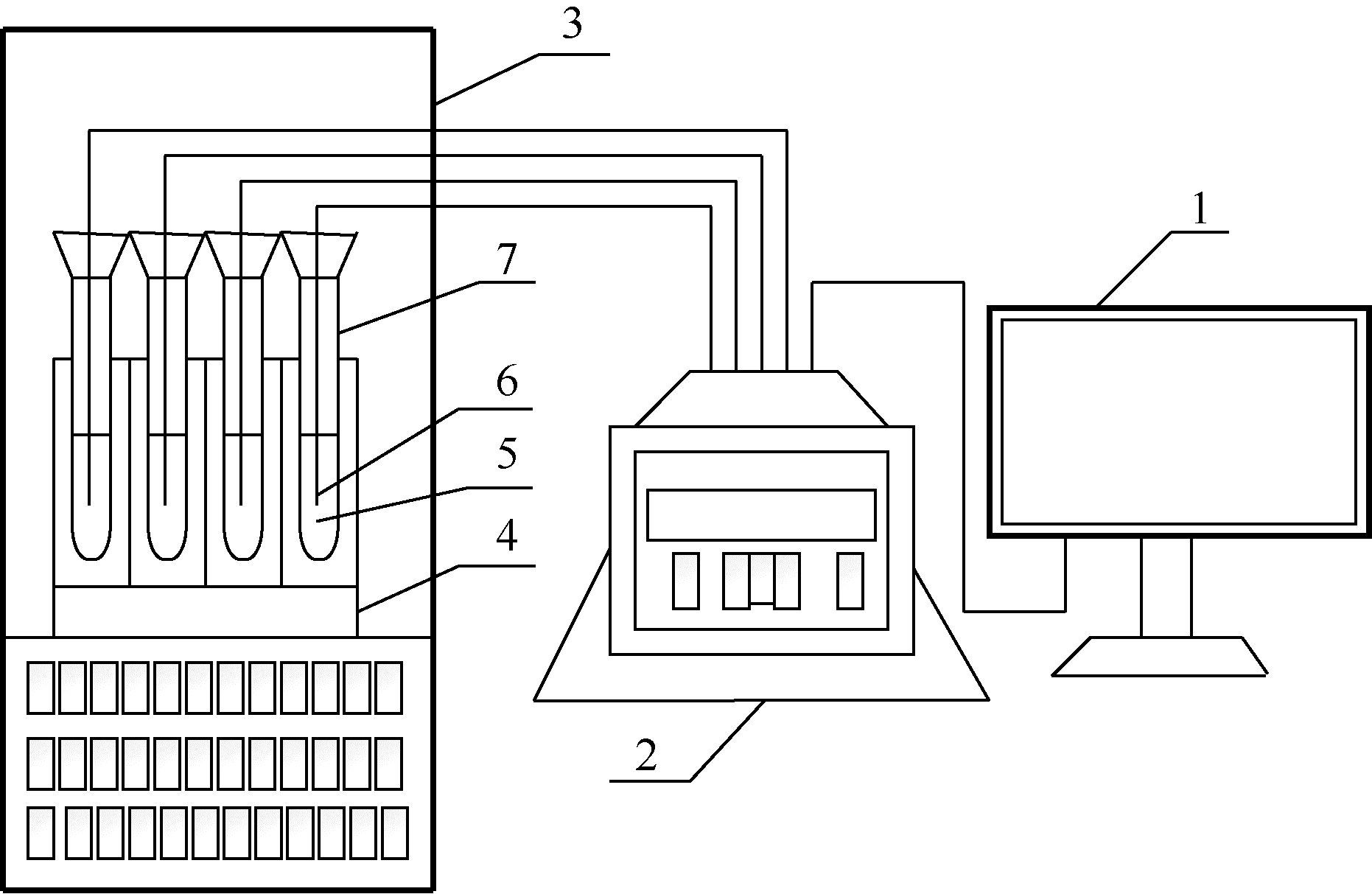
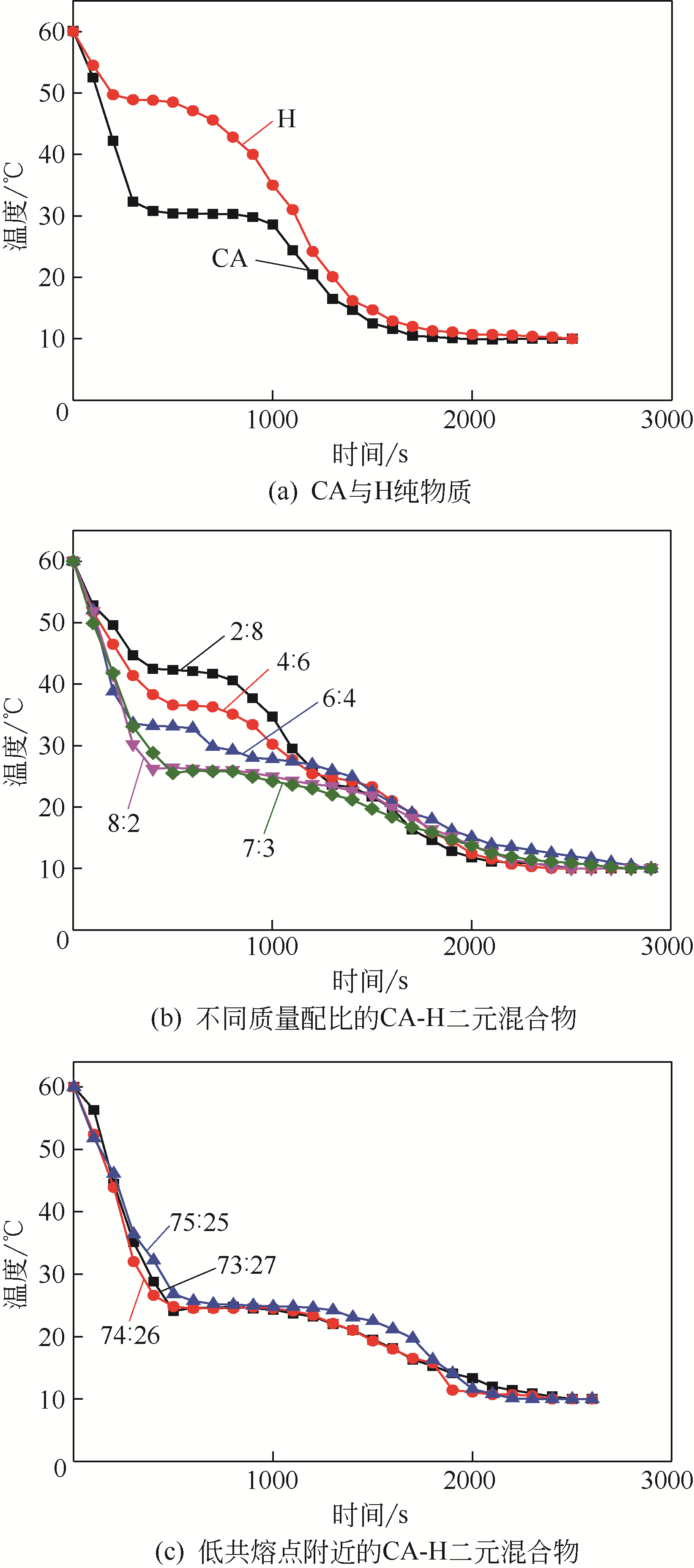
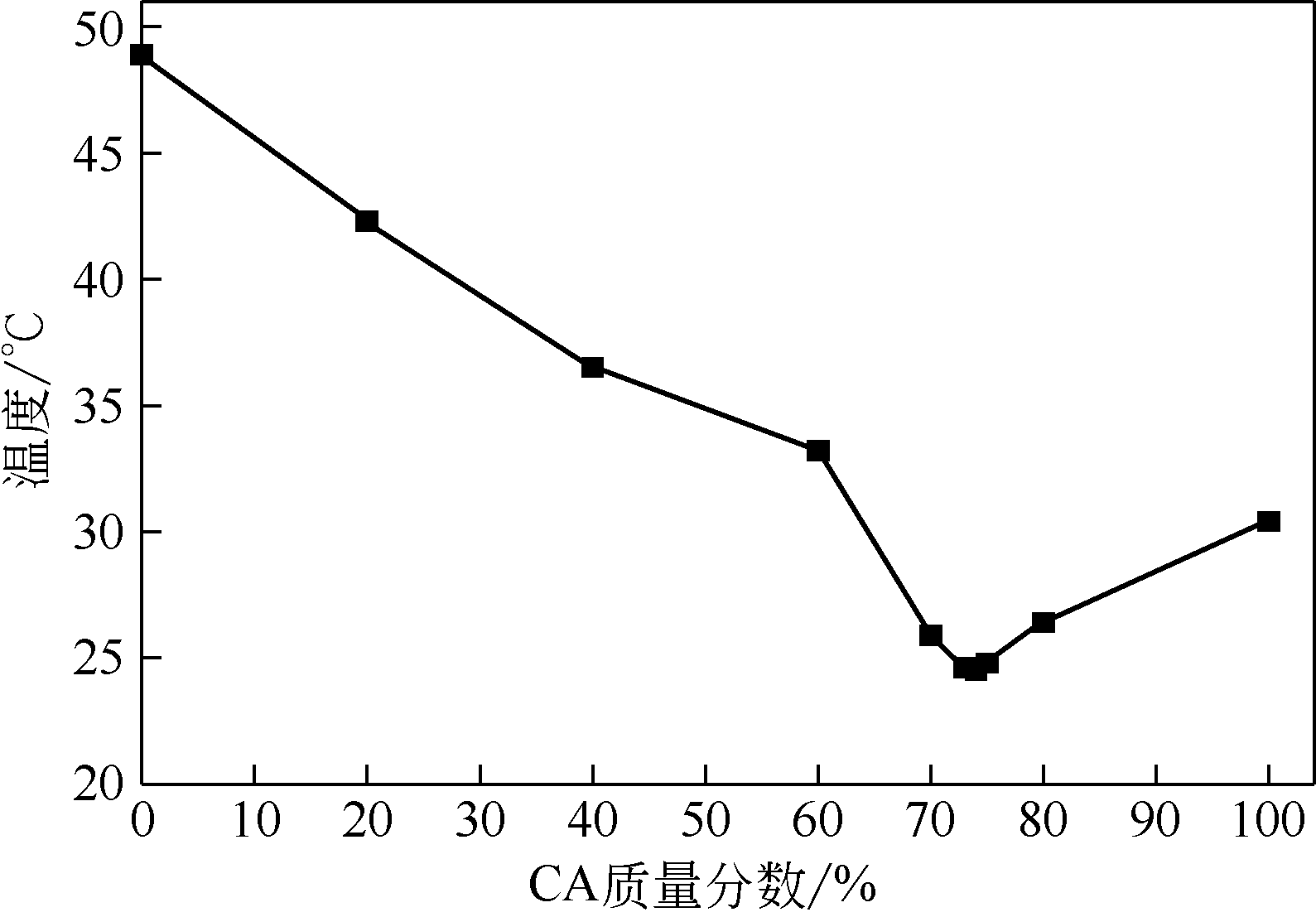
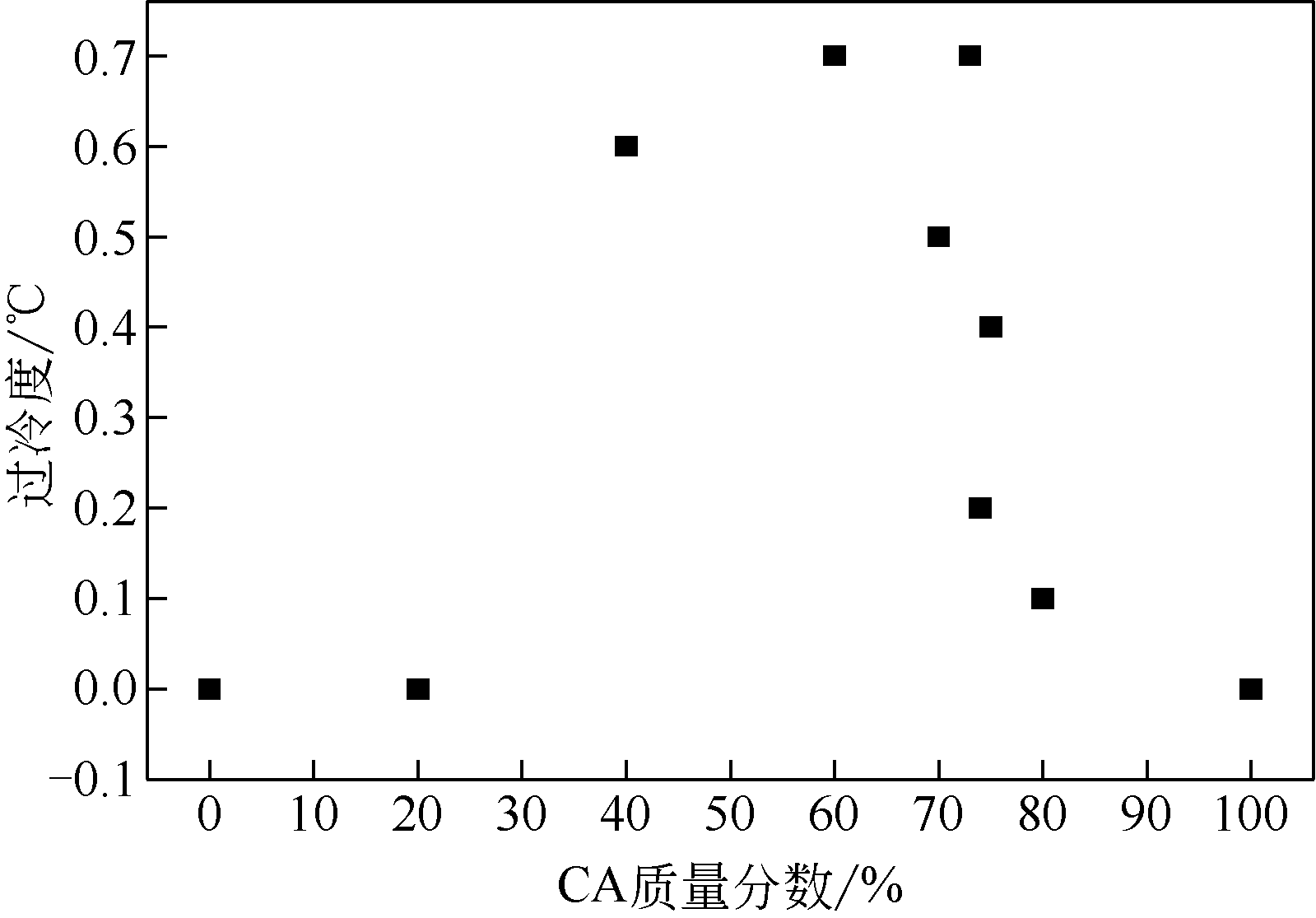
| 1 |
江亿. 我国建筑耗能状况及有效的节能途径[J]. 暖通空调,2005,35(5): 30-40.
|
|
JIANGYi. Current building energy consumption in China and effective energy efficiency measures[J]. HV&AC,2005,35(5): 30-40.
|
| 2 |
XUL Y, LIUJ J, PEIJ J, et al. Building energy saving potential in hot summer and cold winter (HSCW) zone, China-influence of building energy efficiency standards and implications[J]. Energy Policy, 2013, 57: 253-262.
|
| 3 |
谭羽非. 新型相变蓄能墙体的应用探讨[J]. 新型建筑材料, 2003 (2): 3-5.
|
|
TANYufei. Discussion on the application of new phase change energy storage wall[J]. New Building Materials, 2003 (2): 3-5.
|
| 4 |
林坤平, 张寅平, 江亿. 我国不同气候地区夏季相变墙房间热性能模拟和评价[J]. 太阳能学报, 2003, 24(1): 47-52.
|
|
LINKunping, ZHANGYinping, JIANGYi. Simulation and evaluation on the thermal performance of PCM wallboard rooms located in various climate regions of China in summer[J]. Acta Energiae Solaris Sinica, 2003, 24(1): 47-52.
|
| 5 |
清华大学建筑节能研究中心. 中国建筑节能年度发展研究报告[M]. 北京: 中国建筑工业出版社, 2016.
|
|
Tsinghua University Building Energy Conservation Research Center. Annual report on china building energy efficiency[M]. Beijing: China Architecture & Building Press, 2016.
|
| 6 |
TYAGIV V, BUDDHID. PCM thermal storage in buildings: a state of art[J]. Renewable and Sustainable Energy Reviews, 2007, 11(9): 1146-1166.
|
| 7 |
PASUPATHYA, VELRAJR, SEENIRAJR V. Phase change material-based building architecture for thermal management in residential and commercial establishments[J]. Renewable and Sustainable Energy Reviews, 2008, 12(1): 39-64.
|
| 8 |
孟多, 赵康, 王东旭. 二元脂肪酸/硅藻土助滤剂定形相变复合材料的制备及性能[J]. 复合材料学报, 2018, 35(9): 2258-2265.
|
|
MENGDuo, ZHAOKang, WANGDongxu. Preparation and properties of binary fatty acid mixture/diatomite filter aid form-stable phase change material[J]. Acta Materiae Compositae Sinica, 2018, 35(9): 2258-2265.
|
| 9 |
ZHANGZ L, YUANY P, ZHANGN, et al. Experimental investigation on thermophysical properties of capric acidelauric acid phase change slurries for thermal storage system[J]. Energy, 2015, 90: 359-368.
|
| 10 |
袁亚光, 袁艳平, 张楠, 等. 月桂酸-棕榈酸-硬脂酸/膨胀石墨复合相变材料的制备及性能[J]. 化工学报, 2014, 65(s2): 286-292.
|
|
YUANYaguang, YUANYanping, ZHANGNan, et al. Preparation and properties of lauric-palmitic-stearic acid eutectic mixture/expanded graphite composite phase change material for energy storage[J]. CIESC Journal, 2014, 65(s2): 286-292.
|
| 11 |
SARIA, KAYGUSUZK. Some fatty acids used for latent heat storage: thermal stability and corrosion of metals with respect to thermal cycling [J]. Renewable Energy, 2003, 28(6): 939-948.
|
| 12 |
ROZANNAD, CHUAHT G, SALMIAHA, et al. Fatty acids as phase change materials (PCMs) for thermal energy storage: a review[J]. International Journal of Green Energy, 2005, 1(4): 495-513.
|
| 13 |
黄雪, 崔英德, 张步宁, 等. 脂肪酸类相变材料传热及液相渗漏的研究进展[J]. 化工进展, 2014, 33(10): 2676-2680.
|
|
HUANGXue, CUIYingde, ZHANGBuning, et al. Review on heat transfer and liquid phase leakage of fatty acids phase change materials[J]. Chemical Industry and Engineering Progress, 2014, 33(10): 2676-2680.
|
| 14 |
黄艳, 章学来. 十二醇-癸酸-纳米粒子复合相变材料传热性能[J]. 化工学报, 2016, 67(6): 2271-2276.
|
|
HUANGYan, ZHANGXuelai. Heat transfer property of lauryl alcohol-capric acid-nanoparticle composite phase change materials[J]. CIESC Journal, 2016, 67(6): 2271-2276.
|
| 15 |
樊铁林, 陈蜜蜜, 檀星, 等. 脂肪酸/废加气块定形相变储能集料制备及性能[J]. 化工进展, 2017, 36(3): 996-1002.
|
|
FANTielin, CHENMimi, TANXing, et al. Preparation and properties of shape-stabilized phase change aggregate from fatty acid and waste autoclaved aerated concrete[J]. Chemical Industry and Engineering Progress, 2017, 36(3): 996-1002.
|
| 16 |
袁艳平, 白力, 牛犇. 癸酸与棕榈酸低共熔混合物的制备与热特性分析[J]. 材料导报, 2010, 24(4): 113-115.
|
|
YUANYanping, BAILi, NIUBen. Preparation and thermal analysis on eutectic mixture of capric acid and palmitic acid[J]. Materials Review, 2010, 24(4): 113-115.
|
| 17 |
李玉洋, 章学来, 徐笑锋, 等. 正辛酸-肉豆蔻酸低温相变材料的制备和循环性能[J]. 化工进展, 2018, 37(2): 689-693.
|
|
LIYuyang, ZHANGXuelai, XUXiaofeng, et al. Preparation and cyclic properties of low temperature phase change materials of n-caprylic acid and myristic acid[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 689-693.
|
| 18 |
蔡伟, 孙志高, 马鸿凯, 等. 有机复合相变材料性能研究[J]. 化工新型材料, 2015, 43(8): 87-89.
|
|
CAIWei, SUNZhigao, MAHongkai, et al. Research on property of organic composite phase change materials[J]. New Chemical Materials, 2015, 43(8): 87-89.
|
| 19 |
ZUOJ G, LIW Z, WENGL D. Thermal properties of lauric acid/1-tetradecanol binary system for energy storage[J]. Applied Thermal Engineering, 2011, 31(6): 1352-1355.
|
| 20 |
蔡伟, 孙志高, 马鸿凯, 等. 月桂酸-十四醇二元复合相变材料的相变特性[J]. 太阳能学报, 2017, 38(9): 2493-2497.
|
|
CAIWei, SUNZhigao, MAHongkai, et al. Phase transition properties of LA-TD binary composite phase change materials[J].Acta Energiae Solaris Sinica, 2017, 38(9): 2493-2497.
|
| 21 |
HUANGJ, LUS, KONGX, et al. Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: preparation and performance analysis[J]. Materials, 2013, 6(10): 4758-4775.
|
| 22 |
ZUOJ G, LIW Z, WENGL D. Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change material (PCM)[J]. Energy & Buildings, 2011, 43(1): 207-210.
|
 ),Hua FEI(
),Hua FEI( ),Linya WANG,Min FANG,Dahua JIANG,Yunchao ZHAO
),Linya WANG,Min FANG,Dahua JIANG,Yunchao ZHAO