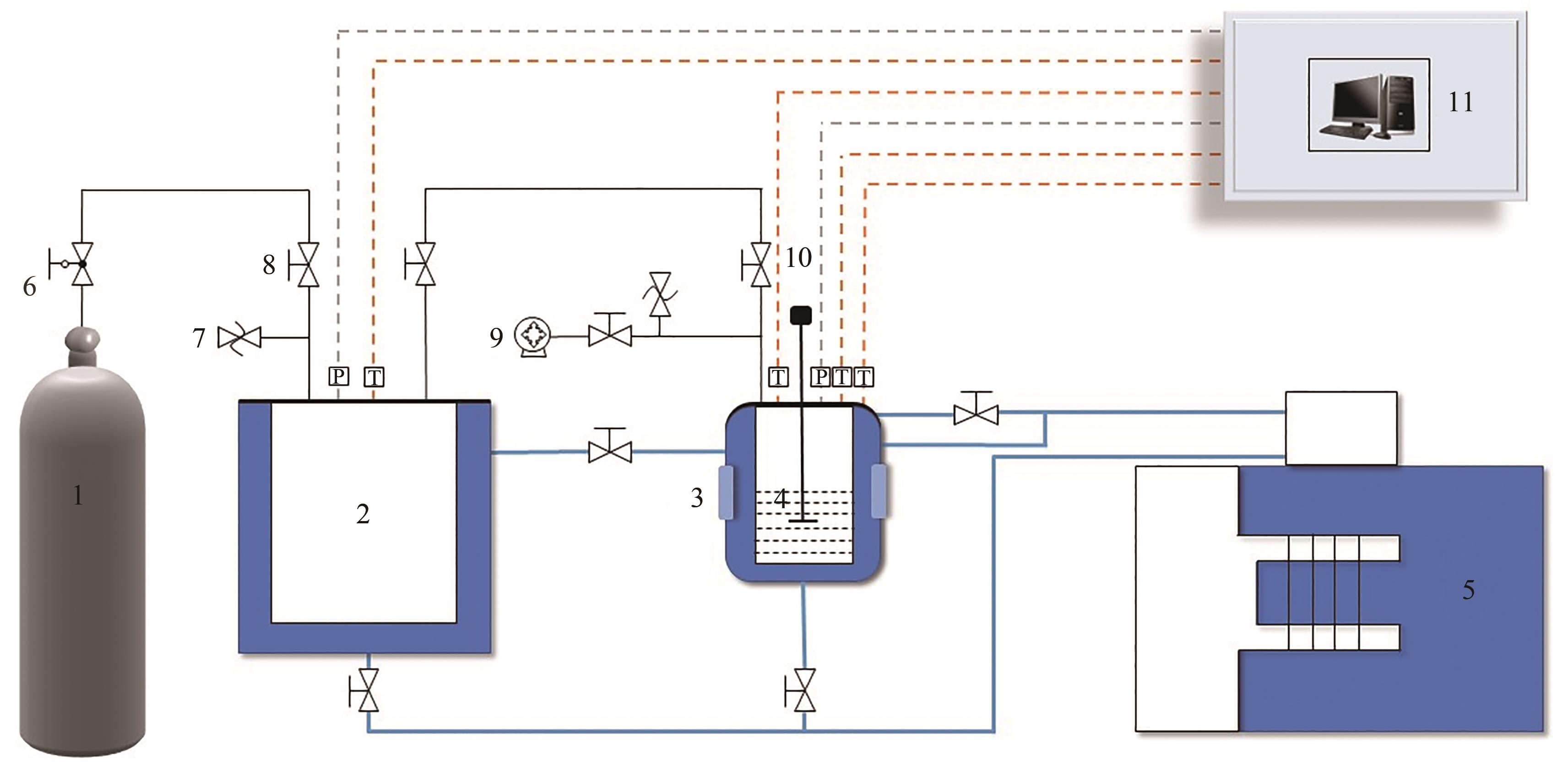
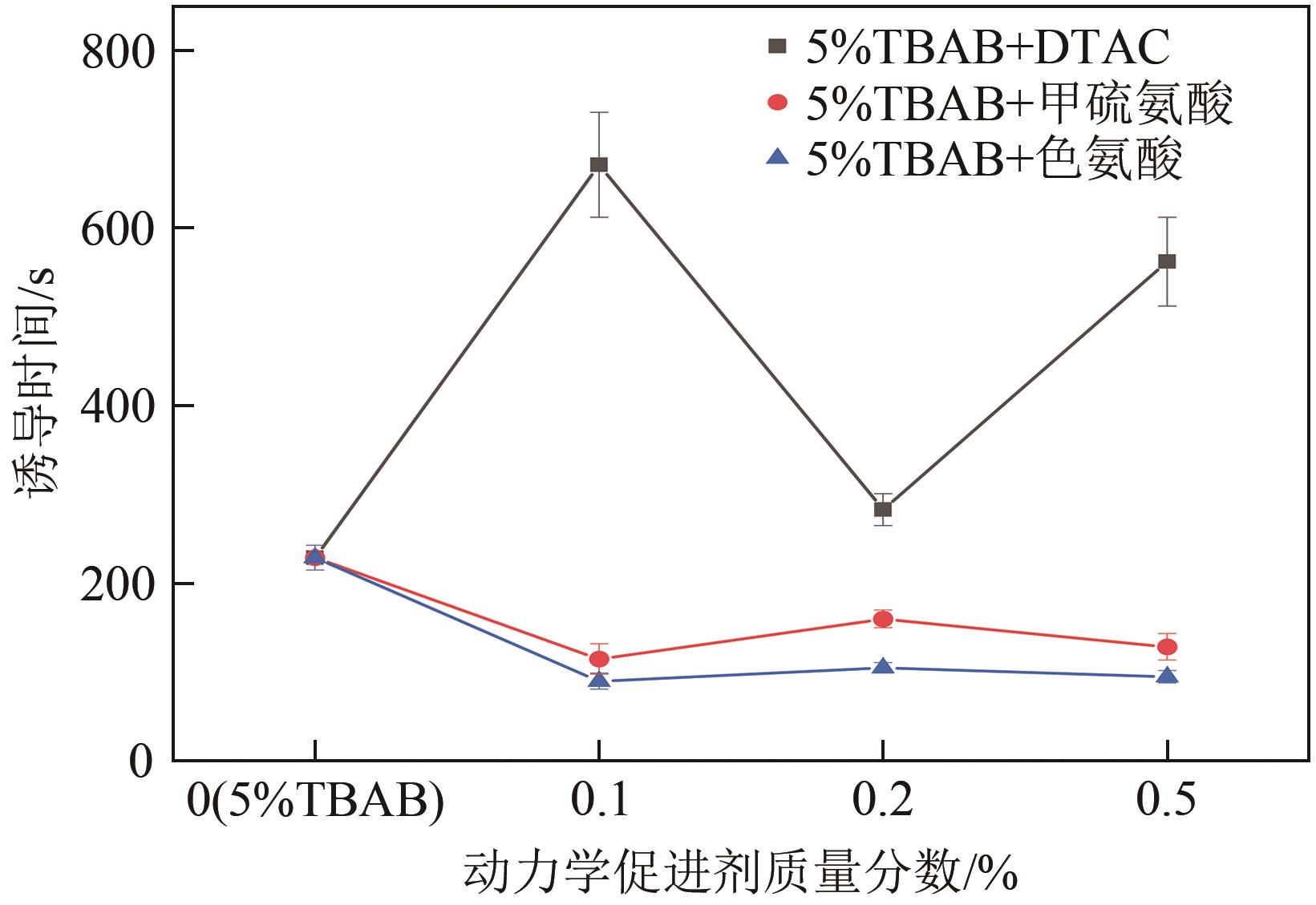
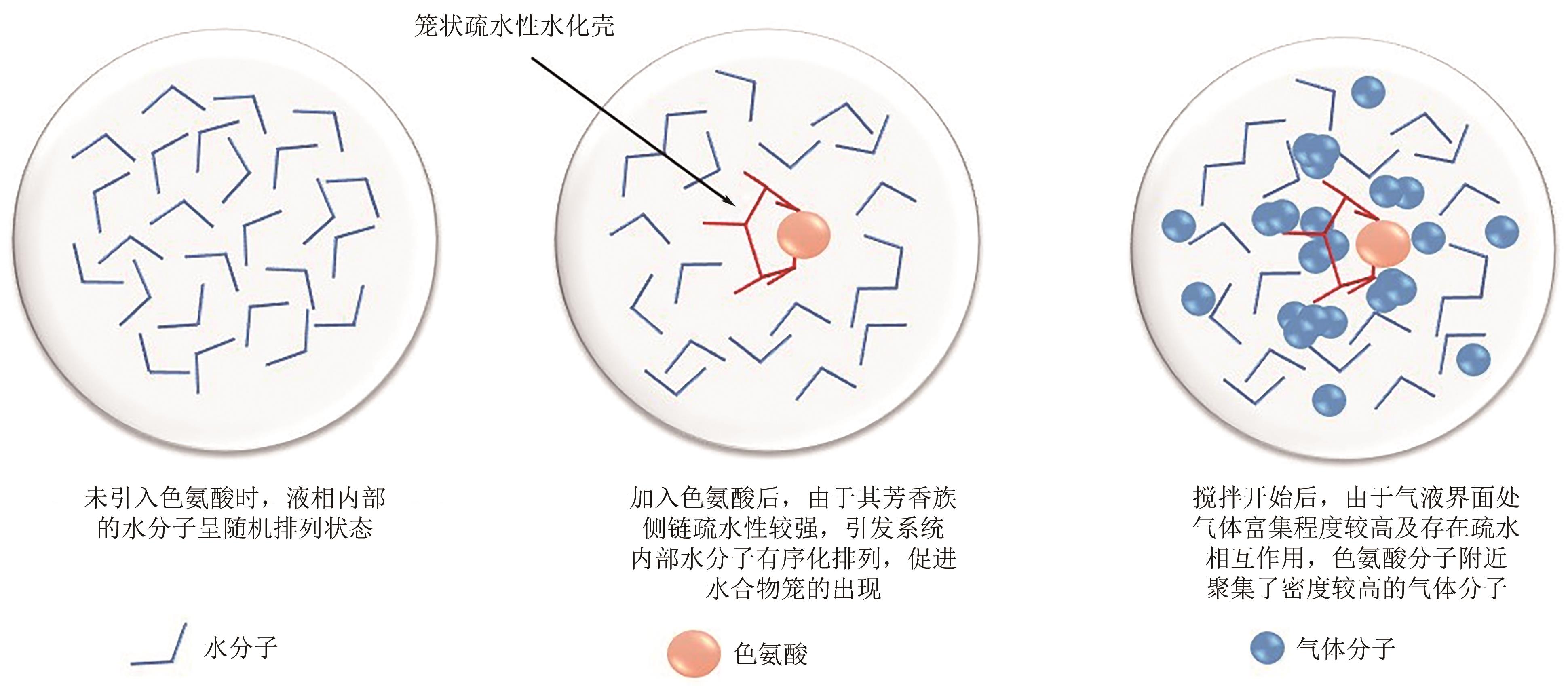
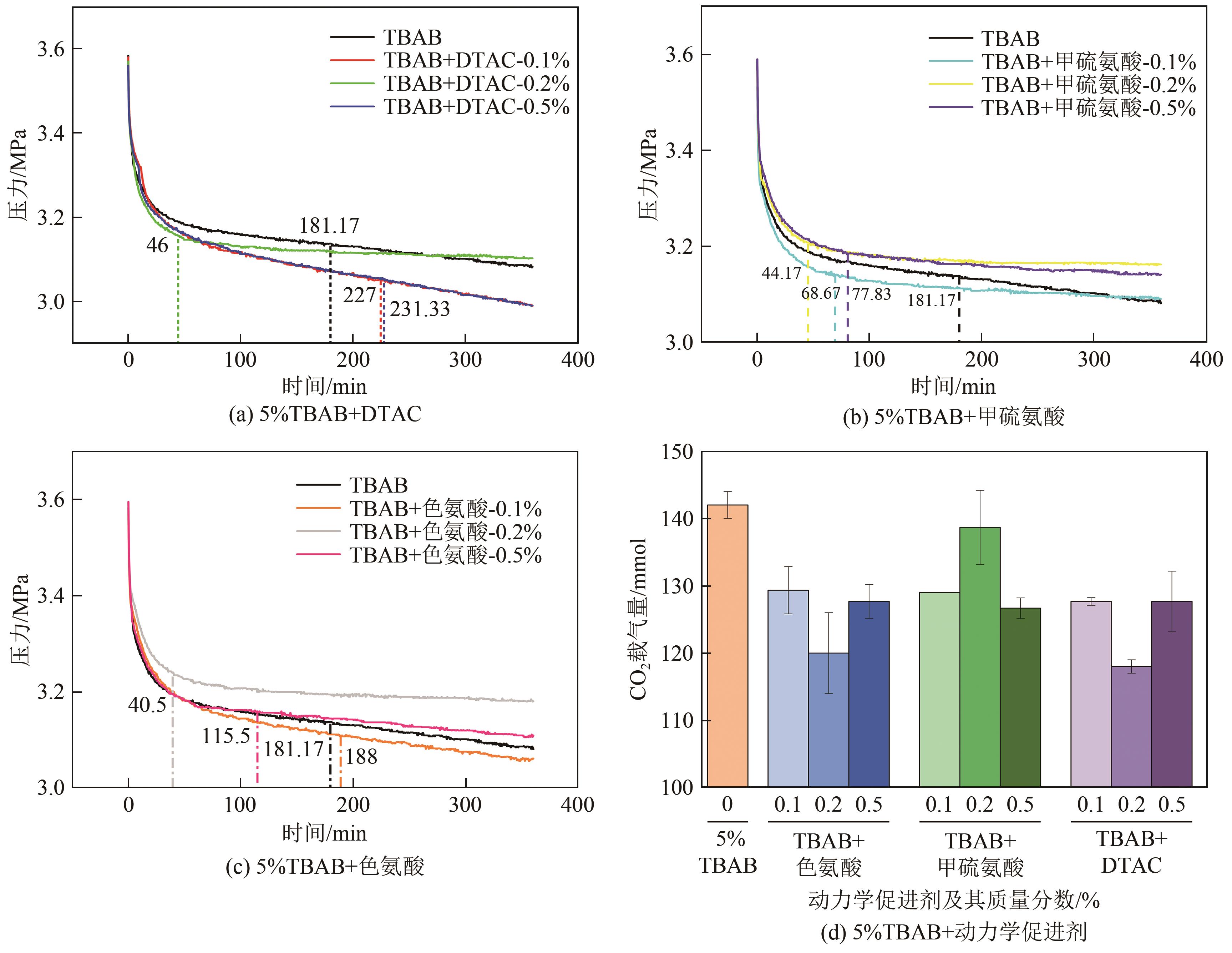
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (10): 5067-5075.DOI: 10.16085/j.issn.1000-6613.2022-2113
• Chemical processes and equipment • Previous Articles Next Articles
Kinetics studies of carbon gas hydrate separation in the presence of amino acids and DTAC
- 1.School of Safety Engineering, Heilongjiang University of Science&Technology, Harbin 150022, Heilongjiang, China
2.Baotailong New Materials Co. , Ltd. , Qitaihe 154600, Heilongjiang, China
-
Received:2022-11-14Revised:2022-11-27Online:2023-11-11Published:2023-10-15 -
Contact:GOU Zenian
氨基酸和DTAC对CO2水合分离动力学影响
- 1.黑龙江科技大学安全工程学院,黑龙江 哈尔滨 150022
2.宝泰隆新材料股份有限公司,黑龙江 七台河 154600
-
通讯作者:苟泽念 -
作者简介:康宇(1982—),男,博士,研究方向为煤与瓦斯突出防治、水合物技术。E-mail:1982kangyu@163.com。 -
基金资助:国家自然科学基金重点项目(51334005);国家自然科学基金(51704102)
CLC Number:
Cite this article
KANG Yu, GOU Zenian. Kinetics studies of carbon gas hydrate separation in the presence of amino acids and DTAC[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5067-5075.
康宇, 苟泽念. 氨基酸和DTAC对CO2水合分离动力学影响[J]. 化工进展, 2023, 42(10): 5067-5075.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2113
| 编号 | TBAB质量分数/% | 动力学促进剂 | 实验条件 | 实验结果 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 试剂 | 质量分数/% | 温度/K | 压力/MPa | 诱导时间/s | t90/min | CO2载气量 /mmol | 水合物相 CO2质量分数/% | 回收率/% | 分离因子 | ||
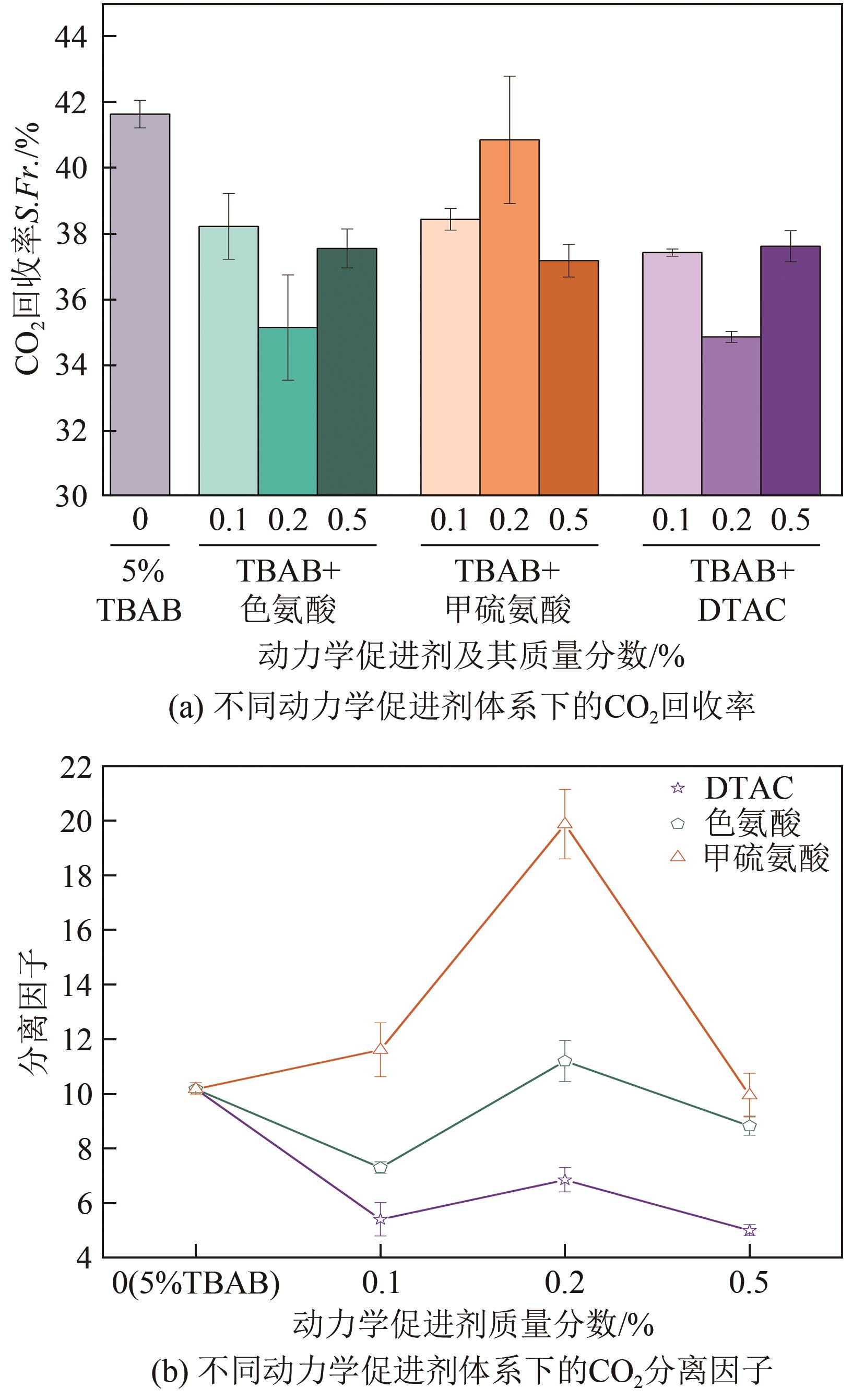
| 1 | 5 | — | — | 278.15 | 3.6 | 229 | 181.17 | 142 | 67.96 | 41.62 | 10.18 |
| 2-1 | 色氨酸 | 0.1 | 90 | 188 | 129 | 62.03 | 38.21 | 7.30 | |||
| 2-2 | 0.2 | 105 | 40.5 | 120 | 71.75 | 35.14 | 11.2 | ||||
| 2-3 | 0.5 | 95 | 115.5 | 128 | 66.30 | 37.54 | 8.83 | ||||
| 3-1 | 甲硫氨酸 | 0.1 | 115 | 68.67 | 129 | 71.51 | 38.42 | 11.61 | |||
| 3-2 | 0.2 | 160 | 44.17 | 139 | 80.21 | 40.84 | 19.86 | ||||
| 3-3 | 0.5 | 128 | 77.83 | 127 | 68.80 | 37.17 | 9.95 | ||||
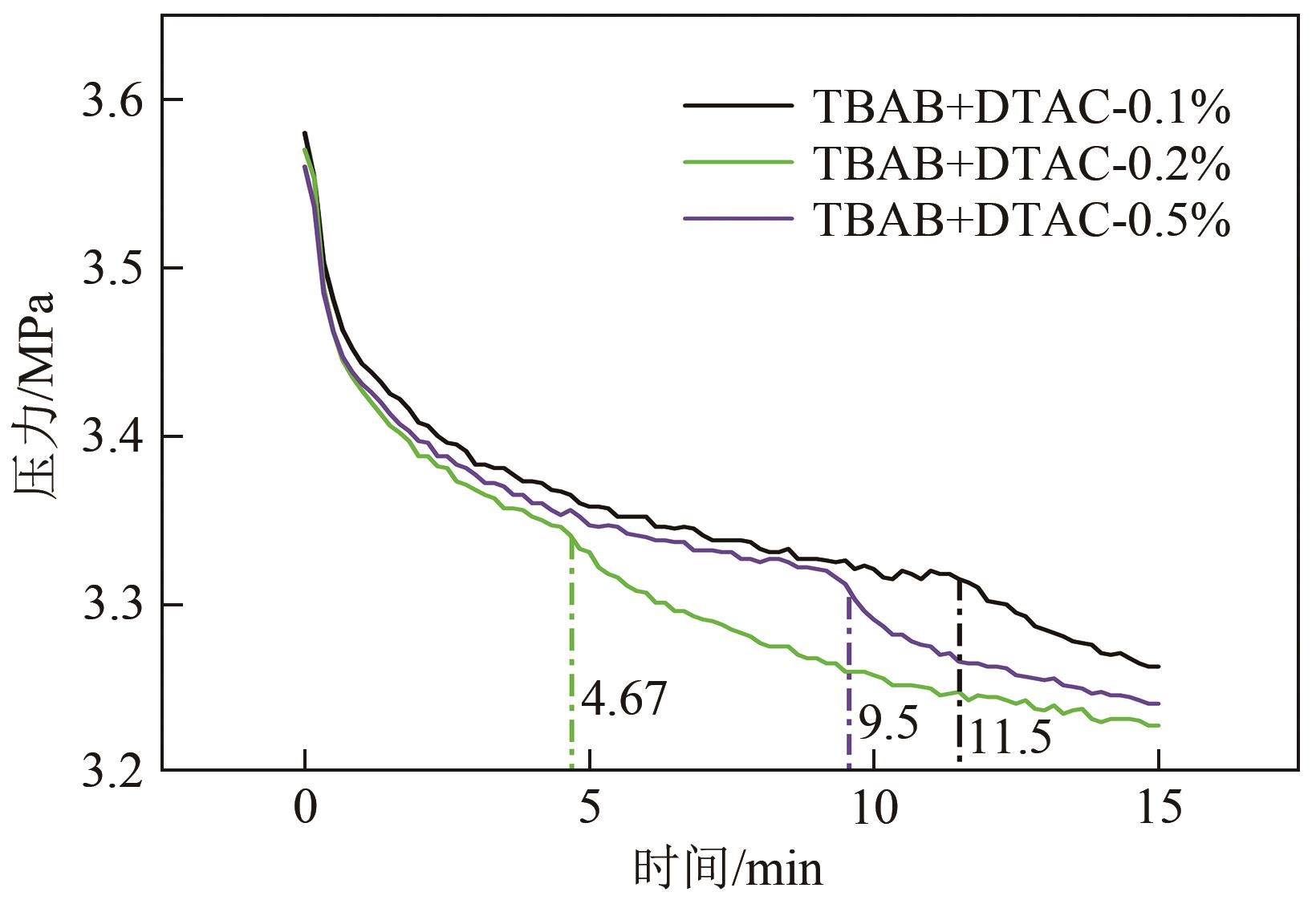
| 4-1 | DTAC | 0.1 | 671 | 227 | 129 | 55.62 | 37.41 | 5.41 | |||
| 4-2 | 0.2 | 283 | 46 | 118 | 61.61 | 34.85 | 6.86 | ||||
| 4-3 | 0.5 | 562 | 231.33 | 128 | 53.87 | 37.60 | 5.01 | ||||
| 5-1 | 10 | — | — | 24 | 214.83 | 150 | 68.84 | 44.14 | 11.07 | ||
| 6-1 | 甲硫氨酸 | 0.1 | 20 | 251.17 | 147 | 69.66 | 42.82 | 11.30 | |||
| 6-2 | 0.2 | 30 | 130.67 | 149 | 74.32 | 43.70 | 14.65 | ||||
| 6-3 | 0.5 | 25 | 191.33 | 149 | 71.40 | 43.47 | 12.48 | ||||
| 编号 | TBAB质量分数/% | 动力学促进剂 | 实验条件 | 实验结果 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 试剂 | 质量分数/% | 温度/K | 压力/MPa | 诱导时间/s | t90/min | CO2载气量 /mmol | 水合物相 CO2质量分数/% | 回收率/% | 分离因子 | ||
| 1 | 5 | — | — | 278.15 | 3.6 | 229 | 181.17 | 142 | 67.96 | 41.62 | 10.18 |
| 2-1 | 色氨酸 | 0.1 | 90 | 188 | 129 | 62.03 | 38.21 | 7.30 | |||
| 2-2 | 0.2 | 105 | 40.5 | 120 | 71.75 | 35.14 | 11.2 | ||||
| 2-3 | 0.5 | 95 | 115.5 | 128 | 66.30 | 37.54 | 8.83 | ||||
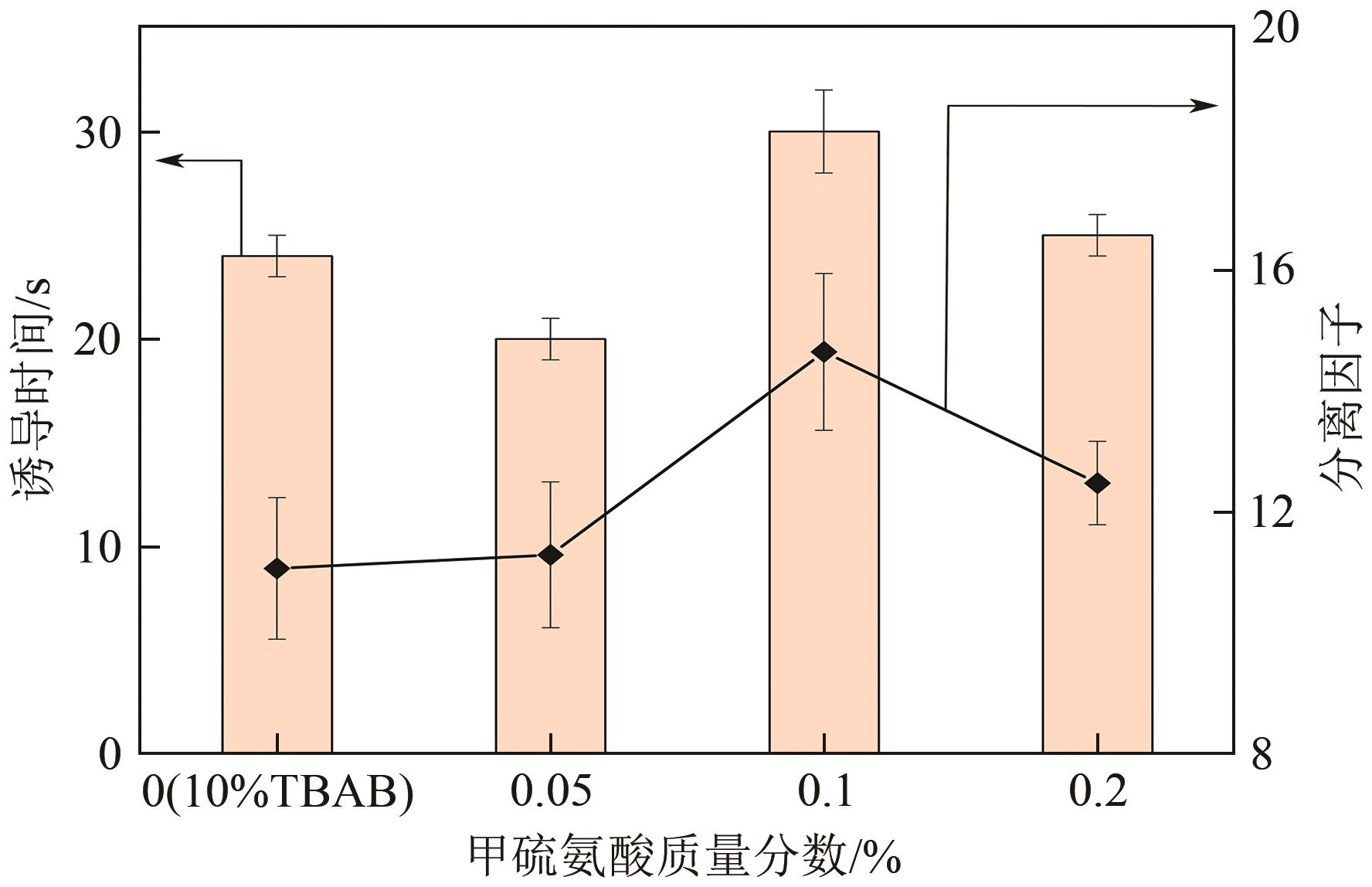
| 3-1 | 甲硫氨酸 | 0.1 | 115 | 68.67 | 129 | 71.51 | 38.42 | 11.61 | |||
| 3-2 | 0.2 | 160 | 44.17 | 139 | 80.21 | 40.84 | 19.86 | ||||
| 3-3 | 0.5 | 128 | 77.83 | 127 | 68.80 | 37.17 | 9.95 | ||||
| 4-1 | DTAC | 0.1 | 671 | 227 | 129 | 55.62 | 37.41 | 5.41 | |||
| 4-2 | 0.2 | 283 | 46 | 118 | 61.61 | 34.85 | 6.86 | ||||
| 4-3 | 0.5 | 562 | 231.33 | 128 | 53.87 | 37.60 | 5.01 | ||||
| 5-1 | 10 | — | — | 24 | 214.83 | 150 | 68.84 | 44.14 | 11.07 | ||
| 6-1 | 甲硫氨酸 | 0.1 | 20 | 251.17 | 147 | 69.66 | 42.82 | 11.30 | |||
| 6-2 | 0.2 | 30 | 130.67 | 149 | 74.32 | 43.70 | 14.65 | ||||
| 6-3 | 0.5 | 25 | 191.33 | 149 | 71.40 | 43.47 | 12.48 | ||||
| 1 | ARNELL N, LOWE J, BROWN S . et al. A global assessment of the effects of climate policy on the impacts of climate change[J]. Nature Climate Change, 2013, 3(5): 512-519. |
| 2 | MEINSHAUSEN Malte, LEWIS Jared, MCGLADE Christophe, et al. Realization of Paris Agreement pledges may limit warming just below 2°C[J]. Nature, 2022, 604(7905): 304-309. |
| 3 | KANG Yu, WU Qiang. Pressure calculation of gas hydrate in the coastal area of the coastal area based on the set pair analysis[J]. Journal of Coastal Research, 2020, 103(S1): 1018. |
| 4 | JIANG Kai, ASHWORTH Peta, ZHANG Shiyi, et al. China’s carbon capture, utilization and storage (CCUS) policy: A critical review[J]. Renewable and Sustainable Energy Reviews, 2020, 119: 109601. |
| 5 | 秦阿宁, 吴晓燕, 李娜娜, 等. 国际碳捕集、利用与封存(CCUS)技术发展战略与技术布局分析[J]. 科学观察, 2022, 17(4): 29-37. |
| QIN Aning, WU Xiaoyan, LI Nana, et al. Analysis on international strategy and technology development of carbon capture, utilization and storage(CCUS)[J]. Science Focus, 2022, 17(4): 29-37. | |
| 6 | YONG X, WXA B, SNA B, et al. Comparative study of A106 steel corrosion in fresh and dirty MEA solutions during the CO2 capture process: Effect of NO3 - [J]. Corrosion Science, 2020, 167: 108521. |
| 7 | KANG S P, LEE H, LEE C S, et al. Hydrate phase equilibria of the guest mixtures containing CO2, N2 and tetrahydrofuran[J]. Fluid Phase Equilibria, 2001, 185(1/2): 101-109. |
| 8 | KANG Seong Pil, LEE Huen. Recovery of CO2 from flue gas using gas hydrate: Thermodynamic verification through phase equilibrium measurements[J]. Environmental Science & Technology, 2000, 34(20): 4397-4400. |
| 9 | LINGA Praveen. A new apparatus to enhance the rate of gas hydrate formation: Application to capture of carbon dioxide[J]. International Journal of Greenhouse Gas Control, 2010, 4(4): 630-637. |
| 10 | LINGA P, KUMAR R, ENGLEZOS P, et al. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures[J]. Chemical Engineering Science, 2007, 62(16): 4268-4276. |
| 11 | LINGA Praveen. The clathrate hydrate process for post and pre-combustion capture of carbon dioxide[J]. Journal of Hazardous Materials, 2007, 149(3): 625-629. |
| 12 | LI Xiaosen. Tetra-n-butyl ammonium bromide semi-clathrate hydrate process for post-combustion capture of carbon dioxide in the presence of dodecyl trimethyl ammonium chloride[J]. Energy, 2010, 35(9): 3902-3908. |
| 13 | XU Chungang, CHEN Zhaoyang, CAI Jing, et al. Study on pilot-scale CO2 separation from flue gas by the hydrate method[J]. Energy & Fuels, 2014, 28(2): 1242-1248. |
| 14 | XIA Z, ZHAO Q, CHEN Z, et al. Review of methods and applications for promoting gas hydrate formation process[J]. Journal of Natural Gas Science and Engineering, 2022, 101: 104528. |
| 15 | YE N, ZHANG P. Equilibrium data and morphology of tetra-n-butyl ammonium bromide semiclathrate hydrate with carbon dioxide[J]. Journal of Chemical & Engineering Data, 2012, 57(5): 1557-1562. |
| 16 | XU Chungang, YU Yisong, DING Yalong, et al. The effect of hydrate promoters on gas uptake[J]. Physical Chemistry Chemical Physics, 2017, 19(32): 21769-21776. |
| 17 | ZHANG Fengyuan. The effect of sodium dodecyl sulfate and dodecyltrimethylammonium chloride on the kinetics of CO2 hydrate formation in the presence of tetra-n-butyl ammonium bromide for carbon capture applications[J]. Energy, 2021, 227: 120424. |
| 18 | 康宇. 低饱和度含瓦斯水合物突出煤体三轴压缩实验研究[J]. 黑龙江科技大学学报, 2016, 26(4): 383-386. |
| KANG Yu. Tri-axial compression test on strength properties of gas hydrate-bearing coal with low concentration using[J]. Journal of Heilongjiang University of Science and Technology, 2016, 26(4): 383-386. | |
| 19 | VELUSWAMY Hari Prakash, HONG Qiwei, LINGA Praveen. Morphology study of methane hydrate formation and dissociation in the presence of amino acid[J]. Crystal Growth & Design, 2016, 16(10): 5932-5945. |
| 20 | LIU Xuejian, REN Junjie, CHEN Daoyi, et al. Comparison of SDS and L-methionine in promoting CO2 hydrate kinetics: Implication for hydrate-based CO2 storage[J]. Chemical Engineering Journal, 2022, 438: 135504. |
| 21 | BHATTACHARJEE Gaurav, LINGA Praveen. Amino acids as kinetic promoters for gas hydrate applications: A mini review[J]. Energy & Fuels, 2021, 35(9): 7553-7571. |
| 22 | 詹昊, 徐纯刚, 李小森, 等. TBAB和CP双添加剂水合物法分离烟气中的CO2 [J]. 化工进展, 2012, 31(7): 1442-1448, 1457. |
| ZHAN Hao, XU Chungang, LI Xiaosen, et al. An experimental study on CO2 hydrate-based separation from flue gas with tetra-n-butyl ammonium bromide/cyclopentane solution[J]. Chemical Industry and Engineering Progress, 2012, 31(7): 1442-1448, 1457. | |
| 23 | 李士凤. 基于水合物技术的模拟电厂烟气中二氧化碳捕获研究[D]. 大连: 大连理工大学, 2010. |
| LI Shifeng. Capture of carbon dioxide from simulated power plant flue gas based on clathrate hydrate technology[D]. Dalian: Dalian University of Technology, 2010. | |
| 24 | NGUYEN Ngoc N, NGUYEN Anh V. Hydrophobic effect on gas hydrate formation in the presence of additives[J]. Energy & Fuels, 2017, 31(10): 10311-10323. |
| 25 | NGUYEN N, NGUYEN A, STEEL K, et al. Interfacial gas enrichment at hydrophobic surfaces and the origin of promotion of gas hydrate formation by hydrophobic solid particles[J]. The Journal of Physical Chemistry C, 121: 3830-3840, 2017. |
| 26 | HE Zhongjin, LINGA Praveen, JIANG Jianwen. What are the key factors governing the nucleation of CO2 hydrate?[J]. Physical Chemistry Chemical Physics, 2017, 19(24): 15657-15661. |
| 27 | 康宇. 三轴压缩下含瓦斯水合物煤体力学特性的离散元研究[J]. 煤炭技术, 2022, 41(10): 106-110. |
| KANG Yu. Discrete element study on mechanical properties of gas hydrate bearing coal under triaxial compression[J]. Coal Technology, 2022, 41(10): 106-110. | |
| 28 | 康宇, 吴强. 含瓦斯水合物突出煤体声发射特征影响因素探讨[J]. 黑龙江科技信息, 2017(6): 55. |
| KANG Yu, WU Qiang. Discussion on the influencing factors of acoustic emission characteristics of gas hydrate outburst coal[J]. Heilongjiang Science and Technology Information, 2017(6): 55. | |
| 29 | Chi LO, ZHANG Junshe, SOMASUNDARAN Ponisseril, et al. Investigations of surfactant effects on gas hydrate formation via infrared spectroscopy[J]. Journal of Colloid and Interface Science, 2012, 376(1): 173-176. |
| 30 | 张青宗, 吕秋楠, 李小森, 等. 玉米棒+四氢呋喃对IGCC合成气水合物生成动力学的影响[J]. 化工进展, 2022, 41(1): 174-181. |
| ZHANG Qingzong, Qiunan LYU, LI Xiaosen, et al. Kinetics studies of IGCC syngas hydrate formation in the presence of corn cobs and tetrahydrofuran[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 174-181. | |
| 31 | HE Yan, SUN Mengting, CHEN Chen, et al. Surfactant-based promotion to gas hydrate formation for energy storage[J]. Journal of Materials Chemistry A, 2019, 7(38): 21634-21661. |
| 32 | KUMAR Asheesh, BHATTACHARJEE Gaurav, KULKARNI B D, et al. Role of surfactants in promoting gas hydrate formation[J]. Industrial & Engineering Chemistry Research, 2015, 54(49): 12217-12232. |
| 33 | 张保勇, 吴强, 王永敬. 表面活性剂对气体水合物生成诱导时间的作用机理[J]. 吉林大学学报(工学版), 2007, 37(1): 239-244. |
| ZHANG Baoyong, WU Qiang, WANG Yongjing. Reaction mechanism between surfactant and induction time of gas hydrate formation[J]. Journal of Jilin University Engineering and Technology Edition, 2007, 37(1): 239-244. | |
| 34 | 杨锐, 杨春花, 王力峰, 等. 不同方法测定两种表面活性剂的临界胶束浓度[J]. 广州化工, 2014, 42(12): 116-118. |
| YANG Rui, YANG Chunhua, WANG Lifeng, et al. Critical micelle concentration of surfactant determined by two different method[J]. Guangzhou Chemical Industry, 2014, 42(12): 116-118. |
| [1] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [2] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [3] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [4] | LI You, WU Yue, ZHONG Yu, LIN Qixuan, REN Junli. Pretreatment of wheat straw with acidic molten salt hydrate for xylose production and its effect on enzymatic hydrolysis efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4974-4983. |
| [5] | YIN Xinyu, PI Pihui, WEN Xiufang, QIAN Yu. Application of special wettability materials for anti-hydrate-nucleation and anti-hydrate-adhesion in oil and gas pipelines [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4076-4092. |
| [6] | WANG Junjie, PAN Yanqiu, NIU Yabin, YU Lu. Molecular level catalytic reforming model construction and application [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3404-3412. |
| [7] | ZHANG Kai, LYU Qiunan, LI Gang, LI Xiaosen, MO Jiamei. Morphology and occurrence characteristics of methane hydrates in the mud of the South China Sea [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3865-3874. |
| [8] | YANG Yang, SUN Zhigao, LI Cuimin, LI Juan, HUANG Haifeng. Promotion on the formation of HCFC-141b hydrate under static conditions by surfactant OP-13 [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2854-2859. |
| [9] | LIU Jia, LIANG Deqing, LI Junhui, LIN Decai, WU Siting, LU Fuqin. A review of flow assurance studies on hydrate slurry in oil-water system [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1739-1759. |
| [10] | GE Weitong, LIAO Yalong, LI Mingyuan, JI Guangxiong, XI Jiajun. Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1885-1894. |
| [11] | LI Yun, CUI Nan, XIONG Xingxing, HUANG Zhiyuan, WANG Dongliang, XU Dan, LI Jun, LI Zebing. Influence of rare earth element Er(Ⅲ) on performance of short-cut nitrification and its inhibition kinetics [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1659-1668. |
| [12] | WANG Wei, ZHANG Dongxu, LI Zunzhao, WANG Xiaolin, HUANG Qiyu. Research progress on the growth behavior of hydrates in water-in-oil emulsion systems [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1155-1166. |
| [13] | SONG Ye, CHEN Yuzhuo, SONG Yuncai, FENG Jie. Catalyst design and reactor analysis for in-situ purification of organic solid waste syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1383-1396. |
| [14] | DUAN Yihang, GAO Ningbo, QUAN Cui. Effect of hydrothermal treatment on pyrolysis characteristics and kinetics of oily sludge [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 603-613. |
| [15] | YUE Zihan, LONG Zhen, ZHOU Xuebing, ZANG Xiaoya, LIANG Deqing. State of the art on hydrogen storage of sⅡ clathrate hydrate [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||