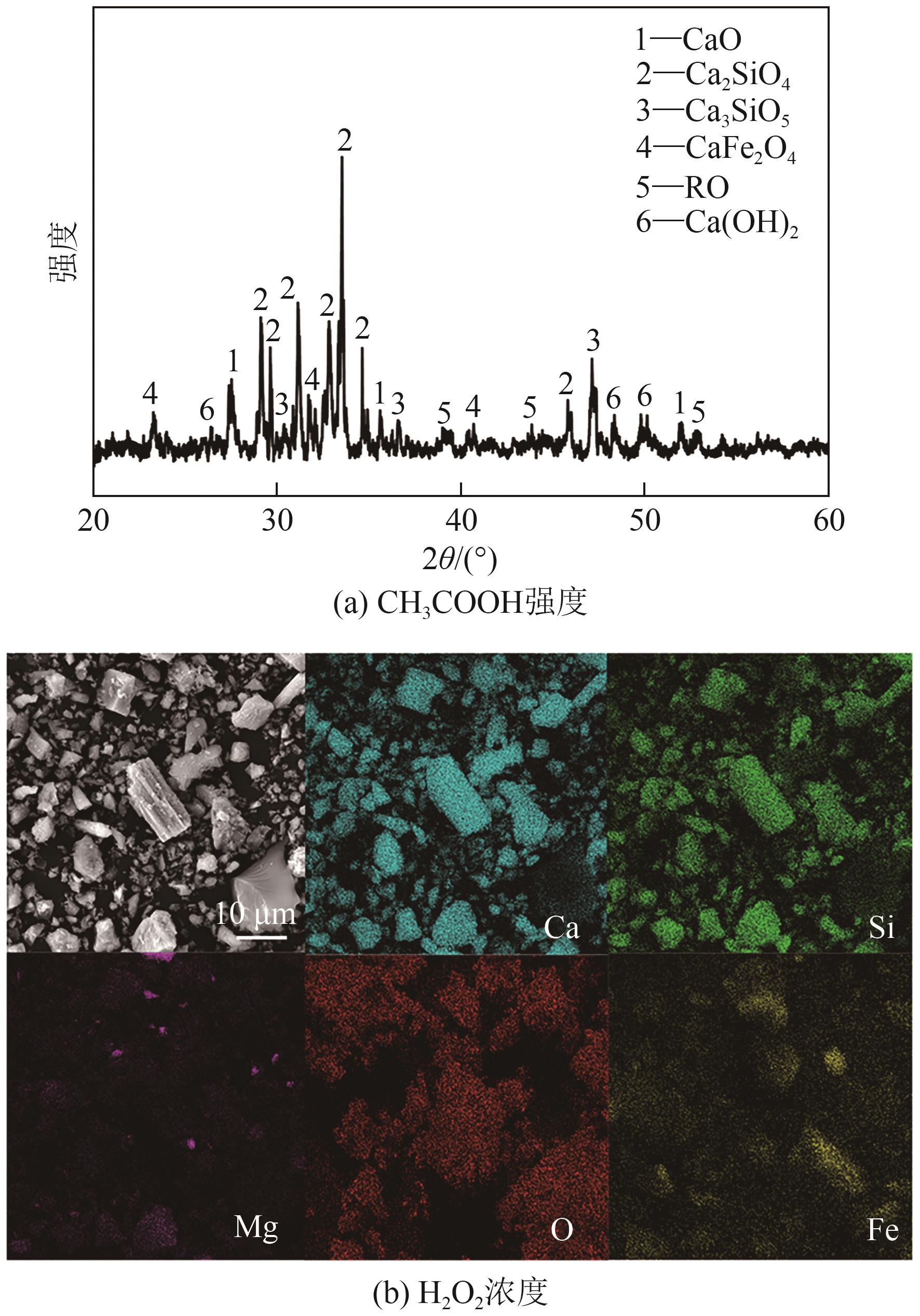
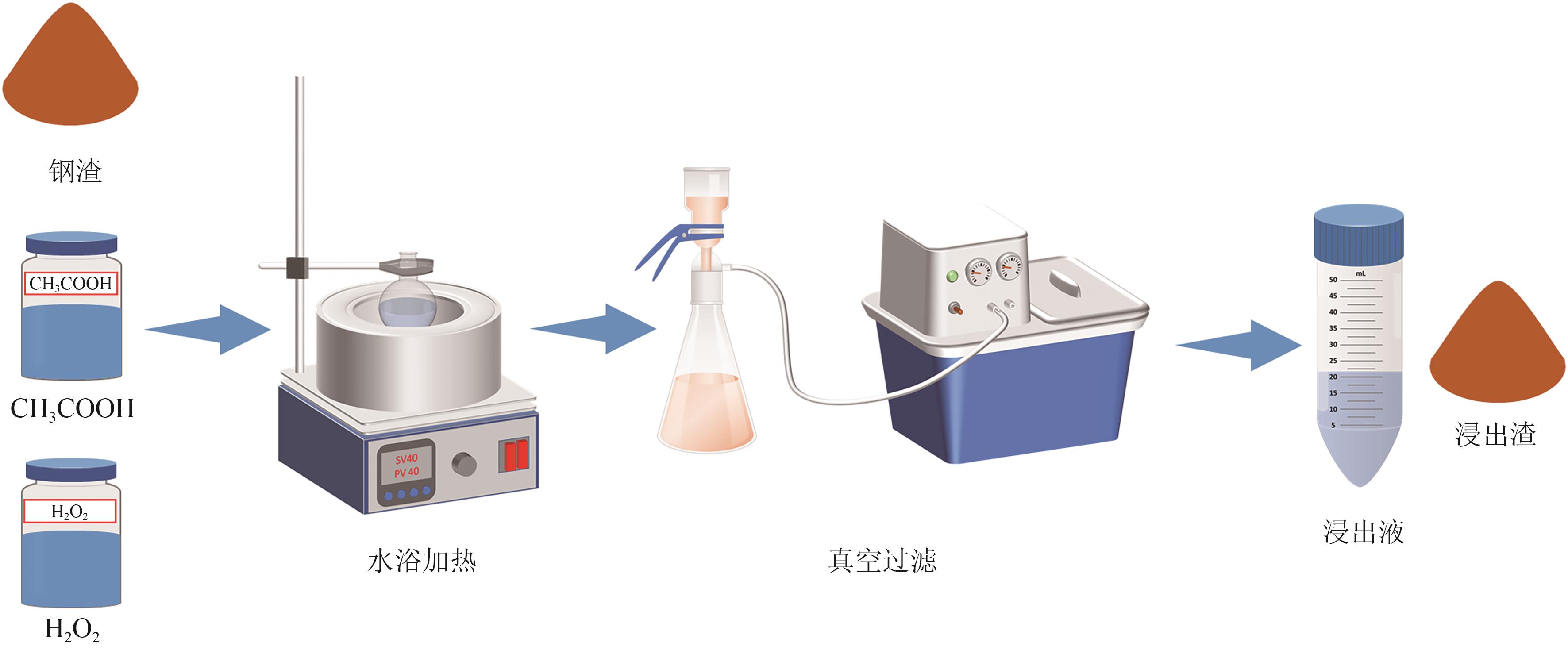
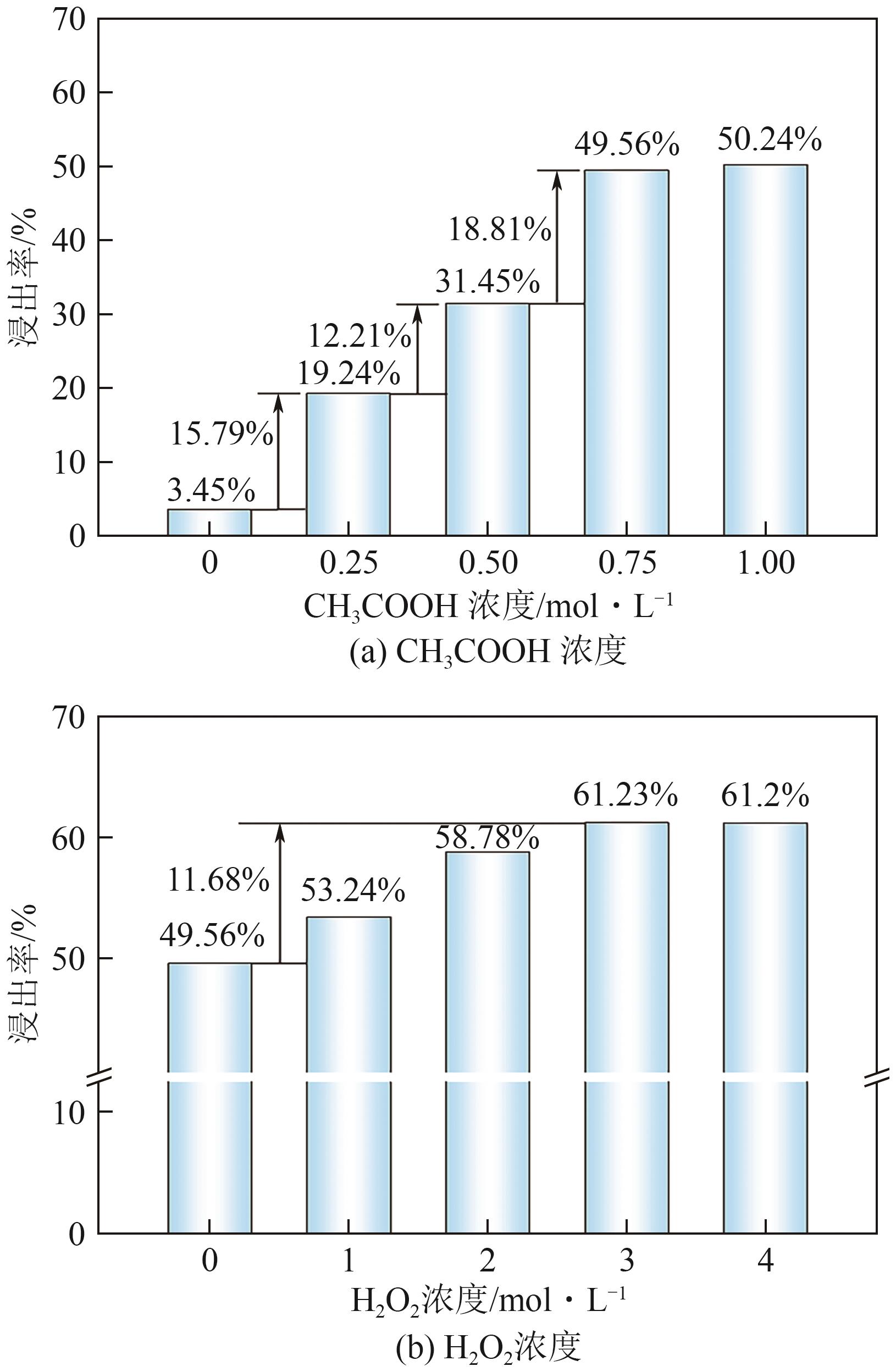
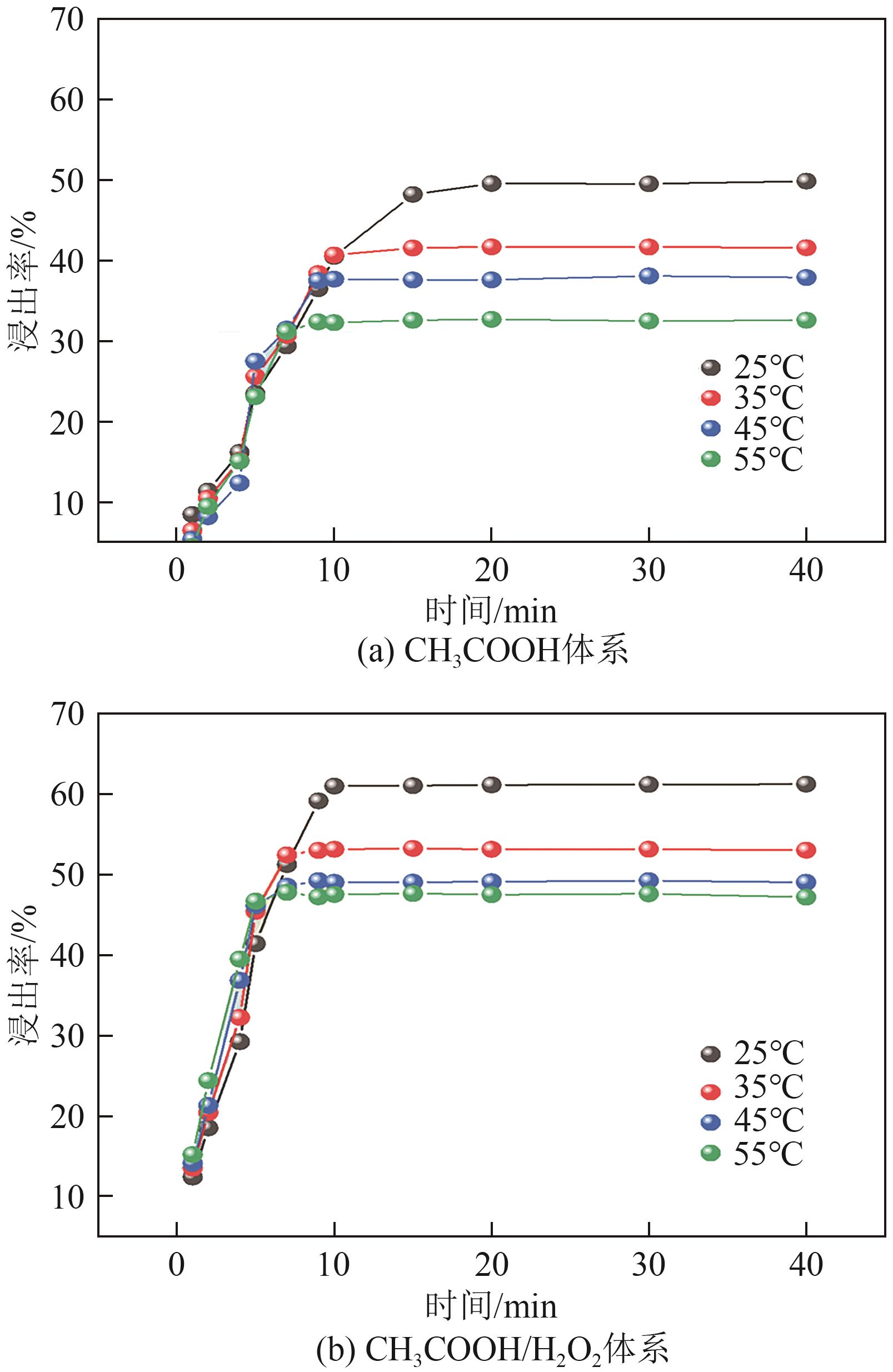
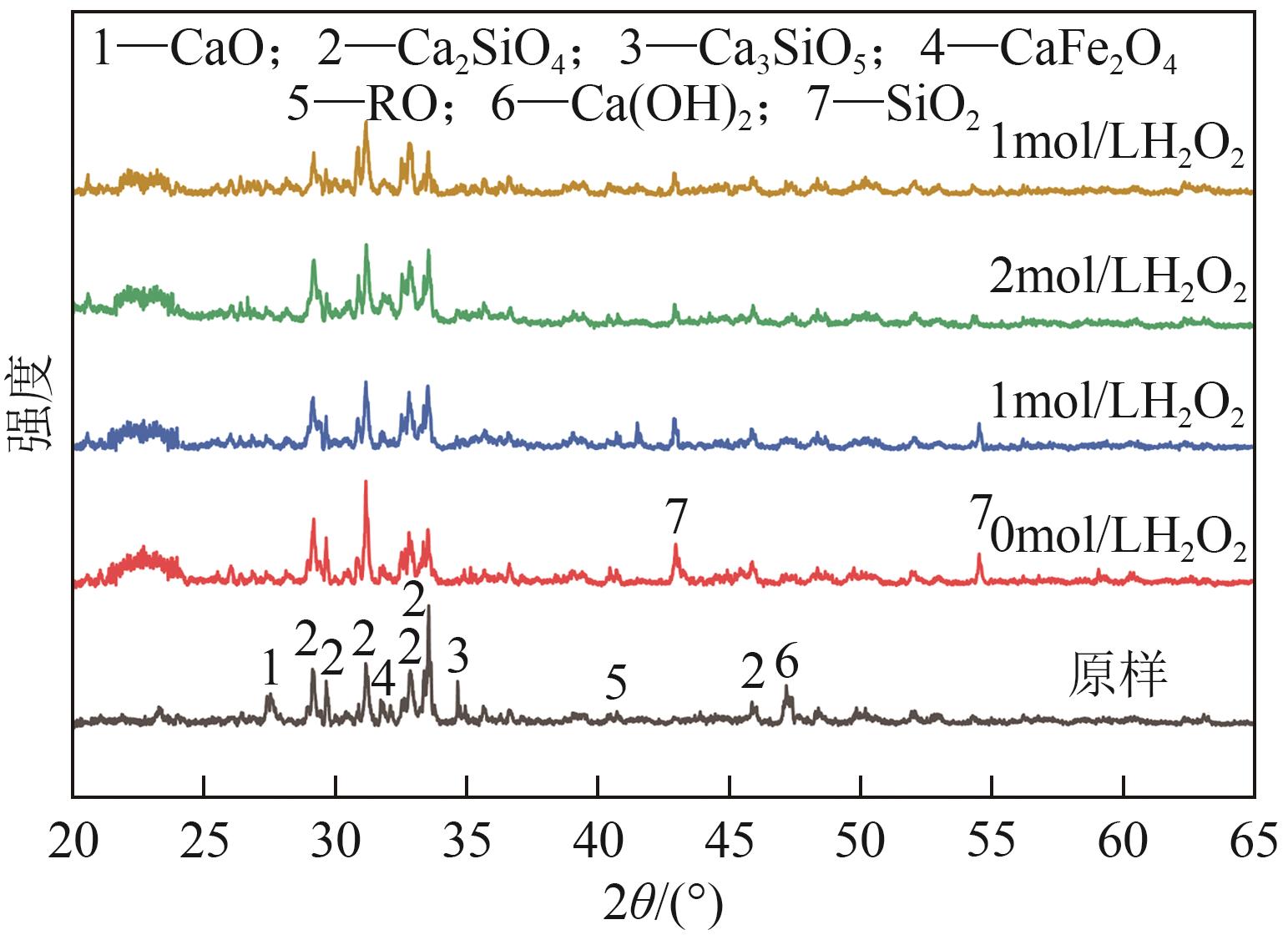
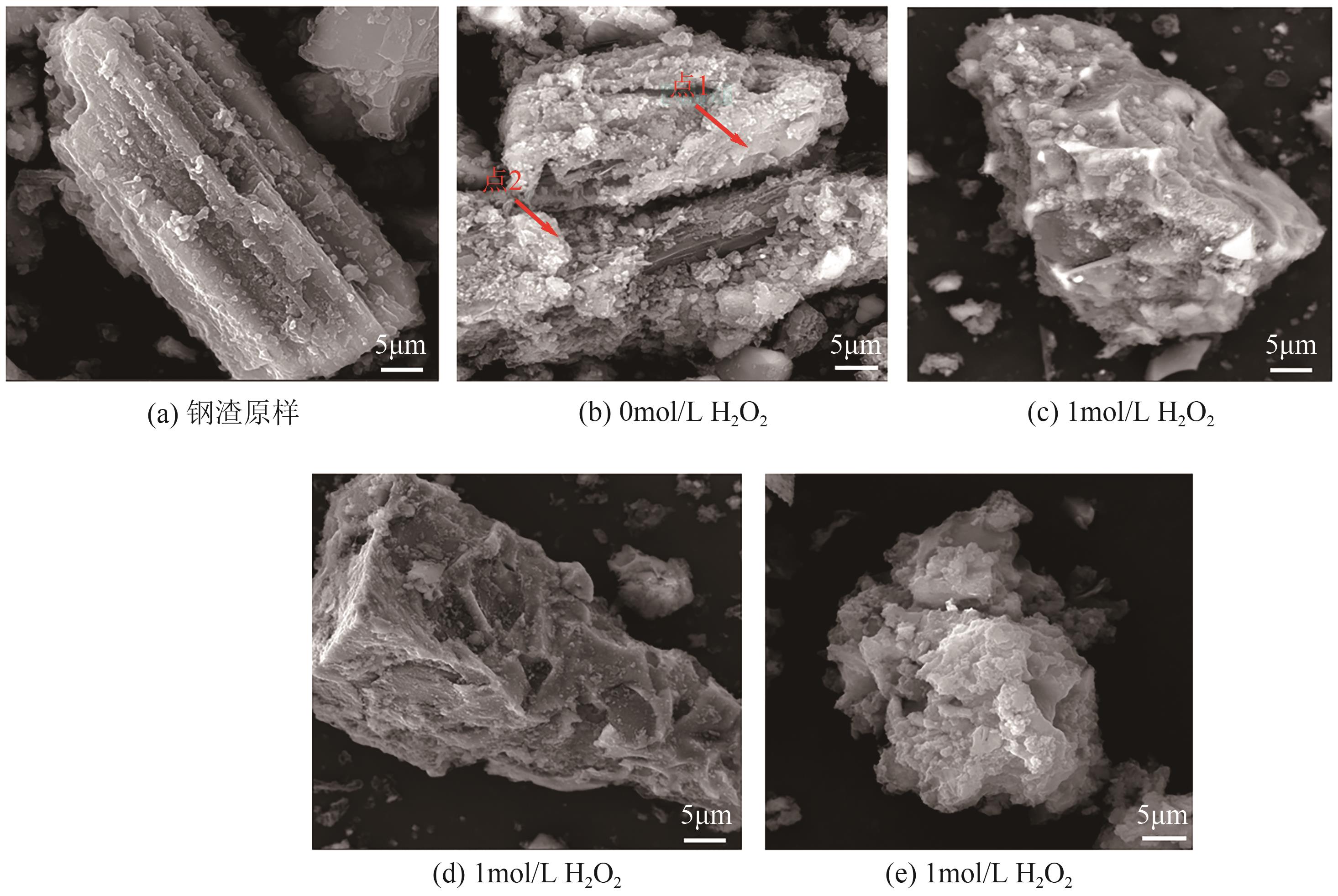
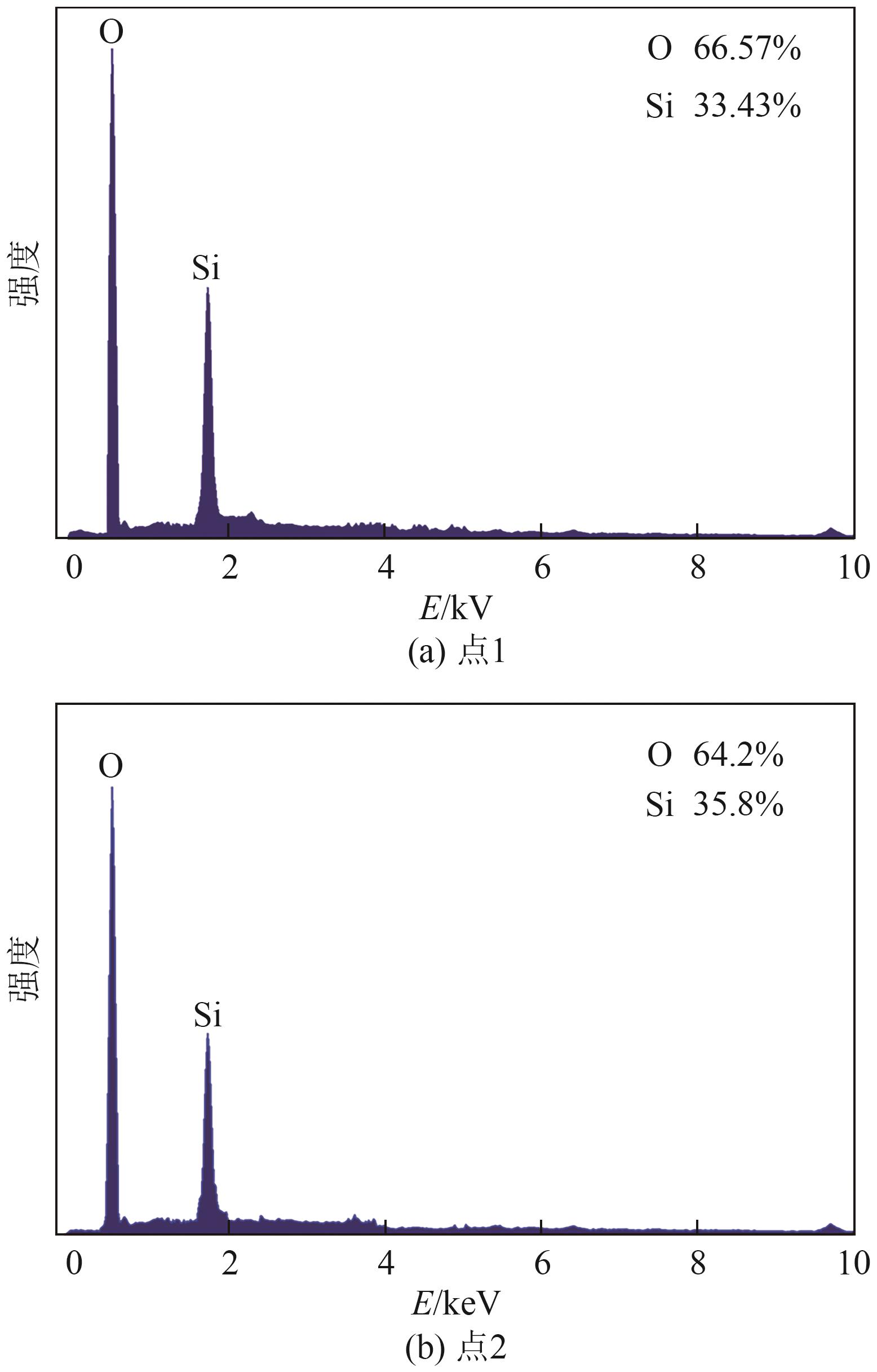
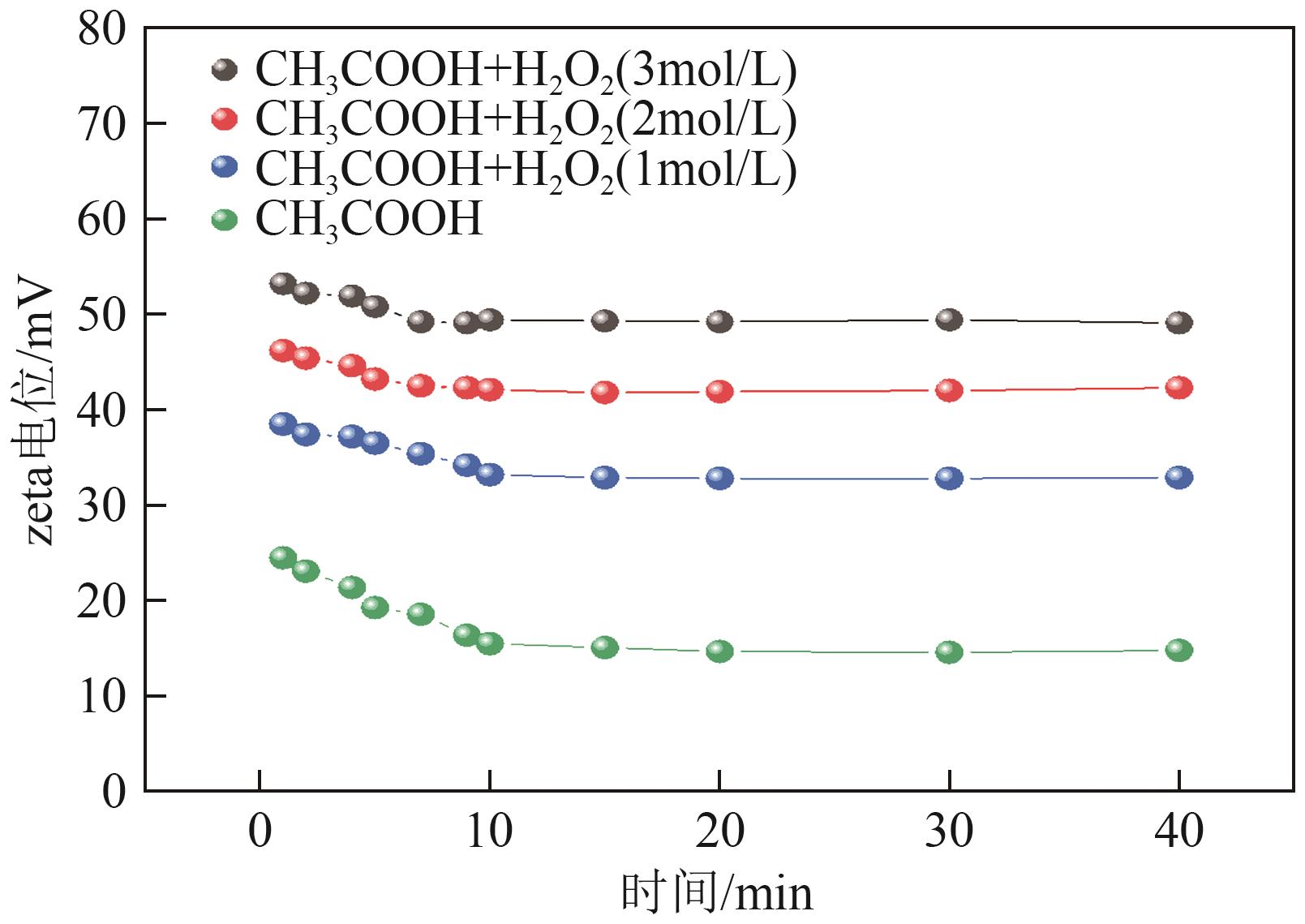
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (9): 5471-5478.DOI: 10.16085/j.issn.1000-6613.2024-1257
• Resources and environmental engineering • Previous Articles
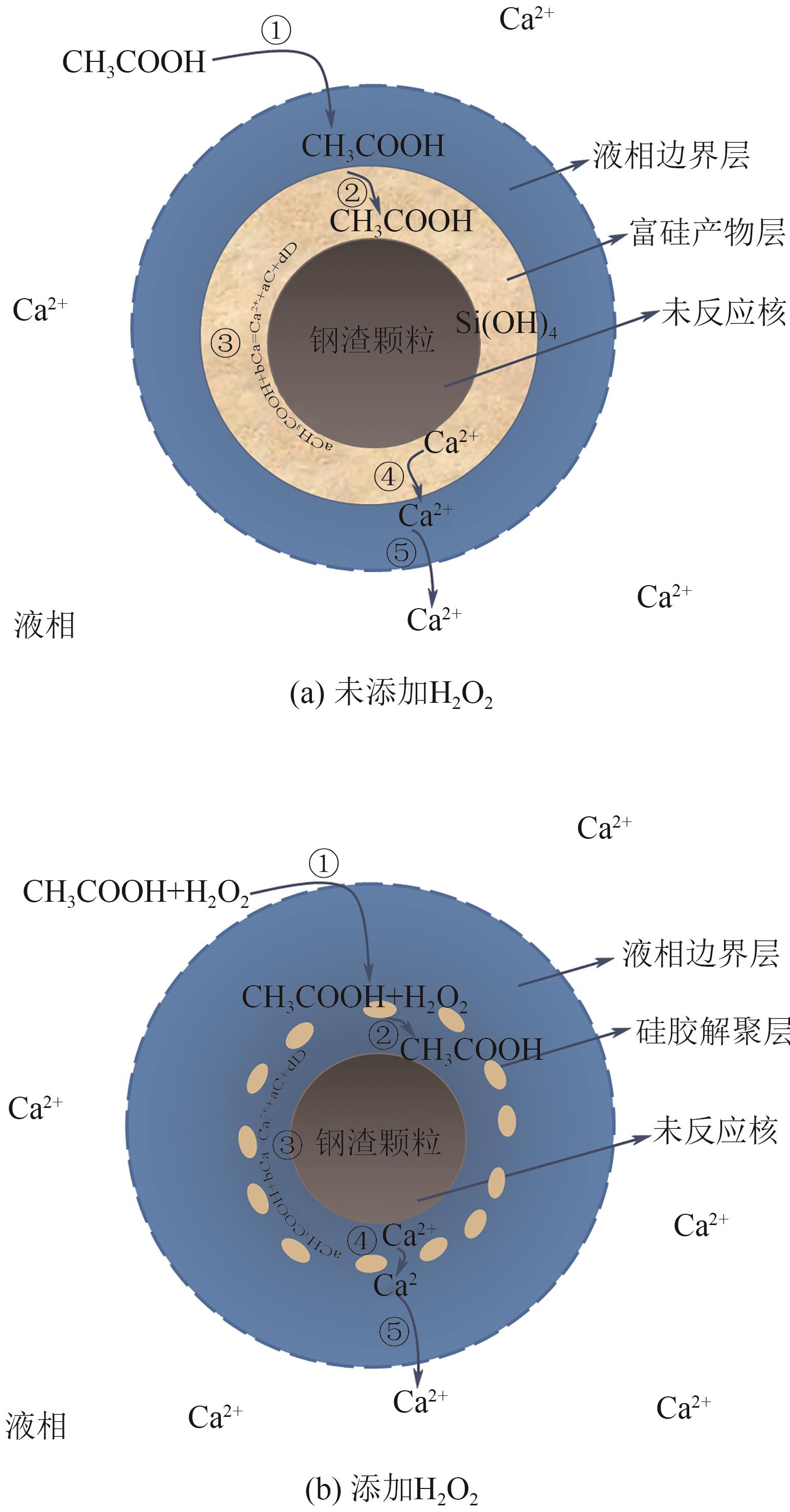
Leaching mechanism of calcium components in steel slag under CH3COOH/H2O2 leaching system
ZHENG Qinsheng1( ), ZHANG Chaohui1, XING Xiangdong1,2, SHE Yuan1,3(
), ZHANG Chaohui1, XING Xiangdong1,2, SHE Yuan1,3( ), LI Jiayu1
), LI Jiayu1
- 1.School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
2.Jiuquan Iron and Steel (Group) Co. , Ltd. , Jiayuguan 735100, Gansu, China
3.Special Steel Co. , Xining 810000, Qinghai, China
-
Received:2024-07-31Revised:2024-09-09Online:2025-09-30Published:2025-09-25 -
Contact:SHE Yuan
CH3COOH/H2O2浸取体系下钢渣钙组分浸出机制
郑钦升1( ), 张朝晖1, 邢相栋1,2, 折媛1,3(
), 张朝晖1, 邢相栋1,2, 折媛1,3( ), 李嘉雨1
), 李嘉雨1
- 1.西安建筑科技大学冶金工程学院,陕西 西安 710055
2.酒泉钢铁(集团)有限公司,甘肃 嘉峪关 735100
3.西宁特殊钢股份有限公司,青海 西宁 810000
-
通讯作者:折媛 -
作者简介:郑钦升(2000—),男,硕士研究生,研究方向为冶金环保与资源利用。E-mail:2204211102@xauat.edu.cn。 -
基金资助:国家自然科学基金(52174325);陕西省创新能力支撑计划(2023-CX-TD-53);陕西省教育厅重点实验室科学研究计划(20JS072)
CLC Number:
Cite this article
ZHENG Qinsheng, ZHANG Chaohui, XING Xiangdong, SHE Yuan, LI Jiayu. Leaching mechanism of calcium components in steel slag under CH3COOH/H2O2 leaching system[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5471-5478.
郑钦升, 张朝晖, 邢相栋, 折媛, 李嘉雨. CH3COOH/H2O2浸取体系下钢渣钙组分浸出机制[J]. 化工进展, 2025, 44(9): 5471-5478.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1257
| 元素 | 质量分数/% |
|---|---|
| Ca | 39.66 |
| Si | 9.14 |
| Mg | 3.44 |
| Fe | 15.17 |
| Al | 2.83 |
| Mn | 0.71 |
| P | 0.29 |
| 元素 | 质量分数/% |
|---|---|
| Ca | 39.66 |
| Si | 9.14 |
| Mg | 3.44 |
| Fe | 15.17 |
| Al | 2.83 |
| Mn | 0.71 |
| P | 0.29 |
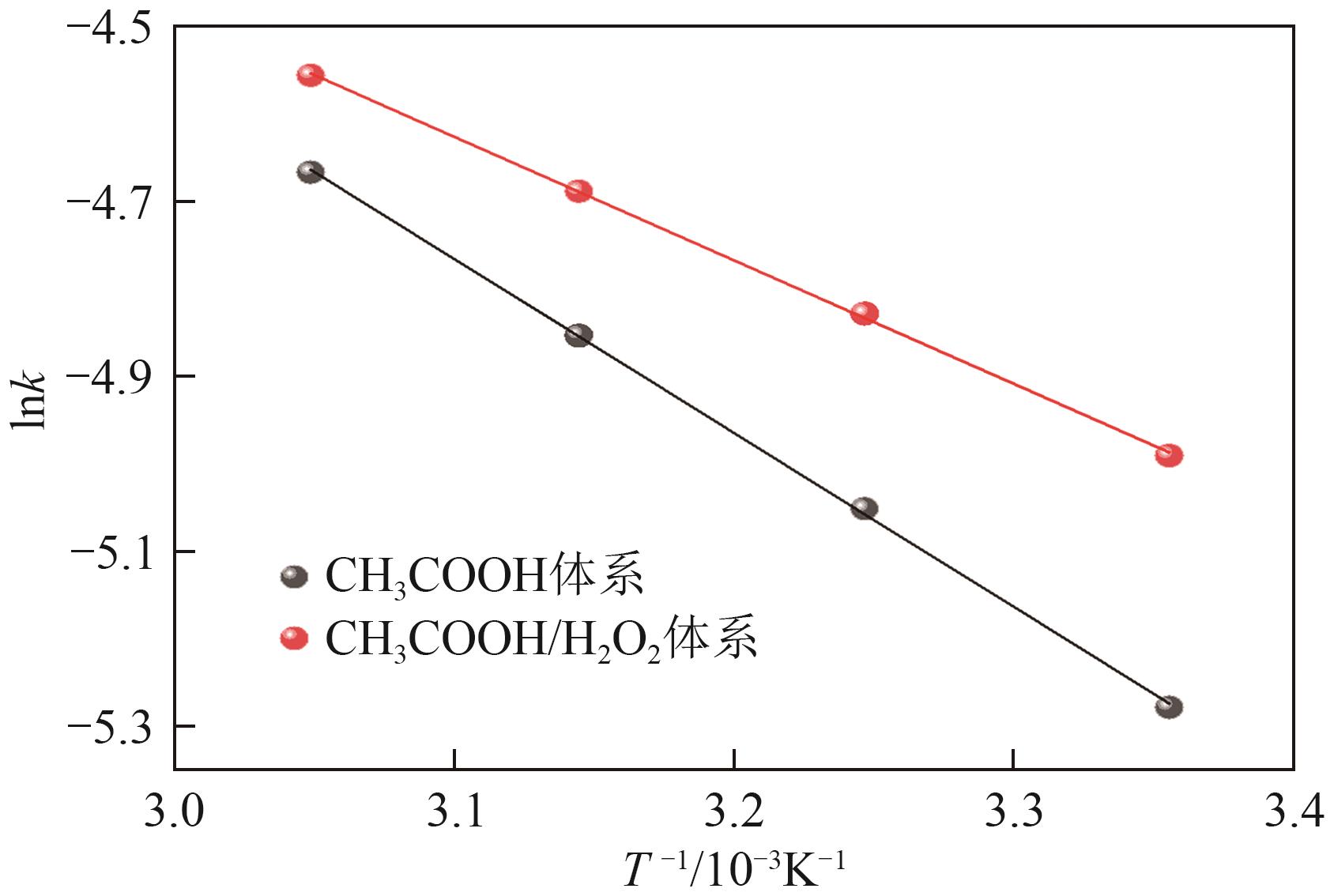
| 浸出条件 | 温度/℃ | 化学反应控制 | 固体产物层控制 | 混合控制 | 富硅层控制 | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | R12 | k2 | R22 | k3 | R32 | k4 | R42 | ||
| CH3COOH体系 | 25 | 0.0195 | 0.8456 | 0.0035 | 0.9256 | 0.0051 | 0.9354 | 0.0065 | 0.9926 |
| 35 | 0.0245 | 0.8759 | 0.0054 | 0.9189 | 0.0064 | 0.9254 | 0.0071 | 0.9943 | |
| 45 | 0.0294 | 0.8412 | 0.0065 | 0.9354 | 0.0078 | 0.9268 | 0.0078 | 0.9946 | |
| 55 | 0.0354 | 0.9014 | 0.0072 | 0.9215 | 0.0094 | 0.9121 | 0.0083 | 0.9927 | |
| CH3COOH/H2O2体系 | 25 | 0.0214 | 0.8062 | 0.0057 | 0.9272 | 0.0068 | 0.9939 | 0.0071 | 0.9314 |
| 35 | 0.0302 | 0.8848 | 0.0071 | 0.9440 | 0.0080 | 0.9903 | 0.0079 | 0.9216 | |
| 45 | 0.0384 | 0.8755 | 0.0076 | 0.9585 | 0.0092 | 0.9955 | 0.0085 | 0.9316 | |
| 55 | 0.0405 | 0.8956 | 0.0081 | 0.9451 | 0.0105 | 0.9941 | 0.0089 | 0.8921 | |
| 浸出条件 | 温度/℃ | 化学反应控制 | 固体产物层控制 | 混合控制 | 富硅层控制 | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | R12 | k2 | R22 | k3 | R32 | k4 | R42 | ||
| CH3COOH体系 | 25 | 0.0195 | 0.8456 | 0.0035 | 0.9256 | 0.0051 | 0.9354 | 0.0065 | 0.9926 |
| 35 | 0.0245 | 0.8759 | 0.0054 | 0.9189 | 0.0064 | 0.9254 | 0.0071 | 0.9943 | |
| 45 | 0.0294 | 0.8412 | 0.0065 | 0.9354 | 0.0078 | 0.9268 | 0.0078 | 0.9946 | |
| 55 | 0.0354 | 0.9014 | 0.0072 | 0.9215 | 0.0094 | 0.9121 | 0.0083 | 0.9927 | |
| CH3COOH/H2O2体系 | 25 | 0.0214 | 0.8062 | 0.0057 | 0.9272 | 0.0068 | 0.9939 | 0.0071 | 0.9314 |
| 35 | 0.0302 | 0.8848 | 0.0071 | 0.9440 | 0.0080 | 0.9903 | 0.0079 | 0.9216 | |
| 45 | 0.0384 | 0.8755 | 0.0076 | 0.9585 | 0.0092 | 0.9955 | 0.0085 | 0.9316 | |
| 55 | 0.0405 | 0.8956 | 0.0081 | 0.9451 | 0.0105 | 0.9941 | 0.0089 | 0.8921 | |
| [1] | 徐润生, 张雨晨, 张建良, 等. 钢铁冶金固废固化CO2研究现状及趋势[J]. 钢铁研究学报, 2023, 35(7): 779-789. |
| XU Runsheng, ZHANG Yuchen, ZHANG Jianliang, et al. Status and trends of steel metallurgical solid waste solidification CO2 research[J]. Journal of Iron and Steel Research, 2023, 35(7): 779-789. | |
| [2] | FAN Jingli, LI Zezheng, HUANG Xi, et al. A net-zero emissions strategy for China’s power sector using carbon-capture utilization and storage[J]. Nature Communications, 2023, 14(1): 5972. |
| [3] | MA Zhuohui, LIAO Hongqiang, WANG Li, et al. Effects of iron/silicon/magnesium/aluminum on CaO carbonation of CO2 in steel slag-based building materials during carbonation curing[J]. Construction and Building Materials, 2021, 298: 123889. |
| [4] | 赵珂萍, 李晓玉, 李瑞红, 等. 固废源CaO基CO2捕集材料的制备与捕集性能研究进展[J]. 硅酸盐通报, 2023, 42(2): 520-530. |
| ZHAO Keping, LI Xiaoyu, LI Ruihong, et al. Research progress on preparation and capture performance of CaO-based CO2 capture materials from solid wastes[J]. Bulletin of the Chinese Ceramic Society, 2023, 42(2): 520-530. | |
| [5] | JIN Peng, ZHANG Siyi, LIU Yu, et al. Application of Bacillus mucilaginosus in the carbonation of steel slag[J]. Applied Microbiology and Biotechnology, 2021, 105(23): 8663-8674. |
| [6] | ANDRADE Humberto Dias, DE CARVALHO José Maria Franco, COSTA Laís Cristina Barbosa, et al. Mechanical performance and resistance to carbonation of steel slag reinforced concrete[J]. Construction and Building Materials, 2021, 298: 123910. |
| [7] | 颜峰, 黄小明, 郭荣鑫, 等. 预处理改善钢渣体积安定性的研究现状[J]. 钢铁, 2022, 57(10): 30-42. |
| YAN Feng, HUANG Xiaoming, GUO Rongxin, et al. Research status of improving volume stability of steel slag by pretreatment[J]. Iron & Steel, 2022, 57(10): 30-42. | |
| [8] | 赵雯涵, 吴水木, 李英杰. 钙基工业固废循环捕集CO2性能研究进展[J]. 煤炭学报, 2022, 47(11): 3926-3935. |
| ZHAO Wenhan, WU Shuimu, LI Yingjie. A review on cyclic CO2 capture performance of calcium-based industrial solid waste[J]. Journal of China Coal Society, 2022, 47(11): 3926-3935. | |
| [9] | PÉREZ-MORENO S M, GÁZQUEZ M J, BOLÍVAR J P. CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments[J]. Chemical Engineering Journal, 2015, 262: 737-746. |
| [10] | 李文秀, 杨宇航, 黄艳, 等. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057. |
| LI Wenxiu, YANG Yuhang, HUANG Yan, et al. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057. | |
| [11] | 王秋华, 吴嘉帅, 张卫风. 碱性工业固废矿化封存二氧化碳研究进展[J]. 化工进展, 2023, 42(3): 1572-1582. |
| WANG Qiuhua, WU Jiashuai, ZHANG Weifeng. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1572-1582. | |
| [12] | HUANG Yi, XIONG Guo. Influence of hydrothermal pretreatment temperature on the hydration properties and direct carbonation efficiency of Al-rich ladle furnace refining slag[J]. Processes, 2021, 9(8): 1458. |
| [13] | 甄常亮, 程翠花, 张巧荣, 等. “两步法” 重构钢渣物相变化特征及黏度调控机制[J]. 钢铁, 2023, 58(7): 144-153. |
| ZHEN Changliang, CHENG Cuihua, ZHANG Qiaorong, et al. Phase change characteristics and viscosity regulation mechanism during two-stepsteel slag reconstruction process[J]. Iron & Steel, 2023, 58(7): 144-153. | |
| [14] | 张俊, 严定鎏, 齐渊洪, 等. 钢铁冶炼渣的处理利用难点分析[J]. 钢铁, 2020, 55(1): 1-5. |
| ZHANG Jun, YAN Dingliu, QI Yuanhong, et al. Difficulty analysis on treatment and utilization of iron and steel smelting slag[J]. Iron & Steel, 2020, 55(1): 1-5. | |
| [15] | 方明航, 伊元荣, 马文青, 等. 温度对精炼渣碳酸化效果影响分析[J]. 硅酸盐通报, 2020, 39(12): 3905-3912. |
| FANG Minghang, YI Yuanrong, MA Wenqing, et al. Influence of temperature on carbonation effect of refining slag[J]. Bulletin of the Chinese Ceramic Society, 2020, 39(12): 3905-3912. | |
| [16] | LUO Yinbo, HE Dongfeng. Indirect carbonation by a two-step leaching process using ammonium chloride and acetic acid[J]. JOM, 2022, 74(5): 1958-1968. |
| [17] | SUN Jia, LUO Sang, WANG Yaozheng, et al. Pre-treatment of steel slag and its applicability in asphalt mixtures for sustainable pavements[J]. Chemical Engineering Journal, 2023, 476: 146802. |
| [18] | HOU Jiwei, CHEN Zhimin, LIU Jiaxiang. Hydration activity and expansibility model for the RO phase in steel slag[J]. Metallurgical and Materials Transactions B, 2020, 51(4): 1697-1704. |
| [19] | TONG Zhibo, SUN Jingting, WANG Jiang, et al. Iron reduction and diopside-based glass ceramic preparation based on mineral carbonation of steel slag[J]. Environmental Science and Pollution Research International, 2021, 28(1): 796-804. |
| [20] | 王中辉, 苏胜, 尹子骏, 等. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| WANG Zhonghui, SU Sheng, YIN Zijun, et al. Research progress of CO2 mineralization and integrated absorption-mineralization(IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327. | |
| [21] | LEKAKH S N, RAWLINS C H, ROBERTSON D G C, et al. Kinetics of aqueous leaching and carbonization of steelmaking slag[J]. Metallurgical and Materials Transactions B, 2008, 39(1): 125-134. |
| [22] | KODAMA Satoshi, NISHIMOTO Taiki, YAMAMOTO Naoki, et al. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution[J]. Energy, 2008, 33(5): 776-784. |
| [23] | 秦松, 李俊国, 王亚军, 等. AOD不锈钢渣中Ca高效浸出及其间接碳酸化[J]. 钢铁, 2024, 59(8): 200-209. |
| QIN Song, LI Junguo, WANG Yajun, et al. Efficient leaching and indirect carbonation of Ca from AOD stainless steel slag[J]. Iron & Steel, 2024, 59(8): 200-209. | |
| [24] | 赵树海, 金永丽, 郭嘉诚, 等. 低钙钢渣中钙、铁分离及回收利用[J]. 中国冶金, 2023, 33(10): 125-132. |
| ZHAO Shuhai, JIN Yongli, GUO Jiacheng, et al. Separation and recovery of calcium and iron from low calcium steel slag[J]. China Metallurgy, 2023, 33(10): 125-132. | |
| [25] | ZHAO Qing, LIU Kun, SUN Lifeng, et al. Towards carbon sequestration using stainless steel slag via phase modification and co-extraction of calcium and magnesium[J]. Process Safety and Environmental Protection, 2020, 133: 73-81. |
| [26] | MARIN RIVERA Rodolfo, VAN GERVEN Tom. Production of calcium carbonate with different morphology by simultaneous CO2 capture and mineralisation[J]. Journal of CO2 Utilization, 2020, 41: 101241. |
| [27] | ZHANG Jing, LUO Guoping, ZHANG Hao, et al. Effect of the carbon mixing ratio on mineral evolution and gasification dephosphorization during the pre-reduction sintering process of Bayan Obo iron ore concentrate[J]. ISIJ International, 2023, 63(3): 455-465. |
| [28] | ARCE Gretta L A F, SOARES NETO Turibio G, ÁVILA I, et al. Leaching optimization of mining wastes with lizardite and brucite contents for use in indirect mineral carbonation through the pH swing method[J]. Journal of Cleaner Production, 2017, 141: 1324-1336. |
| [29] | DUBEY R S, RAJESH Y B R D, MORE M A. Synthesis and characterization of SiO2 nanoparticles via Sol-gel method for industrial applications[J]. Materials Today: Proceedings, 2015, 2(4/5): 3575-3579. |
| [30] | 朱伶俐, 杨章, 赵宇, 等. 钢渣-矿渣复合水泥基材料3D打印性能[J]. 材料导报, 2023, 37(12): 111-116. |
| ZHU Lingli, YANG Zhang, ZHAO Yu, et al. 3D printing performance of composite cement-based materials with blast furnace slag and steel slag[J]. Materials Reports, 2023, 37(12): 111-116. | |
| [31] | XU Runsheng, ZHANG Yuchen, ZHANG Jianliang, et al. Research on the leaching of calcium ions from de-vanadiumized steel slag for indirect CO2 mineral sequestration: Thermodynamics, kinetics, and parameter optimization[J]. Metallurgical and Materials Transactions B, 2024, 55(2): 877-890. |
| [32] | Ningning LYU, CHEN Huifang, SU Chang, et al. Kinetics investigation of phosphorus leaching from steelmaking slag[J]. Mineral Processing and Extractive Metallurgy Review, 2023, 44(8): 571-576. |
| [33] | DE SOUZA GONÇALVES Gabriel Alves, DE CARVALHO Thamyres Cardoso, GARJULLI Franco, et al. Adsorption of niobium and tantalum contained in a tin slag leachate by ion exchange resins: Equilibrium isotherms, kinetic and thermodynamic studies[J]. Journal of Sustainable Metallurgy, 2023, 9(3): 1329-1343. |
| [34] | FENG Guodong, CHENG Peng, YAN Wenfu, et al. Accelerated crystallization of zeolites via hydroxyl free radicals[J]. Science, 2016, 351(6278): 1188-1191. |
| [35] | FENG Guodong, WANG Jianyu, BORONAT Mercedes, et al. Radical-facilitated green synthesis of highly ordered mesoporous silica materials[J]. Journal of the American Chemical Society, 2018, 140(14): 4770-4773. |
| [1] | CHEN Siming, LIU Jingchao, ZHONG Zhixuan, ZHANG Xinzhu, ZHU Tianhao, PENG Yiqing, YOU Sai, WANG Yikai, YUAN Jiajun, ZHANG Yongchun. Development and application of deep eutectic solvents in carbon dioxide capture [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5377-5390. |
| [2] | ZHANG Wenjing, HUANG Zhixin, LI Shiteng, DENG Shuai, LI Shuangjun. Biomass carbon aerogels for CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5018-5032. |
| [3] | WANG Xiaoxiao, KONG Fulin, LI Xiaoyu, REN Yongqiang, XU Shisen. Numerical simulation of CO2 absorbents microscale flow on the surface of structured packings in the presence of perforations [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4311-4321. |
| [4] | HUANG Ke’er, LIU Jiahao, LI Haoming, ZHOU Tianhang, GAO Jinsen, LAN Xingying. Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4352-4364. |
| [5] | WANG Zicheng, ZHANG Haifan, YUAN Peng, SUN Chen, ZOU Weijie, LI Xinze, XING Xiaokai. Coupled hydraulic-thermal calculation model of supercritical/ dense-phase CO2 steady-state pipeline transportation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4701-4708. |
| [6] | MAO Yuanhao, FAN Huifeng, SAYD Sultan, FANG Furong, ZHONG Qi, YU Yunsong, WU Xiaomei, ZHANG Zaoxiao. Research progress in the electrochemically mediated amine regeneration CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4089-4100. |
| [7] | XIAO Li, QI Shaopeng, ZHOU Kun, BO Yanan, WANG Xiulin, YAO Huichao, DAI Ruoyun, SUI Yiyan. Composite structure design of defect state TiO2-x -Au clusters for efficient visible light driven CO2 reduction [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3062-3071. |
| [8] | WANG Yuting, WANG Mengxiang, LI Wenwen, LI Gang, WANG Yajun. Photo-Fenton synergistic degradation of tetracycline by Fe(Ⅲ)/3D conjugated carbon nitride system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3072-3083. |
| [9] | XIE Wuqiang, ZHANG Ling, HE Gang, JIANG Lifeng, ZHENG Xirui, ZHANG Hepeng. Electrocatalytic CO2 reduction to methane by CoTBrPP-PTAB-Cu catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3093-3100. |
| [10] | FU Zijun, SONG Xuehang, SHEN Qun, WANG Xiaobo, GU Jiaming, WANG Danfeng, WEI Wei, SUN Nannan. Carbon footprint analysis of integrated CO2 capture and methanation technology based on life cycle assessment [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2879-2887. |
| [11] | DOU Yu, WANG Wenxuan, FAN Chunlei, MA Jiliang, LIANG Cai, CHEN Xiaoping. Preparation of vaterite CaCO3 by mineralizing CO2 from desulfurized gypsum [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2328-2337. |
| [12] | SU Xiaojie, YAN Qun, LI Xincheng, XUE Wenhui, CHEN Yihao. Activation of potassium persulfate by NiCo2O4@chrysotile to degrade methyl orange [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2352-2364. |
| [13] | ZHU Shiyu, HE Yongjin, WANG Mingzi, CHEN Bilian. Research progress on microalgae to fix CO2 in flue gas from coal-fired power plants [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1666-1682. |
| [14] | LI Letian, LU Shijian, LIU Hanxiao, WU Liming, LIU Ling, KANG Guojun. Progress of desorption and regeneration of organic amine-enriched liquids [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 490-499. |
| [15] | WANG Ning, LU Shijian, LIU Ling, LIANG Jing, LIU Miaomiao, SUN Mengyuan, KANG Guojun. Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 445-464. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||