化工进展 ›› 2022, Vol. 41 ›› Issue (2): 1025-1035.DOI: 10.16085/j.issn.1000-6613.2021-0545
复配醇胺溶液对CO2的吸收解吸性能及其降解性能
李红1( ), 吉轲1, 齐天勤机1, 李晓静1, 万慧慧1, 张永春1, 陈绍云1,2(
), 吉轲1, 齐天勤机1, 李晓静1, 万慧慧1, 张永春1, 陈绍云1,2( )
)
- 1.大连理工大学化工学院精细化工国家重点实验室,辽宁 大连 116024
2.大连理工大学洛阳研究院,河南 洛阳 471000
-
收稿日期:2021-03-17修回日期:2021-05-31出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:陈绍云 -
作者简介:李红(1997—),女,硕士研究生,研究方向为二氧化碳捕集。E-mail:lh970715@hotmail.com 。 -
基金资助:国家重点研发计划(2017YFB0603301)
Properties of CO2 absorption-desorption based on alcohol amines solutions and their degradation
LI Hong1( ), JI Ke1, Tianqinji QI1, LI Xiaojing1, WAN Huihui1, ZHANG Yongchun1, CHEN Shaoyun1,2(
), JI Ke1, Tianqinji QI1, LI Xiaojing1, WAN Huihui1, ZHANG Yongchun1, CHEN Shaoyun1,2( )
)
- 1.State Key Laboratory of Fine Chemistry, School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China
2.Luoyang Research Institute of Dalian University of Technology, Luoyang 471000, Henan, China
-
Received:2021-03-17Revised:2021-05-31Online:2022-02-05Published:2022-02-23 -
Contact:CHEN Shaoyun
摘要:
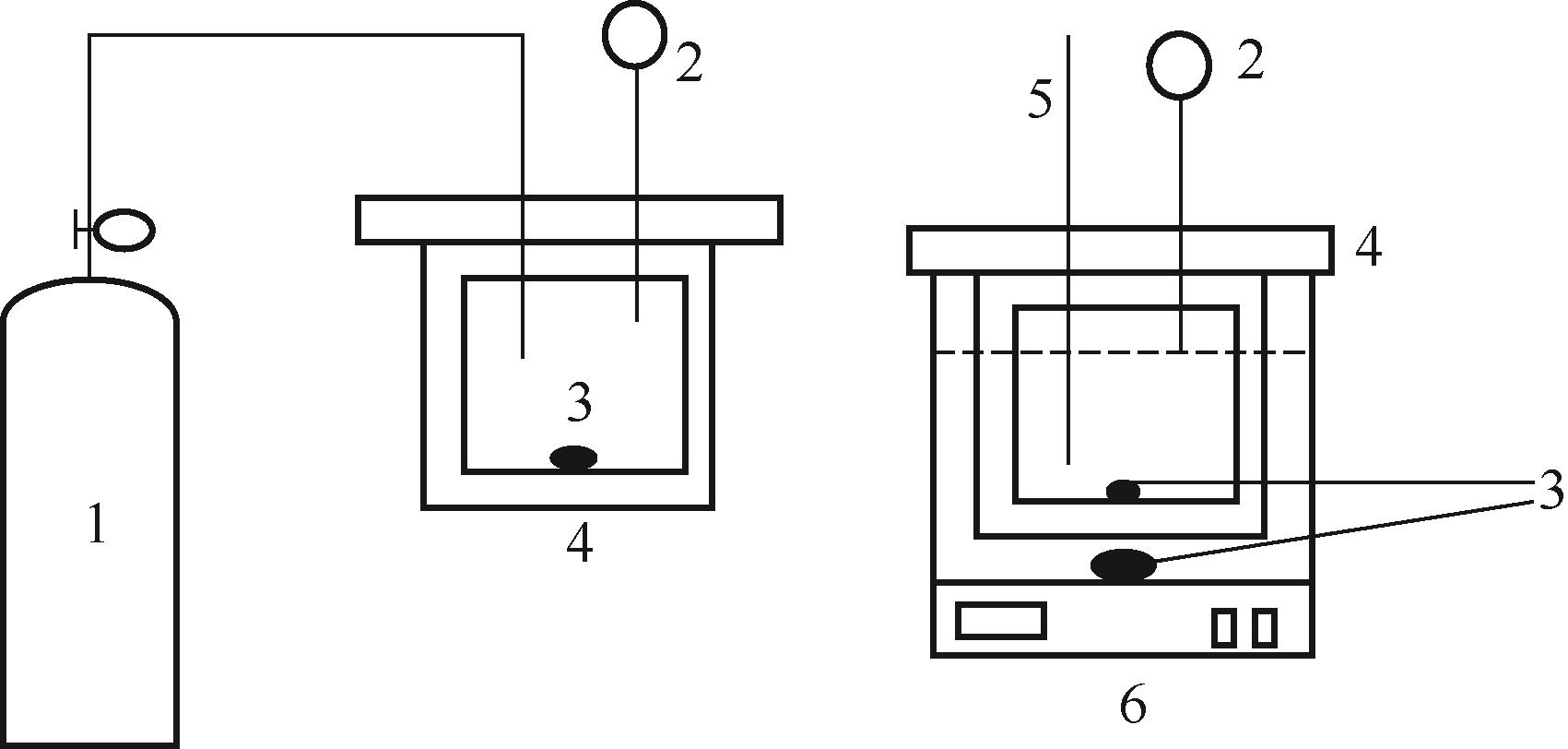
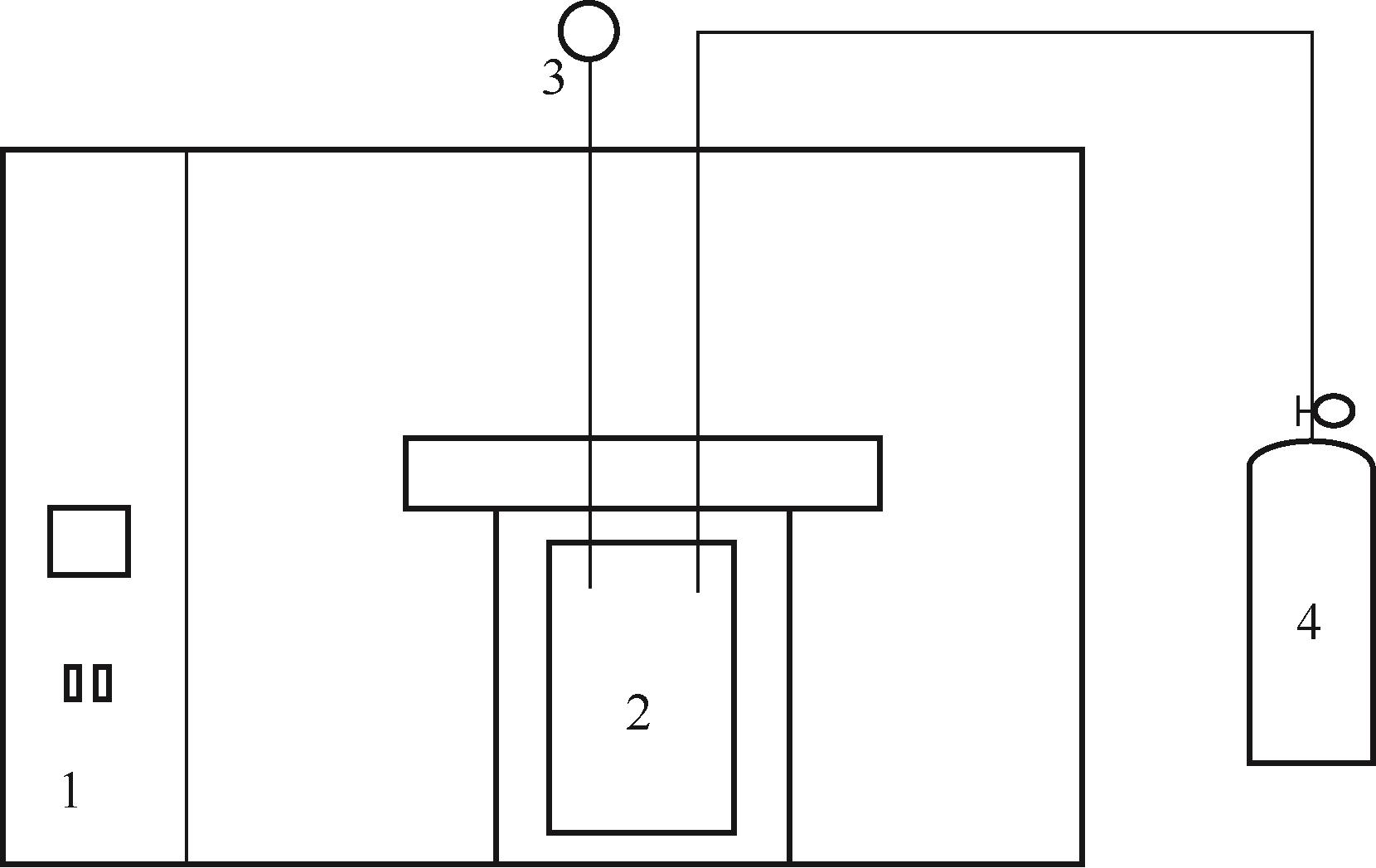
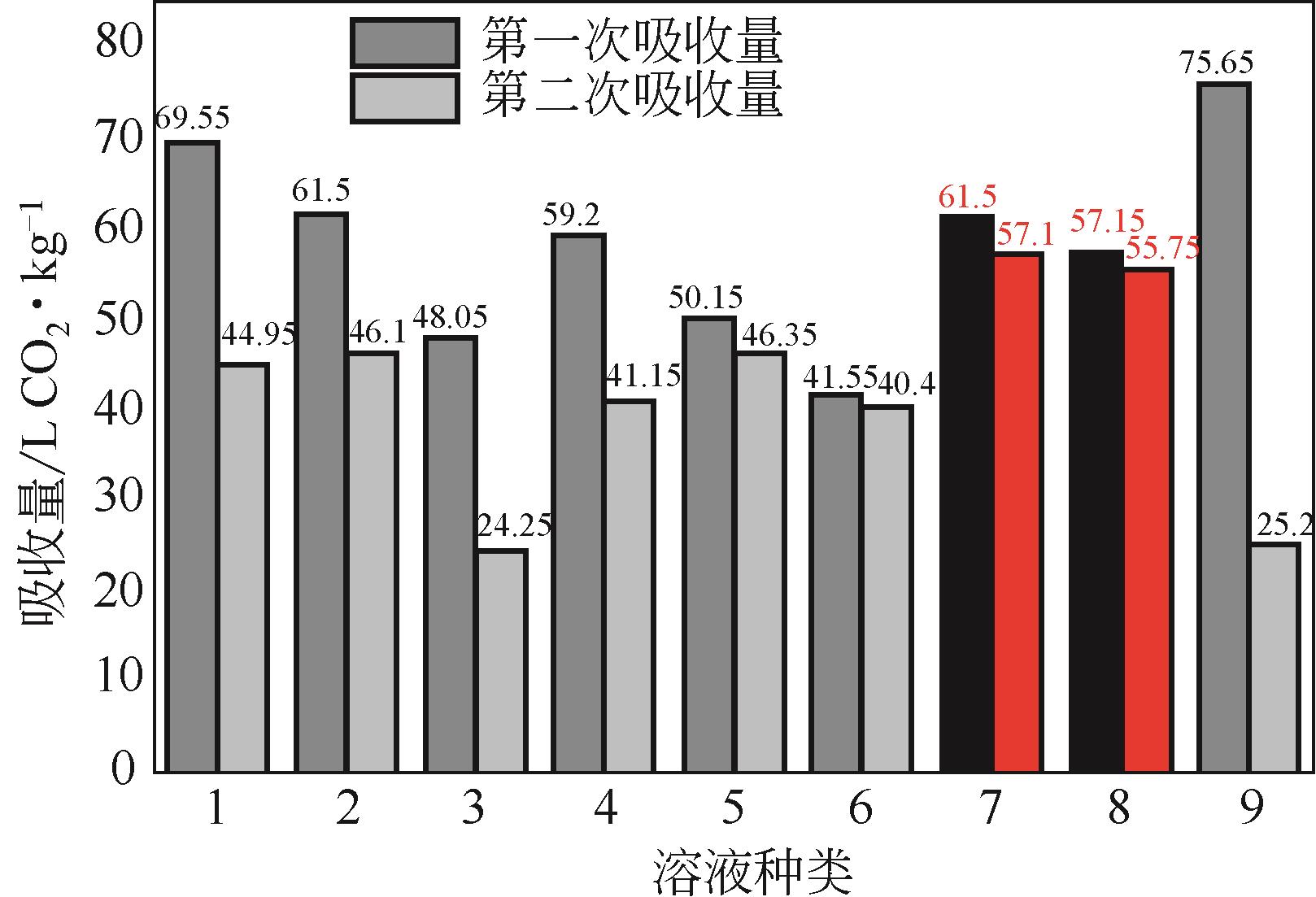
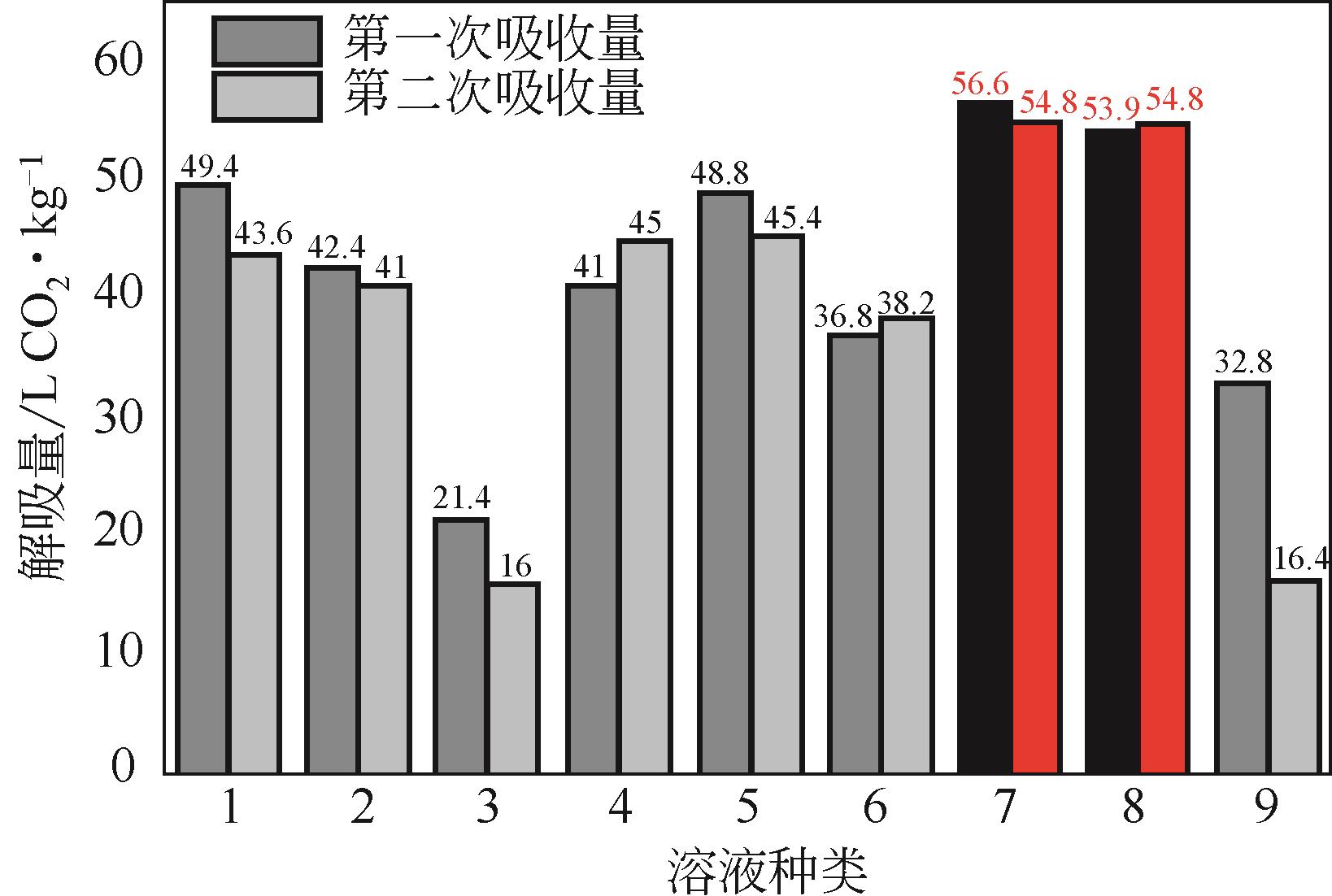
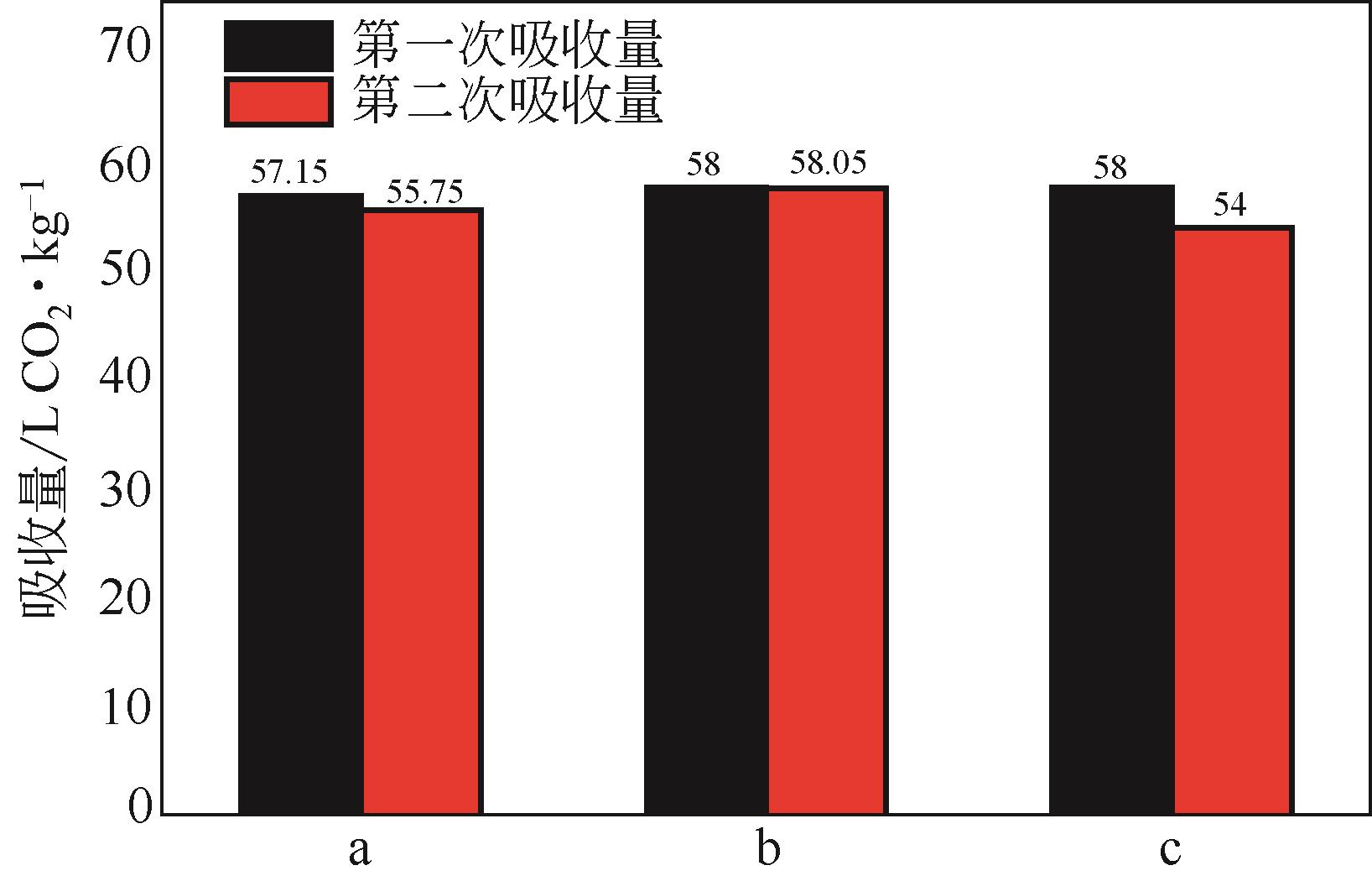
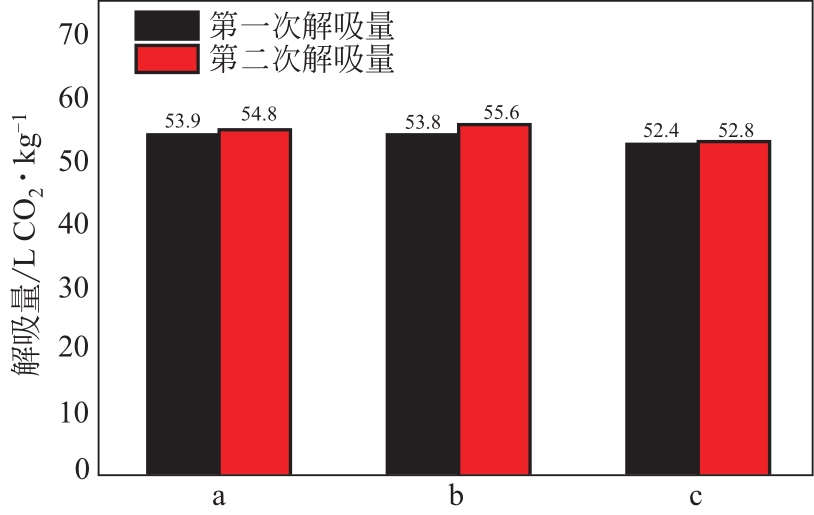
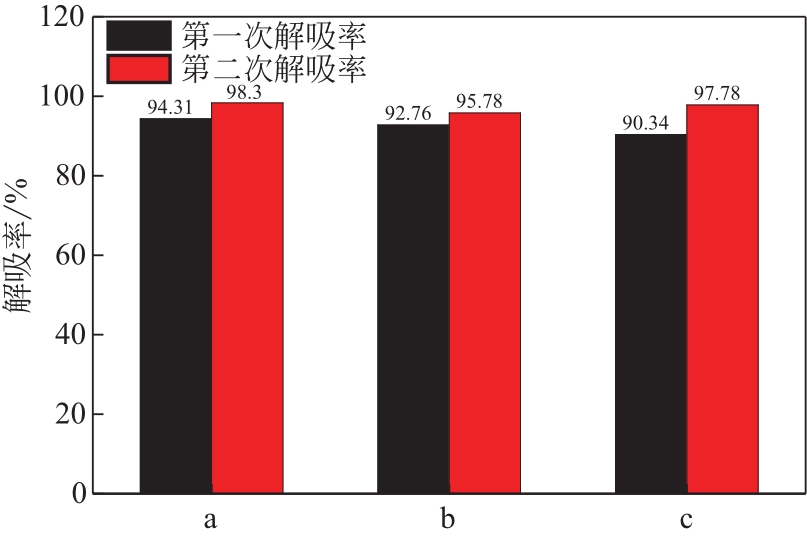
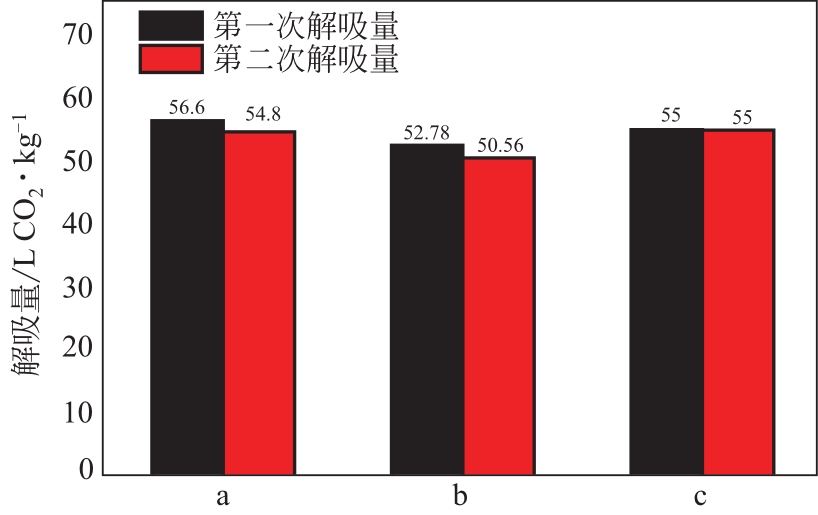
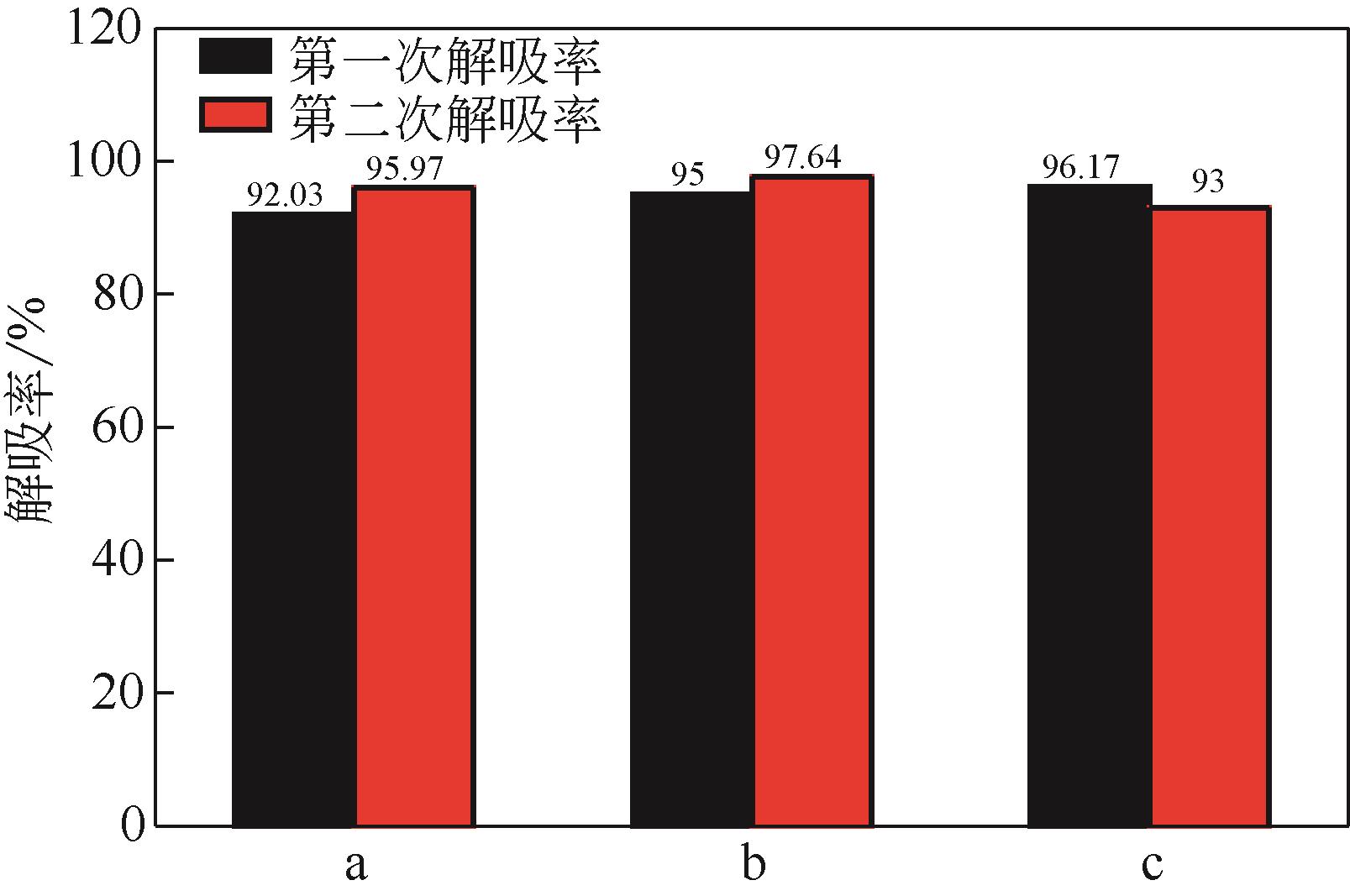
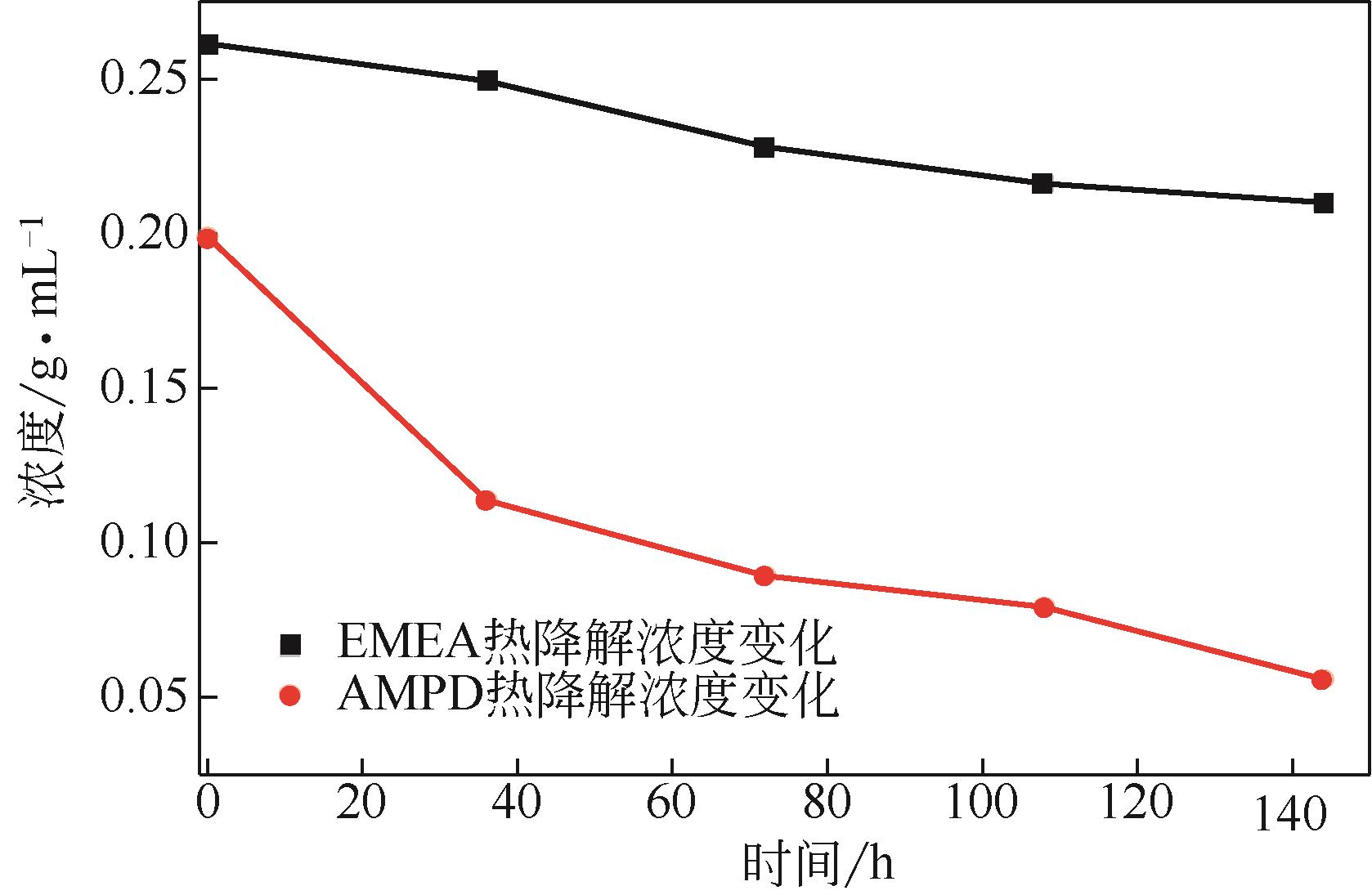
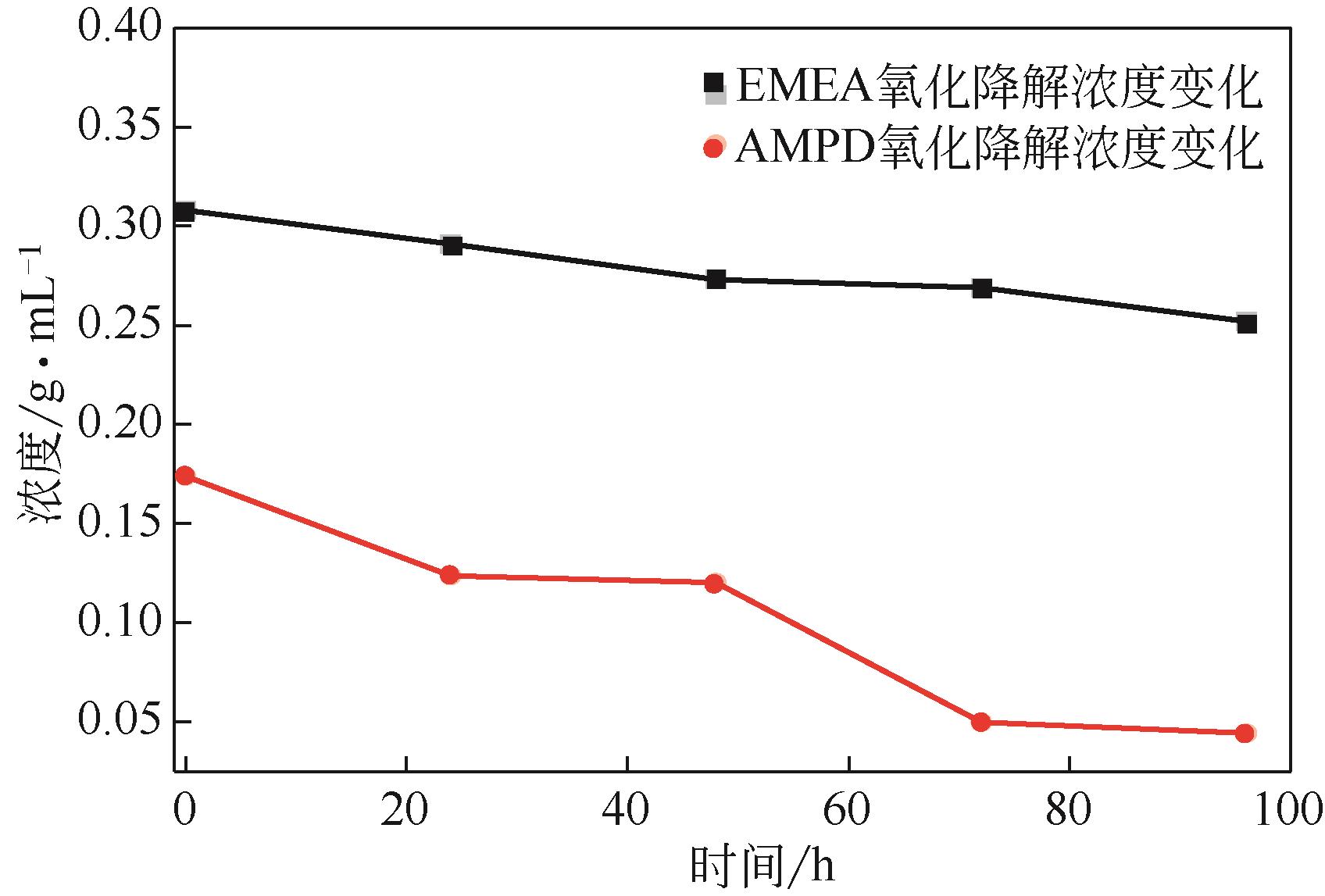
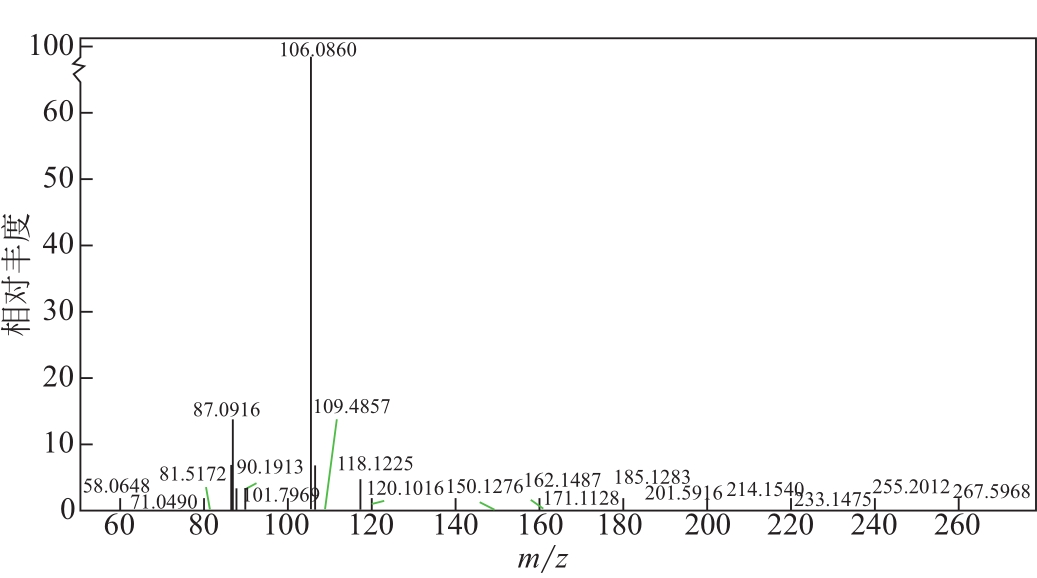
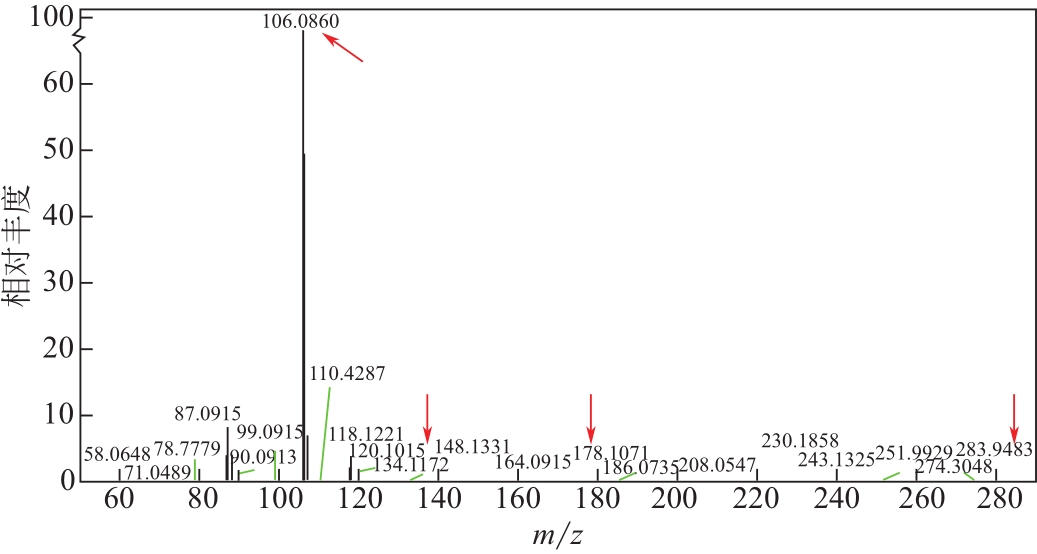
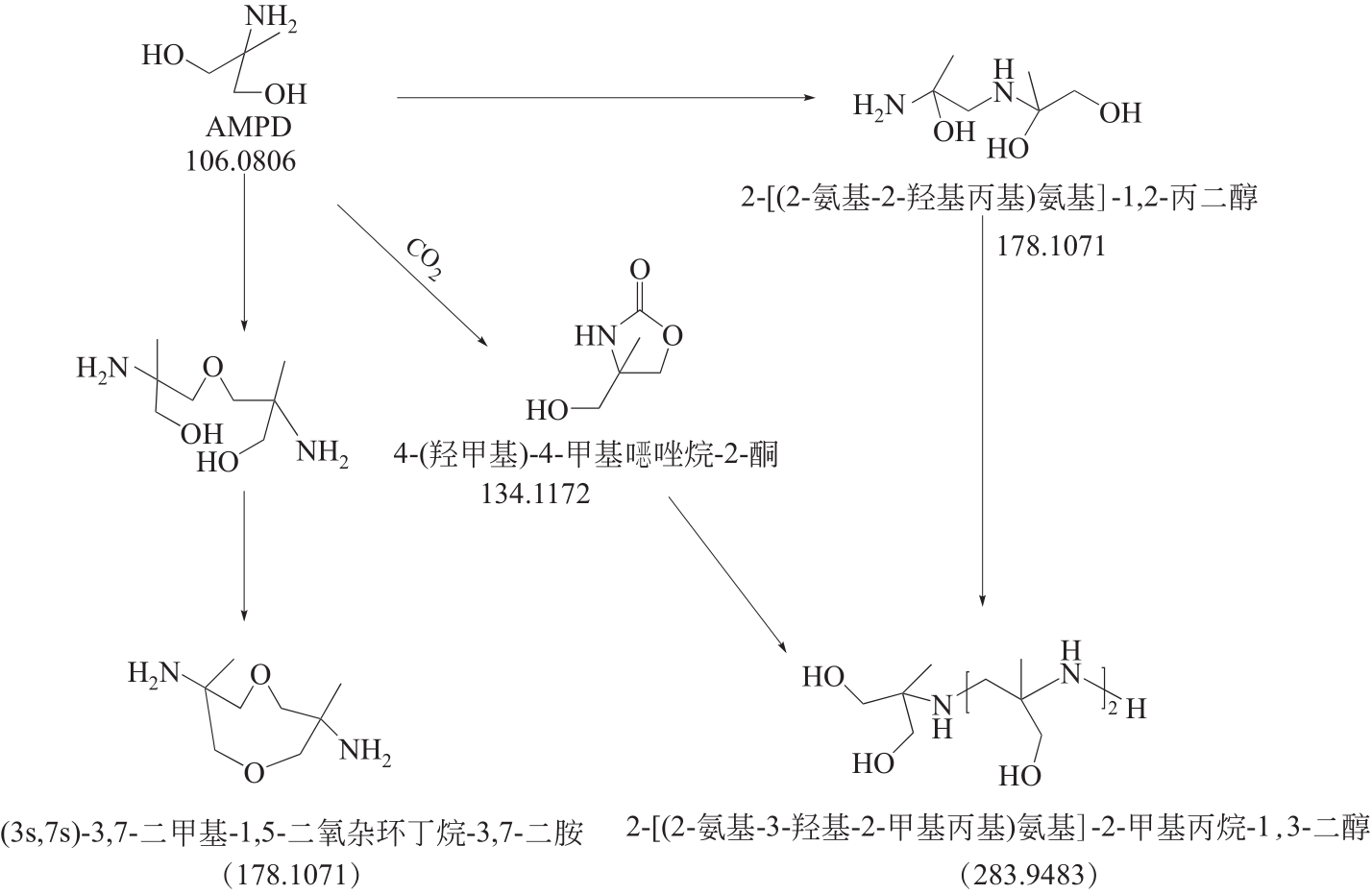
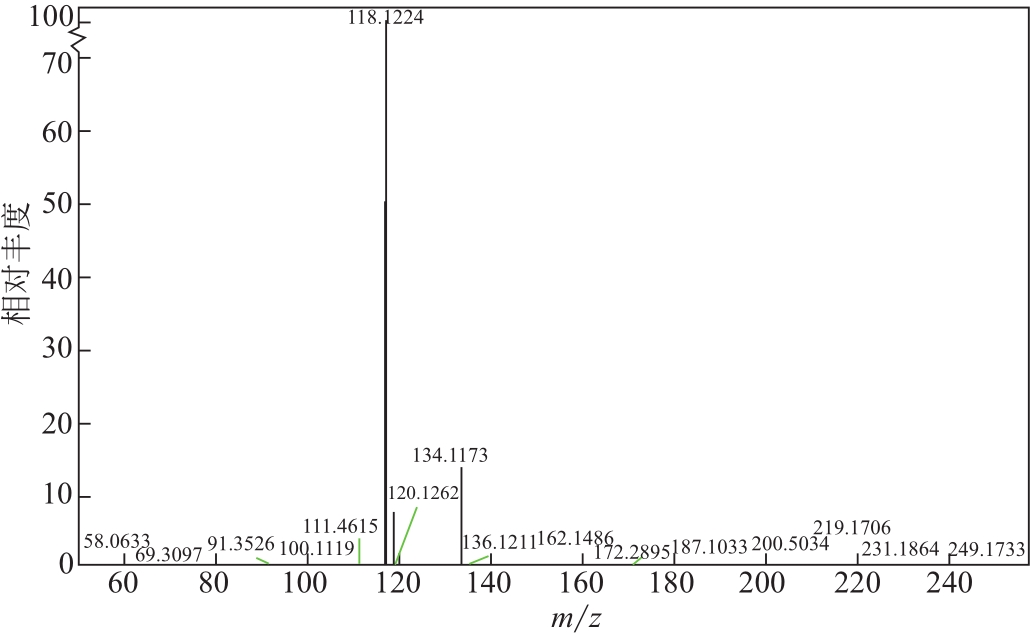
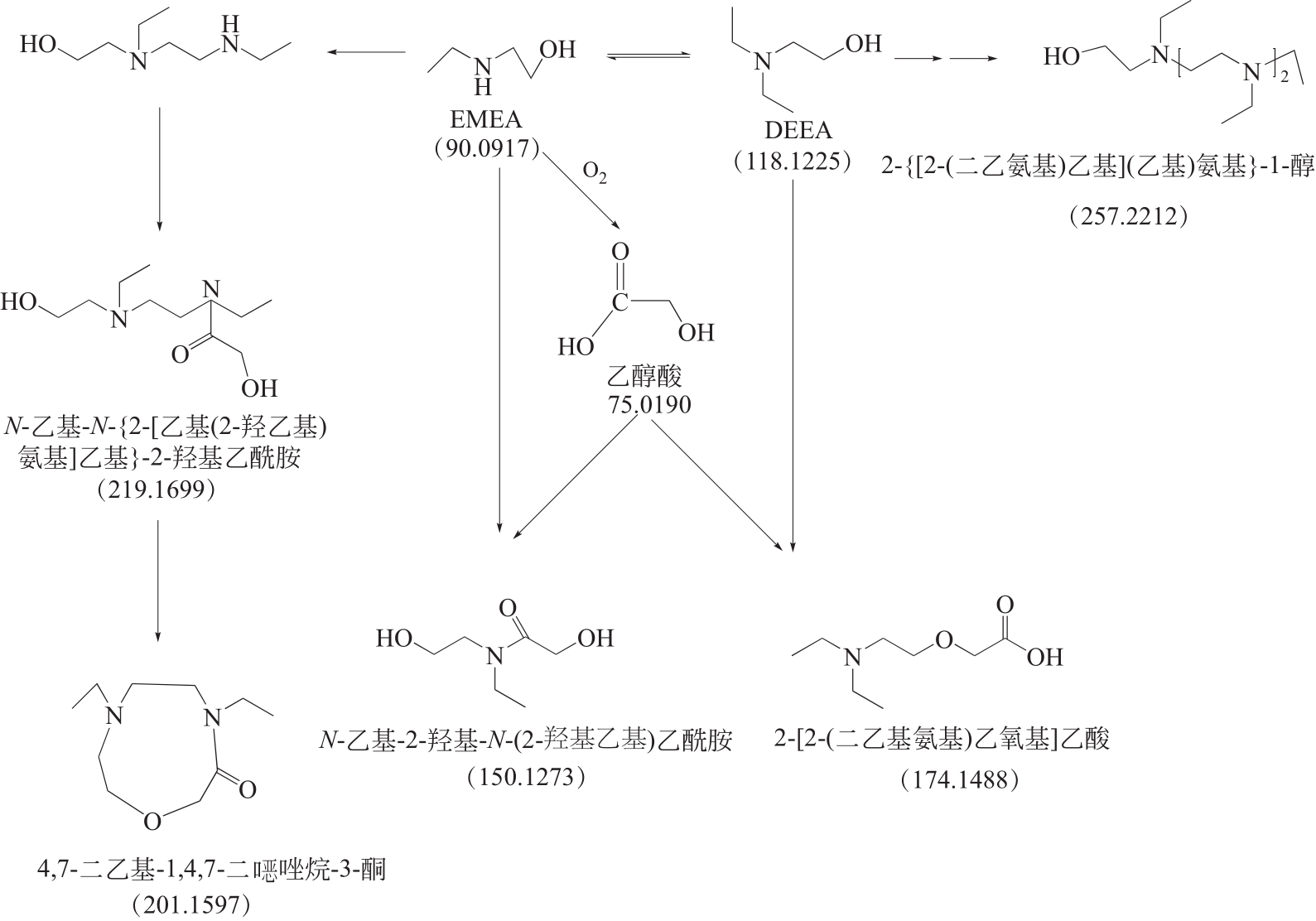
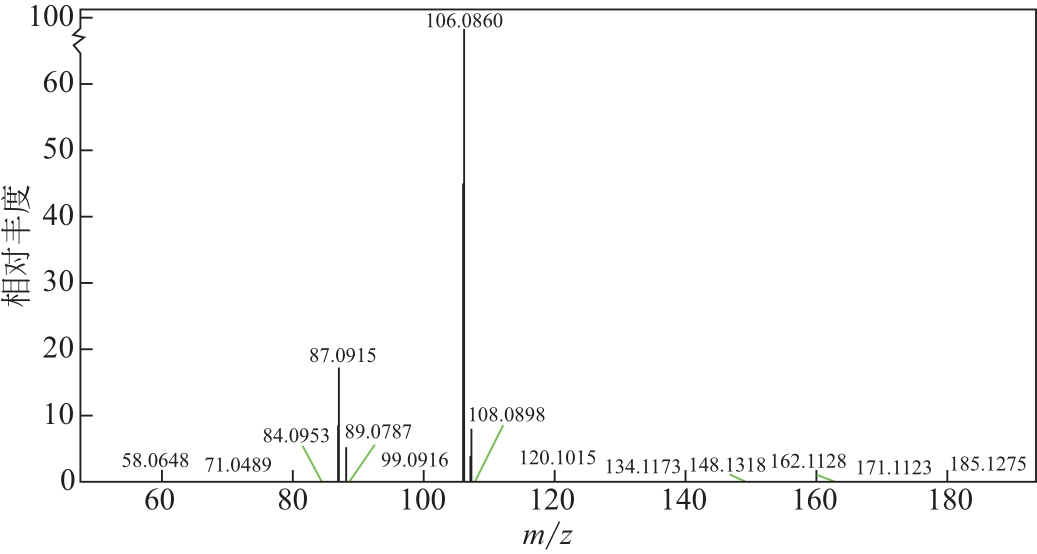
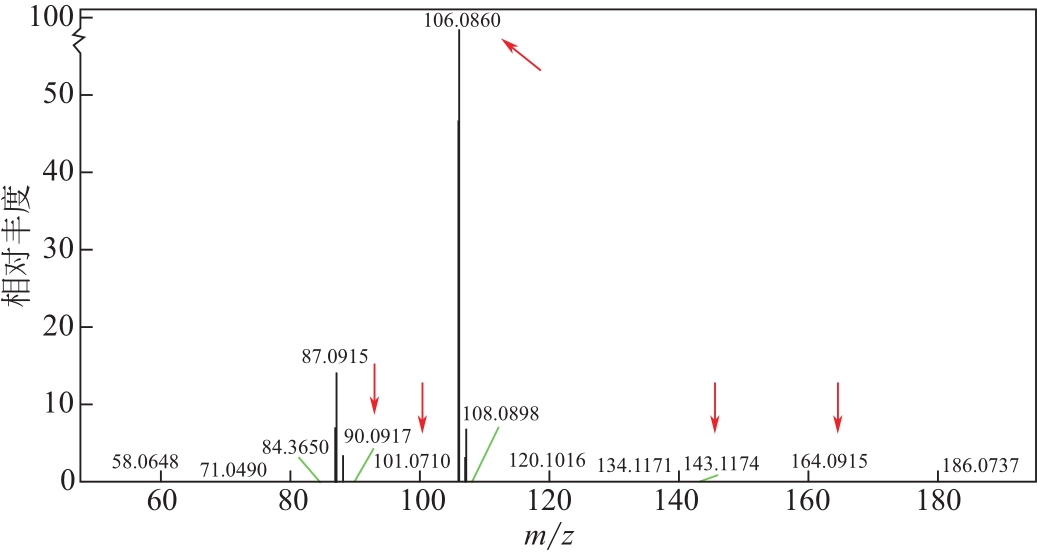
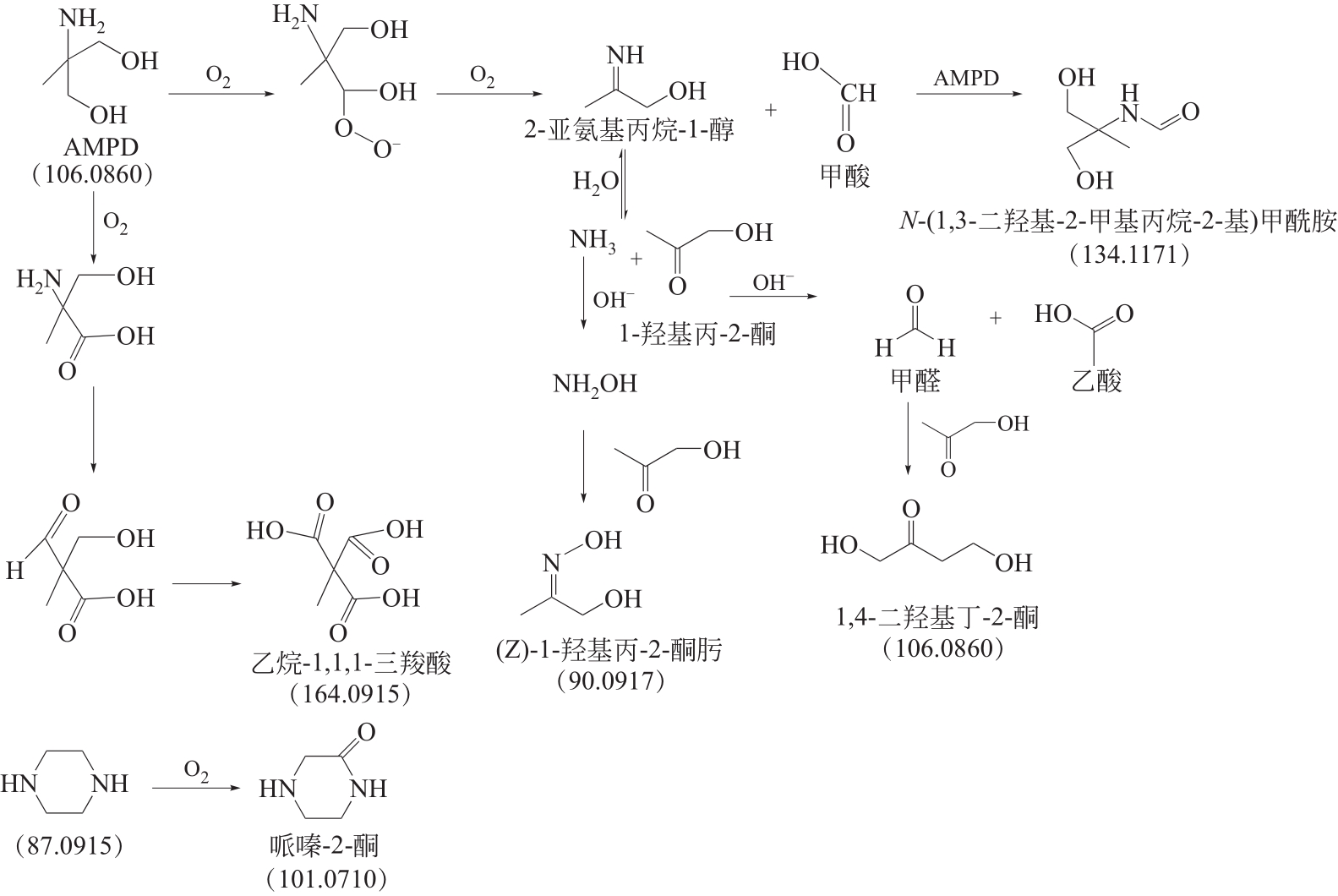
比较了9种不同配方醇胺溶液对CO2的吸收解吸性能。其中,N-乙基乙醇胺(EMEA)+二乙氨基乙醇(DEEA)+哌嗪(PZ)和2-氨基-2-甲基-1,3丙二醇(AMPD)+哌嗪(PZ)+水(H2O)两种溶液的吸收解吸的稳定性最佳,平均解吸率高于94.00%。本文对这两种溶液进行了144h热降解和96h氧化降解实验,结果表明热降解后的EMEA+DEEA+PZ溶液的吸收解吸性能稳定性较高,每千克溶液对CO2的平均吸收量、平均解吸量略有升高,分别升高1.58L、0.35L,平均解吸率下降2.04%;而氧化降解造成每千克EMEA+DEEA+PZ溶液对CO2的平均吸收量、平均解吸量分别下降0.45L、1.75L,平均解吸率下降2.25%;热降解对于AMPD+PZ+H2O溶液吸收解吸性能影响较大,造成每千克该溶液对CO2的平均吸收量、平均解吸量分别下降5.63L、4.03L,平均解吸率升高2.32%;在经过氧化降解之后,每千克AMPD+PZ+H2O溶液对CO2的平均吸收量、平均解吸量分别下降1.05L、0.70L,平均解吸率升高0.59%。液质联用分析结果表明,热降解导致EMEA浓度减小了19.67%,AMPD浓度减小了71.89%;氧化降解导致EMEA浓度减小了18.18%,AMPD浓度减小了74.53%。由此说明,EMEA+DEEA+PZ比AMPD+PZ+H2O具有更佳的抗降解性能。同时,根据电喷雾质谱结果对两种溶液的热降解、氧化降解的机理进行推测,热降解中生成恶唑烷酮类物质,氧化降解则是生成酸类物质。其中,氧化降解对两种溶液都造成了负面影响,而热降解则不然。
中图分类号:
引用本文
李红, 吉轲, 齐天勤机, 李晓静, 万慧慧, 张永春, 陈绍云. 复配醇胺溶液对CO2的吸收解吸性能及其降解性能[J]. 化工进展, 2022, 41(2): 1025-1035.
LI Hong, JI Ke, Tianqinji QI, LI Xiaojing, WAN Huihui, ZHANG Yongchun, CHEN Shaoyun. Properties of CO2 absorption-desorption based on alcohol amines solutions and their degradation[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1025-1035.
| 序号 | 复配溶液 | 质量分数/% |
|---|---|---|
| 1 | AEEA+H2O | 30+70 |
| 2 | AEEA+MEA+DEEA+H2O | 7.5+15+7.5+70 |
| 3 | AEEA+GI+H2O | 30+50+20 |
| 4 | AEEA+SUL+PZ① | 30+60+10 |
| 5 | AMP+DEA+MDEA+H2O | 10+10+10+70 |
| 6 | AMP+DMSO+AEEA② | 27+63+10 |
| 7 | AMPD+PZ+H2O | 20+10+70 |
| 8 | EMEA+DEEA+PZ③ | 27+63+10 |
| 9 | MDEA+MEA+PZ④ | 30+50+20 |
表3 复配溶液的配方
| 序号 | 复配溶液 | 质量分数/% |
|---|---|---|
| 1 | AEEA+H2O | 30+70 |
| 2 | AEEA+MEA+DEEA+H2O | 7.5+15+7.5+70 |
| 3 | AEEA+GI+H2O | 30+50+20 |
| 4 | AEEA+SUL+PZ① | 30+60+10 |
| 5 | AMP+DEA+MDEA+H2O | 10+10+10+70 |
| 6 | AMP+DMSO+AEEA② | 27+63+10 |
| 7 | AMPD+PZ+H2O | 20+10+70 |
| 8 | EMEA+DEEA+PZ③ | 27+63+10 |
| 9 | MDEA+MEA+PZ④ | 30+50+20 |
| 1 | FAN Jingli, XU Mao, LI Fengyu, et al. Carbon capture and storage (CCS) retrofit potential of coal-fired power plants in China: the technology lock-in and cost optimization perspective[J]. Applied Energy, 2018, 229: 326-334. |
| 2 | DESIDERI U. Advanced absorption processes and technology for carbon dioxide (CO2) capture in power plants[M]//Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology. Amsterdam: Elsevier, 2010: 155-182. |
| 3 | 李函珂, 党成雄, 杨光星, 等. 面向二氧化碳捕集的过程强化技术进展[J]. 化工进展, 2020, 39(12): 4919-4939. |
| LI Hanke, DANG Chengxiong, YANG Guangxing, et al. Process intensification techniques towards carbon dioxide capture: a review[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 4919-4939. | |
| 4 | 吴涛, 桑圣欢, 祁亚军, 等. 水泥厂碳捕集工艺技术[J]. 水泥技术, 2020(4): 90-95. |
| WU Tao, SANG Shenghuan, QI Yajun, et al. Carbon capture technology in cement plant[J]. Cement Technology, 2020(4): 90-95. | |
| 5 | 邬高翔, 田瑞. 二氧化碳捕集技术研究进展[J]. 云南化工, 2020, 47(4): 22-23. |
| WU Gaoxiang, TIAN Rui. Research progress of carbon dioxide capture technology[J]. Yunnan Chemical Technology, 2020, 47(4): 22-23. | |
| 6 | 王建行, 赵颖颖, 李佳慧, 等. 二氧化碳的捕集、固定与利用的研究进展[J]. 无机盐工业, 2020, 52(4): 12-17. |
| WANG Jianhang, ZHAO Yingying, LI Jiahui, et al. Research progress of carbon dioxide capture, fixation and utilization[J]. Inorganic Chemicals Industry, 2020, 52(4): 12-17. | |
| 7 | 周旭健, 李清毅, 陈瑶姬, 等. 化学吸收法在燃后区CO2捕集分离中的研究和应用[J]. 能源工程, 2019(3): 58-66. |
| ZHOU Xujian, LI Qingyi, CHEN Yaoji, et al. Chemical solvents for post-combustion CO2 capture: a review[J]. Energy Engineering, 2019(3): 58-66. | |
| 8 | 陈鸿伟, 张泽, 孙玮, 等. 介孔材料CO2吸附性能的研究进展[J]. 材料导报, 2016, 30(5): 63-68. |
| CHEN Hongwei, ZHANG Ze, SUN Wei, et al. Review on CO2 adsorption performance of mesoporous materials[J]. Materials Review, 2016, 30(5): 63-68. | |
| 9 | 刘丽影, 宫赫, 王哲, 等. 捕集高湿烟气中CO2的变压吸附技术[J]. 化学进展, 2018, 30(6): 872-878. |
| LIU Liying, GONG He, WANG Zhe, et al. Application of pressure swing adsorption technology to capture CO2 in highly humid flue gas[J]. Progress in Chemistry, 2018, 30(6): 872-878. | |
| 10 | 时飞, 李奕帆. 混合基质膜在碳捕集领域的研究进展[J]. 化工进展, 2020, 39(6): 2453-2462. |
| SHI Fei, LI Yifan. Advances of mixed matrix membrane for CO2 capture[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2453-2462. | |
| 11 | 华东阳, 李睿, 马梦桐, 等. 基于膜分离法的沼气脱CO2和H2S工艺研究[J]. 中国沼气, 2020, 38(4): 34-38. |
| HUA Dongyang, LI Rui, MA Mengtong, et al. Parameter optimization of CO2 and H2S removal from biogas based on membrane separation[J]. China Biogas, 2020, 38(4): 34-38. | |
| 12 | 伍勇东, 赵丹, 任吉中, 等. Pebax/TPP共混膜的制备及CO2分离性能研究[J]. 膜科学与技术, 2020, 40(1): 37-44. |
| WU Yongdong, ZHAO Dan, REN Jizhong, et al. CO2 separation property of Pebax/TPP blend membranes[J]. Membrane Science and Technology, 2020, 40(1): 37-44. | |
| 13 | 李鑫, 王永洪, 张新儒, 等. 分子量和NaY添加量对炭分子筛膜CO2分离性能的影响[J]. 现代化工, 2020, 40(S1): 159-165. |
| LI Xin, WANG Yonghong, ZHANG Xinru, et al. Effects of molecular weight and NaY addition on separation performance of carbon molecular sieve membrane for CO2[J]. Modern Chemical Industry, 2020, 40(S1): 159-165. | |
| 14 | 喻忠厚. 日本用超临界二氧化碳提纯法分离天然成分成功[N]. 化学工业日报, 1985-09-21. |
| YU Zhonghou. Japan succeeds in separating natural ingredients by supercritical carbon dioxide purification[N]. Japan Chemical Daily, 1985-09-21. | |
| 15 | SHARIF M, ZHANG T T, WU X M, et al. Evaluation of CO2 absorption performance by molecular dynamic simulation for mixed secondary and tertiary amines[J]. International Journal of Greenhouse Gas Control, 2020, 97: 103059. |
| 16 | 唐婧. 海螺集团在高质量发展道路上笃定前行[J]. 中国水泥, 2020(2): 50-55. |
| TANG Jing. Conch moves forward with determination on the road of high-quality development[J]. China Cement, 2020(2): 50-55. | |
| 17 | KARNWIBOON K, SAIWAN C, IDEM R, et al. Solvent extraction of degradation products in amine absorption solution for CO2 capture in flue gases from coal combustion: effect of amines[J]. Energy Procedia, 2017, 114: 1980-1985. |
| 18 | KARNWIBOON K, KRAJANGPIT W, SUPAP T, et al. Solvent extraction based reclaiming technique for the removal of heat stable salts (HSS) and neutral degradation products from amines used during the capture of carbon dioxide (CO2) from industrial flue gases[J]. Separation and Purification Technology, 2019, 228: 115744. |
| 19 | LIU Fei, FANG Mengxiang, DONG Wenfeng, et al. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation[J]. Applied Energy, 2019, 233/234: 468-477. |
| 20 | NWAOHA C, SAIWAN C, TONTIWACHWUTHIKUL P, et al. Carbon dioxide (CO2) capture: absorption-desorption capabilities of 2-amino-2-methyl-1-propanol (AMP), piperazine (PZ) and monoethanolamine (MEA) tri-solvent blends[J]. Journal of Natural Gas Science and Engineering, 2016, 33: 742-750. |
| 21 | BIHONG Lv, YANG Kexuan, ZHOU Xiaobin, et al. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy efficient and non-corrosive carbon dioxide capture[J]. Applied Energy, 2020, 264: 114703. |
| 22 | KARLSSON H K, DRABO P, SVENSSON H. Precipitating non-aqueous amine systems for absorption of carbon dioxide using 2-amino-2-methyl-1-propanol[J]. International Journal of Greenhouse Gas Control, 2019, 88: 460-468. |
| 23 | DUATEPE F P G, ORHAN O Y, ALPER E. Kinetics of carbon dioxide absorption by nonaqueous solutions of promoted sterically hindered amines[J]. Energy Procedia, 2017, 114: 57-65. |
| 24 | GORDESLI F P, UME C S, ALPER E. Mechanism and kinetics of carbon dioxide capture using activated 2-amino-2-methyl-1,3-propanediol[J]. International Journal of Chemical Kinetics, 2013, 45(9): 566-573. |
| 25 | ZHANG Rui, ZHANG Xiaowen, YANG Qi, et al. Analysis of the reduction of energy cost by using MEA-MDEA-PZ solvent for post-combustion carbon dioxide capture (PCC)[J]. Applied Energy, 2017, 205: 1002-1011. |
| 26 | CLOSMANN F, NGUYEN T, ROCHELLE G T. MDEA/piperazine as a solvent for CO2 capture[J]. Energy Procedia, 2009, 1(1): 1351-1357. |
| 27 | 储可弘, 陈绍云, 李强, 等. 基于N-乙基乙醇胺非水CO2吸收剂的抗氧化剂[J]. 化工进展, 2019, 38(12): 5565-5571. |
| CHU Kehong, CHEN Shaoyun, LI Qiang, et al. Oxidation inhibitor for thylethanolamine based non-aqueous CO2 absorbent[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5565-5571. | |
| 28 | LEPAUMIER H, PICQ D, CARRETTE P L. New amines for CO2 capture. Ⅱ. Oxidative degradation mechanisms[J]. Ind. Eng. Chem. Res., 2009, 48(20): 9068-9075. |
| 29 | LEPAUMIER H, PICQ D, CARRETTE P L. New amines for CO2 capture. Ⅰ. Mechanisms of amine degradation in the presence of CO2[J]. Ind. Eng. Chem. Res., 2009, 48(20): 9061-9067. |
| 30 | GOUEDARD C, PICQ D, LAUNAY F, et al. Amine degradation in CO2 capture. I. A review[J]. International Journal of Greenhouse Gas Control, 2012, 10: 244-270. |
| 31 | VEGA F, CANO M, SANNA A, et al. Oxidative degradation of a novel AMP/AEP blend designed for CO2 capture based on partial oxy-combustion technology[J]. Chemical Engineering Journal, 2018, 350: 883-892. |
| 32 | WANG T L, JENS K J. Oxidative degradation of AMP/MEA blends for post-combustion CO2 capture[J]. Energy Procedia, 2013, 37: 306-313. |
| 33 | WANG T L, JENS K J. Towards an understanding of the oxidative degradation pathways of AMP for post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 37: 354-361. |
| [1] | 李季桐, 王刚, 熊亚选, 徐钱. 不同工质单效吸收式制冷系统的能量和㶲分析[J]. 化工进展, 2023, 42(S1): 104-112. |
| [2] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [3] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [6] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [7] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [8] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [9] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [10] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [11] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [12] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [13] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [14] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [15] | 杨红梅, 高涛, 鱼涛, 屈撑囤, 高家朋. 高铁酸盐处理难降解有机物磺化酚醛树脂[J]. 化工进展, 2023, 42(6): 3302-3308. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||