaner Production, 2019, 222: 36-46. |
| 12 | 易蔓, 李婷婷, 李海红, 等. Ca/Mg负载改性沼渣生物炭对水中磷的吸附特性[J]. 环境科学, 2019, 40(3): 1318-1327. |
| YI Man, LI Tingting, LI Haihong, et al. Characteristics of phosphorus adsorption in aqueous solution by Ca/Mg loaded biogas residue biochar[J]. Environmental Science, 2019, 40(3): 1318-1327. | |
| 13 | CCPETTI D, FINSTERLE K, MARZIALI L, et al. Eutrophication management in surface waters using lanthanum modified bentonite: a review[J]. Water Research, 2016, 97: 162-174. |
| 14 | D’HAESE P C, DOUGLAS G, VERHULST A, et al. Human health risk associated with the management of phosphorus in freshwaters using lanthanum and aluminium[J]. Chemosphere, 2019, 220: 286-299. |
| 15 | 罗元, 谢坤, 张克强, 等. 镧(La)改性吸附材料脱除水体磷酸盐研究进展[J]. 化工进展, 2019, 38(11): 5005-5014. |
| LUO Yuan, XIE Kun, ZHANG Keqiang, et al. Research progress on removal phosphate in aqueous solution by lanthanum modified adsorption materials[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5005-5014. | |
| 16 | CUI W, KAMRAN M, SONG Q, et al. Lanthanum chloride improves maize grain yield by promoting photosynthetic characteristics, antioxidants enzymes and endogenous hormone at reproductive stages[J]. Journal of Rare Earths, 2019, 37(7): 781-790. |
| 17 | 张有林, 原双进, 王小纪, 等. 基于中国核桃发展战略的核桃加工业的分析与思考[J]. 农业工程学报, 2015, 31(21): 1-8. |
| ZHANG Youlin, YUAN Shuangjin, WANG Xiaofei, et al. Analysis and reflection on development strategy of walnut processing industry in China [J]. Transactions of the Chinese Society of Agricultural Engineering, 2015, 31(21): 1-8. | |
| 18 | YU Y, AN Q, ZHOU Y, et al. Highly synergistic effects on ammonium removal by the co-system of Pseudomonas stutzeri XL-2 and modified walnut shell biochar[J]. Bioresource Technology, 2019, 280: 239-246. |
| 19 | 张静雪, 梁晓怿, 贾倩. 核桃壳基活性炭的制备及其在超级电容器中的应用[J]. 现代化工, 2020, 40(1): 180-184. |
| ZHANG Jingxue, LIANG Xiaoyi, JIA Qian. Preparation of walnut shell-based activated carbon and its application in supercapacitor[J]. Modern Chemical Industry, 2020, 40(1): 180-184. | |
| 20 | 赵阳, 高建民, 郝新敏, 等. 基于KOH活化法的核桃壳基活性炭制备及其表征[J]. 安全与环境学报, 2016, 16(2): 262-266. |
| ZHAO Yang, GAO Jianmin, HAO Xinmin, et al. Preparation and characterization of the carbon purified from the walnut shells activated chemically with KOH[J]. Journal of Safety and Environment, 2016, 16(2): 262-266. | |
| 21 | YAO Y, GAO B, INYANG M, et al. Biochar derived from anaerobically digested sugar beet tailings: characterization and phosphate removal potential[J]. Bioresource Technology, 2011, 102(10): 6273-6278. |
| 22 | 宋小宝, 何世颖, 冯彦房, 等. 载镧磁性水热生物炭的制备及其除磷性能[J]. 环境科学, 2020, 41(2): 773-783. |
| SONG Xiaobao, HE Shiyan, FENG Yanfang, et al. Fabrication of La-MHTC composites for phosphate removal: adsorption behavior and mechanism[J]. Environmental Science, 2020, 41(2): 773-783. | |
| 23 | WANG Z, ZHENG H, LUO Y, et al. Characterization and influence of biochars on nitrous oxide emission from agricultural soil[J]. Environmental Pollution, 2013, 174: 289-296. |
| 24 | MCDEVITT N T, BAUN W L. Infrared absorption study of metal oxides in the low frequency region (700-240cm-1)[J]. Spectrochimica Acta, 1964, 20(5): 799-808. |
| 25 | LI R, WANG J J, ZHOU B, et al. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute[J]. Bioresource Technology, 2016, 215: 209-214. |
| 26 | YANG Q, WANG X, LUO W, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge[J]. Bioresource Technology, 2018, 247: 537-544. |
| 27 | 马锋锋, 赵保卫, 钟金魁, 等. 牛粪生物炭对磷的吸附特性及其影响因素研究[J]. 中国环境科学, 2015, 35(4): 1156-1163. |
| MA Fengfeng, ZHAO Baowei, ZHONG Jinkui, et al. Characteristics phosphate adsorption onto biochars derived from dairy manure and its influencing factors[J]. China Environmental Science, 2015, 35(4): 1156-1163. | |
| 28 | 王彤彤, 马江波, 曲东, 等. 两种木材生物炭对铜离子的吸附特性及其机制[J]. 环境科学, 2017, 38(5): 2161-2171. |
| WANG Tongtong, MA Jiangbo, QU Dong, et al. Characteristics and mechanism of copper adsorption from aqueous solutions on biochar produced from sawdust and apple branch[J]. Environmental Science, 2017, 38(5): 2161-2171. | |
| 29 | GU W, LI X, XING M, et al. Removal of phosphate from water by amine-functionalized copper ferrite chelated with La(III)[J]. Science of the Total Environment, 2018, 619/620: 42-48. |
| 30 | HUANG W, LI D, LIU Z, et al. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents[J]. Chemical Engineering Journal, 2014, 236:191-201. |
| 31 | ZHOU J, YANG S, YU J, et al. Novel hollow microspheres of hierarchical zinc-aluminum layered double hydroxides and their enhanced adsorption capacity for phosphate in water[J]. Journal of Hazardous Materials, 2011, 192: 1114-1121. |
| 32 | LIU X, SHEN F, SMITH R L JR, et al. Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions[J]. Bioresour Technology, 2019, 294: 122198. |
| 33 | 付军, 范芳, 李海宁, 等. 铁锰复合氧化物/壳聚糖珠: 一种环境友好型除磷吸附剂[J]. 环境科学, 2016, 37(12): 4882-4890. |
| FU Jun, FAN Fang, LI Haining, et al. Fe-Mn binary oxide impregnated chitosan bead (FMCB): an environmental friendly sorbent for phosphate removal[J]. Environmental Science, 2016, 37(12): 4882-4890. | |
| 34 | TANG Q, SHI C, SHI W, et al. Preferable phosphate removal by nano-La (Ⅲ) hydroxides modified mesoporous rice husk biochars: role of the host pore structure and point of zero charge[J]. Science of the Total Environment, 2019, 662: 511-520. |
| 35 | WANG Z, GUO H, SHEN F, et al. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3-), and phosphate (PO43-)[J]. Chemosphere, 2015, 119: 646-653. |
| 36 | LIAO T, LI T, SU X, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology, 2018, 263: 207-213. |
| 37 | HAGHSERESHT F, WANG S, DO D D. A novel lanthanum-modified bentonite, phoslock, for phosphate removal from wastewaters[J]. Applied Clay Science, 2009, 46(4): 369-375. |
| 38 | TIAN S, JIANG P, NING P, et al. Enhanced adsorption removal of phosphate from water by mixed lanthanum/aluminum pillared montmorillonite[J]. Chemical Engineering Journal, 2009, 151(1/2/3): 141-148. |
| 39 | SHIN E W, KARTHIKEYYAN K G, TSHABALALA M A. Orthophosphate sorption onto lanthanum-treated lignocellulosic sorbents[J]. Environmental Science & Technology, 2005, 39(16): 6273-6279. |
| 40 | LI H, RU J, YIN W, et al. Removal of phosphate from polluted water by lanthanum doped vesuvianite [J]. Journal of Hazardous Materials, 2009, 168(1): 326-330. |
| [1] | 徐晨阳, 都健, 张磊. 基于图神经网络的化学反应优劣评价[J]. 化工进展, 2023, 42(S1): 205-212. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [5] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [6] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [7] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [8] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [9] | 陈林, 徐培渊, 张晓慧, 陈杰, 徐振军, 陈嘉祥, 密晓光, 冯永昌, 梅德清. 液化天然气绕管式换热器壳侧混合工质流动及传热特性[J]. 化工进展, 2023, 42(9): 4496-4503. |
| [10] | 张帆, 陶少辉, 陈玉石, 项曙光. 基于改进恒热传输模型的精馏模拟初始化[J]. 化工进展, 2023, 42(9): 4550-4558. |
| [11] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [14] | 王浩然, 殷全玉, 方明, 侯建林, 李军, 何斌, 张明月. 近临界水处理废弃烟梗工艺优化[J]. 化工进展, 2023, 42(9): 5019-5027. |
| [15] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||