化工进展 ›› 2021, Vol. 40 ›› Issue (1): 273-281.DOI: 10.16085/j.issn.1000-6613.2020-0501
杂原子掺杂碳材料活化过硫酸盐技术的研究进展
李小娟1( ), 叶兰妹1, 廖凤珍1, 叶梓瑜1, 叶礼志1,2
), 叶兰妹1, 廖凤珍1, 叶梓瑜1, 叶礼志1,2
- 1.福州大学环境与资源学院,福建 福州 350108
2.高雄大学土木与环境工程学系,台湾 高雄 81148
-
收稿日期:2020-04-01出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:李小娟 -
作者简介:李小娟(1982—),女,博士,副教授,研究方向为高效环境材料的制备与应用。E-mail:lixiaojuan@fzu.edu.cn 。 -
基金资助:国家自然科学基金(21407026)
Research progress in the application of heteroatom-doped carbonaceous materials for persulfate activation
Xiaojuan LI1( ), Lanmei YE1, Fengzhen LIAO1, Ziyu YE1, Lizhi YEH1,2
), Lanmei YE1, Fengzhen LIAO1, Ziyu YE1, Lizhi YEH1,2
- 1.College of Environment and Resources, Fuzhou University, Fuzhou 350108, Fujian, China
2.Department of Civil and Environmental Engineering, University of Kaohsiung, Kaohsiung 81148, Taiwan, China
-
Received:2020-04-01Online:2021-01-05Published:2021-01-12 -
Contact:Xiaojuan LI
摘要:
碳材料因其比表面积高、吸附性能佳,并且能克服加热、紫外光照射、超声等传统活化方式能耗高、金属催化材料产生二次污染的弊端而在活化过硫酸盐降解有机污染物应用中具有潜力。杂原子(N、S、B、P等)掺杂不仅能打破碳材料网络惰性、提高电导率,还能增加反应活性位点,是提升碳材料活化过硫酸盐性能的有效途径。本文介绍了碳材料活化过硫酸盐的机理,主要包括自由基途径、单线态氧途径及表面电子传递,并进一步总结了杂原子掺杂碳材料活化过硫酸盐的机理;然后综述了杂原子碳材料的种类、制备方法及其在有机污染物降解中的应用,最后指出了已有研究存在的不足,并提出杂原子掺杂碳材料稳定性及可重复利用性的提升和降解机制的深入探索是未来研究的方向。
中图分类号:
引用本文
李小娟, 叶兰妹, 廖凤珍, 叶梓瑜, 叶礼志. 杂原子掺杂碳材料活化过硫酸盐技术的研究进展[J]. 化工进展, 2021, 40(1): 273-281.
Xiaojuan LI, Lanmei YE, Fengzhen LIAO, Ziyu YE, Lizhi YEH. Research progress in the application of heteroatom-doped carbonaceous materials for persulfate activation[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 273-281.
| 单一杂原子 掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐投加量 | 污染物浓度 | 降解效果 | |||||||
| N-SWCNT | 液相 | SWCNT | N | 尿素 | 200 | 2.0g·L-1 PDS | 20mg·kg-1 硝基苯 | 60min,100% | R | [ |
| NMC-850 | 液相 | SBA-15 | N | 乙二胺和 双氰胺 | 50 | 1.25g·L-1 PMS | 30mg·L-1 邻苯基苯酚 | 150min,81% | 1O2 | [ |
| NCNT-550 | 液相 | CNT | N | 尿素 | 100 | 8mmol·L-1 PMS | 0.106mmol·L-1 苯酚 | 20min,100% | 1O2 | [ |
| N-ND/PDDA/GO | 液相 | ND/PDDA/GO | N | NH3 | 100 | 1mmol·L-1 PMS | 0.1mmol·L-1 4-CP | 60min,100% | E | [ |
| N-RGO | 液相 | rGO | N | 氨溶液 | 120 | 0.8mmol·L-1 PMS | 88mg·L-1 BPA | 7min,100% | R | [ |
| N-rGO | 液相 | rGO | N | 三聚氰胺 | 50 | 2g·L-1 PMS | 20mg·L-1 IBP | 180min,90% | R | [ |
| N-rGO-N2 | 液相 | rGO | N | 尿素 | 400 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+1O2 | [ |
| N-AND | 液相 | AND | N | 三聚氰胺 | 200 | 6.5mmol·L-1 PMS | 20mg·L-1 苯酚 | 45min,100% | R | [ |
| N-CNT-35 | 液相 | CNT | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | R | [ |
| NoCNT-700 | 液相 | SWCNTs | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+E | [ |
| NG350 | 液相 | rGO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 180min,85% | R | [ |
| G-N | 液相 | GO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | — | [ |
| NG-700 | 液相 | rGO | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 30min,100% | R | [ |
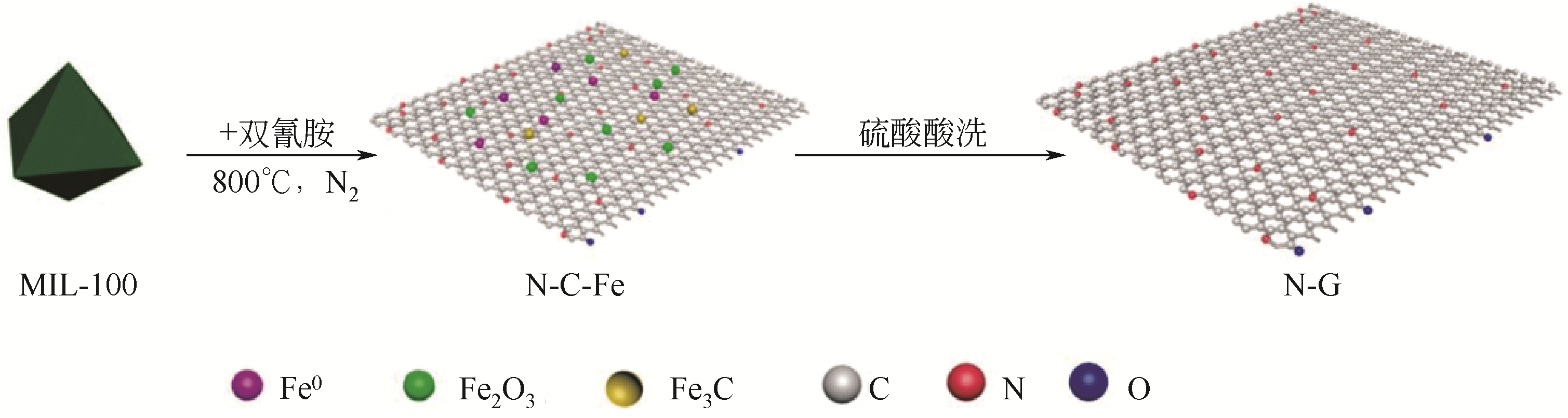
N-G(D)N-G(M) N-G(M) | 液相 | MIL-100 | N | 双氰胺 三聚氰胺 尿素 | 100 | 3.25mmol·L-1 PMS | 20mg·kg-1 PHBA | 20min,100% 30min,100% 90min,100% | 1O2 | [ |
| N-G(D) | 液相 | MIL-100 | N | 双氰胺 | 100 | 3.25mmol·L-1 PMS | 50mg·kg-1 苯酚 | 30min,100% | 1O2 | [ |
| NH4NO3-CNT-OH | 固相 | CNT-OH (0.5∶1) | N | 硝酸 | 100 | 污染物∶[PDS]= 1∶500 | 43.48mmol·L-1 2,4,4-HBP | 120min,100% | R | [ |
| N-IrGO | 固相 | IrGO | N | 尿素 | 50 | 500mg·L-1 PMS | 5mg·L-1 二苯甲酮-1 | 60min,100% | 1O2 | [ |
| CPPy-F-8 | 原位 | PPy-F | N | 聚吡咯 | 100 | 3.25mmol·L-1 PMS | 20mg·L-1 苯酚 | 120min,97% | R+1O2 | [ |
| NPC-800 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 0.8g·L-1 PMS | 25mg·L-1 苯酚 | 60min,86.1% | R | [ |
| N-C-900 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 150 | 5mmol·L-1 PDS | 0.3mmol·L-1 PCA | 120min, >96.3% | R+1O2+E | [ |
| ACS-800 | 原位 | 聚噻吩 | S | 噻吩 | 50 | 8mmol·L-1 PDS | 40mg·L-1 4-CP | 60min,100% | R | [ |
| SDAC-800 | 原位 | 聚噻吩 | S | 噻吩 | 100 | 15mmol·L-1 PDS | 80mg·L-1 4-CP | 90min,100% | R+E | [ |
| B-OMC | 液相 | 酚醛树脂 | B | 硼酸 | 200 | 1?mmol·L-1 PMS | 20mg·L-1 BPA | 60min,91% | 1O2 | [ |
| NPCs | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 1.6mmol·L-1 PMS | 20mg·kg-1 苯酚 | 60min,100% | R | [ |
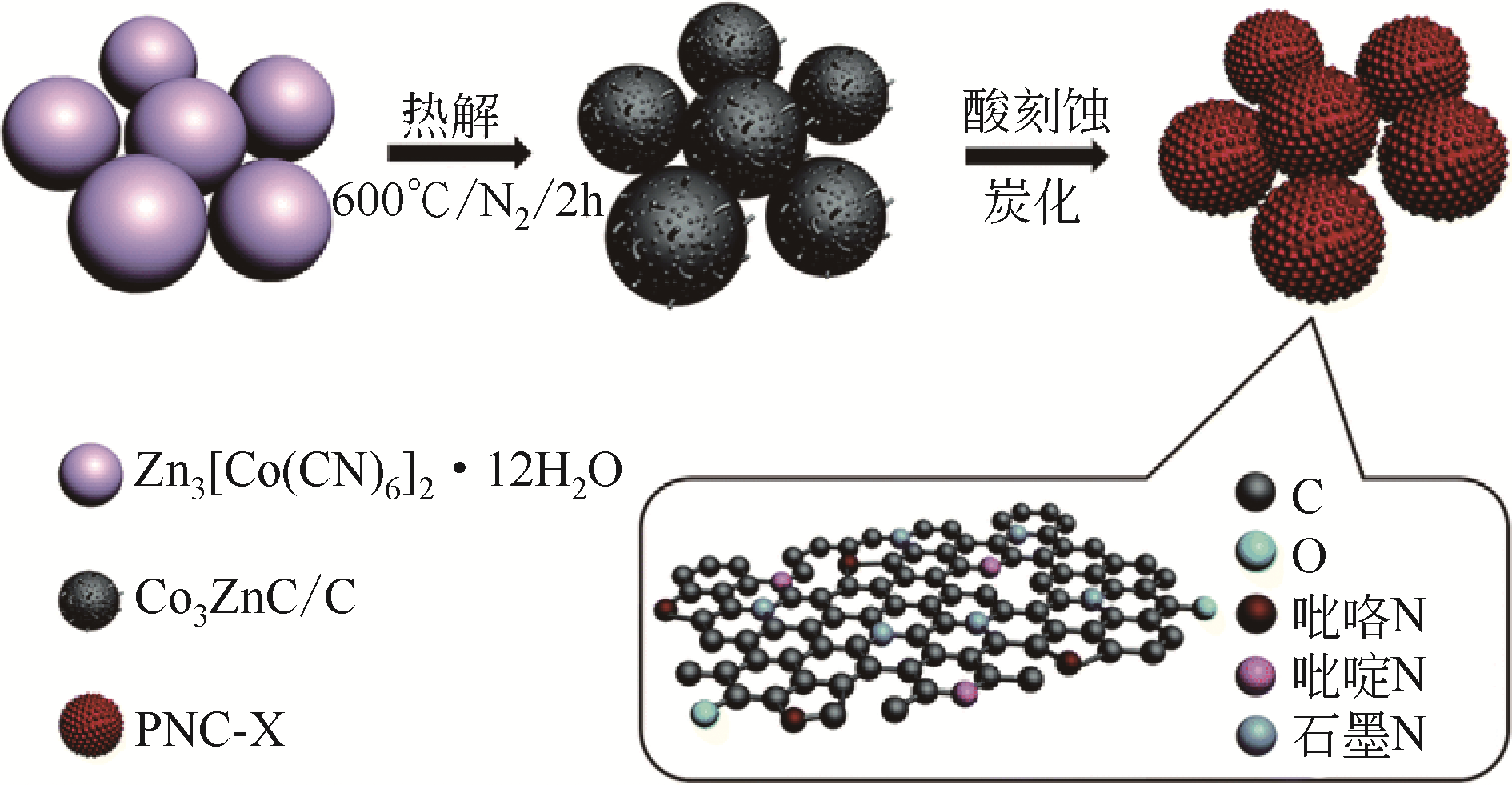
| PNC-800 | 原位 | Zn-Co PBAs | N | 钴氰化钾 | 100 | 1.0g·L-1 PMS | 100mg·L-1 MB | 10min,100% | R+1O2 | [ |
| CBs@NCCs | 原位 | Co-Fe PBAs | N | 钴氰化钾 | 60 | 1.0g·L-1 PMS | 100mg·L-1 MB | 60min, >95% | R+1O2 | [ |
表1 单一杂原子掺杂碳材料及其制备方法、过硫酸盐活化性能和机理
| 单一杂原子 掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐投加量 | 污染物浓度 | 降解效果 | |||||||
| N-SWCNT | 液相 | SWCNT | N | 尿素 | 200 | 2.0g·L-1 PDS | 20mg·kg-1 硝基苯 | 60min,100% | R | [ |
| NMC-850 | 液相 | SBA-15 | N | 乙二胺和 双氰胺 | 50 | 1.25g·L-1 PMS | 30mg·L-1 邻苯基苯酚 | 150min,81% | 1O2 | [ |
| NCNT-550 | 液相 | CNT | N | 尿素 | 100 | 8mmol·L-1 PMS | 0.106mmol·L-1 苯酚 | 20min,100% | 1O2 | [ |
| N-ND/PDDA/GO | 液相 | ND/PDDA/GO | N | NH3 | 100 | 1mmol·L-1 PMS | 0.1mmol·L-1 4-CP | 60min,100% | E | [ |
| N-RGO | 液相 | rGO | N | 氨溶液 | 120 | 0.8mmol·L-1 PMS | 88mg·L-1 BPA | 7min,100% | R | [ |
| N-rGO | 液相 | rGO | N | 三聚氰胺 | 50 | 2g·L-1 PMS | 20mg·L-1 IBP | 180min,90% | R | [ |
| N-rGO-N2 | 液相 | rGO | N | 尿素 | 400 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+1O2 | [ |
| N-AND | 液相 | AND | N | 三聚氰胺 | 200 | 6.5mmol·L-1 PMS | 20mg·L-1 苯酚 | 45min,100% | R | [ |
| N-CNT-35 | 液相 | CNT | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | R | [ |
| NoCNT-700 | 液相 | SWCNTs | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 20min,100% | R+E | [ |
| NG350 | 液相 | rGO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 180min,85% | R | [ |
| G-N | 液相 | GO | N | 硝酸铵 | 200 | 2g·L-1 PMS | 20mg·kg-1 苯酚 | 45min,100% | — | [ |
| NG-700 | 液相 | rGO | N | 三聚氰胺 | 100 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 30min,100% | R | [ |
N-G(D)N-G(M) N-G(M) | 液相 | MIL-100 | N | 双氰胺 三聚氰胺 尿素 | 100 | 3.25mmol·L-1 PMS | 20mg·kg-1 PHBA | 20min,100% 30min,100% 90min,100% | 1O2 | [ |
| N-G(D) | 液相 | MIL-100 | N | 双氰胺 | 100 | 3.25mmol·L-1 PMS | 50mg·kg-1 苯酚 | 30min,100% | 1O2 | [ |
| NH4NO3-CNT-OH | 固相 | CNT-OH (0.5∶1) | N | 硝酸 | 100 | 污染物∶[PDS]= 1∶500 | 43.48mmol·L-1 2,4,4-HBP | 120min,100% | R | [ |
| N-IrGO | 固相 | IrGO | N | 尿素 | 50 | 500mg·L-1 PMS | 5mg·L-1 二苯甲酮-1 | 60min,100% | 1O2 | [ |
| CPPy-F-8 | 原位 | PPy-F | N | 聚吡咯 | 100 | 3.25mmol·L-1 PMS | 20mg·L-1 苯酚 | 120min,97% | R+1O2 | [ |
| NPC-800 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 0.8g·L-1 PMS | 25mg·L-1 苯酚 | 60min,86.1% | R | [ |
| N-C-900 | 原位 | ZIF-8 | N | 2-甲基咪唑 | 150 | 5mmol·L-1 PDS | 0.3mmol·L-1 PCA | 120min, >96.3% | R+1O2+E | [ |
| ACS-800 | 原位 | 聚噻吩 | S | 噻吩 | 50 | 8mmol·L-1 PDS | 40mg·L-1 4-CP | 60min,100% | R | [ |
| SDAC-800 | 原位 | 聚噻吩 | S | 噻吩 | 100 | 15mmol·L-1 PDS | 80mg·L-1 4-CP | 90min,100% | R+E | [ |
| B-OMC | 液相 | 酚醛树脂 | B | 硼酸 | 200 | 1?mmol·L-1 PMS | 20mg·L-1 BPA | 60min,91% | 1O2 | [ |
| NPCs | 原位 | ZIF-8 | N | 2-甲基咪唑 | 200 | 1.6mmol·L-1 PMS | 20mg·kg-1 苯酚 | 60min,100% | R | [ |
| PNC-800 | 原位 | Zn-Co PBAs | N | 钴氰化钾 | 100 | 1.0g·L-1 PMS | 100mg·L-1 MB | 10min,100% | R+1O2 | [ |
| CBs@NCCs | 原位 | Co-Fe PBAs | N | 钴氰化钾 | 60 | 1.0g·L-1 PMS | 100mg·L-1 MB | 60min, >95% | R+1O2 | [ |
| 杂原子 共掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐 投加量 | 污染物浓度 | 降解效果 | |||||||
| SNG | 液相 | rGO | N,S | N:硝酸铵 S:二甲基亚砜 | 200 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 90min,100% | R | [ |
| 2sNBG-800 | 液相 | rGO | N,B | N:尿素 B:硼酸 | 200 | 0.5mmol·L-1 PMS | 10mg·L-1 SAM | 20min,100% | R+E | [ |
| N-S-PCs | 液相 | 葡萄糖 | N,S | NS:硫脲 | 50 | 6.5mmol·L-1 PDS | 20mg·L-1 磺胺氯哒嗪 | 20min,100% | R+E | [ |
| NPSC-700 | 液相 | ZIF-8 | N,P,S | NPS:聚丙腈 | 60 | 0.4g·L-1 PMS | 25mg·kg-1 BPA | 30min,90.10% | R | [ |
| SNCs | 液相 | 咖啡粉 | N,S | NS:L-半胱氨酸 | 400 | 2mmol·L-1 PDS | 0.02mmol·L-1 TeC | 60min,100% | 1O2+E | [ |
| i-RGO-NS | 固相 | rGO | N,S | NS:硫脲 | 20 | 307mg·L-1 PMS | 15mg·L-1 MP | 20min,100% | 1O2 | [ |
| N,S-rGO | 固相 | rGO | N,S | NS:硫脲 | 50 | 0.9mmol·L-1 PDS | 2mg·L-1 BPA | 20min,100% | R | [ |
| NS-CNT-COOH | 固相 | CNT | N,S | NS:硫脲 | 100 | 1.0g·L-1 PMS | 0.01g·L-1 BP-4 | 30min,100% | E | [ |
表2 杂原子共掺杂碳材料及其制备方法、过硫酸盐活化性能和机理
| 杂原子 共掺杂碳材料 | 掺杂方法 | 碳基体 /前体 | 掺杂 元素 | 杂原子源 | 活化过硫酸盐降解有机污染物效果 | 机理① | 文献 | |||
|---|---|---|---|---|---|---|---|---|---|---|
催化剂浓度 /mg·L-1 | 过硫酸盐 投加量 | 污染物浓度 | 降解效果 | |||||||
| SNG | 液相 | rGO | N,S | N:硝酸铵 S:二甲基亚砜 | 200 | 6.5mmol·L-1 PMS | 20mg·kg-1 苯酚 | 90min,100% | R | [ |
| 2sNBG-800 | 液相 | rGO | N,B | N:尿素 B:硼酸 | 200 | 0.5mmol·L-1 PMS | 10mg·L-1 SAM | 20min,100% | R+E | [ |
| N-S-PCs | 液相 | 葡萄糖 | N,S | NS:硫脲 | 50 | 6.5mmol·L-1 PDS | 20mg·L-1 磺胺氯哒嗪 | 20min,100% | R+E | [ |
| NPSC-700 | 液相 | ZIF-8 | N,P,S | NPS:聚丙腈 | 60 | 0.4g·L-1 PMS | 25mg·kg-1 BPA | 30min,90.10% | R | [ |
| SNCs | 液相 | 咖啡粉 | N,S | NS:L-半胱氨酸 | 400 | 2mmol·L-1 PDS | 0.02mmol·L-1 TeC | 60min,100% | 1O2+E | [ |
| i-RGO-NS | 固相 | rGO | N,S | NS:硫脲 | 20 | 307mg·L-1 PMS | 15mg·L-1 MP | 20min,100% | 1O2 | [ |
| N,S-rGO | 固相 | rGO | N,S | NS:硫脲 | 50 | 0.9mmol·L-1 PDS | 2mg·L-1 BPA | 20min,100% | R | [ |
| NS-CNT-COOH | 固相 | CNT | N,S | NS:硫脲 | 100 | 1.0g·L-1 PMS | 0.01g·L-1 BP-4 | 30min,100% | E | [ |
| 38 | DUAN X G, SUN H Q, WANG Y X, et al. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation[J]. ACS Catalysis, 2015, 5(2): 553-559. |
| 39 | MA W J, DU Y C, WANG N, et al. ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation[J]. Environmental Science and Pollution Research, 2017, 24(19): 16276-16288. |
| 40 | LIU Y, MIAO W, FANG X, et al. MOF-derived metal-free N-doped porous carbon mediated peroxydisulfate activation via radical and non-radical pathways: role of graphitic N and C-O[J]. Chemical Engineering Journal, 2020, 380: 122584. |
| 41 | GUO Y P, ZENG Z Q, LI Y L, et al. In-situ sulfur-doped carbon as a metal-free catalyst for persulfate activated oxidation of aqueous organics[J]. Catalysis Today, 2018, 307: 12-19. |
| 42 | GUO Y P, ZENG Z Q, ZHU Y C, et al. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene[J]. Applied Catalysis B: Environmental, 2018, 220: 635-644. |
| 43 | WANG Y B, LIU M, ZHAO X, et al. Insights into heterogeneous catalysis of peroxymonosulfate activation by boron-doped ordered mesoporous carbon[J]. Carbon, 2018, 135: 238-247. |
| 44 | DUAN X G, INDRAWIRAWAN S, SUN H Q, et al. Effects of nitrogen-, boron-, and phosphorus-doping or codoping on metal-free graphene catalysis[J]. Catalysis Today, 2015, 249: 184-191. |
| 45 | YIN R L, GUO W Q, DU J S, et al. Heteroatoms doped graphene for catalytic ozonation of sulfamethoxazole by metal-free catalysis: performances and mechanisms[J]. Chemical Engineering Journal, 2017, 317: 632-639. |
| 46 | WANG Q, LI L, LUO L, et al. Activation of persulfate with dual-doped reduced graphene oxide for degradation of alkylphenols[J]. Chemical Engineering Journal, 2019, 376: 120891. |
| 47 | TIAN W J, ZHANG H Y, DUAN X G, et al. Nitrogen-and sulfur-codoped hierarchically porous carbon for adsorptive and oxidative removal of pharmaceutical contaminants[J]. ACS Applied Materials and Interfaces, 2016, 8(11): 7184-7193. |
| 48 | SUN H Q, WANG Y X, LIU S Z, et al. Facile synthesis of nitrogen doped reduced graphene oxide as a superior metal-free catalyst for oxidation[J]. Chemical Communications, 2013, 49(85): 9914-9916. |
| 49 | MA W J, WANG N, TONG T Z, et al. Nitrogen, phosphorus, and sulfur tri-doped hollow carbon shells derived from ZIF-67@poly (cyclotriphosphazene-co-4,4′-sulfonyldiphenol) as a robust catalyst of peroxymonosulfate activation for degradation of bisphenol A[J]. Carbon, 2018, 137: 291-303. |
| 1 | WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 2 | CHEN X, OH W D, LIM T T. Graphene- and CNTs-based carbocatalysts in persulfates activation: material design and catalytic mechanisms[J]. Chemical Engineering Journal, 2018, 354: 941-976. |
| 3 | GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 50 | WANG G L, CHEN S, QUAN X, et al. Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants[J]. Carbon, 2017, 115: 730-739. |
| 51 | DUAN X G, AO Z M, SUN H Q, et al. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis[J]. ACS Applied Materials and Interfaces, 2015, 7(7): 4169-4178. |
| 52 | LIANG P, ZHANG C, DUAN X G, et al. N-doped graphene from metal-organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism[J]. ACS Sustainable Chemistry and Engineering, 2017, 5(3): 2693-2701. |
| 53 | LIANG P, ZHANG C, DUAN X G, et al. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: formation mechanism and generation of singlet oxygen from peroxymonosulfate[J]. Environmental Science: Nano, 2017, 4(2): 315-324. |
| 54 | LIU H, SUN P, FENG M B, et al. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals[J]. Applied Catalysis B: Environmental, 2016, 187: 1-10. |
| 55 | YANG L, ZENG X F, WANG W C, et al. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells[J]. Advanced Functional Materials, 2018, 28(7): 1704537. |
| 56 | REN Q, WANG H, LU X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science, 2018, 5(3): 1700515. |
| 57 | WANG N, MA W J, REN Z Q, et al. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradation of organic pollutants[J]. Journal of Materials Chemistry A, 2018, 6(3): 884-895. |
| 58 | WANG N, MA W J, REN Z Q, et al. Template synthesis of nitrogen-doped carbon nanocages-encapsulated carbon nanobubbles as catalyst for activation of peroxymonosulfate[J]. Inorganic Chemistry Frontiers, 2018, 5(8): 1849-1860. |
| 59 | HUO X W, ZHOU P, ZHANG J, et al. N, S-doped porous carbons for persulfate activation to remove tetracycline: nonradical mechanism[J]. Journal of Hazardous Materials, 2020, 391: 122055. |
| 60 | SUN P, LIU H, ZHAI Z C, et al. Degradation of UV filter BP-1 with nitrogen-doped industrial graphene as a metal-free catalyst of peroxymonosulfate activation[J]. Chemical Engineering Journal, 2019, 356: 262-271. |
| 4 | DUAN X G, INDRAWIRAWAN S, KANG J, et al. Synergy of carbocatalytic and heat activation of persulfate for evolution of reactive radicals toward metal-free oxidation[J], Catalysis Today, 2020, 355: 319-324. |
| 5 | HUANG K C, ZHAO Z Q, HOAG G E, et al. Degradation of volatile organic compounds with thermally activated persulfate oxidation[J]. Chemosphere, 2005, 61(4): 551-560. |
| 6 | AO X W, LIU W J. Degradation of sulfamethoxazole by medium pressure UV and oxidants: peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 2017, 313: 629-637. |
| 7 | XU L J, WANG X T, SUN Y, et al. Mechanistic study on the combination of ultrasound and peroxymonosulfate for the decomposition of endocrine disrupting compounds[J]. Ultrasonics Sonochemistry, 2020, 60: 104749. |
| 8 | CHUA C K, PUMERA M. Carbocatalysis: the state of “metal-free” catalysis[J]. Chemistry, 2015, 21(36): 12550-12562. |
| 9 | HOU J F, YANG S S, WAN H Q, et al. Highly effective catalytic peroxymonosulfate activation on N-doped mesoporous carbon for o-phenylphenol degradation[J]. Chemosphere, 2018, 197: 485-493. |
| 10 | MANDAL S, BERA T, DUBEY G, et al. Uses of K2S2O8 in metal catalyzed and metal free oxidative transformations[J]. ACS Catalysis, 2018, 8(6): 5085-5144. |
| 11 | DUAN X G, SUN H Q, KANG J, et al. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons[J]. ACS Catalysis, 2015, 5(8): 4629-4636. |
| 12 | 陈一萍, 夏管商, 郑朝洪, 等. CNTs/PMS高级氧化体系去除水中的环丙沙星[J]. 化工进展, 2019, 38(4): 2037-2045. |
| CHEN Y P, XIA G S, ZHENG C H, et al. Degradation of ciprofloxacin by advanced oxidation process with carbon nanotubes/peroxymonosulfate CNTs/PMS[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 2037-2045. | |
| 13 | YUN E T, YOO H Y, BAE H, et al. Exploring the role of persulfate in the activation process: radical precursor versus electron acceptor[J]. Environmental Science and Technology, 2017, 51(17): 10090-10099. |
| 14 | CHENG X, GUO H G, ZHANG Y L, et al. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: activation performance and structure-function relationship[J]. Water Research, 2019, 157: 406-414. |
| 15 | ZHAO Q, MAO Q, ZHOU Y, et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: a review on heterogeneous catalysts and applications[J]. Chemosphere, 2017, 189: 224-238. |
| 16 | OH W D, DONG Z L, Q, LIM T T, et al. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects[J]. Applied Catalysis B: Environmental, 2016, 194: 169-201. |
| 17 | LI Y, LIU L D, LIU L, et al. Efficient oxidation of phenol by persulfate using manganite as a catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2016, 411, 264-271. |
| 18 | SUN B J, MA W J, WANG N, et al. Polyaniline: a new metal-free catalyst for peroxymonosulfate activation with highly efficient and durable removal of organic pollutants[J]. Environmental Science and Technology, 2019, 53: 9771-9780. |
| 19 | RESHETNYAK O V, KOVAL’CHUK E P, SKURSKI P, et al. The origin of luminescence accompanying electrochemical reduction or chemical decomposition of peroxydisulfates[J]. Journal of Luminescence, 2003, 105(1): 27-34. |
| 20 | HUANG B C, JIANG J, HUANG G X, et al. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate[J]. Journal of Materials Chemistry A, 2018, 6(19): 8978-8985. |
| 21 | WEI M Y, GAO L, LI J, et al. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation[J]. Journal of Hazardous Materials, 2016, 316: 60-68. |
| 22 | SUN P, LIU H, FENG M B, et al. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: singlet oxygen-dominated catalytic degradation of organic contaminants[J]. Applied Catalysis B: Environmental, 2019, 251: 335-345. |
| 23 | CHENG X, GUO H G, ZHANG Y L, et al. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 2017, 113: 80-88. |
| 24 | HOU J F, XU L X, HAN Y X, et al. Deactivation and regeneration of carbon nanotubes and nitrogen-doped carbon nanotubes in catalytic peroxymonosulfate activation for phenol degradation: variation of surface functionalities[J]. RSC Advances, 2019, 9(2): 974-983. |
| 25 | HU P D, SU H R, CHEN Z Y, et al. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation[J]. Environmental Science and Technology, 2017, 51(19): 11288-11296. |
| 26 | HAN C, DUAN X G, ZHANG M J, et al. Role of electronic properties in partition of radical and nonradical processes of carbocatalysis toward peroxymonosulfate activation[J]. Carbon, 2019, 153: 73-80. |
| 27 | LEE H, LEE H J, JEONG J, et al. Activation of persulfates by carbon nanotubes: oxidation of organic compounds by nonradical mechanism[J]. Chemical Engineering Journal, 2015, 266: 28-33. |
| 28 | TANG L, LIU Y N, WANG J J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: electron transfer mechanism[J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. |
| 29 | DUAN X G, O'DONNELL K, SUN H Q, et al. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions[J]. Small, 2015, 11(25): 3036-3044. |
| 30 | YUN E T, MOON G H, LEE H, et al. Oxidation of organic pollutants by peroxymonosulfate activated with low-temperature-modified nanodiamonds: understanding the reaction kinetics and mechanism[J]. Applied Catalysis B: Environmental, 2018, 237: 432-441. |
| 31 | WANG X B, TANG P, DING C, et al. Simultaneous enhancement of adsorption and peroxymonosulfate activation of nitrogen-doped reduced graphene oxide for bisphenol A removal[J]. Journal of Environmental Chemical Engineering, 2017, 5(5): 4291-4297. |
| 32 | WANG J, DUAN X G, DONG Q, et al. Facile synthesis of N-doped 3D graphene aerogel and its excellent performance in catalytic degradation of antibiotic contaminants in water[J]. Carbon, 2019, 144: 781-790. |
| 33 | LI D G, DUAN X G, SUN H Q, et al. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: the effects of precursors and annealing ambience on metal-free catalytic oxidation[J]. Carbon, 2017, 115: 649-658. |
| 34 | DUAN X G, AO Z M, LI D G, et al. Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation[J]. Carbon, 2016, 103: 404-411. |
| 35 | CHEN X, DUAN X G, OH W D, et al. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: maneuverable N-B bonding configurations and oxidation pathways[J]. Applied Catalysis B: Environmental, 2019, 253: 419-432. |
| 36 | PAN X X, CHEN J, WU N N, et al. Degradation of aqueous 2,4,4″-trihydroxybenzophenone by persulfate activated with nitrogen doped carbonaceous materials and the formation of dimer products[J]. Water Research, 2018, 143: 176-187. |
| 37 | SUN H Q, KWAN C K, SUVOROVA A, et al. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals[J]. Applied Catalysis B: Environmental, 2014, 154/155: 134-141. |
| [1] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [2] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [3] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [4] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [5] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [6] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [7] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [8] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [9] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [10] | 杨家添, 唐金铭, 梁恣荣, 黎胤宏, 胡华宇, 陈渊. 新型淀粉基高吸水树脂抑尘剂的制备及其应用[J]. 化工进展, 2023, 42(6): 3187-3196. |
| [11] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [12] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [13] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| [14] | 吕学东, 罗发亮, 林海涛, 宋丹青, 刘义, 牛瑞雪, 郑柳春. 聚丁二酸丁二醇酯的合成工艺及气体阻隔性最新进展[J]. 化工进展, 2023, 42(5): 2546-2554. |
| [15] | 陈韶云, 周贤太, 纪红兵. 金属卟啉/碳纳米管仿生催化剂的制备及其在Baeyer-Villiger氧化反应中的催化机理[J]. 化工进展, 2023, 42(3): 1332-1340. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||