Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (6): 2854-2859.DOI: 10.16085/j.issn.1000-6613.2022-1428
• Chemical processes and equipment • Previous Articles Next Articles
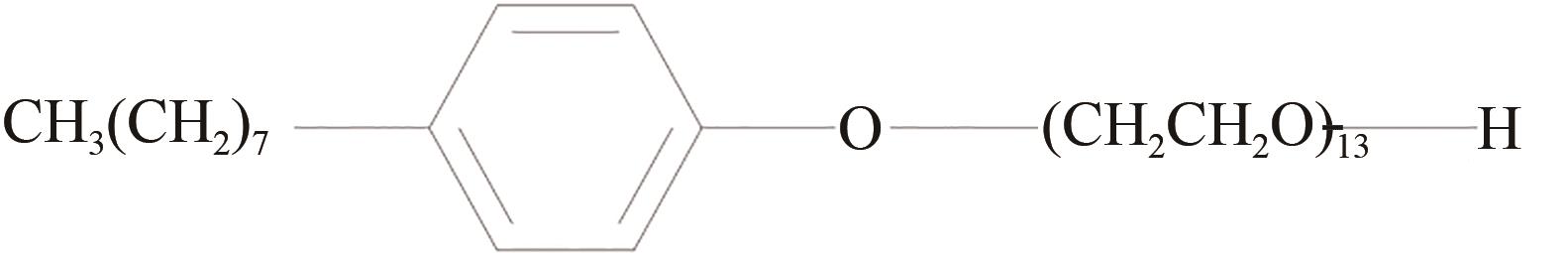
Promotion on the formation of HCFC-141b hydrate under static conditions by surfactant OP-13
YANG Yang( ), SUN Zhigao(
), SUN Zhigao( ), LI Cuimin, LI Juan, HUANG Haifeng
), LI Cuimin, LI Juan, HUANG Haifeng
- School of Environment Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, Jiangsu, China
-
Received:2022-07-29Revised:2022-11-04Online:2023-06-29Published:2023-06-25 -
Contact:SUN Zhigao
静态条件下表面活性剂OP-13促进HCFC-141b水合物生成
- 苏州科技大学环境科学与工程学院,江苏 苏州 215009
-
通讯作者:孙志高 -
作者简介:杨扬(1996—),男,硕士研究生,主要从事储能技术研究。E-mail:869058167@qq.com。 -
基金资助:江苏省高校自然科学研究重大项目(16KJA480001);江苏省自然科学基金(BK20170382);苏州市科技计划(SS202149)
CLC Number:
Cite this article
YANG Yang, SUN Zhigao, LI Cuimin, LI Juan, HUANG Haifeng. Promotion on the formation of HCFC-141b hydrate under static conditions by surfactant OP-13[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2854-2859.
杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1428
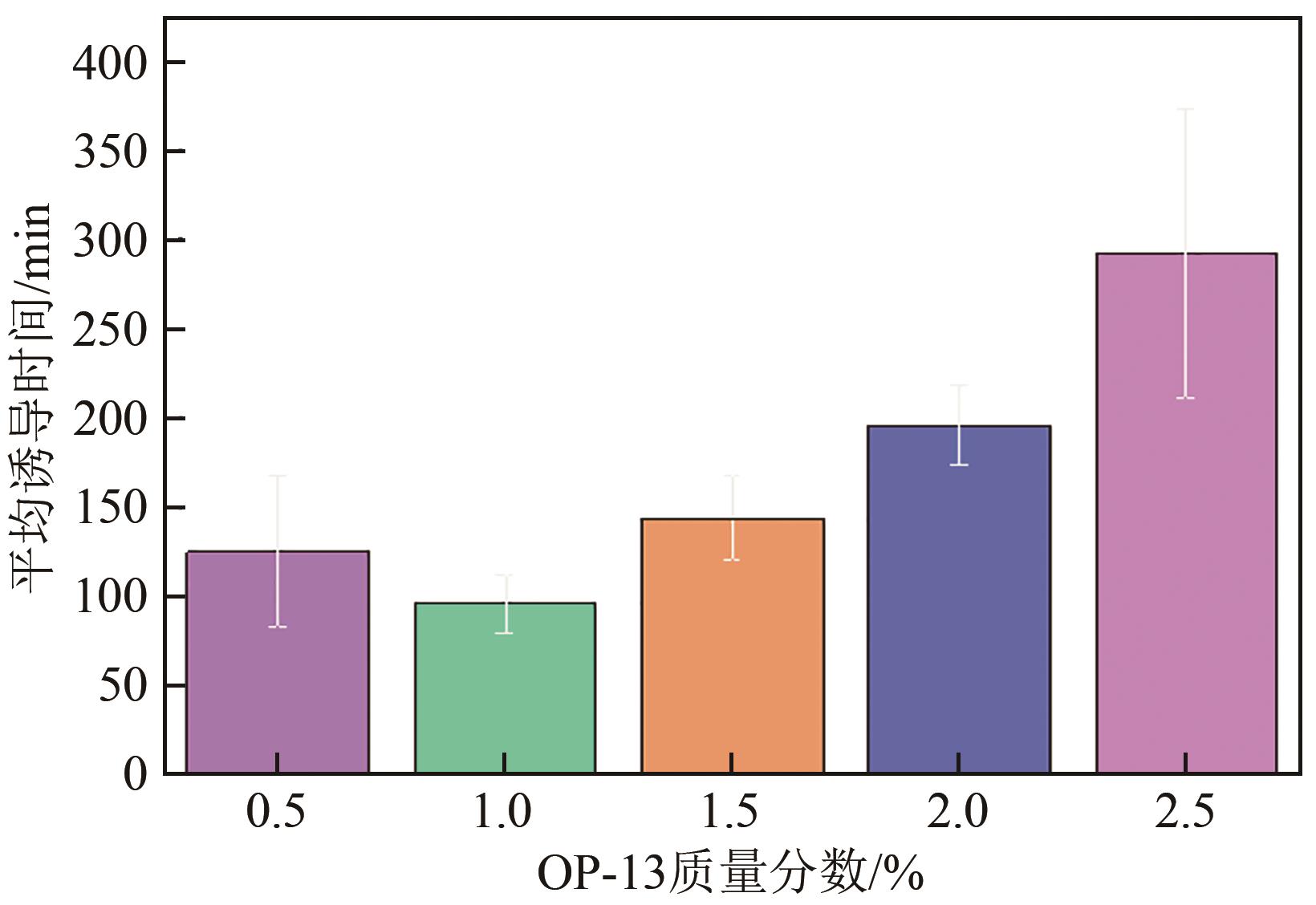
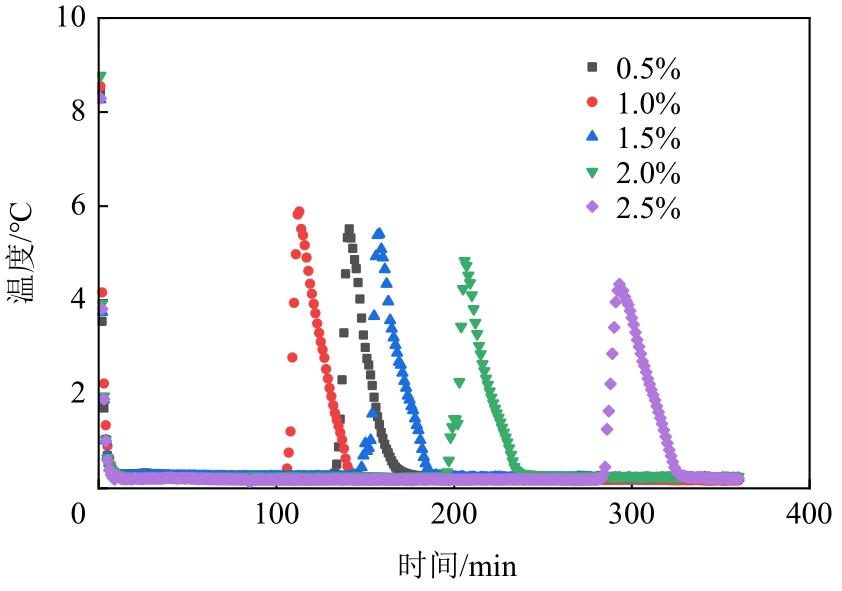
| OP-13质量分数/% | 诱导时间/min | 平均诱导时间/min | 诱导时间方差 | 形成持续时间/min | 平均持续时间/min |
|---|---|---|---|---|---|
| 0.5 | 129,157,79,69,193 | 125 | 42.65 | 41,44,50,56,49 | 48 |
| 1.0 | 70,80,109,117,104 | 96 | 16.36 | 53,42,44,45,38 | 44 |
| 1.5 | 133,142,110,146,189 | 144 | 23.48 | 43,44,50,45,45 | 45 |
| 2.0 | 204,235,186,162,195 | 196 | 21.76 | 42,54,41,49,42 | 46 |
| 2.5 | 201,351,198,428,284 | 292 | 80.78 | 36,64,40,47,43 | 46 |
| OP-13质量分数/% | 诱导时间/min | 平均诱导时间/min | 诱导时间方差 | 形成持续时间/min | 平均持续时间/min |
|---|---|---|---|---|---|
| 0.5 | 129,157,79,69,193 | 125 | 42.65 | 41,44,50,56,49 | 48 |
| 1.0 | 70,80,109,117,104 | 96 | 16.36 | 53,42,44,45,38 | 44 |
| 1.5 | 133,142,110,146,189 | 144 | 23.48 | 43,44,50,45,45 | 45 |
| 2.0 | 204,235,186,162,195 | 196 | 21.76 | 42,54,41,49,42 | 46 |
| 2.5 | 201,351,198,428,284 | 292 | 80.78 | 36,64,40,47,43 | 46 |
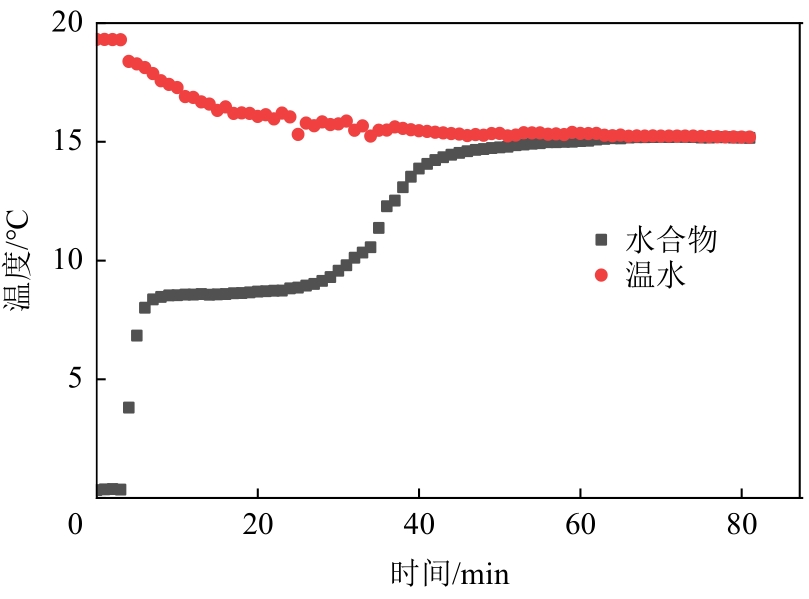
| 项目 | 实验1 | 实验2 | 实验3 |
|---|---|---|---|
| 冰质量/g | 9.98 | 10.02 | 10.01 |
| 冰融化前后的温度/℃ | -4.9/21.3 | -4.7/18.2 | -4.7/16.6 |
| 温水质量/g | 239.47 | 215.88 | 208.21 |
| 温水放热前/后的温度/℃ | 26.4/21.3 | 23.6/18.2 | 22.0/16.6 |
| 冰蓄冷密度/kJ·kg-1 | 327.05 | 326.48 | 321.23 |
| 相对误差/% | -2.4 | -2.5 | -4.1 |
| 项目 | 实验1 | 实验2 | 实验3 |
|---|---|---|---|
| 冰质量/g | 9.98 | 10.02 | 10.01 |
| 冰融化前后的温度/℃ | -4.9/21.3 | -4.7/18.2 | -4.7/16.6 |
| 温水质量/g | 239.47 | 215.88 | 208.21 |
| 温水放热前/后的温度/℃ | 26.4/21.3 | 23.6/18.2 | 22.0/16.6 |
| 冰蓄冷密度/kJ·kg-1 | 327.05 | 326.48 | 321.23 |
| 相对误差/% | -2.4 | -2.5 | -4.1 |
| OP-13质量分数/% | 实验1 | 实验2 | 实验3 | 平均值 |
|---|---|---|---|---|
| 0.5 | 246.68 | 240.72 | 255.21 | 247.54 |
| 1.0 | 250.42 | 243.35 | 251.49 | 248.42 |
| 1.5 | 234.82 | 238.03 | 230.47 | 234.44 |
| 2.0 | 235.21 | 225.54 | 229.80 | 230.18 |
| 2.5 | 223.53 | 215.34 | 210.40 | 216.42 |
| OP-13质量分数/% | 实验1 | 实验2 | 实验3 | 平均值 |
|---|---|---|---|---|
| 0.5 | 246.68 | 240.72 | 255.21 | 247.54 |
| 1.0 | 250.42 | 243.35 | 251.49 | 248.42 |
| 1.5 | 234.82 | 238.03 | 230.47 | 234.44 |
| 2.0 | 235.21 | 225.54 | 229.80 | 230.18 |
| 2.5 | 223.53 | 215.34 | 210.40 | 216.42 |
| 1 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 北京: 化学工业出版社, 2008. |
| CHEN Guangjin, SUN Changyu, MA Qinglan. Gas hydrate science and technology[M]. Beijing: Chemical Industry Press, 2008. | |
| 2 | SUN Shicai, LI Yanmin, GU Linlin, et al. Experimental study on carbon dioxide hydrate formation in the presence of static magnetic field[J]. The Journal of Chemical Thermodynamics, 2022, 170: 106764. |
| 3 | 方书起, 张欣悦, 李思齐, 等. 撞击流式反应器内水合物法分离沼气中CO2研究[J]. 化工学报, 2020, 71(5): 2099-2108, 2457. |
| FANG Shuqi, ZHANG Xinyue, LI Siqi, et al. Investigation on separation of CO2 from biogas by hydrate method in impinging stream reactor[J]. CIESC Journal, 2020, 71(5):2099-2108, 2457. | |
| 4 | 宋光春, 施政灼, 李玉星, 等. 油水体系内水合物的生成: 温度、压力和搅拌速率影响[J]. 化工进展, 2019, 38(3): 1338-1345. |
| SONG Guangchun, SHI Zhengzhuo, LI Yuxing, et al. Hydrate formation in oil-water systems: investigations of the influences of temperature, pressure and rotation rate[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1338-1345. | |
| 5 | PARK S S, KIM N J. Study on methane hydrate formation using ultrasonic waves[J]. Journal of Industrial and Engineering Chemistry, 2013, 19(5): 1668-1672. |
| 6 | LI Airong, JIANG Lele, TANG Siyao. An experimental study on carbon dioxide hydrate formation using a gas-inducing agitated reactor[J]. Energy, 2017, 134: 629-637. |
| 7 | 申小冬, 任俊杰, 梁德青. 微型鼓泡器中甲烷水合物的生成特性[J]. 石油化工, 2018, 47(5): 431-436. |
| SHEN Xiaodong, REN Junjie, LIANG Deqing. Formation characteristics of methane hydrate in a micro-bubbler[J]. Petrochemical Technology, 2018, 47(5): 431-436. | |
| 8 | Eduardo ANDRES-GARCIA, DIKHTIARENKO Alla, FAUTH Francois, et al. Methane hydrates: Nucleation in microporous materials[J]. Chemical Engineering Journal, 2019, 360: 569-576. |
| 9 | HEYDARI Atousa, PEYVANDI Kiana. Study of biosurfactant effects on methane recovery from gas hydrate by CO2 replacement and depressurization[J]. Fuel, 2020, 272: 117681. |
| 10 | ASADI F, METAXAS P J, LIM V W, et al. Cyclodextrins as eco-friendly nucleation promoters for methane hydrate[J]. Chemical Engineering Journal, 2021, 417: 127932. |
| 11 | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| Wenxin MEN, PENG Qingshou, GUI Xia. Phase equilibrium of CO2 hydrate in the presence of four different quaternary ammonium salts[J]. CIESC Journal, 2022, 73(4): 1472-1482. | |
| 12 | 王英梅, 董世强, 展静, 等. 石英砂粒径大小对甲烷水合物形成及分布的影响[J]. 化工进展, 2020, 39(8): 3049-3056. |
| WANG Yingmei, DONG Shiqiang, ZHAN Jing, et al. Effect of quartz sand particle size on the formation and distribution of methane hydrate[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3049-3056. | |
| 13 | SAID S, GOVINDARAJ V, HERRI J M, et al. A study on the influence of nanofluids on gas hydrate formation kinetics and their potential: Application to the CO2 capture process[J]. Journal of Natural Gas Science and Engineering, 2016, 32: 95-108. |
| 14 | 李文昭, 潘振, 马贵阳, 等. 表面活性剂吸附对促进甲烷水合物生成效果的影响[J]. 化工学报, 2017, 68(4): 1542-1549. |
| LI Wenzhao, PAN Zhen, MA Guiyang, et al. Promotion effects of surfactant adsorption on formation of methane hydrates[J].CIESC Journal, 2017, 68(4): 1542-1549. | |
| 15 | RAJABI F S, BONYADI M. A comparative study on the effects of Fe3O4 nanofluid, SDS and CTAB aqueous solutions on the CO2 hydrate formation[J]. Journal of Molecular Liquids, 2020, 300: 112251. |
| 16 | 周麟晨, 孙志高, 陆玲, 等. 静态条件下表面活性剂促进HCFC-141b水合物生成[J]. 高校化学工程学报, 2020, 34(2): 402-410. |
| ZHOU Linchen, SUN Zhigao, LU Ling, et al. Enhancement of HCFC-141b hydrate formation with surfactants in a static system[J]. Journal of Chemical Engineering of Chinese Universities, 2020, 34(2): 402-410. | |
| 17 | 张学民, 李洋, 姚泽, 等. 表面活性剂对气体水合物生成过程的定量影响[J]. 过程工程学报, 2018, 18(2): 356-360. |
| ZHANG Xuemin, LI Yang, YAO Ze, et al. Quantitative influence of surfactant on the formation process for gas hydrate[J]. The Chinese Journal of Process Engineering, 2018, 18(2): 356-360. | |
| 18 | 李荣, 孙志高, 宋佳. 氨基酸侧链对HCFC-141b水合物形成的影响[J]. 储能科学与技术, 2022, 11(7): 2126-2132. |
| LI Rong, SUN Zhigao, SONG Jia. Effect of amino acid side chains on HCFC-141b hydrate formation[J]. Energy Storage Science and Technology, 2022, 11(7): 2126-2132. | |
| 19 | 张龙明, 李璞, 李娜, 等. 混合量热法测定水合物浆体蓄冷密度[J]. 制冷学报, 2014, 35(6): 47-52. |
| ZHANG Longming, LI Pu, LI Na, et al. Determination of hydrate slurry’s cool-storage density with mixing calorimetry method[J]. Journal of Refrigeration, 2014, 35(6): 47-52. | |
| 20 | 敬加强, 杨航, 张帅, 等. 水合物生成诱导期研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(6): 24-32. |
| JING Jiaqiang, YANG Hang, ZHANG Shuai, et al. Research progress of hydrate formation induction period[J]. Natural Gas Chemical Industry, 2021, 46(6): 24-32. | |
| 21 | KACHCHIEV Dimo. Nucleation-basic theory with applications[M]. Oxford: Butterworth Heinemann, 2001. |
| 22 | 马鸿凯, 孙志高, 蔡伟, 等. HCFC-141b水合物静态生成促进技术的试验研究[J]. 流体机械, 2016, 44(1): 66-70. |
| MA Hongkai, SUN Zhigao, CAI Wei, et al. Experimental study on promot technology of HCFC-141b hydration formation in quiescent system[J]. Fluid Machinery, 2016, 44(1): 66-70. | |
| 23 | 闫明月, 王岳, 刘嘉琪, 等. 表面活性剂对甲烷水合物生成影响研究进展[J]. 应用化工, 2021, 50(8): 2317-2325. |
| YAN Mingyue, WANG Yue, LIU Jiaqi, et al. Research progress on influence factors of methane hydrate formation in surfactant solution[J]. Applied Chemical Industry, 2021, 50(8): 2317-2325. | |
| 24 | ANDO N, KUWABARA Y, MORI Y H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using methane and micelle-forming surfactants[J]. Chemical Engineering Science, 2012, 73(1): 79-85. |
| 25 | WANG Fei, GUO Gang, LIU Guoqiang, et al. Effects of surfactant micelles and surfactant-coated nanospheres on methane hydrate growth pattern[J]. Chemical Engineering Science, 2016, 144(1): 108-115. |
| 26 | NGUYEN N N, NGUYEM A V. Hydrophobic effect on gas hydrate formation in the presence of additives[J]. Energy and Fuels, 2017, 31(9): 10311-10323. |
| 27 | 闫乐乐, 梁生康, 宋丹丹, 等. 鼠李糖脂生物表面活性剂胶束性质研究[J]. 中国海洋大学学报(自然科学版), 2016, 46(12): 68-72. |
| YAN Lele, LIANG Shengkang, SONG Dandan, et al. Studies on some micelle properties of rhamnolipid biosurfactant[J]. Periodical of Ocean University of China, 2016, 46(12): 68-72. |
| [1] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [2] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [3] | LI You, WU Yue, ZHONG Yu, LIN Qixuan, REN Junli. Pretreatment of wheat straw with acidic molten salt hydrate for xylose production and its effect on enzymatic hydrolysis efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4974-4983. |
| [4] | YIN Xinyu, PI Pihui, WEN Xiufang, QIAN Yu. Application of special wettability materials for anti-hydrate-nucleation and anti-hydrate-adhesion in oil and gas pipelines [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4076-4092. |
| [5] | ZHANG Kai, LYU Qiunan, LI Gang, LI Xiaosen, MO Jiamei. Morphology and occurrence characteristics of methane hydrates in the mud of the South China Sea [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3865-3874. |
| [6] | LIU Jia, LIANG Deqing, LI Junhui, LIN Decai, WU Siting, LU Fuqin. A review of flow assurance studies on hydrate slurry in oil-water system [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1739-1759. |
| [7] | WANG Wei, ZHANG Dongxu, LI Zunzhao, WANG Xiaolin, HUANG Qiyu. Research progress on the growth behavior of hydrates in water-in-oil emulsion systems [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1155-1166. |
| [8] | KANG Yu, GOU Zenian. Kinetics studies of carbon gas hydrate separation in the presence of amino acids and DTAC [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5067-5075. |
| [9] | YUE Zihan, LONG Zhen, ZHOU Xuebing, ZANG Xiaoya, LIANG Deqing. State of the art on hydrogen storage of sⅡ clathrate hydrate [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134. |
| [10] | DENG Quanlong, DING Houcheng, XU Yuandi, JIANG Zhong’an, YANG Lan, SUN Xuefei. Synergistic dust removal performance of surfactant droplets combined with metal mesh grid [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 546-552. |
| [11] | ZHANG Qingqing, BI Haipu, SHU Zhongjun, OU Hongxiang, WANG Shangbin, WANG Junqi, PAN Yi. Research progress on control behaviors and substitutes of PFOS in foam extinguishing agents [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 340-350. |
| [12] | SONG Chao, YE Xuemin, LI Chunxi. Molecular dynamics study on the influence of self-assembly behaviors of nanoparticles and surfactants on the properties of silicone oil/water interface [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 366-375. |
| [13] | WANG Yinmei, ZHANG Zhaohui, LIU Shenghao, JIAO Wenze, WANG Lijin, TENG Yadong, LIU Jie. Atmospheric pressure decomposition of carbon dioxide hydrate in accelerator system [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 141-149. |
| [14] | WANG Yunfei, SUN Changyu, YU Xichong, LI Qingping, CHEN Guangjin. Analysis of the methane hydrate decomposition kinetics through depressurization method by using a pilot-scale reactor [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4111-4119. |
| [15] | HUANG Ting, LI Qingping, LI Rui, PANG Weixin, CHEN Guangjin. Experimental simulation of depressurization mining of the class 1 hydrate reservoir [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4120-4128. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||