Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (1): 373-385.DOI: 10.16085/j.issn.1000-6613.2022-0233
• Fine chemicals • Previous Articles Next Articles
Progress on separation and purification for organic compounds by melt crystallization
- 1.School of Chemistry and Chemical Engineering, Xi'an University of Architecture and Technology, Xi'an 710055, Shaanxi, China
2.Quality Assurance Department, Zhejiang Xianfeng Technologies Co. , Ltd. , Taizhou 317000, Zhejiang, China
-
Received:2022-02-14Revised:2022-04-08Online:2023-02-20Published:2023-01-25 -
Contact:QI Yabing
熔融结晶技术分离纯化有机化合物的研究进展
- 1.西安建筑科技大学化学与化工学院,陕西 西安 710055
2.浙江先锋科技股份有限公司质保部,浙江 台州 317000
-
通讯作者:齐亚兵 -
作者简介:齐亚兵(1983—),男,博士,讲师,研究方向为结晶与分离技术。E-mail: qiyabing123@163.com。 -
基金资助:西安市碑林区科技计划(GX2134);西安建筑科技大学人才科技基金(RC1714);西安建筑科技大学青年科技基金(QN1509)
CLC Number:
Cite this article
QI Yabing, JIA Honglei. Progress on separation and purification for organic compounds by melt crystallization[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 373-385.
齐亚兵, 贾宏磊. 熔融结晶技术分离纯化有机化合物的研究进展[J]. 化工进展, 2023, 42(1): 373-385.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0233
| 熔融结晶方式 | 过程 | 优点 | 缺点 | 适用情况 |
|---|---|---|---|---|
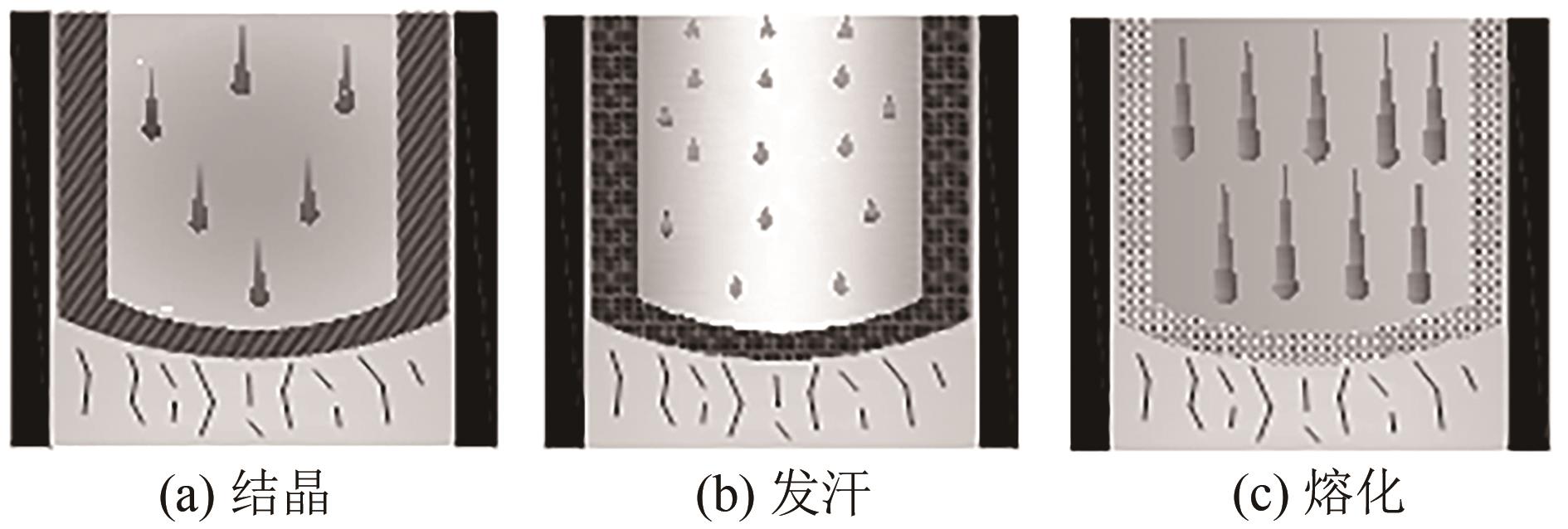
| 层式熔融结晶 | 先结晶、再发汗、后熔化 | 生长速率快、装置简单、无结垢、固液分离容易、装置易放大 | 间歇操作、能耗高、处理能力小、效率低、固液相界面积小 | 不太关注能耗和效率时,为熔融结晶法制备精细化学品的首选 |
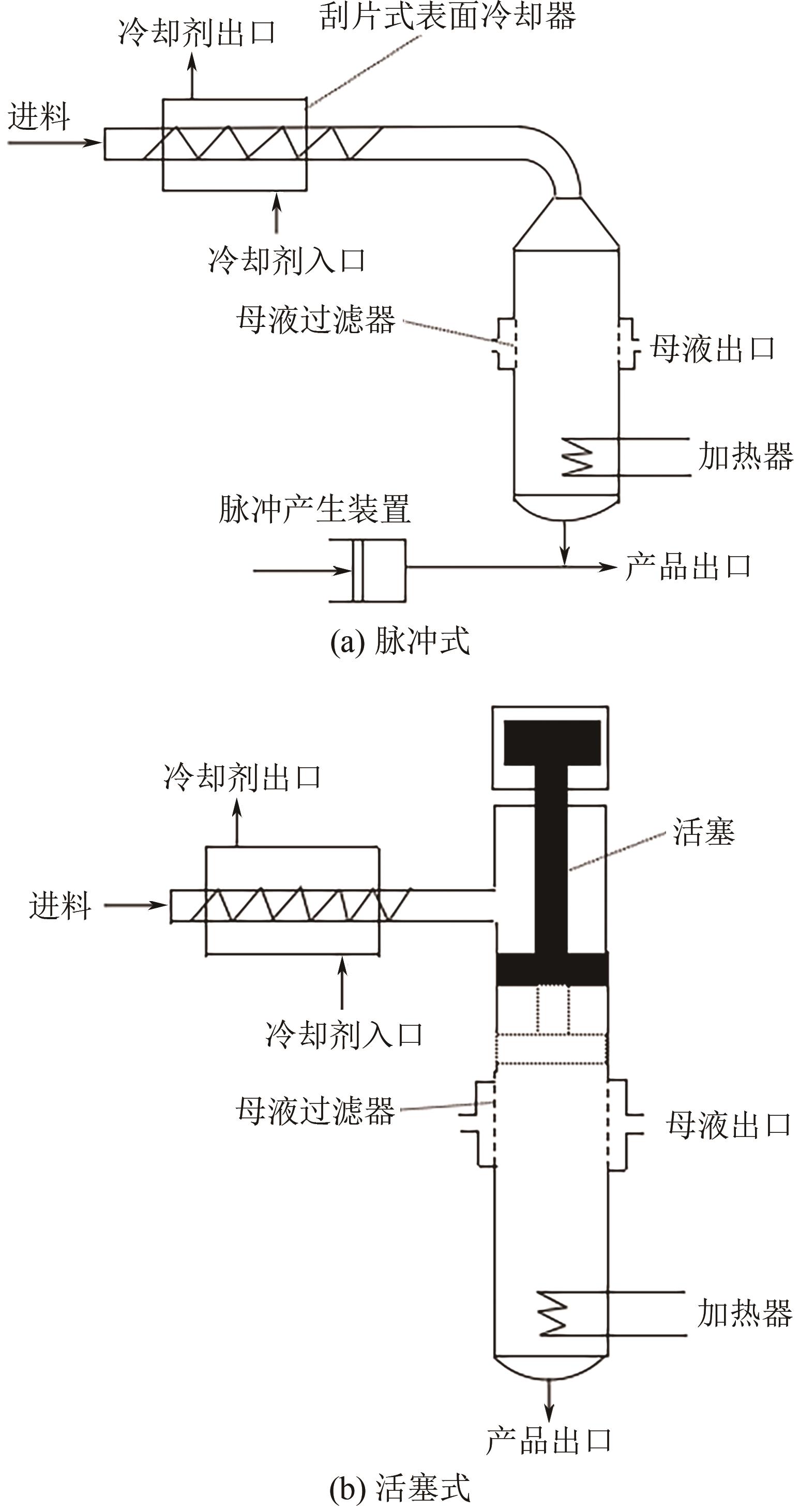
| 悬浮熔融结晶 | 结晶、洗涤、发汗和熔化同时进行 | 固液相界面积大、传热传质性能好、连续操作、处理能力大、效率高 | 固液分离难、易结垢、易堵塞、设备复杂、稳定运行周期短 | 固液分离和结垢问题能较好解决的情况下,采用此法 |
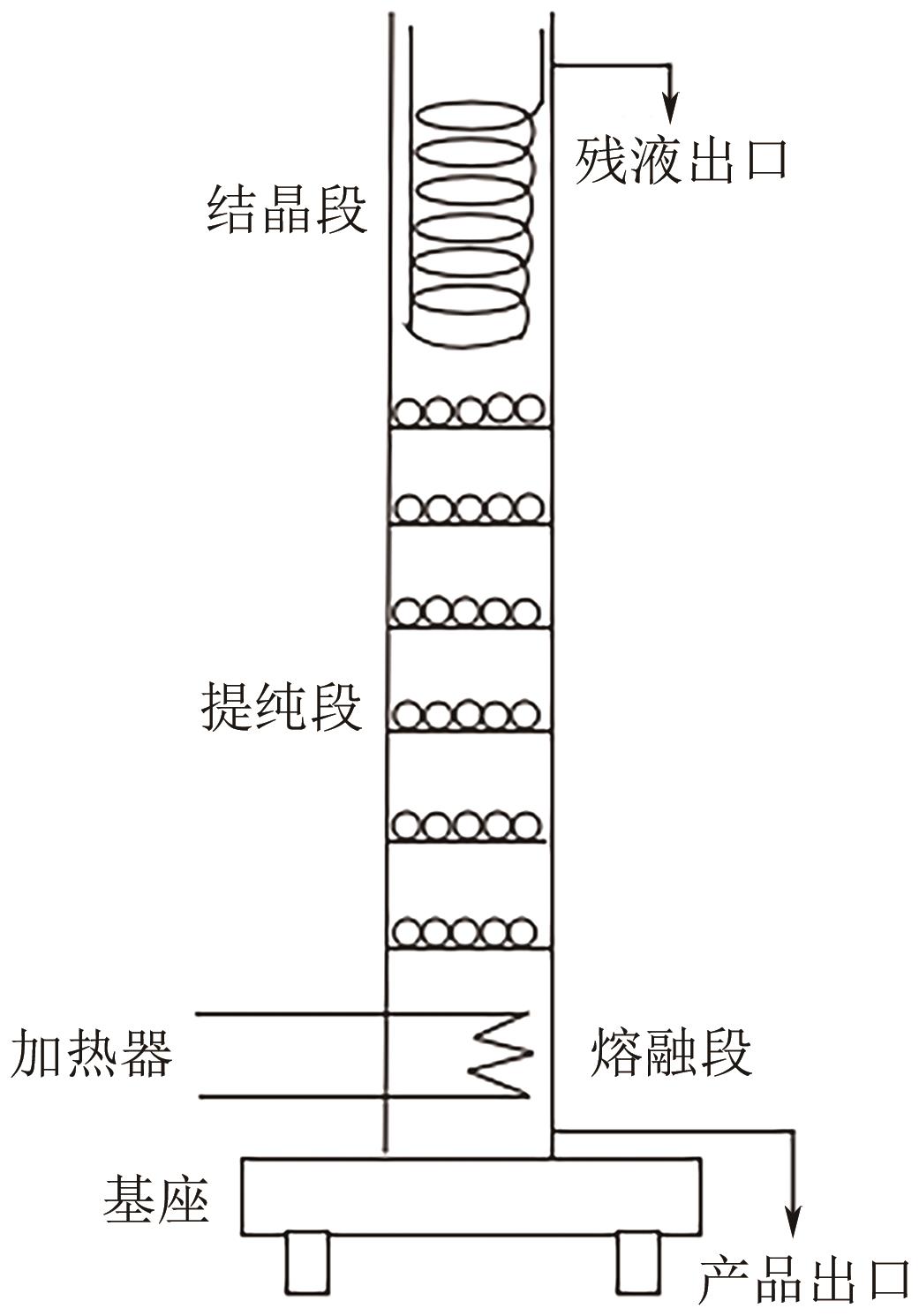
| 区域熔融结晶 | 熔区多次从材料棒一端移动到另一端,材料棒中间段为高纯产品 | 结晶设备体积小、操作简单、产品纯度高 | 处理量小、分离时间长、效率低 | 适合高附加值、高纯度精细化学品和药物的制备 |
| 层式熔融结晶与悬浮熔融结晶的耦合 | 原料经造粒、发汗、洗涤后得到纯度较高、粒度较大的结晶产品 | 能耗较低、产品纯度较高、粒度较大 | 适合对产品纯度和粒度要求较高的情况 | |
| 精馏与熔融结晶的耦合 | 精馏塔顶串联降膜结晶器,精馏塔顶轻组分依次结晶、发汗、熔化 | 能耗较低、收率较高、产量较高、分离因子较高 | 适合混合液中轻重组分熔点差异大、沸点差异小,需获得高纯度轻组分的情况 |
| 熔融结晶方式 | 过程 | 优点 | 缺点 | 适用情况 |
|---|---|---|---|---|
| 层式熔融结晶 | 先结晶、再发汗、后熔化 | 生长速率快、装置简单、无结垢、固液分离容易、装置易放大 | 间歇操作、能耗高、处理能力小、效率低、固液相界面积小 | 不太关注能耗和效率时,为熔融结晶法制备精细化学品的首选 |
| 悬浮熔融结晶 | 结晶、洗涤、发汗和熔化同时进行 | 固液相界面积大、传热传质性能好、连续操作、处理能力大、效率高 | 固液分离难、易结垢、易堵塞、设备复杂、稳定运行周期短 | 固液分离和结垢问题能较好解决的情况下,采用此法 |
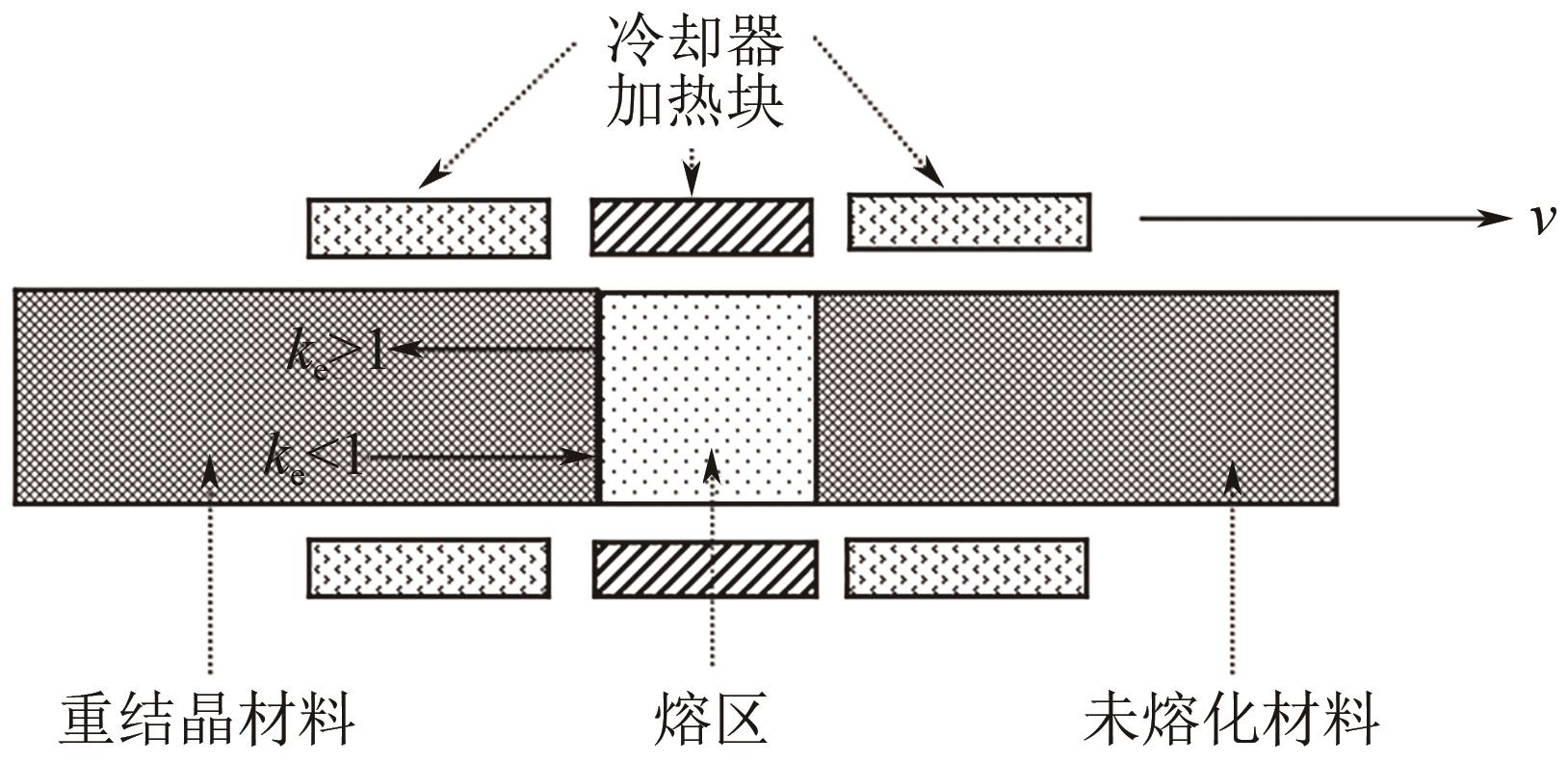
| 区域熔融结晶 | 熔区多次从材料棒一端移动到另一端,材料棒中间段为高纯产品 | 结晶设备体积小、操作简单、产品纯度高 | 处理量小、分离时间长、效率低 | 适合高附加值、高纯度精细化学品和药物的制备 |
| 层式熔融结晶与悬浮熔融结晶的耦合 | 原料经造粒、发汗、洗涤后得到纯度较高、粒度较大的结晶产品 | 能耗较低、产品纯度较高、粒度较大 | 适合对产品纯度和粒度要求较高的情况 | |
| 精馏与熔融结晶的耦合 | 精馏塔顶串联降膜结晶器,精馏塔顶轻组分依次结晶、发汗、熔化 | 能耗较低、收率较高、产量较高、分离因子较高 | 适合混合液中轻重组分熔点差异大、沸点差异小,需获得高纯度轻组分的情况 |
| 结晶器类型 | 结晶器型号 | 特点 | 分离精制应用 |
|---|---|---|---|
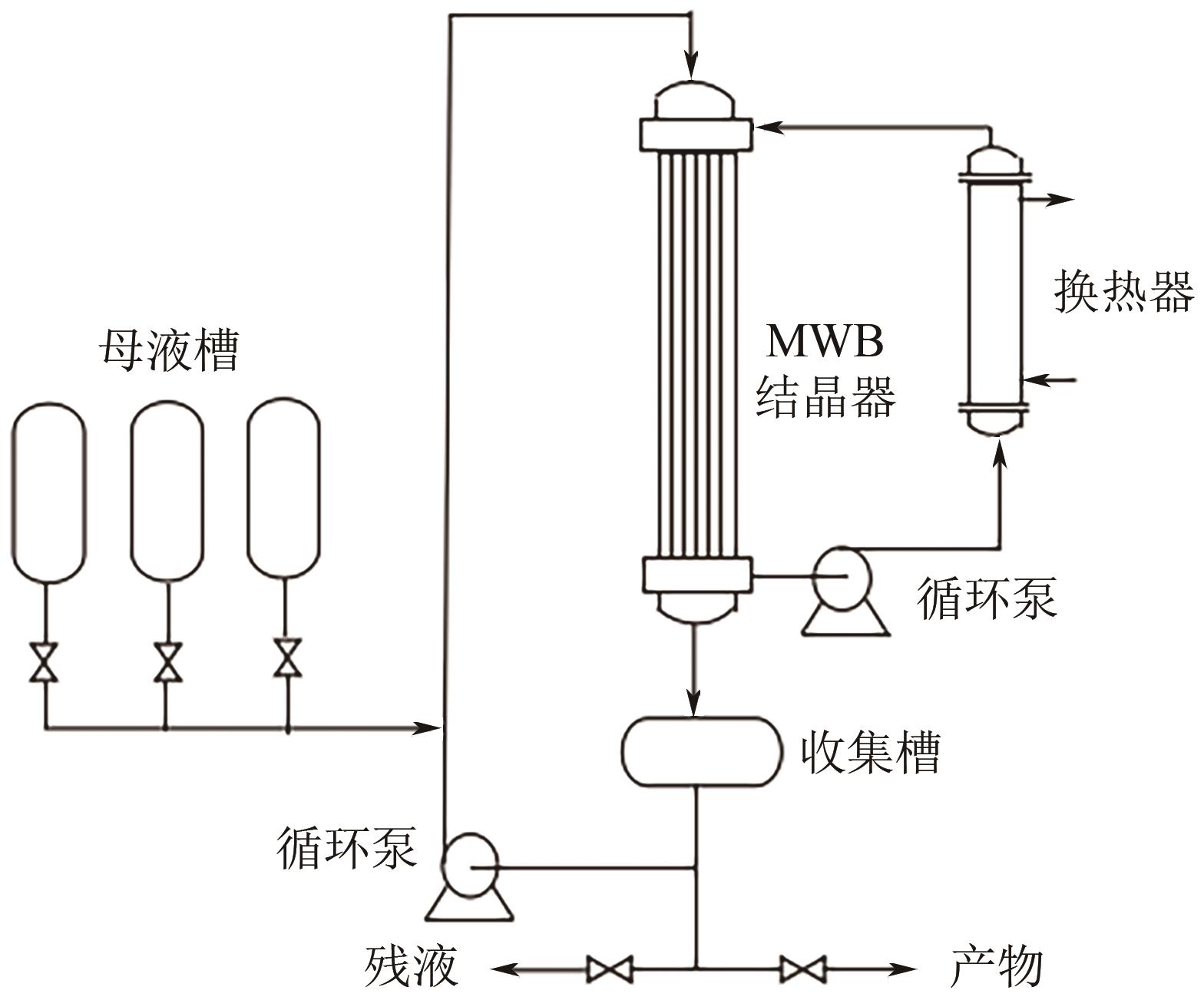
| 层式熔融结晶器 | MWB结晶器 | 结构简单、无运转件、开停车容易 | 苯甲酸、己内酰胺、苯酚、医药中间体 |
| FFC结晶器 | 可随时开停车、操作灵活,能耗低、应用面广、配有计算机辅助控制系统 | 醚醛、萘、对二氯苯、对硝基氯苯 | |
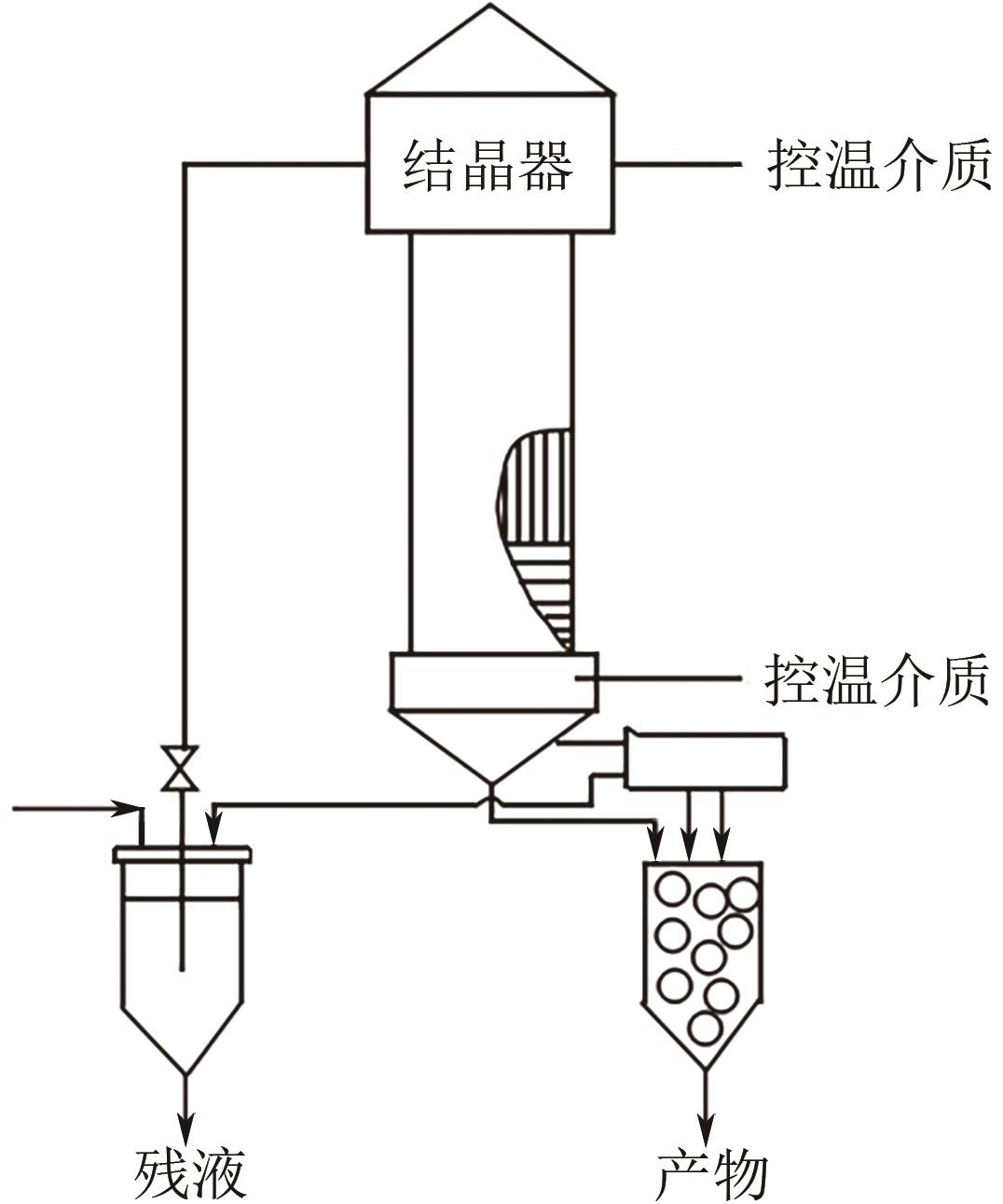
| 悬浮熔融结晶器 | Brodie结晶器 | 投资少、操作费用低、处理量大、操作弹性大、产品纯度高、腐蚀小;设备结构复杂、维修要求高、操作难度大、回收率不高 | 萘、苯、对二氯苯、对二甲苯、对硝基氯苯 |
| Phillips结晶器 | 提纯塔轴向温差较大、能量消耗大、生产经济性较低、产品收率较低 | 二甲苯异构体 | |
| TNO结晶器 | 结晶塔需要振动、工业放大困难、产品纯度高 | 二甲苯、苯并噻吩 | |
| CCCC结晶器 | 运转件简单、容时生产能力较大、操作控制难度大、具有运转件 | 苯、对二甲苯、对二氯苯、萘 | |
| KCP结晶器 | 子设备较多,提纯设备结构复杂并带有运转件,维修及控制要求较高 | 二氯苯、二甲苯、萘 | |
| 倾斜塔式结晶器 | 结晶塔处理能力大、稳定性好、产品纯度高 | 对二氯苯 | |
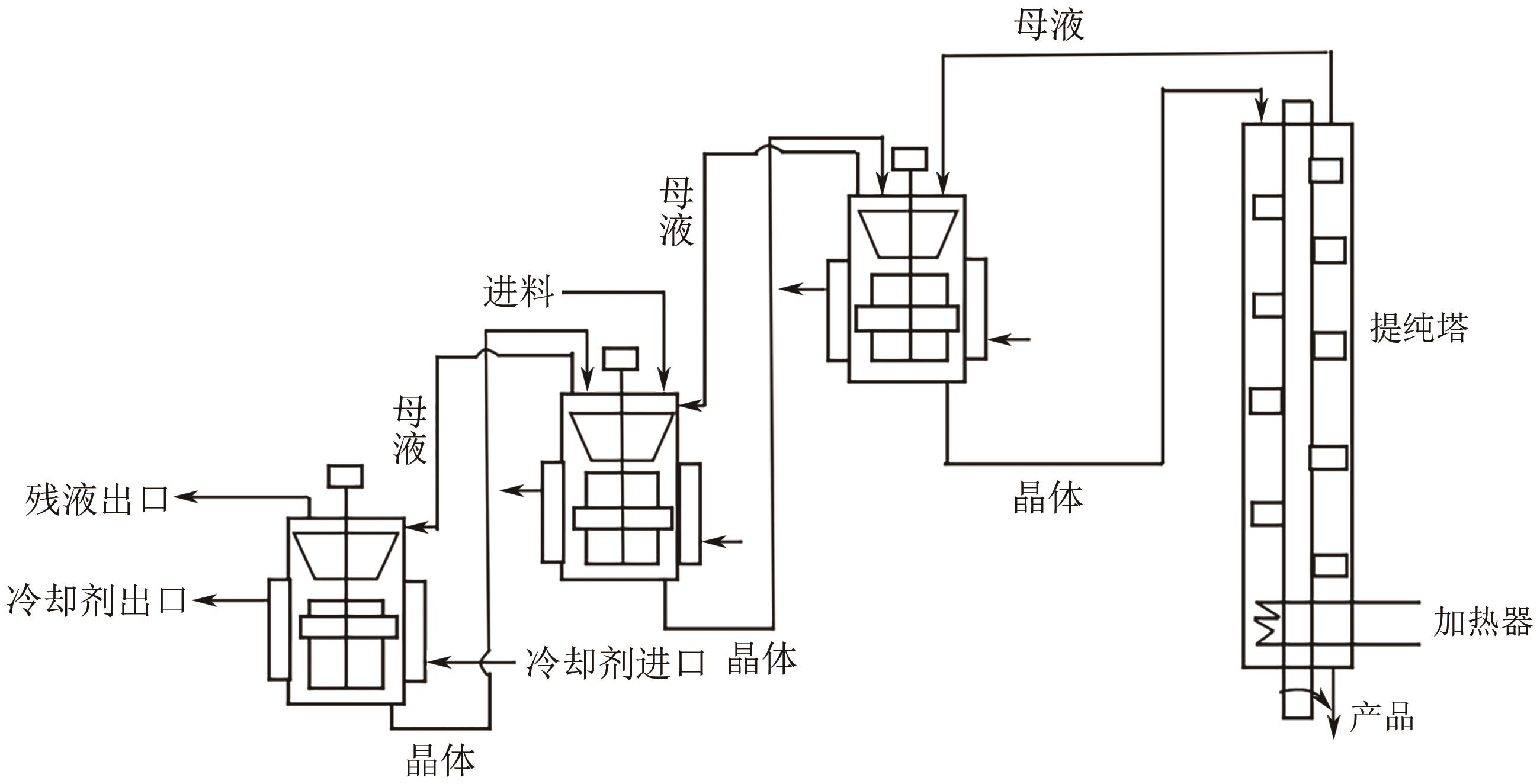
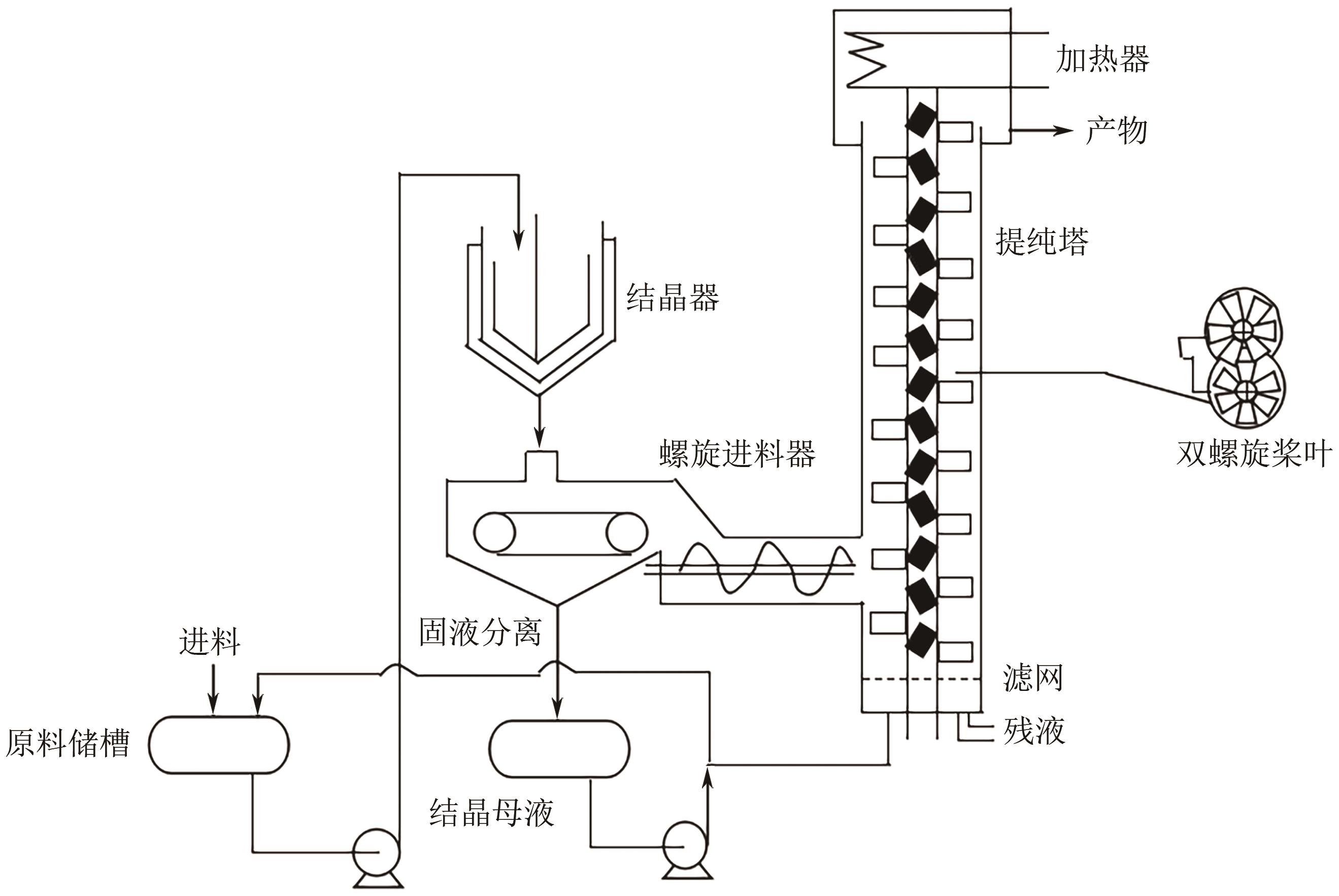
| 区域熔融结晶器 | 箱式熔融结晶器 | 设备体积小、能耗低、产品纯度高、产量低、效率低 | 1, 2-二苯乙烷 |
| 结晶器类型 | 结晶器型号 | 特点 | 分离精制应用 |
|---|---|---|---|
| 层式熔融结晶器 | MWB结晶器 | 结构简单、无运转件、开停车容易 | 苯甲酸、己内酰胺、苯酚、医药中间体 |
| FFC结晶器 | 可随时开停车、操作灵活,能耗低、应用面广、配有计算机辅助控制系统 | 醚醛、萘、对二氯苯、对硝基氯苯 | |
| 悬浮熔融结晶器 | Brodie结晶器 | 投资少、操作费用低、处理量大、操作弹性大、产品纯度高、腐蚀小;设备结构复杂、维修要求高、操作难度大、回收率不高 | 萘、苯、对二氯苯、对二甲苯、对硝基氯苯 |
| Phillips结晶器 | 提纯塔轴向温差较大、能量消耗大、生产经济性较低、产品收率较低 | 二甲苯异构体 | |
| TNO结晶器 | 结晶塔需要振动、工业放大困难、产品纯度高 | 二甲苯、苯并噻吩 | |
| CCCC结晶器 | 运转件简单、容时生产能力较大、操作控制难度大、具有运转件 | 苯、对二甲苯、对二氯苯、萘 | |
| KCP结晶器 | 子设备较多,提纯设备结构复杂并带有运转件,维修及控制要求较高 | 二氯苯、二甲苯、萘 | |
| 倾斜塔式结晶器 | 结晶塔处理能力大、稳定性好、产品纯度高 | 对二氯苯 | |
| 区域熔融结晶器 | 箱式熔融结晶器 | 设备体积小、能耗低、产品纯度高、产量低、效率低 | 1, 2-二苯乙烷 |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率 | 文献 |
|---|---|---|---|---|---|
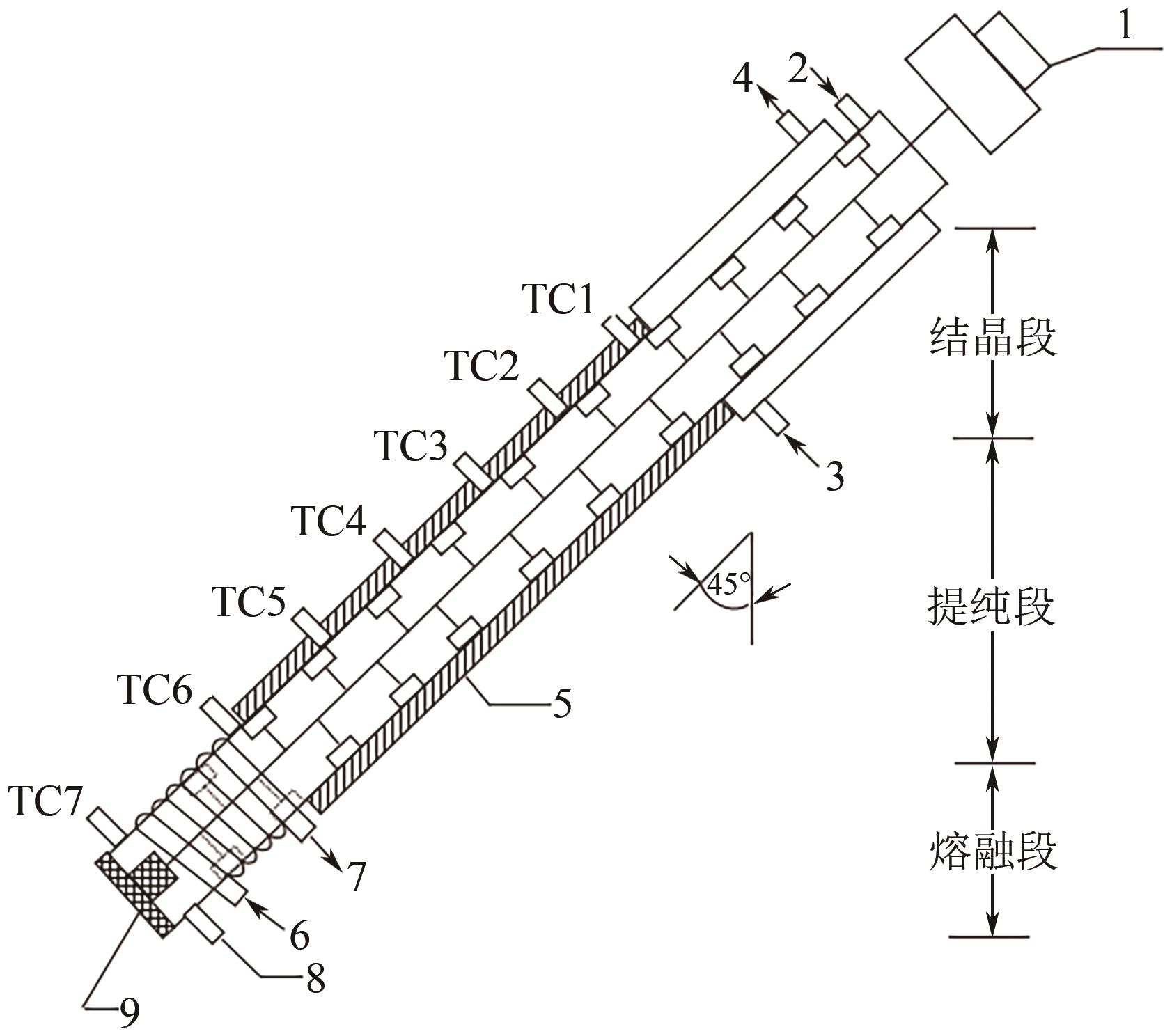
| 悬浮熔融结晶 | 倾斜塔式熔融结晶器 | 对二氯苯+邻二氯苯 | C对二氯苯=80%~93%(质量分数,本表下同), θ=45°, n=15r/min, Tc=25~30℃, τ=48h | C对二氯苯=99.997% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | α-甲基萘+β-甲基萘 | Cα-甲基萘=5.69%, Cβ-甲基萘=84.7% | Cβ-甲基萘=96% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2,4’-MDI+4, 4’-MDI | C4,4’-MDI=92.1% | C4,4’-MDI>99% (四次熔融结晶) | [ |
| 层式熔融结晶 | 鼓泡降膜熔融结晶器 | 对甲酚+间甲酚 | C对甲酚=97%~98%, Q氮气=90L/min, Tci=40℃, tc=40min, Rc=0.6~0.8℃/min, Ts=25~35℃, Rs=0.2~0.3℃/min, ts=40min | C对甲酚=99.42% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 6-叔丁基间甲酚+ 2-叔丁基对甲酚 | C6-叔丁基间甲酚=95.8% | C6-叔丁基间甲酚=99.9%,η=72.4%(两次结晶) | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 1,2,4,5-四甲基苯+ 1,2,3,4-四甲基苯+ 1,2,3,5-四甲基苯 | C1,2,4,5-四甲基苯=94.02%, Tc=73℃,Rc=0.03℃/min, Ts=77℃, ts=30min | C1,2,4,5-四甲基苯=99.06%,η=75.29% | [ |
| 悬浮结晶+区域熔融结晶 | MSMPR结晶器+箱式区域熔融结晶器 | 2,4-TDI+2,6-TDI | 悬浮结晶原料:C2,4-TDI=80%, C2,6-TDI=20%; 区域熔融结晶原料: C2,4-TDI=90.1% | C2,4-TDI=90.1%, η悬浮结晶=12.61%(3次悬浮结晶);C2,4-TDI>98.5%, η熔融结晶=60%(6次熔融结晶) | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 2,4-DNT+2,6-DNT | C2, 4-DNT=76.2%, C2,6-DNT=19.8%, Rc=0.12~0.2℃/min, Tc=25~30℃, Rs<0.1℃/min,Ts=66~68℃, τ=10~15h | C2,4-DNT=98.5%,η=25%~35% | [ |
| 悬浮熔融结晶 | 塔式悬浮熔融结晶器 | 对二甲苯+间二甲苯 | C对二甲苯=93.8% | C对二甲苯>99% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 2,4-TDI+2,6-TDI | C2,4-TDI=80% | C2,4-TDI>95%(一级熔融结晶),C2,4-TDI>99%(二级熔融结晶),η总>40% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对硝基氯苯+间硝基氯苯 | C对硝基氯苯=60%~68% | C对硝基氯苯>99% (两级结晶) | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2,4-二硝基氯苯+ 2,6-二硝基氯苯 | C2,4-二硝基氯苯=84.77%, Tci=49℃, Tcf=47℃, Rc=0.1℃/min, Tsf=49℃, Rs=0.05℃/min | C2,4-二硝基氯苯=99.94% (三级熔融结晶) | [ |
| 层式熔融结晶 | 鼓泡降膜熔融结晶器 | L-丙交酯+M-丙交酯 | CL-丙交酯=96.63%, CM-丙交酯=1.71%,C乳酸=1.2%, ∆T=20℃, tc=60min, ts=50min | CL-丙交酯=97.72% | [ |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率 | 文献 |
|---|---|---|---|---|---|
| 悬浮熔融结晶 | 倾斜塔式熔融结晶器 | 对二氯苯+邻二氯苯 | C对二氯苯=80%~93%(质量分数,本表下同), θ=45°, n=15r/min, Tc=25~30℃, τ=48h | C对二氯苯=99.997% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | α-甲基萘+β-甲基萘 | Cα-甲基萘=5.69%, Cβ-甲基萘=84.7% | Cβ-甲基萘=96% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2,4’-MDI+4, 4’-MDI | C4,4’-MDI=92.1% | C4,4’-MDI>99% (四次熔融结晶) | [ |
| 层式熔融结晶 | 鼓泡降膜熔融结晶器 | 对甲酚+间甲酚 | C对甲酚=97%~98%, Q氮气=90L/min, Tci=40℃, tc=40min, Rc=0.6~0.8℃/min, Ts=25~35℃, Rs=0.2~0.3℃/min, ts=40min | C对甲酚=99.42% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 6-叔丁基间甲酚+ 2-叔丁基对甲酚 | C6-叔丁基间甲酚=95.8% | C6-叔丁基间甲酚=99.9%,η=72.4%(两次结晶) | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 1,2,4,5-四甲基苯+ 1,2,3,4-四甲基苯+ 1,2,3,5-四甲基苯 | C1,2,4,5-四甲基苯=94.02%, Tc=73℃,Rc=0.03℃/min, Ts=77℃, ts=30min | C1,2,4,5-四甲基苯=99.06%,η=75.29% | [ |
| 悬浮结晶+区域熔融结晶 | MSMPR结晶器+箱式区域熔融结晶器 | 2,4-TDI+2,6-TDI | 悬浮结晶原料:C2,4-TDI=80%, C2,6-TDI=20%; 区域熔融结晶原料: C2,4-TDI=90.1% | C2,4-TDI=90.1%, η悬浮结晶=12.61%(3次悬浮结晶);C2,4-TDI>98.5%, η熔融结晶=60%(6次熔融结晶) | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 2,4-DNT+2,6-DNT | C2, 4-DNT=76.2%, C2,6-DNT=19.8%, Rc=0.12~0.2℃/min, Tc=25~30℃, Rs<0.1℃/min,Ts=66~68℃, τ=10~15h | C2,4-DNT=98.5%,η=25%~35% | [ |
| 悬浮熔融结晶 | 塔式悬浮熔融结晶器 | 对二甲苯+间二甲苯 | C对二甲苯=93.8% | C对二甲苯>99% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 2,4-TDI+2,6-TDI | C2,4-TDI=80% | C2,4-TDI>95%(一级熔融结晶),C2,4-TDI>99%(二级熔融结晶),η总>40% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对硝基氯苯+间硝基氯苯 | C对硝基氯苯=60%~68% | C对硝基氯苯>99% (两级结晶) | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2,4-二硝基氯苯+ 2,6-二硝基氯苯 | C2,4-二硝基氯苯=84.77%, Tci=49℃, Tcf=47℃, Rc=0.1℃/min, Tsf=49℃, Rs=0.05℃/min | C2,4-二硝基氯苯=99.94% (三级熔融结晶) | [ |
| 层式熔融结晶 | 鼓泡降膜熔融结晶器 | L-丙交酯+M-丙交酯 | CL-丙交酯=96.63%, CM-丙交酯=1.71%,C乳酸=1.2%, ∆T=20℃, tc=60min, ts=50min | CL-丙交酯=97.72% | [ |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率 | 文献 |
|---|---|---|---|---|---|
| 层式熔融结晶 | 降膜熔融结晶器 | 对二甲苯粗品 | C二甲苯=95%(质量分数,本表下同), C二甲苯=5%, Rc=8℃/h, Tcf=-1℃, Rs=2.5℃·h-1, Tsf=13℃ | C二甲苯=99.52%,η=65.88% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对苯二甲酰氯粗品 | C对苯二甲酰氯=96%, Rc=0.05℃/min, Tcf=-64℃,Rs=0.1℃/min, Tsf=77℃ | C对苯二甲酰氯>99.9% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 丁二腈粗品 | C丁二腈=99.84%, Tc=-47℃, Rc=0.017℃/min,Ts=56℃, Rs=0.03℃/min | C丁二腈=99.959%, η=61.9% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2, 6-二叔丁基对甲酚粗品 | CBHT=90%, Rc=4℃/min, Tcf=50℃, tc=20min,Ts=67℃, ts=1h | CBHT=99.95%,η=75.91% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 芴粗品 | C芴=95%, C甲基氧芴=2.8%, Rc=0.067℃/min,Tcf=108℃, Rs=0.02℃/min, Ts=113℃ | C芴=97.14%,η=49.2% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 乙二醇+乙二醇单甲醚; 乙二醇+1,2-丁二醇; 乙二醇+1,2-丙二醇 | C乙二醇=94.66%(乙二醇+乙二醇单甲醚体系),C乙二醇=93.42%(乙二醇+1, 2-丁二醇体系),C乙二醇=94.87%(乙二醇+1, 2-丙二醇体系) | C乙二醇≥99.8% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对苯二胺粗品 | C对苯二胺=92.35%, Tc1=125℃, Rc1=4℃·h-1,Tsf1=134℃, Rs1=3℃·h-1; Tc2=128℃, Rc2=2℃·h-1, Tsf2=139℃, Rs2=2℃·h-1 | C对苯二胺=99.7%,η=77.21%(两级熔融结晶) | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 异丙苯脱苯塔塔顶馏出液 | C苯=55.554%, Tcf=-28℃, tcg=90min,Rs=0.1℃/min, Tsf=-15℃, tss=90min | C苯=99.5% (两级熔融结晶) | [ |
| 层式熔融结晶 | 降膜结晶器 | 2-吡咯酮粗品 | C2-吡咯酮=99.5%, Tpre=25℃, QF=1.5L·h-1, Rc=6℃·h-1, Tcf=8℃, Rs=4℃·h-1, Tsf=19℃ | C2-吡咯酮>99.9%,η>73.3% | [ |
| 层式熔融结晶 | MWB结晶器 | N-乙烯基-2-吡咯烷酮粗品 | CNVP=99.27%, Rc=0.07℃/min, Tcf=11℃,Rs=0.4℃/min, Tsf=14℃, ts=20min | CNVP>99.99% (两级熔融结晶) | [ |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率 | 文献 |
|---|---|---|---|---|---|
| 层式熔融结晶 | 降膜熔融结晶器 | 对二甲苯粗品 | C二甲苯=95%(质量分数,本表下同), C二甲苯=5%, Rc=8℃/h, Tcf=-1℃, Rs=2.5℃·h-1, Tsf=13℃ | C二甲苯=99.52%,η=65.88% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对苯二甲酰氯粗品 | C对苯二甲酰氯=96%, Rc=0.05℃/min, Tcf=-64℃,Rs=0.1℃/min, Tsf=77℃ | C对苯二甲酰氯>99.9% | [ |
| 层式熔融结晶 | 降膜熔融结晶器 | 丁二腈粗品 | C丁二腈=99.84%, Tc=-47℃, Rc=0.017℃/min,Ts=56℃, Rs=0.03℃/min | C丁二腈=99.959%, η=61.9% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2, 6-二叔丁基对甲酚粗品 | CBHT=90%, Rc=4℃/min, Tcf=50℃, tc=20min,Ts=67℃, ts=1h | CBHT=99.95%,η=75.91% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 芴粗品 | C芴=95%, C甲基氧芴=2.8%, Rc=0.067℃/min,Tcf=108℃, Rs=0.02℃/min, Ts=113℃ | C芴=97.14%,η=49.2% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 乙二醇+乙二醇单甲醚; 乙二醇+1,2-丁二醇; 乙二醇+1,2-丙二醇 | C乙二醇=94.66%(乙二醇+乙二醇单甲醚体系),C乙二醇=93.42%(乙二醇+1, 2-丁二醇体系),C乙二醇=94.87%(乙二醇+1, 2-丙二醇体系) | C乙二醇≥99.8% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 对苯二胺粗品 | C对苯二胺=92.35%, Tc1=125℃, Rc1=4℃·h-1,Tsf1=134℃, Rs1=3℃·h-1; Tc2=128℃, Rc2=2℃·h-1, Tsf2=139℃, Rs2=2℃·h-1 | C对苯二胺=99.7%,η=77.21%(两级熔融结晶) | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 异丙苯脱苯塔塔顶馏出液 | C苯=55.554%, Tcf=-28℃, tcg=90min,Rs=0.1℃/min, Tsf=-15℃, tss=90min | C苯=99.5% (两级熔融结晶) | [ |
| 层式熔融结晶 | 降膜结晶器 | 2-吡咯酮粗品 | C2-吡咯酮=99.5%, Tpre=25℃, QF=1.5L·h-1, Rc=6℃·h-1, Tcf=8℃, Rs=4℃·h-1, Tsf=19℃ | C2-吡咯酮>99.9%,η>73.3% | [ |
| 层式熔融结晶 | MWB结晶器 | N-乙烯基-2-吡咯烷酮粗品 | CNVP=99.27%, Rc=0.07℃/min, Tcf=11℃,Rs=0.4℃/min, Tsf=14℃, ts=20min | CNVP>99.99% (两级熔融结晶) | [ |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率、杂质含量 | 文献 |
|---|---|---|---|---|---|
| 区域熔融结晶 | 箱式区域熔融结晶器 | 联苄粗品 | C联苄=98.35%(质量分数,本表下同), v=8.8mm·h-1, N=8, Th=60℃, Tc=15℃, Z=0.13 | C联苄=99.84%, η=50% | [ |
| 层式熔融结晶 | 指型结晶器 | 苯甲酸粗品 | C邻苯二甲酸=1335.59mg·kg-1, Rc=1.5℃·h-1,Tcf=110℃, Rs=6℃·h-1, Tsf=122℃ | C邻苯二甲酸=97.83mg·kg-1, η=56.08% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2-氯-5-三氟 甲基吡啶粗品 | C2-氯-5-三氟甲基吡啶=89%, C3-氯-5-三氟甲基吡啶=2.8%, C2-氯-3-三氟甲基吡啶=1.7%, Rc=0.071℃/min, Tcf=16~19℃, Rs=0.062~0.083℃/min,Tsf=28~30℃ | C2-氯-5-三氟甲基吡啶=99%,η=40% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 邻碘苯胺粗品 | C邻碘苯胺=70.95%, Rc=0.06℃/min, Tcf=28℃, tcg=2h, Rs=0.04℃/min, Tsf=40℃ | C邻碘苯胺=99.07%,η=61.75% | [ |
| 减压精馏+层式熔融结晶 | 降膜熔融结晶器 | 人造麝香粗品 | CDDHI=85%, Rc=4℃·h-1, tc=2h, Rs=6℃·h-1, ts=30min, 通氮气, 二级结晶 | CDDHI=99.2%, η=63.2% | [ |
| 层式熔融结晶 | 翅片式降膜结晶器 | 苯甲酸粗品 | C苯甲酸=94.5% | C苯甲酸=99.5% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 棕榈油 | 硬酯:C棕榈酸=42.63%, C油酸=34.71%; 软酯:C棕榈酸=50.02%, C油酸=30.89% | [ | |
| 区域熔融结晶 | 菲粗品 | C菲=98%, N=30 | C菲>98.5% | [ | |
| 区域熔融结晶 | 箱式区域熔融结晶器 | 联苄粗品 | C联苄=98.35%, v=4.97mm·h-1或12.35mm·h-1, Th=60℃, Tc=10~30℃ | C联苄(变熔区)>C联苄(恒熔区), C联苄(双熔区)>C联苄(单熔区) | [ |
| 分离方式 | 结晶器 | 原料 | 工艺参数 | 产品纯度、收率、杂质含量 | 文献 |
|---|---|---|---|---|---|
| 区域熔融结晶 | 箱式区域熔融结晶器 | 联苄粗品 | C联苄=98.35%(质量分数,本表下同), v=8.8mm·h-1, N=8, Th=60℃, Tc=15℃, Z=0.13 | C联苄=99.84%, η=50% | [ |
| 层式熔融结晶 | 指型结晶器 | 苯甲酸粗品 | C邻苯二甲酸=1335.59mg·kg-1, Rc=1.5℃·h-1,Tcf=110℃, Rs=6℃·h-1, Tsf=122℃ | C邻苯二甲酸=97.83mg·kg-1, η=56.08% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 2-氯-5-三氟 甲基吡啶粗品 | C2-氯-5-三氟甲基吡啶=89%, C3-氯-5-三氟甲基吡啶=2.8%, C2-氯-3-三氟甲基吡啶=1.7%, Rc=0.071℃/min, Tcf=16~19℃, Rs=0.062~0.083℃/min,Tsf=28~30℃ | C2-氯-5-三氟甲基吡啶=99%,η=40% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 邻碘苯胺粗品 | C邻碘苯胺=70.95%, Rc=0.06℃/min, Tcf=28℃, tcg=2h, Rs=0.04℃/min, Tsf=40℃ | C邻碘苯胺=99.07%,η=61.75% | [ |
| 减压精馏+层式熔融结晶 | 降膜熔融结晶器 | 人造麝香粗品 | CDDHI=85%, Rc=4℃·h-1, tc=2h, Rs=6℃·h-1, ts=30min, 通氮气, 二级结晶 | CDDHI=99.2%, η=63.2% | [ |
| 层式熔融结晶 | 翅片式降膜结晶器 | 苯甲酸粗品 | C苯甲酸=94.5% | C苯甲酸=99.5% | [ |
| 层式熔融结晶 | 静态熔融结晶器 | 棕榈油 | 硬酯:C棕榈酸=42.63%, C油酸=34.71%; 软酯:C棕榈酸=50.02%, C油酸=30.89% | [ | |
| 区域熔融结晶 | 菲粗品 | C菲=98%, N=30 | C菲>98.5% | [ | |
| 区域熔融结晶 | 箱式区域熔融结晶器 | 联苄粗品 | C联苄=98.35%, v=4.97mm·h-1或12.35mm·h-1, Th=60℃, Tc=10~30℃ | C联苄(变熔区)>C联苄(恒熔区), C联苄(双熔区)>C联苄(单熔区) | [ |
| 结晶类型 | 提纯体系 | 模型方程 | 模型假设 | 文献 |
|---|---|---|---|---|
| 动态层式熔融结晶 | 对二甲苯 | jl=αl∆Tl, ∆Tl=(Tl0-T0)exp(-lαl/cplΓ) | ①过程温度的改变对液膜物性的影响可以忽略。②晶层与液膜之间的接触面温度等于结晶温度T0,液膜的平均浓度Cl决定T0。③液膜的平均浓度和流速在结晶管轴向方向上数值一致。④局部对流传热系数ɑl不随着管长变化。⑤过程喷淋密度Γ的变化忽略不计 | [ |
| 悬浮熔融结晶 | 对二甲苯 | 沿塔高的浓度分布: WL=a+(WL,0-a)exp(-bz), | ①提纯段为稳定状态。②径向无浓度差。③晶体相和回流熔融液以及其他参数沿提纯段不变 | [ |
| 区域熔融结晶 | 联苄、TDI | 一次区熔:(1)区域1(0≤x<1-z),C1(x)/C0=1-(1-ke)exp(-kex/z);(2) 区域2(1-z<x≤1),C1(x)/C0={1-(1-ke)exp[-ke(1-z)/z]}×{1-[x-(1-z)]/z} 多次区熔: (1) 区域1(x=0), (2) 区域2(0<x≤1-z), (3) 区域3(1-z<x<1), (4) 区域4(x=1), | ①恒定的熔区长度。②恒定的熔区移动速度。③恒定和相等的切面面积。④恒定的密度(在固体和液体中)。⑤均一的起始浓度。⑥冷凝界面上处于平衡状态。⑦平整的结晶界面。⑧恒定的扩散系数(在熔化物中)。⑨固体中没有扩散 | [ |
| 结晶类型 | 提纯体系 | 模型方程 | 模型假设 | 文献 |
|---|---|---|---|---|
| 动态层式熔融结晶 | 对二甲苯 | jl=αl∆Tl, ∆Tl=(Tl0-T0)exp(-lαl/cplΓ) | ①过程温度的改变对液膜物性的影响可以忽略。②晶层与液膜之间的接触面温度等于结晶温度T0,液膜的平均浓度Cl决定T0。③液膜的平均浓度和流速在结晶管轴向方向上数值一致。④局部对流传热系数ɑl不随着管长变化。⑤过程喷淋密度Γ的变化忽略不计 | [ |
| 悬浮熔融结晶 | 对二甲苯 | 沿塔高的浓度分布: WL=a+(WL,0-a)exp(-bz), | ①提纯段为稳定状态。②径向无浓度差。③晶体相和回流熔融液以及其他参数沿提纯段不变 | [ |
| 区域熔融结晶 | 联苄、TDI | 一次区熔:(1)区域1(0≤x<1-z),C1(x)/C0=1-(1-ke)exp(-kex/z);(2) 区域2(1-z<x≤1),C1(x)/C0={1-(1-ke)exp[-ke(1-z)/z]}×{1-[x-(1-z)]/z} 多次区熔: (1) 区域1(x=0), (2) 区域2(0<x≤1-z), (3) 区域3(1-z<x<1), (4) 区域4(x=1), | ①恒定的熔区长度。②恒定的熔区移动速度。③恒定和相等的切面面积。④恒定的密度(在固体和液体中)。⑤均一的起始浓度。⑥冷凝界面上处于平衡状态。⑦平整的结晶界面。⑧恒定的扩散系数(在熔化物中)。⑨固体中没有扩散 | [ |
| 1 | 李婷, 侯经纬, 李潇, 等. 熔融结晶在重芳烃分离中的应用[J]. 过程工程学报, 2020, 20(6): 628-637. |
| LI Ting, HOU Jingwei, LI Xiao, et al. Application of melt crystallization in the purification of heavy aromatics[J]. The Chinese Journal of Process Engineering, 2020, 20(6): 628-637. | |
| 2 | 刘海岛, 尹秋响. 熔融结晶及其耦合技术研究的进展[J]. 化学工业与工程, 2004, 21(5): 367-371. |
| LIU Haidao, YIN Qiuxiang. Progress in melt crystallization and its hybrid technique[J]. Chemical Industry and Engineering, 2004, 21(5): 367-371. | |
| 3 | 张国荣, 陈慧萍, 王国安. 结晶技术在医药生产中的应用[J]. 应用化工, 2015, 44(1): 154-158. |
| ZHANG Guorong, CHEN Huiping, WANG Guo’an. Application of crystallization technology in pharmaceuticals production[J]. Applied Chemical Industry, 2015, 44(1): 154-158. | |
| 4 | 刘海岛. 辛基酚熔融结晶过程的研究[D]. 天津: 天津大学, 2004. |
| LIU Haidao. Study of melt crystallization of octyl phenol[D]. Tianjin: Tianjin University, 2004. | |
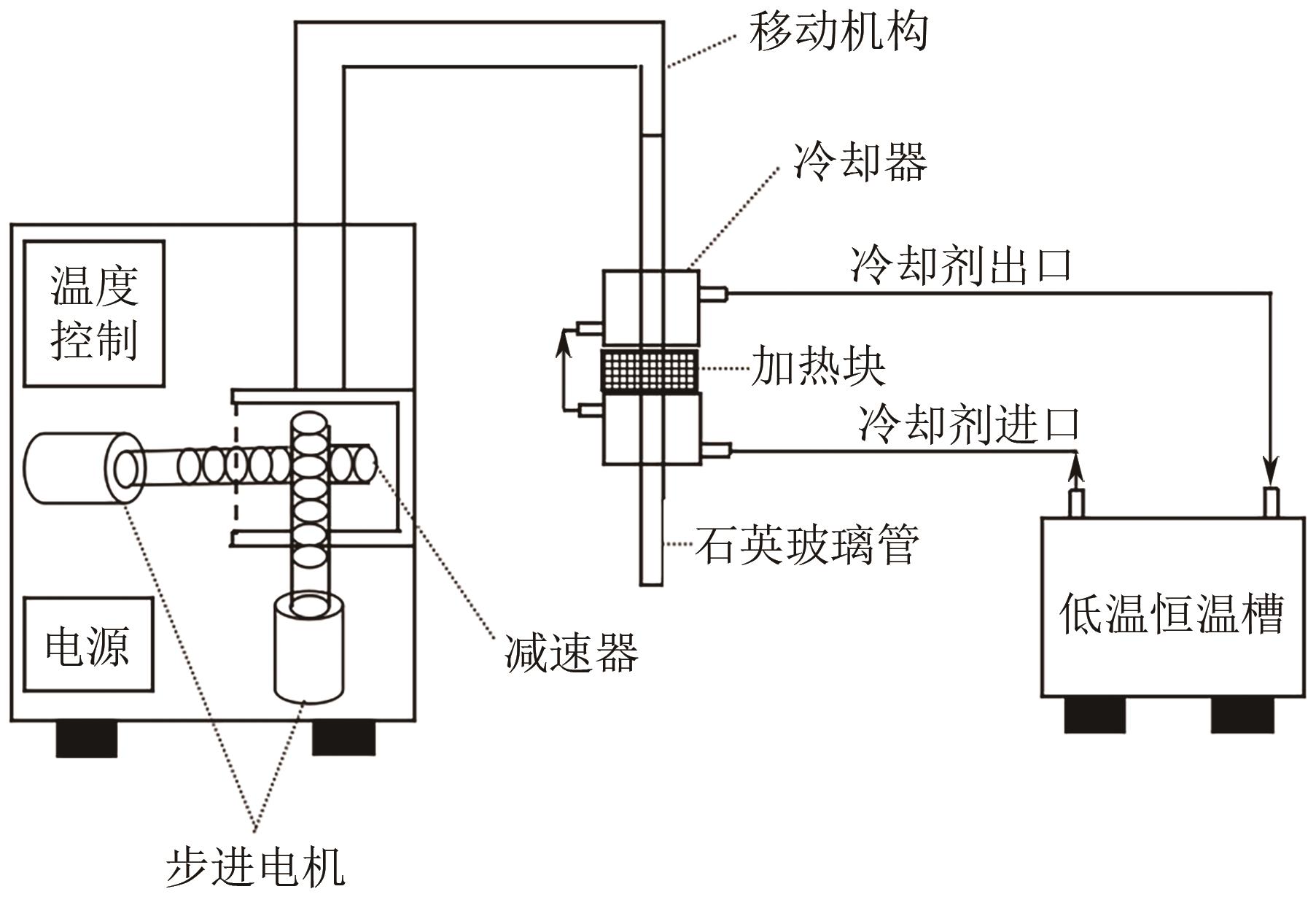
| 5 | 齐亚兵. 联苄的区域熔融结晶纯化过程研究[D]. 成都: 四川大学,2013. |
| QI Yabing. Investigationon on purification of bibenzyl by zone refining[D]. Chengdu: Sichuan University, 2013. | |
| 6 | ARKENBOUT F G. Melt crystallization technology [M]. Phoenix: Technomic Publishing Co., Inc., 1995. |
| 7 | WYNN N P. Separate organics by melt crystallization[J].Chemical Engineering Progress, 1992, 88(3): 52-60 |
| 8 | REN Yongsheng, LI Jun, DUAN Xiaoxiao. Determination of equilibrium distribution coefficients of impurities in phosphorus by vertical zone-melting technique[J]. Chinese Journal of Chemical Engineering, 2011, 19(2): 223-226. |
| 9 | BULAU H C, ULRICH J. On the purification potential of a new continuous melt crystallization process[A]// Proceedings of the international symposium on industrial crystallization (ISIC’1998)[C]. Beijing: Chemical Industrial Press, 1998. |
| 10 | MYASNIKOV S K, UTESHINSKY A D, KULOV N N. Hybrid of pervaporation and condensation-distillation crystallization: a new combined separation technology[J]. Theoretical Foundations of Chemical Engineering, 2003, 37(6): 527-532. |
| 11 | 叶青, 王车礼, 裘兆蓉. 精馏-熔融结晶耦合工艺[J]. 江苏石油化工学院学报, 2000, 12(3): 46-49. |
| YE Qing, WANG Cheli, QIU Zhaorong. Distillation-melt crystallization hybrid process[J]. Journal of Jiangsu Institute of Petrochemical Technology, 2000, 12(3): 46-49. | |
| 12 | 景博, 常泽伟, 贾晟哲, 等. 熔融结晶的过程强化[J]. 化工学报, 2021, 72(8): 3907-3918. |
| JING Bo, CHANG Zewei, JIA Shengzhe, et al. Process intensification of melt crystallization[J]. CIESC Journal, 2021, 72(8): 3907-3918. | |
| 13 | LI Chunli, ZHOU Yiwei, SU Weiyi, et al. Research progress of hybrid distillation/crystallization technology[J]. Chemical Engineering & Technology, 2018, 41(10): 1894-1904. |
| 14 | BEIERLING T, MICOVIC J, LUTZE P, et al. Using complex layer melt crystallization models for the optimization of hybrid distillation/melt crystallization processes[J]. Chemical Engineering and Processing: Process Intensification, 2014, 85: 10-23. |
| 15 | MOLINAR J G D, DODGSON B V. Theory and practice related to the Brodie purifier[J]. The Chemical Engineer, 1974, 7/8: 460-464. |
| 16 | BRODIE J A. A continuous multi-stage melt purification process[J]. Mechanical & Chemical Engineering Transaction, 1971, 5: 37-44. |
| 17 | BRODIE J A. Solid-liquid continuous countercurrent purifier method and apparatus: US3645699[P]. 1972-02-29. |
| 18 | MCKAY D L, GOARD H W. Continuous fractional crystallization[J]. Chemical Engineering Progress, 1965, 61(11): 99-104. |
| 19 | MARWILL S J, KOLNER S J. Pulsed column purification of para-xylene[J]. Chemical Engineering Progress, 1963, 59(2): 60-65. |
| 20 | MCKAY D L, DALE G H, WEEDMAN J A. A bench-scale crystallization purification column[J]. Industrial & Engineering Chemistry, 1960, 52(3): 197-200. |
| 21 | MCKAY D L, DALE G H, TABLER D C. Para-xylene via fractional crystallization[J]. Chemical Engineering Progress, 1966, 62: 104-112. |
| 22 | ARKENBOUT G J, VAN KUIJK A, VAN DER MEER J, et al. Pulsed crystallization column and method of countercurrent crystallization: US4400189[P]. 1983-08-23. |
| 23 | ARKENBOUT G J. Crystallization column: US4257796[P]. 1981-03-24. |
| 24 | ARKENBOUT G J, KUIJK A V, SMIT W M. New simple crystallization column[J]. Chemistry and Industry, 1973, 3(2): 139-142. |
| 25 | SAXER K, PAPP A. The MWB Crystallization Process[J]. Chemical Engineering Progress, 1980, 76(4): 64-66. |
| 26 | 王静康. 工业结晶的现在与未来[J]. 化学工程, 1992, 20(2): 57-63. |
| WANG Jingkang. Today and tomorrow of industrial crystallization[J]. Chemical Engineering (China), 1992, 20(2): 57-63. | |
| 27 | TAKEGAMI K, MORITA M, NAKAMARU K, et al. Countercurrent, cooling crystallization and purification method for multi-component molten mixture: US4588414[P]. 1986-05-13. |
| 28 | HAYASHI Y, KODAMA K, OHTA M, et al. Apparatus for purifying crystals comprising a stirrer with fins forming a discontinuous spiral: US3770386[P]. 1973-11-06. |
| 29 | 陈亮. 倾斜结晶塔内有机物结晶纯化过程的研究[D]. 成都: 四川大学, 2007. |
| CHEN Liang. An investigation on the purification process of organic compounds in an inclined column crystallizer[D]. Chengdu: Sichuan University, 2007. | |
| 30 | QI Yabing, LI Jun, ZHOU Kun, et al. Study on segregation process and equilibrium distribution coefficients of impurities during zone refining of bibenzyl[J]. Separation Science and Technology, 2013, 48(5): 820-826. |
| 31 | 朱政. 熔融结晶法提纯β-甲基萘研究[D]. 天津: 天津大学, 2007. |
| ZHU Zheng. A study on the purification of β-methylnaphthalene by melt crystallization[D]. Tianjin: Tianjin University, 2007. | |
| 32 | 姜福美. 结晶-精馏耦合分离MDI同分异构体的研究[D]. 青岛: 中国海洋大学, 2008. |
| JIANG Fumei. Crystal-distillation hybrid to separate MDI isomeric[D]. Qingdao: Ocean University of China, 2008. | |
| 33 | CONG Shan, LI Xingang, WU Jun, et al. Optimization of parameters for melt crystallization of p-cresol[J]. Chinese Journal of Chemical Engineering, 2012, 20(4): 649-653. |
| 34 | 李智勇. 6-叔丁基间甲酚熔融结晶过程研究[D]. 天津: 天津大学, 2012. |
| LI Zhiyong. A study on the melt crystallization process of 6-tert-buty-3-methylphenol[D]. Tianjin: Tianjin University, 2012. | |
| 35 | CONG Shan, LIU Ying, LI Hong, et al. Purification and separation of durene by static melt crystallization[J]. Chinese Journal of Chemical Engineering, 2015, 23(3): 505-509. |
| 36 | 颜跃勇. 区域熔融法纯化TDI过程研究[D]. 成都: 成都理工大学, 2016. |
| YAN Yueyong. The investigation on purification process of TDI by zone melting[D]. Chengdu: Chengdu University of Technology, 2016. | |
| 37 | 雍亚兰. 结晶发汗法分离2, 4-二硝基甲苯的研究[D]. 北京: 北京理工大学, 2016. |
| YONG Yalan. The research on the separation of 2, 4-dinitrotoluene by crystallization and sweating process[D]. Beijing: Beijing Institute of Technology, 2016. | |
| 38 | 宋书恒. 对二甲苯熔融结晶过程实验及数学模型研究[D]. 湘潭: 湘潭大学, 2016. |
| SONG Shuheng. The crystallization purification process and mathematical model of para-xylene[D]. Xiangtan: Xiangtan University, 2016. | |
| 39 | 杨博. 降膜结晶法制备TDI-100[D]. 青岛: 青岛科技大学, 2019. |
| YANG Bo. Preparation of TDI-100 by falling film crystallization[D]. Qingdao: Qingdao University of Science & Technology, 2019. | |
| 40 | 林韬, 张卫江, 肖梦然, 等. 熔融结晶分离提纯对间硝基氯苯的研究[J]. 天津大学学报, 2007, 40(4): 444-448. |
| LIN Tao, ZHANG Weijiang, XIAO Mengran, et al. Purification of p-nitrochlorobenzene and m-nitrochlorobenzene by melt crystallization[J]. Journal of Tianjin University, 2007, 40(4): 444-448. | |
| 41 | JIA Shengzhe, JING Bo, HONG Wei, et al. Purification of 2, 4-dinitrochlorobenzene using layer melt crystallization: model and experiment[J]. Separation and Purification Technology, 2021, 270: 118806. |
| 42 | 张涛, 石建明, 张绍军, 等. 熔融分步结晶提纯L-丙交酯[J]. 化学工程, 2010, 38(12): 22-25, 59. |
| ZHANG Tao, SHI Jianming, ZHANG Shaojun, et al. Purification of L-lactide by melt fractional crystallization[J]. Chemical Engineering (China), 2010, 38(12): 22-25, 59. | |
| 43 | 沈澍. 熔融结晶法纯化对二甲苯研究[D]. 天津: 天津大学, 2017. |
| SHEN Shu. Study on the purification of para-xylene by melt crystallization[D]. Tianjin: Tianjin University, 2017. | |
| 44 | 李改真. 熔融结晶法提纯对苯二甲酰氯[D]. 开封: 河南大学, 2017. |
| LI Gaizhen. Purification of terephthaloyl chloride by melt crystallization[D]. Kaifeng: Henan University, 2017. | |
| 45 | 王冬冬. 丁二腈熔融结晶过程的研究[D]. 天津:天津大学,2018. |
| WANG Dongdong. Study on the melting crystallization process of succinonitrile[D]. Tianjin: Tianjiin University, 2018. | |
| 46 | 侯亚伟. 熔融结晶法提纯BHT研究[D]. 天津: 天津科技大学, 2012. |
| HOU Yawei. Study on purification of BHT with melt crystallization[D]. Tianjin: Tianjin University of Science & Technology, 2012. | |
| 47 | 贾春燕, 尹秋响, 张美景, 等. 利用熔融结晶法进行芴的提纯[J]. 化工学报, 2007, 58(9): 2266-2269. |
| JIA Chunyan, YIN Qiuxiang, ZHANG Meijing, et al. Purification of fluorene by melt crystallization[J]. CIESC, 2007, 58(9): 2266-2269. | |
| 48 | WANG Tiefeng, DONG Jinxiang. Ethylene glycol purification by melt crystallization: removal of 2-methoxyethanol impurity[J]. Chinese Journal of Chemical Engineering, 2021, 37: 39-45. |
| 49 | WANG Tiefeng, LI Xu, DONG Jinxiang. Ethylene glycol purification by melt crystallization: removal of short-chain glycol impurities[J]. Industrial & Engineering Chemistry Research, 2020, 59(18): 8805-8812. |
| 50 | 和树宝. 熔融结晶法分离胺类化合物的研究[D]. 青岛: 青岛科技大学, 2012. |
| HE Shubao. Study on separation of amine compounds by melt crystallization[D]. Qingdao: Qingdao University of Science & Technology, 2012. | |
| 51 | 高松, 张傑, 张可. 熔融结晶法从异丙苯装置污苯中回收苯的研究[J]. 现代化工, 2018, 38(2): 144-148. |
| GAO Song, ZHANG Jie, ZHANG Ke. Recovery of benzene from light benzene of cumene production facility by melt crystallization[J]. Modern Chemical Industry, 2018, 38(2): 144-148. | |
| 52 | CHEN Wei, LI Siqi, LI Sifang. Purification of 2-pyrrolidone by falling film melt crystallization[J]. Industrial & Engineering Chemistry Research, 2021, 60(36): 13286-13292. |
| 53 | KIM S H, SEO M D, TAK M S, et al. Effects of sweating time and cooling strategy on purification of N-vinyl-2-pyrrolidinone using a melt crystallizer[J]. Korean Journal of Chemical Engineering, 2013, 30(11): 1997-2000. |
| 54 | 李亚楠. 苯甲酸提纯工艺的研究[D]. 天津: 天津科技大学, 2017. |
| LI Yanan. Study on the purification technology of benzoic acid[D]. Tianjin: Tianjin University of Science & Technology, 2017. | |
| 55 | 胡猛, 于万金, 董青青, 等. 熔融结晶法提纯2-氯-5-三氟甲基吡啶[J]. 现代化工, 2015, 35(4): 65-67, 69. |
| HU Meng, YU Wanjin, DONG Qingqing, et al. Purification of 2-chloro-5-(trifluoromethyl) pyridine by melt crystallization[J]. Modern Chemical Industry, 2015, 35(4): 65-67, 69. | |
| 56 | 许奎, 朱静, 胡雪, 等. 熔融结晶法提纯邻碘苯胺工艺[J]. 现代化工, 2019, 39(3): 136-138, 140. |
| XU Kui, ZHU Jing, HU Xue, et al. Purification of o-iodoaniline by melt crystallization[J]. Modern Chemical Industry, 2019, 39(3): 136-138, 140. | |
| 57 | 叶青, 裘兆蓉, 王车礼, 等. 用熔融结晶技术提纯人造麝香DDHI[J]. 精细化工, 2001, 18(5): 260-261, 277. |
| YE Qing, QIU Zhaorong, WANG Cheli, et al. Refinement of musk DDHI by melt crystallization[J]. Fine Chemicals, 2001, 18(5): 260-261, 277. | |
| 58 | 段晓宇, 赵风云, 李飞龙, 等. 利用熔融结晶法进行苯甲酸的提纯[J]. 现代化工, 2015, 35(1): 92-94. |
| DUAN Xiaoyu, ZHAO Fengyun, LI Feilong, et al. Purification of benzoic acid by melt crystallization[J]. Modern Chemical Industry, 2015, 35(1): 92-94. | |
| 59 | 陆超. 棕榈油熔融层结晶分离技术的研究[D]. 天津: 天津大学, 2020. |
| LU Chao. The study on fractionation of palm oil using melt layer crystallization[D]. Tianjin: Tianjin University, 2020. | |
| 60 | COUVRAT N, BUREL A, TISSE S, et al. Combining zone melting and preparative chromatography to purify phenanthrene[J]. Journal of Thermal Analysis and Calorimetry, 2013, 112(1): 293-300. |
| 61 | QI Yabing, LI Jun. Process parameters influence on zone refining and thermodynamics analysis of 1,2-diphenylethane[J]. Chinese Journal of Chemical Engineering, 2022, 42: 338-343. |
| 62 | WANG Shui, ZHAO Guojing, DU Yizhen, et al. Experimental and theoretical investigation of a new multistage countercurrent melt crystallizer with inclined sieve plates[J]. Korean Journal of Chemical Engineering, 2015, 32(6): 1151-1157. |
| 63 | QI Yabing, LI Jun, WANG Baoming, et al. Numerical modeling and optimization of zone refining in high purification of bibenzyl[J]. Journal of Chemical Engineering of Japan, 2012, 45(8): 571-576. |
| 64 | ZHOU K, YAN Y Y, AN L Y, et al. Numerical modeling for simulation and experimental investigation on purification process of TDI by zone melting [J]. Revue Roumaine De Chimie, 2017, 62(12): 947-955. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [3] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [4] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [5] | ZHANG Tingting, ZUO Xuqian, TIAN Lingdi, WANG Shimeng. Construction method of volatile organic compounds emission inventory and factor database in chemical industry park [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 549-557. |
| [6] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [7] | SHENG Weiwu, CHENG Yongpan, CHEN Qiang, LI Xiaoting, WEI Jia, LI Linge, CHEN Xianfeng. Operating condition analysis of the microbubble and microdroplet dual-enhanced desulfurization reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 142-147. |
| [8] | HE Meijin. Application and development trend of molecular management in separation technology in petrochemical field [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 260-266. |
| [9] | LIAO Zhixin, LUO Tao, WANG Hong, KONG Jiajun, SHEN Haiping, GUAN Cuishi, WANG Cuihong, SHE Yucheng. Application and progress of solvent deasphalting technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4573-4586. |
| [10] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [11] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [12] | PAN Yichang, ZHOU Rongfei, XING Weihong. Advanced microporous membranes for efficient separation of same-carbon-number hydrocarbon mixtures: State-of-the-art and challenges [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3926-3942. |
| [13] | ZHOU Longda, ZHAO Lixin, XU Baorui, ZHANG Shuang, LIU Lin. Advances in electrostatic-cyclonic coupling enhanced multiphase media separation research [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3443-3456. |
| [14] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [15] | CHEN Xiangli, LI Qianqian, ZHANG Tian, LI Biao, LI Kangkang. Research progress on self-healing oil/water separation membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3600-3610. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||