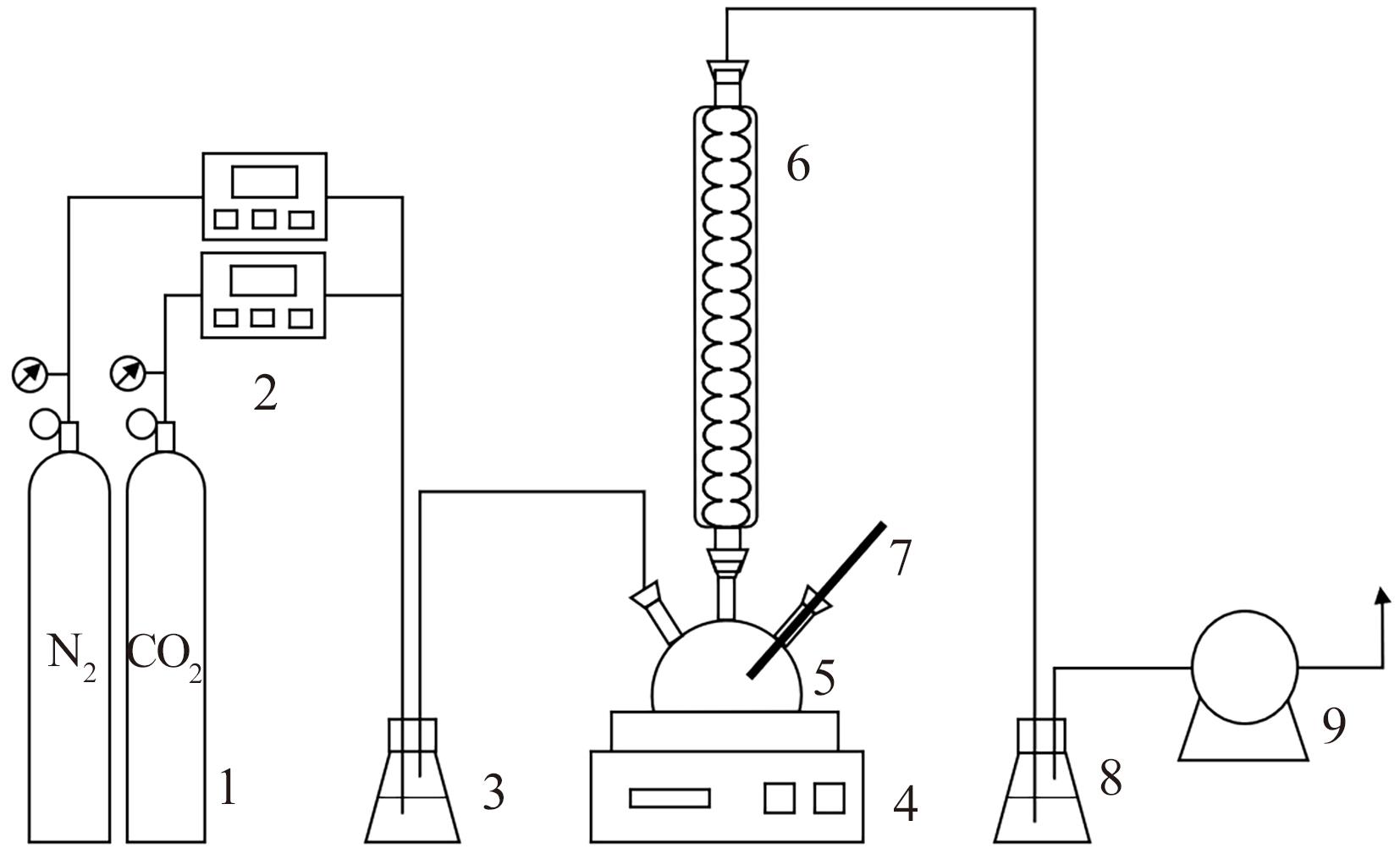
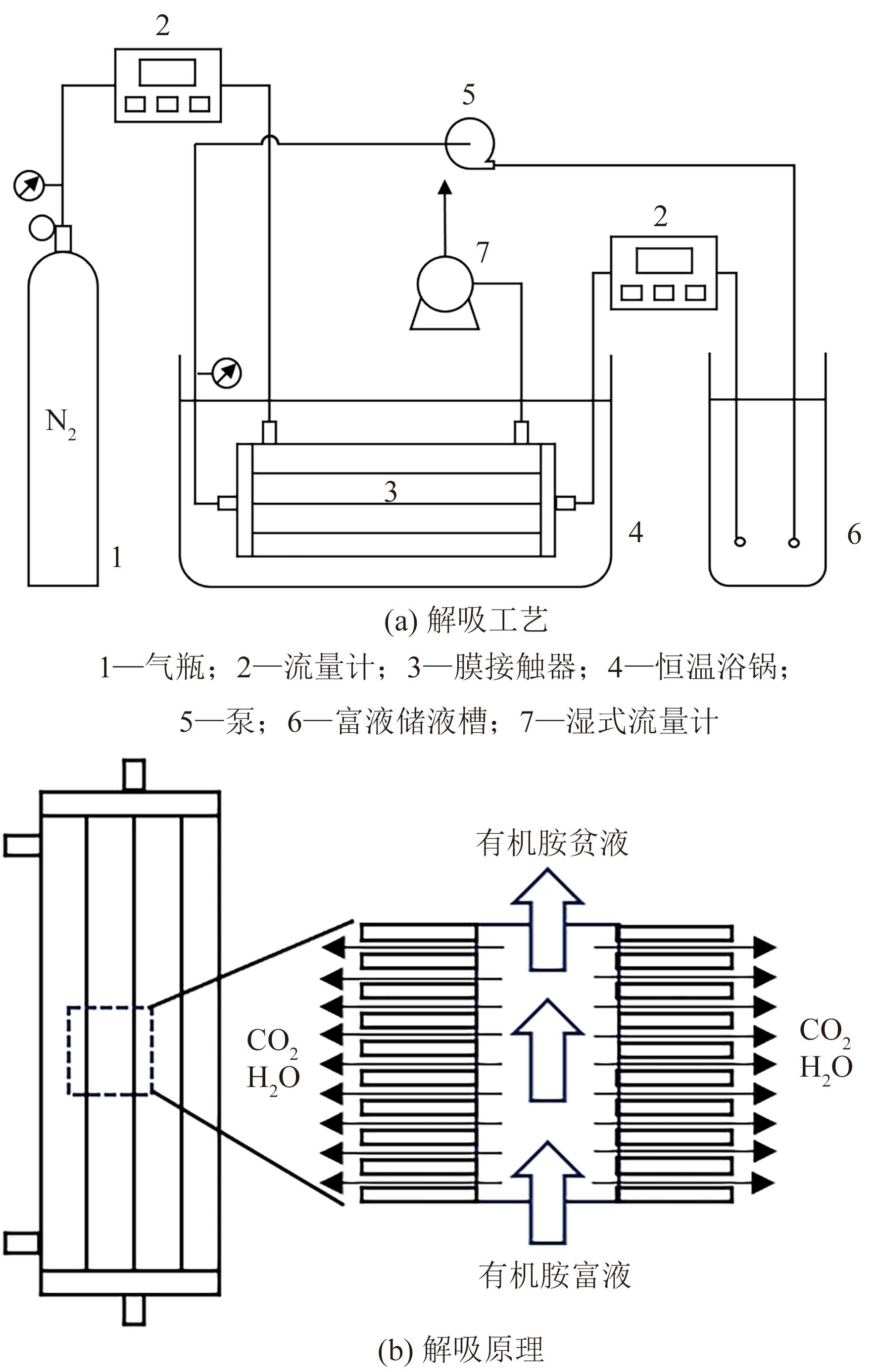
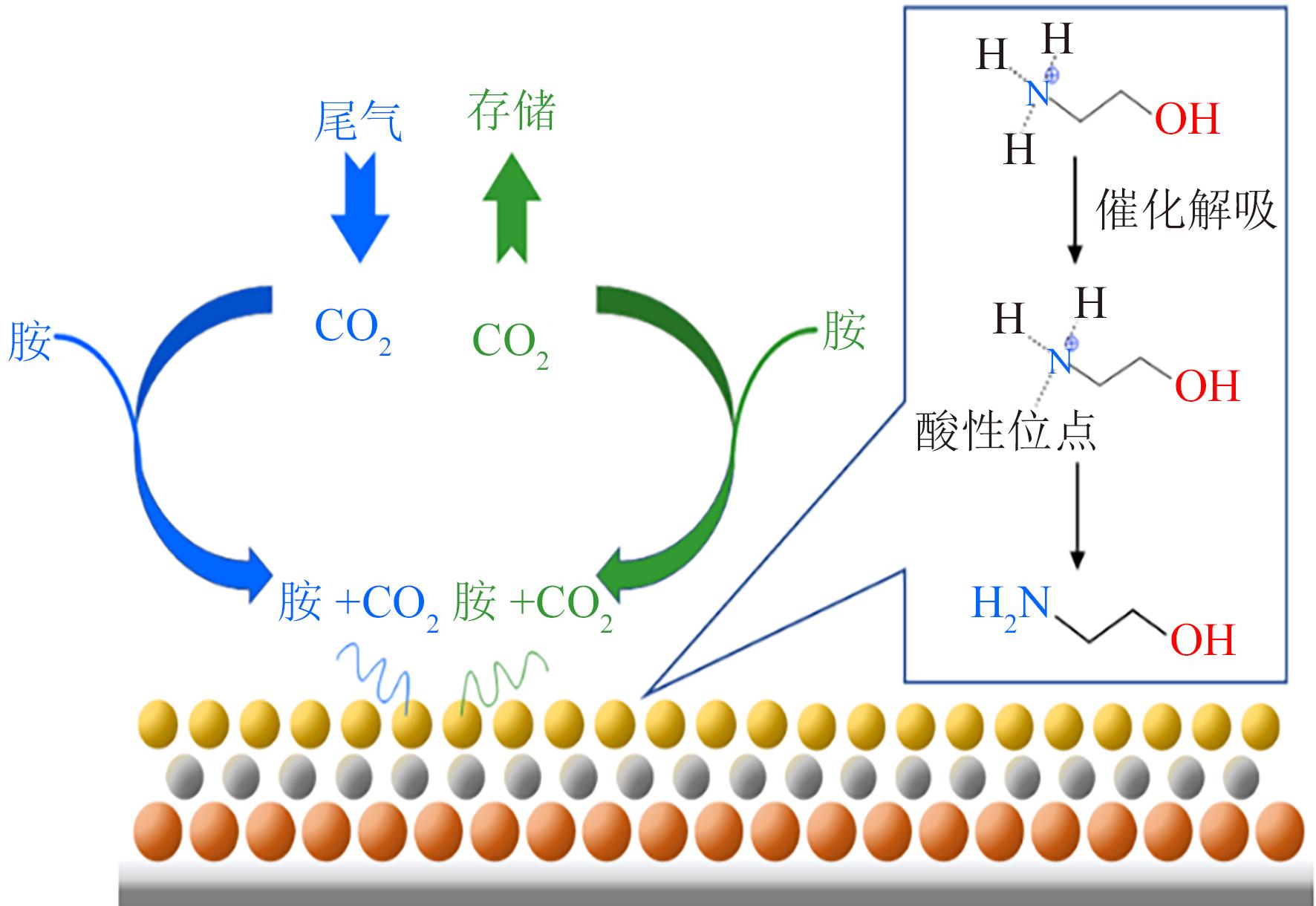
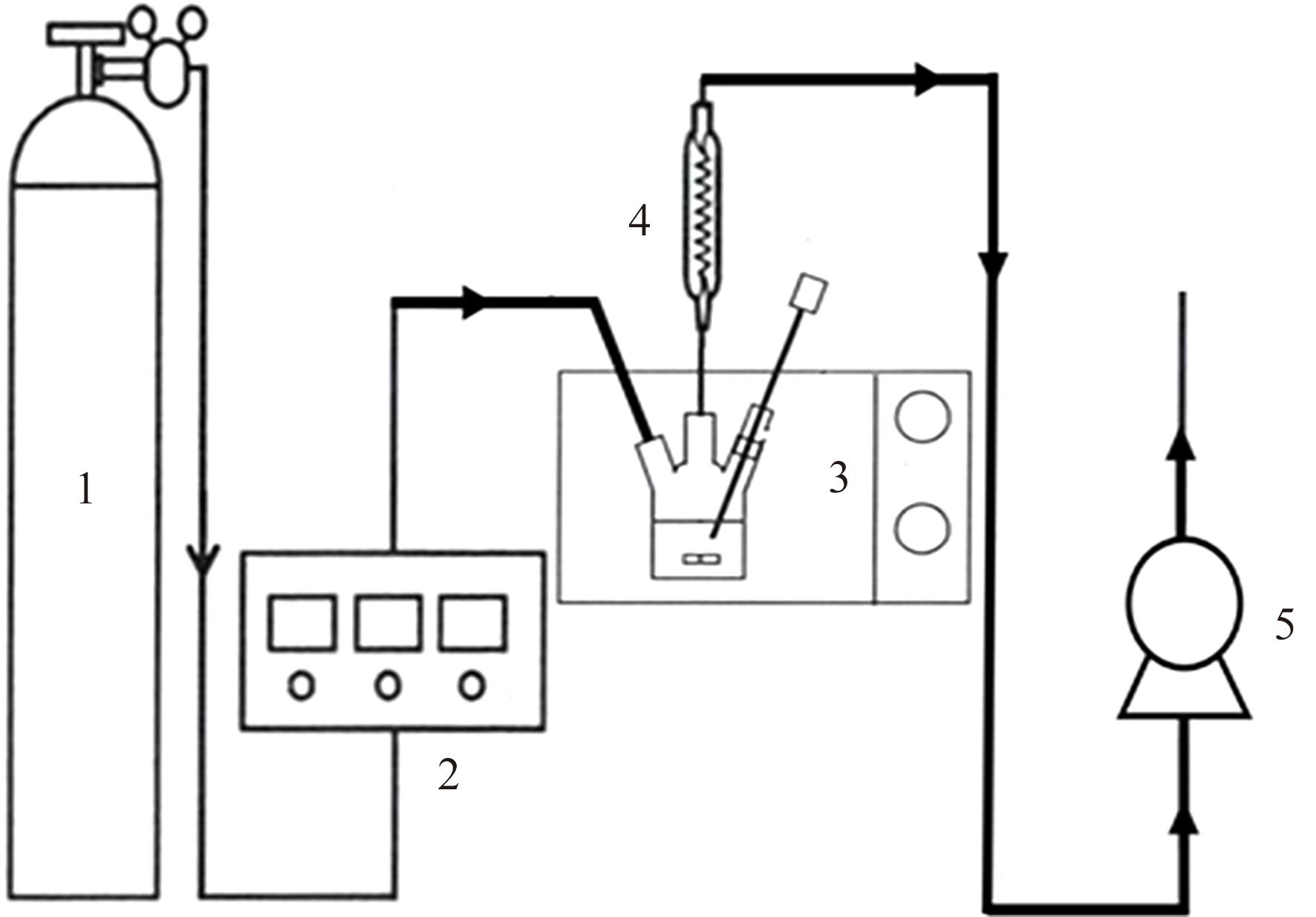
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (1): 490-499.DOI: 10.16085/j.issn.1000-6613.2024-0082
• Resources and environmental engineering • Previous Articles Next Articles
Progress of desorption and regeneration of organic amine-enriched liquids
LI Letian1,2( ), LU Shijian1,2(
), LU Shijian1,2( ), LIU Hanxiao3, WU Liming3,4, LIU Ling1,2, KANG Guojun1,2
), LIU Hanxiao3, WU Liming3,4, LIU Ling1,2, KANG Guojun1,2
- 1.Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization, China University of Mining and Technology, Xuzhou 221008, Jiangsu, China
2.Carbon Neutrality Institute, China University of Mining and Technology, Xuzhou 221008, Jiangsu, China
3.Zhejiang Feida Environmental Protection Technology Co. , Ltd. , Zhuji 311800, Zhejiang, China
4.Hangzhou Iron & Steel Group Co. , Ltd. , Hangzhou 310000, Zhejiang, China
-
Received:2024-01-11Revised:2024-04-08Online:2025-02-13Published:2025-01-15 -
Contact:LU Shijian
有机胺富液解吸再生研究进展
李乐天1,2( ), 陆诗建1,2(
), 陆诗建1,2( ), 刘含笑3, 吴黎明3,4, 刘玲1,2, 康国俊1,2
), 刘含笑3, 吴黎明3,4, 刘玲1,2, 康国俊1,2
- 1.中国矿业大学,江苏省煤基温室气体减排与资源化利用重点实验室,江苏 徐州 221008
2.中国矿业大学,碳中和研究院,江苏 徐州 221008
3.浙江菲达环保科技股份有限公司,浙江 诸暨 311800
4.杭州钢铁集团有限公司,浙江 杭州 310000
-
通讯作者:陆诗建 -
作者简介:李乐天(1999—),男,硕士研究生,研究方向为CO2捕集技术。E-mail:ts23040042a31@cumt.edu.cn。 -
基金资助:国家重点研发计划(2022YFE0115800);中央高校基本科研业务费专项(2023KYJD1004);江苏省科技厅科技项目(BE2023852)
CLC Number:
Cite this article
LI Letian, LU Shijian, LIU Hanxiao, WU Liming, LIU Ling, KANG Guojun. Progress of desorption and regeneration of organic amine-enriched liquids[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 490-499.
李乐天, 陆诗建, 刘含笑, 吴黎明, 刘玲, 康国俊. 有机胺富液解吸再生研究进展[J]. 化工进展, 2025, 44(1): 490-499.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0082
| 种类 | 黏度(20℃) /mPa·s | 介电常数 (20℃,2.45GHz) | 沸点 /℃ |
|---|---|---|---|
| 水 | 1.00 | 80.1 | 100 |
| 乙醇 | 1.17 | 25.7 | 78 |
| 乙二醇 | 20.5 | 38.7 | 198 |
| 正丙醇 | 2.26 | 22.2(25℃) | 97 |
| 正丁醇 | 2.95 | 17.1(25℃) | 118 |
| PEG200 | 62.2 | 6.4 | >250 |
| N,N-二甲基乙酰胺 | 1.37 | 39.1 | 166 |
| 种类 | 黏度(20℃) /mPa·s | 介电常数 (20℃,2.45GHz) | 沸点 /℃ |
|---|---|---|---|
| 水 | 1.00 | 80.1 | 100 |
| 乙醇 | 1.17 | 25.7 | 78 |
| 乙二醇 | 20.5 | 38.7 | 198 |
| 正丙醇 | 2.26 | 22.2(25℃) | 97 |
| 正丁醇 | 2.95 | 17.1(25℃) | 118 |
| PEG200 | 62.2 | 6.4 | >250 |
| N,N-二甲基乙酰胺 | 1.37 | 39.1 | 166 |
| 解吸再生工艺 | 优点 | 缺点 | 适用有机胺类型 |
|---|---|---|---|
| 传统热解吸再生 | 工艺成熟、设备简单廉价 | 能耗较大、设备占地较大、易造成氧化和胺逃逸 | 比热容小、挥发性小 |
| 热解吸+催化解吸再生 | 催化剂用量少、可在传统热解吸的 设备基础上使用 | 催化剂的筛选较难、成本高 | 均可 |
| 热解吸+膜解吸再生 | 设备投资小、设备紧凑,结构简单、操作简单、维修保养容易、能耗低 | 解吸速率慢、膜成本高、稳定性低 | 单胺 |
| 微波解吸再生 | 能量利用效率较高、有机胺挥发较少、 加热速率快 | 微波设备较小、工业化推广受阻 | 极性基团多、介电常数大 |
| 矿化再生 | 原料来源广泛、产物经济价值较高、 矿化反应为放热反应,反应能耗低 | 有机胺再生效率低、吸收-矿化一体化工业 设备较少 | 叔胺 |
| 电化学介导胺解吸再生 | 可以模块化设计、解吸效率快、 有机胺挥发较少、热降解较少 | 解吸时需消耗阳极材料、解吸能耗受压力影响 较大、解吸CO2气体压力低 | 多胺 |
| 解吸再生工艺 | 优点 | 缺点 | 适用有机胺类型 |
|---|---|---|---|
| 传统热解吸再生 | 工艺成熟、设备简单廉价 | 能耗较大、设备占地较大、易造成氧化和胺逃逸 | 比热容小、挥发性小 |
| 热解吸+催化解吸再生 | 催化剂用量少、可在传统热解吸的 设备基础上使用 | 催化剂的筛选较难、成本高 | 均可 |
| 热解吸+膜解吸再生 | 设备投资小、设备紧凑,结构简单、操作简单、维修保养容易、能耗低 | 解吸速率慢、膜成本高、稳定性低 | 单胺 |
| 微波解吸再生 | 能量利用效率较高、有机胺挥发较少、 加热速率快 | 微波设备较小、工业化推广受阻 | 极性基团多、介电常数大 |
| 矿化再生 | 原料来源广泛、产物经济价值较高、 矿化反应为放热反应,反应能耗低 | 有机胺再生效率低、吸收-矿化一体化工业 设备较少 | 叔胺 |
| 电化学介导胺解吸再生 | 可以模块化设计、解吸效率快、 有机胺挥发较少、热降解较少 | 解吸时需消耗阳极材料、解吸能耗受压力影响 较大、解吸CO2气体压力低 | 多胺 |
| 1 | MASSON-DELMOTTE V P, ZHAI P, PIRANI S L, et al. Climate change 2021: The physical science basis. contribution of working group i to the sixth assessment report of the intergovernmental panel on climate change[R]. 2021. |
| 2 | 戴厚良, 苏义脑, 刘吉臻, 等. 碳中和目标下我国能源发展战略思考[J]. 石油科技论坛, 2022, 41(1): 1-8. |
| DAI Houliang, SU Yinao, LIU Jizhen, et al. Thinking of China’s energy development strategy under carbon neutrality goal[J]. Petroleum Science and Technology Forum, 2022, 41(1): 1-8. | |
| 3 | PALES Araceli Fernández, BENNETT S. Energy technology perspectives 2020[R]: International Energy Agency, 2020. |
| 4 | 熊波, 陈健, 李克兵, 等. 工业排放气二氧化碳捕集与利用技术进展[J]. 低碳化学与化工, 2023, 48(1): 9-18. |
| XIONG Bo, CHEN Jian, LI Kebing, et al. Technical progress in carbon dioxide capture and utilization of industrial vent gas[J]. Low-Carbon Chemistry and Chemical Engineering, 2023, 48(1): 9-18. | |
| 5 | LIU Meishen, GADIKOTA Greeshma. Single-step, low temperature and integrated CO2 capture and conversion using sodium glycinate to produce calcium carbonate[J]. Fuel, 2020, 275: 117887. |
| 6 | ROSEN Jonathan, GEARY Timothy, HILMI Abdelkader, et al. Molten carbonate fuel cell performance for CO2 capture from natural gas combined cycle flue gas[J]. Journal of the Electrochemical Society, 2020, 167(6): 064505. |
| 7 | VEGA F, BAENA-MORENO F M, GALLEGO FERNÁNDEZ L M, et al. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale[J]. Applied Energy, 2020, 260: 114313. |
| 8 | LIANG Zhiwu, FU Kaiyun, IDEM Raphael, et al. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chinese Journal of Chemical Engineering, 2016, 24(2): 278-288. |
| 9 | MOTA-MARTINEZ Maria T, BRANDL Patrick, HALLETT Jason P, et al. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture[J]. Molecular Systems Design & Engineering, 2018, 3(3): 560-571. |
| 10 | 陈玮琪. 离子液体非水相变吸收体系CO2捕集性能及其反应机理研究[D]. 杭州: 浙江大学, 2023. |
| CHEN Weiqi. CO2 capture performance and reaction mechanism of ionic liquid non-aqueous phase change absorption systems[D]. Hangzhou: Zhejiang University, 2023. | |
| 11 | LIU Hongyan, FU Zuobao, HUANG Qingshan, et al. Experimental investigation on continuous reaction crystallizer for CO2 capture by phase-change method[J]. Chemical Engineering Journal, 2023, 466: 143345. |
| 12 | BIAN Yangyang, SHEN Shufeng. CO2 absorption into a phase change absorbent: Water-lean potassium prolinate/ethanol solution[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2318-2326. |
| 13 | LI Qiangwei, WANG Yi, AN Shanlong, et al. Kinetics of CO2 absorption in concentrated K2CO3/PZ mixture using a wetted-wall column[J]. Energy & Fuels, 2016, 30(9): 7496-7502. |
| 14 | NWAOHA Chikezie, SAIWAN Chintana, TONTIWACHWUTHIKUL Paitoon, et al. Carbon dioxide (CO2) capture: Absorption-desorption capabilities of 2-amino-2-methyl-1-propanol (AMP), piperazine (PZ) and monoethanolamine (MEA) tri-solvent blends[J]. Journal of Natural Gas Science Engineering, 2016, 33: 742-750. |
| 15 | ZHANG Weidong, JIN Xianhang, TU Weiwei, et al. Development of MEA-based CO2 phase change absorbent[J]. Applied Energy, 2017, 195: 316-323. |
| 16 | Bihong LYU, YANG Kexuan, ZHOU Xiaobin, et al. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture[J]. Applied Energy, 2020, 264: 114703. . |
| 17 | WANG Rujie, JIANG Lei, LI Qiangwei, et al. Energy-saving CO2 capture using sulfolane-regulated biphasic solvent[J]. Energy, 2020, 211: 118667. |
| 18 | SIMONS Katja, NIJMEIJER Kitty, WESSLING Matthias. Gas-liquid membrane contactors for CO2 removal[J]. Journal of Membrane Science, 2009, 340(1/2): 214-220. |
| 19 | 张卫风, 邓兆雄, 邱雪霏, 等. 基于MDEA混合胺CO2富液的膜解吸法试验研究[J]. 动力工程学报, 2020, 40(9): 750-756. |
| ZHANG Weifeng, DENG Zhaoxiong, QIU Xuefei, et al. Experimental study on membrane desorption of CO2-rich solutions with MDEA mixed amine[J]. Journal of Chinese Society of Power Engineering, 2020, 40(9): 750-756. | |
| 20 | DIBROV G A, VOLKOV V V, VASILEVSKY V P, et al. Robust high-permeance PTMSP composite membranes for CO2 membrane gas desorption at elevated temperatures and pressures[J]. Journal of Membrane Science, 2014, 470: 439-450. |
| 21 | CHEN Guangying, CHEN Guangjie, CAO Fan, et al. Mass transfer performance and correlation for CO2 absorption into aqueous 3-diethylaminopropylamine solution in a hollow fiber membrane contactor[J]. Chemical Engineering Processing-Process Intensification, 2020, 152: 107932. |
| 22 | LIN Haiqing, FREEMAN Benny D. Materials selection guidelines for membranes that remove CO2 from gas mixtures[J]. Journal of Molecular Structure, 2005, 739(1/2/3): 57-74. |
| 23 | 贺志敏, 汤志刚, Ebrahim Ataeivarjovi, 等. 用于从CO2-DMC体系分离CO2的PDMS复合膜制备与分离性能[J]. 高校化学工程学报, 2017, 31(5): 1042-1051. |
| HE Zhimin, TANG Zhigang, EBRAHIM Ataeivarjovi, et al. Preparation and separation performance of PDMS composite membranes for CO2 separation from CO2-DMC system[J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(5): 1042-1051. | |
| 24 | BHATTI Umair H, SHAH Abdul K, HUSSAIN Amjad, et al. Catalytic activity of facilely synthesized mesoporous HZSM-5 catalysts for optimizing the CO2 desorption rate from CO2-rich amine solutions[J]. Chemical Engineering Journal, 2020, 389: 123439. |
| 25 | LI Tianci, YANG Congning, TANTIKHAJORNGOSOL Puttipong, et al. Experimental investigations of CO2 absorption and catalyst-aided CO2 desorption performance of several different amines blending with a promoter[J]. Chemical Engineering Science, 2022, 264: 118177. |
| 26 | SHI Huancong, PENG Jiacheng, CHENG Xiaofang, et al. The CO2 desorption analysis of tri-solvent MEA+ BEA+ DEEA with several commercial solid acid catalysts[J]. International Journal of Greenhouse Gas Control, 2022, 116: 103647. |
| 27 | TAN Zhan, ZHANG Shangshang, ZHAO Fangfang, et al. SnO2/ATP catalyst enabling energy-efficient and green amine-based CO2 capture[J]. Chemical Engineering Journal, 2023, 453: 139801. |
| 28 | IDEM Raphael, SHI Huancong, GELOWITZ Don, et al. Catalytic method and apparatus for separating a gaseous component from an incoming gas stream: US20130108532[P]. 2013-05-02. |
| 29 | SHI Huancong, NAAMI Abdulaziz, IDEM Raphael, et al. Catalytic and non catalytic solvent regeneration during absorption-based CO2 capture with single and blended reactive amine solvents[J]. International Journal of Greenhouse Gas Control, 2014, 26: 39-50. |
| 30 | ZHANG Xin, LIU Helei, LIANG Zhiwu. CO2 desorption in single and blended amine solvents with and without catalyst[J]. Energy Procedia, 2017, 114: 1862-1868. |
| 31 | Benjamin DECARDI-NELSON, AKACHUKU Ananda, OSEI Priscilla, et al. Catalyst performance and experimental validation of a rigorous desorber model for low temperature catalyst-aided desorption of CO2 in single and blended amine solutions[J]. Journal of Environmental Chemical Engineering, 2017, 5(4): 3865-3872. |
| 32 | LI Xiaojing, XU Qian, LIU Zhishan, et al. Nonacid carbon materials as catalysts for monoethanolamine energy-efficient regeneration[J]. Environmental Science & Technology, 2023, 57(27): 9975-9983. |
| 33 | BHATTI Umair H, ALIVAND Masood S, BARZAGLI Francesco, et al. Functionalized carbon spheres for energy-efficient CO2 capture: Synthesis, application, and reaction mechanism[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(32): 11955-11964. |
| 34 | LI Yuchen, CHEN Zhen, ZHAN Guoxiong, et al. Inducing efficient proton transfer through Fe/Ni@COF to promote amine-based solvent regeneration for achieving low-cost capture of CO2 from industrial flue gas[J]. Separation and Purification Technology, 2022, 298: 121676. |
| 35 | CHEN Weixuan, WANG Weize, WANG Junjun, et al. Construction of stable MOFs integrated with open metal sites and amine groups for CO2 capture and conversion[J]. Journal of Solid State Chemistry, 2023, 317: 123729. |
| 36 | GUO Yunzhao, ZHANG Huiping, FU Kaiyun, et al. Integration of solid acid catalyst and ceramic membrane to boost amine-based CO2 desorption[J]. Energy, 2023, 274: 127329. |
| 37 | MCGURK Stephen J, MARTíN Claudia F, BRANDANI Stefano, et al. Microwave swing regeneration of aqueous monoethanolamine for post-combustion CO2 capture[J]. Applied Energy, 2017, 192: 126-133. |
| 38 | Yamid GOMEZ-RUEDA, VEROUGSTRAETE Brieuc, RANGA Chanakya, et al. Rapid temperature swing adsorption using microwave regeneration for carbon capture[J]. Chemical Engineering Journal, 2022, 446: 137345. |
| 39 | 李洪, 崔俊杰, 李鑫钢, 等. 微波场强化化工分离过程研究进展[J]. 化工进展, 2016, 35(12): 3735-3745. |
| LI Hong, CUI Junjie, LI Xingang, et al. Recent developments in microwave-assisted chemical separation processes[J]. Chemical Industry and Engineering Progress, 2016, 35(12): 3735-3745. | |
| 40 | 高金哲. 三乙烯四胺非水吸收剂捕集CO2的微波再生过程研究[D]. 杭州: 浙江大学, 2019. |
| GAO Jinzhe. The study on microwave regeneration process of triethylenetetramine nonaqueous absorbents for CO2 capture[D]. Hangzhou: Zhejiang University, 2019. | |
| 41 | BOUGIE Francis, FAN Xianfeng Microwave regeneration of monoethanolamine aqueous solutions used for CO 2 capture[J]. International Journal of Greenhouse Gas Control, 2018, 79: 165-172. |
| 42 | LI Yu, GAO Jinzhe, LI Jinxiu, et al. Screening and performance evaluation of triethylenetetramine nonaqueous solutions for CO2 capture with microwave regeneration[J]. Energy & Fuels, 2020, 34(9): 11270-11281. |
| 43 | TAO Mengna, GAO Jinzhe, ZHANG Wei, et al. A novel phase-changing nonaqueous solution for CO2 capture with high capacity, thermostability, and regeneration efficiency[J]. Industrial & Engineering Chemistry Research, 2018, 57(28): 9305-9312. |
| 44 | LI Jinxiu, LI Yu, LI Chen, et al. CO2 absorption and microwave regeneration with high-concentration TETA nonaqueous absorbents[J]. Greenhouse Gases: Science and Technology, 2022, 12(3): 362-375. |
| 45 | JEMAA N, H-J WALLS, R-D NOBLE, et al. Continuous electrochemically modulated complexation separations process[J]. AIChE Journal, 1993, 39(5): 867-875. |
| 46 | DJURDJEVIC Predrag, BJERRUM Jannik, MELANDER Lars, et al. Metal ammine formation in solution. XXIV. The copper(Ⅱ)- and some other metal(Ⅱ)- mono- and diethanolamine systems[J]. Acta Chemica Scandinavica, 1983, 37a: 881-890. |
| 47 | STERN Michael C, SIMEON Fritz, HERZOG Howard, et al. An electrochemically-mediated gas separation process for carbon abatement[J]. Energy Procedia, 2013, 37: 1172-1179. |
| 48 | 徐楠, 张永春, 毛庆, 等. 有机胺电化学再生系统性能与影响因素[J]. 煤炭学报, 2023, 48(7): 2737-2747. |
| XU Nan, ZHANG Yongchun, MAO Qing, et al. Performance and influencing factors of organic amine electrochemical regeneration system[J]. Journal of China Coal Society, 2023, 48(7): 2737-2747. | |
| 49 | LIU Guangxin, YU Yunsong, HONG Yingting, et al. Identifying electrochemical effects in a thermal-electrochemical co-driven system for CO2 capture[J]. Physical Chemistry Chemical Physics, 2017, 19(20): 13230-13244. |
| 50 | 范会峰, 吴小梅, 刘广鑫, 等. 电化学介导胺再生CO2捕集系统电化学性能研究[J]. 高校化学工程学报, 2022, 36(2): 235-241. |
| FAN Huifeng, WU Xiaomei, LIU Guangxin, et al. Electrochemical performance of electrochemically-mediated amine regeneration CO2 capture systems [J]. Journal of Chemical Engineering of Chinese Universities, 2022, 36(2): 235-241. | |
| 51 | WU Xiaomei, FAN Huifeng, SHARIF Maimoona, et al. Electrochemically-mediated amine regeneration of CO2 capture: From electrochemical mechanism to bench-scale visualization study[J]. Applied Energy, 2021, 302: 117554. |
| 52 | WANG Miao, RAHIMI Mohammad, KUMAR Amit, et al. Flue gas CO2 capture via electrochemically mediated amine regeneration: System design and performance[J]. Applied Energy, 2019, 255: 113879. |
| 53 | WANG Miao, HERZOG Howard J, Alan HATTON T. CO2 capture using electrochemically mediated amine regeneration[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 7087-7096. |
| 54 | 王磊, 蒋勇, 钟达忠, 等. 碳化的MOF用于电催化还原二氧化碳制备乙烯和乙醇[J]. 化工学报, 2022, 73(8): 3576-3585. |
| WANG Lei, JIANG Yong, ZHONG Dazhong, et al. Carbonized metal-organic framework for carbon dioxide reduction to ethylene and ethanol[J]. CIESC Journal, 2022, 73(8): 3576-3585. | |
| 55 | PAN Shu-Yuan, CHEN Yi-Hung, FAN Liang-Shih, et al. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction[J]. Nature Sustainability, 2020, 3(5): 399-405. |
| 56 | LI Kangkang, LEIGH Wardhaugh, FERON Paul, et al. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: Techno-economic assessment of the MEA process and its improvements[J]. Applied Energy, 2016, 165: 648-659. |
| 57 | PARK Sangwon, MIN Jaehong, LEE Mingu, et al. Characteristics of CO2 fixation by chemical conversion to carbonate salts[J]. Chemical Engineering Journal, 2013, 231: 287-293. |
| 58 | 王中辉, 苏胜, 尹子骏, 等. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| WANG Zhonghui, SU Sheng, YIN Zijun, et al. Research progress of CO2 mineralization and integrated absorption-mineralization (IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327. | |
| 59 | ARTI Murnandari, YOUN Min Hye, PARK Ki Tae, et al. Single process for CO2 capture and mineralization in various alkanolamines using calcium chloride[J]. Energy & Fuels, 2017, 31(1): 763-769. |
| 60 | KANG Ji min, MURNANDARI Arti, YOUN Min Hye, et al. Energy-efficient chemical regeneration of AMP using calcium hydroxide for operating carbon dioxide capture process[J]. Chemical Engineering Journal, 2018, 335: 338-344. |
| [1] | ZHOU Yu, TANG Tian, XIONG Ziyou, WEI Qi. Methanol to olefin wastewater treatment based on a two-stage microchannel separation process [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 100-108. |
| [2] | WANG Ning, LU Shijian, LIU Ling, LIANG Jing, LIU Miaomiao, SUN Mengyuan, KANG Guojun. Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 445-464. |
| [3] | LI Lei, ZHAO Yanmin, TIAN Haiyang, LI Jiangwei, ZHOU Qiang, HE Jiani, WU Wanyue. Simulation and optimization of low energy consumption and high efficiency capture process for low concentration CO2 in flue gas [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 581-589. |
| [4] | LI Lin, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Chunxia, WANG Junlian, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Recovery and regeneration preparation of aluminum-based spent catalyst support [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 640-649. |
| [5] | CAO Shuyang, SHI Jingbo, DONG Youming, LYU Jianxiong. Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5095-5105. |
| [6] | LI Hongyan, XIE Shuhan, ZHANG Yanru, WANG Yongjing, WANG Yonghao, LYU Yuancai, LIN Chunxiang, LI Xiaojuan. Research progress on the direct regeneration technology for cathode materials from spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5207-5216. |
| [7] | HU Tingxia, ZHAO Lixin, YAO Zonglu, HUO Lili, JIA Jixiu, XIE Teng. Research progress of bimetallic catalysts in catalytic steam reforming of biomass tar [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4354-4365. |
| [8] | LU Shijian, ZHANG Juanjuan, YANG Fei, LIU Ling, CHEN Siming, KANG Guojun, FANG Qinqin. Research progress of amine escape control technology by chemical absorption method [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4562-4570. |
| [9] | LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613. |
| [10] | LIU Jingang, LIU Qingwang, FAN Zhenzhong, WANG Yangyang, ZHOU Ming. Evaluation of flocculation effect of hyperbranched flocculant on waste oil-based drilling fluid [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4738-4747. |
| [11] | LIU Kefeng, LIU Taoran, CAI Yong, HU Xuesheng, DONG Weigang, ZHOU Huaqun, GAO Fei. Progress in research and engineering demonstration of CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2901-2914. |
| [12] | ZHI Yuan, MA Jiliang, CHEN Xiaoping, LIU Daoyin, LIANG Cai. Decarbonization capability of supported Na-based CO2 adsorbents prepared by fluidized bed spray impregnation [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2961-2967. |
| [13] | WANG Houran, LI Denian, DONG Nanhang, YANG Jizhang, NI Xuanyuan, YE Jiahong, YUAN Haoran, CHEN Yong. Advances in direct repair of cathode materials from retired lithium iron phosphate battery and ternary lithium battery [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3336-3346. |
| [14] | YAO Xue, WU Shuhui, YANG Yang, WANG Xiao, FENG Lei, FENG Xuedong, MA Yanfei. Treatment of oily wastewater by oily sludge-based biochar [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3398-3409. |
| [15] | GAO Fanxiang, LIU Yang, ZHANG Guiquan, QIN Feng, YAO Jiantao, JIN Hui, SHI Jinwen. Research progress of wet process synergistic desulfurization and decarbonization technology for coal-fired flue gas [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2324-2342. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||