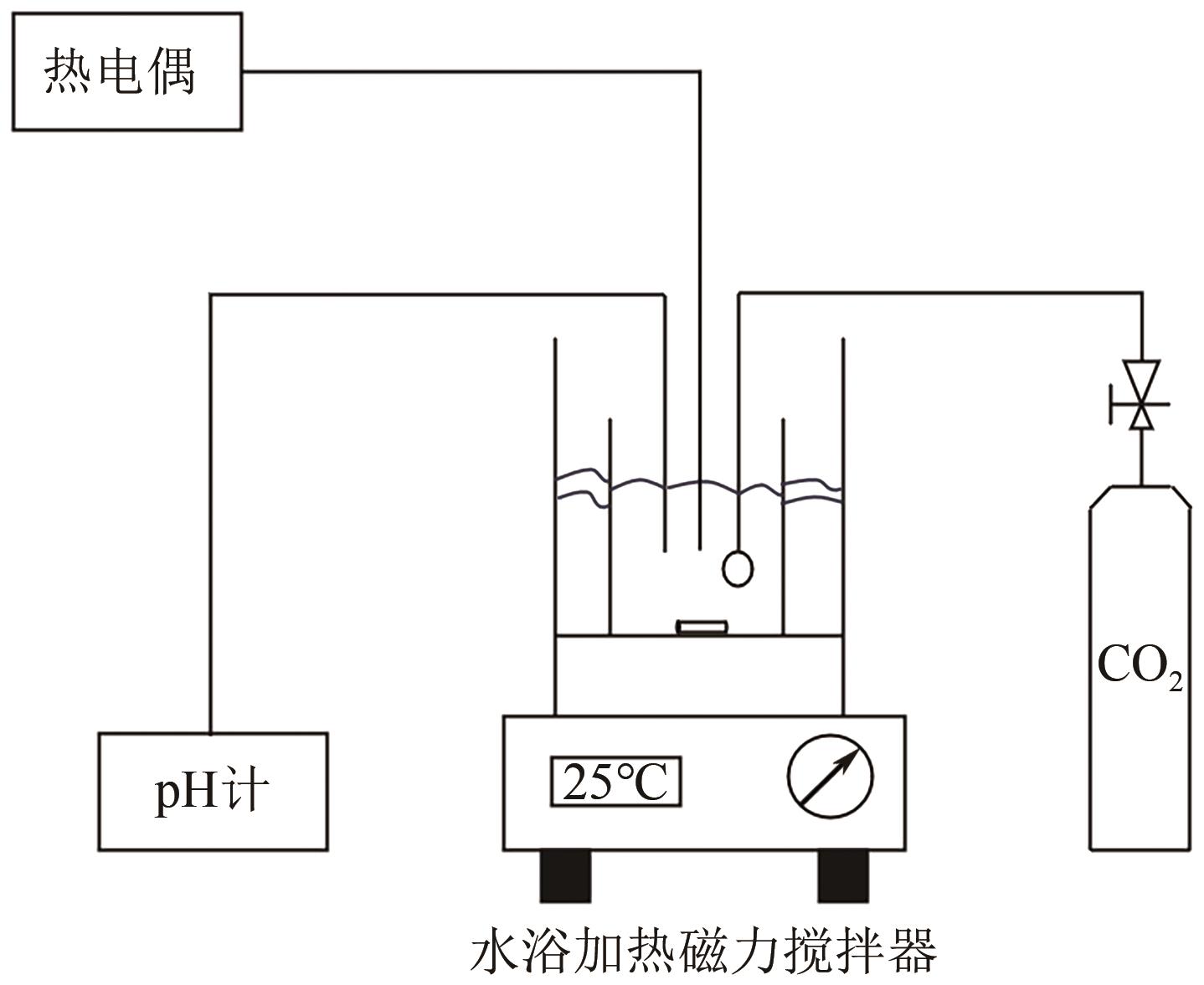
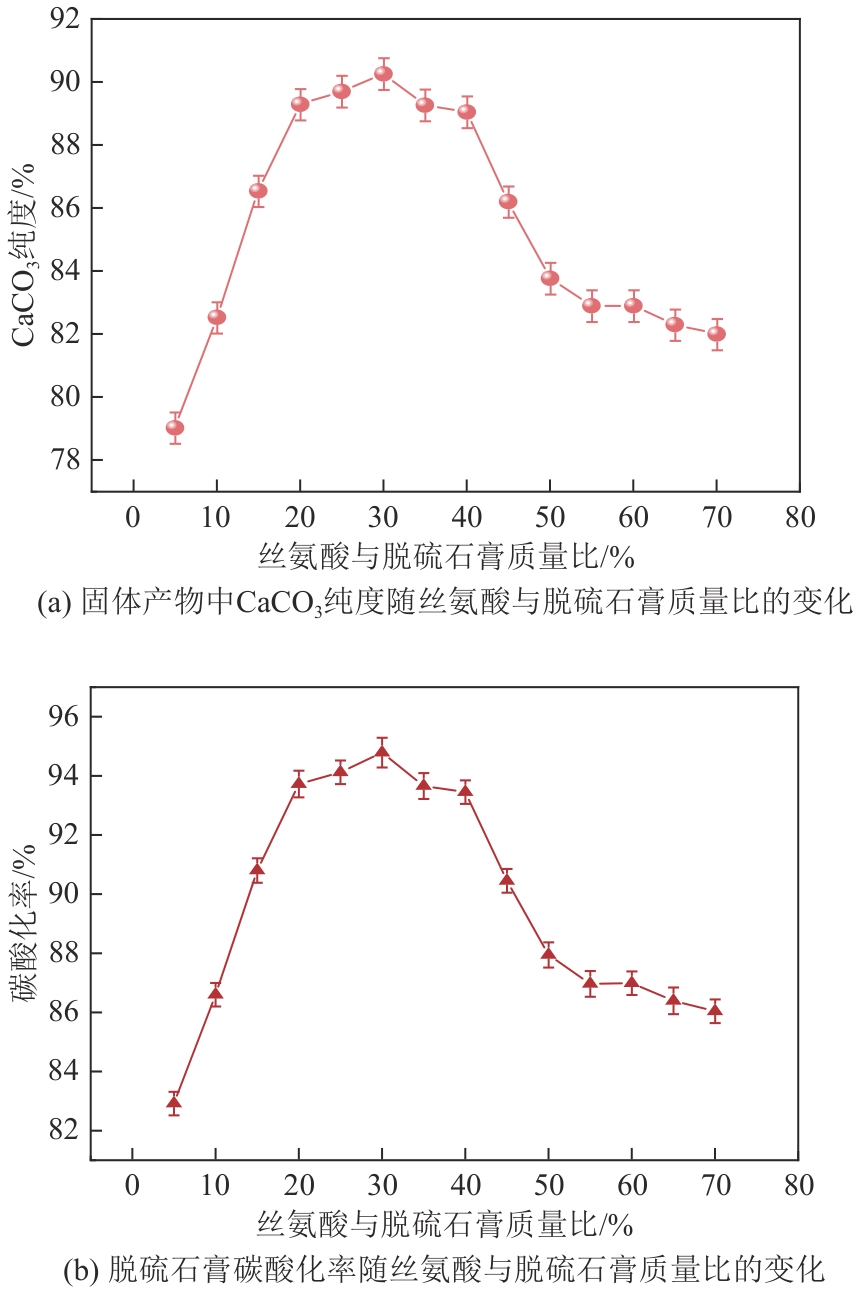
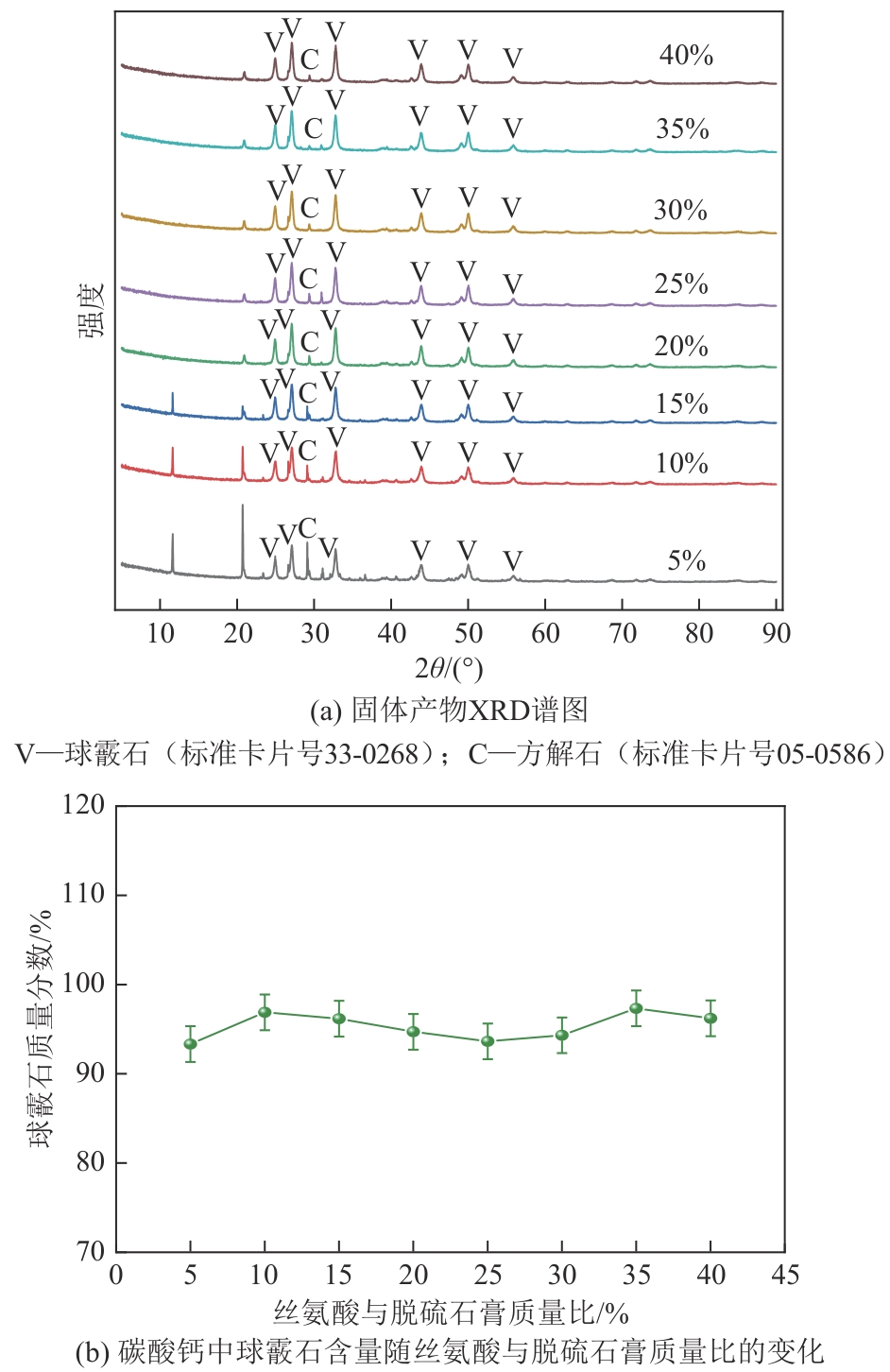
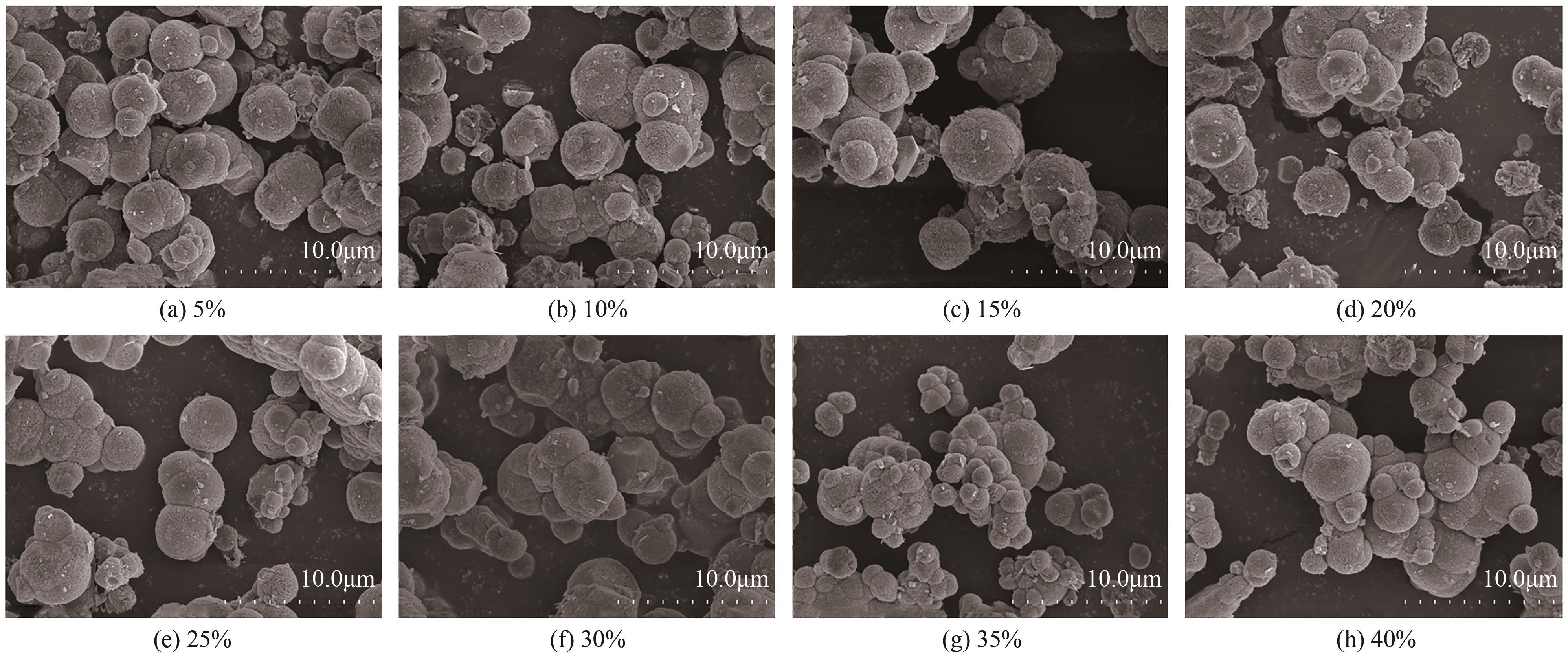
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (4): 2328-2337.DOI: 10.16085/j.issn.1000-6613.2024-0483
• Resources and environmental engineering • Previous Articles Next Articles
Preparation of vaterite CaCO3 by mineralizing CO2 from desulfurized gypsum
DOU Yu1,2( ), WANG Wenxuan3, FAN Chunlei4, MA Jiliang1,2, LIANG Cai1,2, CHEN Xiaoping1,2(
), WANG Wenxuan3, FAN Chunlei4, MA Jiliang1,2, LIANG Cai1,2, CHEN Xiaoping1,2( )
)
- 1.School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
2.Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University, Nanjing 210096, Jiangsu, China
3.Fengye Technology & Environment Group Co. , Ltd. , Yangzhou 225263, Jiangsu, China
4.Huaneng Nanjing Jinling Power Generation Co. , Ltd. , Nanjing 210096, Jiangsu, China
-
Received:2024-03-25Revised:2024-06-24Online:2025-05-07Published:2025-04-25 -
Contact:CHEN Xiaoping
脱硫石膏矿化CO2制备球霰石碳酸钙
窦玉1,2( ), 王文选3, 范春雷4, 马吉亮1,2, 梁财1,2, 陈晓平1,2(
), 王文选3, 范春雷4, 马吉亮1,2, 梁财1,2, 陈晓平1,2( )
)
- 1.东南大学能源与环境学院,江苏 南京 210096
2.东南大学能源热转换及其过程测控教育部重点实验室,江苏 南京 210096
3.江苏峰业环境科技集团股份有限公司,江苏 扬州 225236
4.华能南京金陵发电有限公司,江苏 南京 210096
-
通讯作者:陈晓平 -
作者简介:窦玉(1999—),男,硕士研究生,研究方向为CO2减排技术与固废资源化利用。E-mail:2281396412@qq.com。 -
基金资助:国家重点研发计划(2020YFC1908700);江苏省碳达峰碳中和专项(BK20220001)
CLC Number:
Cite this article
DOU Yu, WANG Wenxuan, FAN Chunlei, MA Jiliang, LIANG Cai, CHEN Xiaoping. Preparation of vaterite CaCO3 by mineralizing CO2 from desulfurized gypsum[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2328-2337.
窦玉, 王文选, 范春雷, 马吉亮, 梁财, 陈晓平. 脱硫石膏矿化CO2制备球霰石碳酸钙[J]. 化工进展, 2025, 44(4): 2328-2337.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0483
| 成分 | 质量分数/% |
|---|---|
| CaO | 36.40 |
| SO3 | 38.00 |
| SiO2 | 2.65 |
| Fe2O3 | 0.22 |
| Al2O3 | 0.61 |
| K2O | 0.08 |
| MgO | 0.36 |
| 结晶水 | 20.91 |
| 成分 | 质量分数/% |
|---|---|
| CaO | 36.40 |
| SO3 | 38.00 |
| SiO2 | 2.65 |
| Fe2O3 | 0.22 |
| Al2O3 | 0.61 |
| K2O | 0.08 |
| MgO | 0.36 |
| 结晶水 | 20.91 |
| 元素 | 质量分数/% |
|---|---|
| S | 1.38 |
| Si | 1.36 |
| F | 0.82 |
| Al | 0.64 |
| Fe | 0.60 |
| Na | 0.25 |
| K | 0.15 |
| Mg | 0.13 |
| Ti | 0.08 |
| Sr | 0.08 |
| Cl | 0.03 |
| Zn | 0.02 |
| P | 0.02 |
| 元素 | 质量分数/% |
|---|---|
| S | 1.38 |
| Si | 1.36 |
| F | 0.82 |
| Al | 0.64 |
| Fe | 0.60 |
| Na | 0.25 |
| K | 0.15 |
| Mg | 0.13 |
| Ti | 0.08 |
| Sr | 0.08 |
| Cl | 0.03 |
| Zn | 0.02 |
| P | 0.02 |
| 取样 质量/g | 定容 体积/mL | 稀释 系数 | 所测 元素 | 仪器读数 /mg·L-1 | 换算含量 /mg·kg-1 | 国家标准 /mg·kg-1 |
|---|---|---|---|---|---|---|
| 0.1058 | 25 | 1 | As | 0.0033 | 0.8 | ≤10 |
| 0.1058 | 25 | 1 | Cd | 0.0004 | 0.1 | ≤10 |
| 0.1058 | 25 | 1 | Co | 0.0114 | 2.7 | |
| 0.1058 | 25 | 1 | Cr | 0.0361 | 8.5 | ≤50 |
| 0.1058 | 25 | 1 | Cu | 0.0057 | 1.3 | |
| 0.1058 | 25 | 1 | Ni | 0.0023 | 0.5 | |
| 0.1058 | 25 | 1 | Pb | 0.0019 | 0.4 | ≤50 |
| 0.1058 | 25 | 1 | Sb | 0.0027 | 0.6 | |
| 0.1058 | 25 | 1 | Tl | 0.0013 | 0.3 | |
| 0.1058 | 25 | 1 | V | 0.0009 | 0.2 |
| 取样 质量/g | 定容 体积/mL | 稀释 系数 | 所测 元素 | 仪器读数 /mg·L-1 | 换算含量 /mg·kg-1 | 国家标准 /mg·kg-1 |
|---|---|---|---|---|---|---|
| 0.1058 | 25 | 1 | As | 0.0033 | 0.8 | ≤10 |
| 0.1058 | 25 | 1 | Cd | 0.0004 | 0.1 | ≤10 |
| 0.1058 | 25 | 1 | Co | 0.0114 | 2.7 | |
| 0.1058 | 25 | 1 | Cr | 0.0361 | 8.5 | ≤50 |
| 0.1058 | 25 | 1 | Cu | 0.0057 | 1.3 | |
| 0.1058 | 25 | 1 | Ni | 0.0023 | 0.5 | |
| 0.1058 | 25 | 1 | Pb | 0.0019 | 0.4 | ≤50 |
| 0.1058 | 25 | 1 | Sb | 0.0027 | 0.6 | |
| 0.1058 | 25 | 1 | Tl | 0.0013 | 0.3 | |
| 0.1058 | 25 | 1 | V | 0.0009 | 0.2 |
| 1 | 曹建宗, 刘琦, 陈文通,等. 典型湿法脱硫系统存在的问题及人工智能在优化运行中的应用[J]. 化工进展, 2020, 39(S1): 242-249 |
| CAO Jianzong, LIU Qi, CHEN Wentong, et al. Problems of typical wet desulfurization system and application of artificial intelligence in optimal operation[J]. Chemical Industry and Engineering Progress, 2020, 39(S1): 242-249. | |
| 2 | AAKRITI, MAITI Soumitra, JAIN Neeraj, et al. A comprehensive review of flue gas desulphurized gypsum: Production, properties, and applications[J]. Construction and Building Materials, 2023, 393: 131918. |
| 3 | 王文飚, 许月阳, 薛建明,等. 燃煤电厂脱硫技术研究进展及建议[J]. 电力科技与环保, 2020, 36(3): 1-5. |
| WANG Wenbiao, XU Yueyang, XUE Jianming, et al. The research progress and suggestions of desulfurization technology in coal-fired power plants[J]. Electric Power Technology and Environmental Protection, 2020, 36(3): 1-5. | |
| 4 | 郭程程. 小型热电厂烟气脱硫系统改造设计优化与实践[J]. 电力科技与环保, 2020, 36(3): 42-44. |
| GUO Chengcheng. Optimization and practice about desulfurization reform in small thermal power plants[J]. Electric Power Technology and Environmental Protection, 2020, 36(3): 42-44. | |
| 5 | WANG Guodong, HOU Zhiwei, ZHAN Li, et al. Integrated numerical simulation of CO2 flooding, heat recovery and storage in high temperature reservoir[J]. Petroleum Science and Technology, 2023, 41(12): 1250-1271. |
| 6 | FREEMAN C L, HARDING J H. The transformation of amorphous calcium carbonate to calcite and classical nucleation theory[J]. Journal of Crystal Growth, 2023, 603: 126978. |
| 7 | Anett LÁZÁR, Zsombor MOLNÁR, Attila DEMÉNY, et al. Insights into the amorphous calcium carbonate (ACC) → ikaite → calcite transformations[J]. CrystEngComm, 2023, 25(5): 738-750. |
| 8 | ZHANG Shuheng, NAHI Ouassef, CHEN Li, et al. Magnesium ions direct the solid-state transformation of amorphous calcium carbonate thin films to aragonite, magnesium-calcite, or dolomite[J]. Advanced Functional Materials, 2022, 32(25): 2201394. |
| 9 | LUO Mingzhi, ZHANG Guoquan, FANG Yuguo, et al. Calcium carbonate crystallization process from the mineralization of calcium chloride waste[J]. Separation and Purification Technology, 2023, 319: 124066. |
| 10 | MARMO Vitor Luca Moura, AMBRÓSIO Jéssica A R, GONÇALVES Erika Peterson, et al. Vaterite microparticle-loaded methylene blue for photodynamic activity in macrophages infected with Leishmania braziliensis [J]. Photochemical & Photobiological Sciences, 2023, 22(8): 1977-1989. |
| 11 | WILLIAMS Jonah M, ZHAO Diandian, MOON Seokyoon, et al. Stabilization of pure vaterite during carbon mineralization: Defining critical activities, additive concentrations, and gas flow conditions for carbon utilization[J]. Crystal Growth & Design, 2023, 23(11): 8103-8115. |
| 12 | Xingyuan SAN, HU Junwei, CHEN Mingyi, et al. Unlocking the mysterious polytypic features within vaterite CaCO3 [J]. Nature Communications, 2023, 14(1): 7858. |
| 13 | 丁文金, 姚金, 乔静怡, 等. 磷石膏矿化CO2制备高纯碳酸钙及产物调控[J]. 矿产保护与利用, 2022, 42(4): 104-112. |
| DING Wenjin, YAO Jin, QIAO Jingyi, et al. Preparation of high-purity CaCO3 from phosphogypsum by CO2 mineralization and product control[J]. Conservation and Utilization of Mineral Resources, 2022, 42(4): 104-112. | |
| 14 | 李文秀, 杨宇航, 黄艳, 等. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057. |
| LI Wenxiu, YANG Yuhang, HUANG Yan, et al. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057. | |
| 15 | SASAMOTO Ryo, KANDA Yasuharu, YAMANAKA Shinya. Difference in cadmium chemisorption on calcite and vaterite porous particles[J]. Chemosphere, 2022, 297: 134057. |
| 16 | FEBRIDA Renny, CAHYANTO Arief, HERDA Ellyza, et al. Synthesis and characterization of porous CaCO3 vaterite particles by simple solution method[J]. Materials, 2021, 14(16): 4425. |
| 17 | TRUSHINA Daria B, BORODINA Tatiana N, BELYAKOV Sergei, et al. Calcium carbonate vaterite particles for drug delivery: Advances and challenges[J]. Materials Today Advances, 2022, 14: 100214. |
| 18 | LUO Wenli, LI Zhaojian, ZHANG Ling, et al. Polyethylenimine-CO2 adduct templated CaCO3 nanoparticles as anticancer drug carrier[J]. Cancer Nanotechnology, 2023, 14(1): 7. |
| 19 | ABAKUMOVA Tatiana O, GUSLIAKOVA Olga I, CVJETINOVIC Julijana, et al. Barnase-loaded vaterite nanoparticles functionalized by EpCAM targeting vectors for the treatment of lung diseases[J]. ACS Applied Nano Materials, 2022, 5(8): 10744-10754. |
| 20 | Donata KONOPACKA-ŁYSKAWA. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review[J]. Crystals, 2019, 9(4): 223. |
| 21 | 王宇轩, 徐颖, 王东平, 等. 球霰石的性质及其应用进展[J]. 安徽理工大学学报(自然科学版), 2017, 37(2): 76-80. |
| WANG Yuxuan, XU Ying, WANG Dongping, et al. Properties and applications of vaterite[J]. Journal of Anhui University of Science and Technology (Natural Science), 2017, 37(2): 76-80. | |
| 22 | LAI Yonghua, CHEN Liangsen, BAO Weichao, et al. Glycine-mediated, selective preparation of monodisperse spherical vaterite calcium carbonate in various reaction systems[J]. Crystal Growth & Design, 2015, 15(3): 1194-1200. |
| 23 | GUO Yuming, WANG Feifei, ZHANG Jie, et al. Biomimetic synthesis of calcium carbonate with different morphologies under the direction of different amino acids[J]. Research on Chemical Intermediates, 2013, 39(6): 2407-2415. |
| 24 | 王波. 脱硫石膏矿化CO2制备均一球霰石型CaCO3机理研究[D]. 太原: 山西大学, 2021. |
| WANG Bo. Study on mechanism of preparation of uniform vaterite-type CaCO3 via CO2 mineralization by flue gas desulfurization gypsum[D]. Taiyuan: Shanxi University, 2021. | |
| 25 | SARKAR Arpita, DUTTA Kingshuk, MAHAPATRA Samiran. Polymorph control of calcium carbonate using insoluble layered double hydroxide[J]. Crystal Growth & Design, 2013, 13(1): 204-211. |
| 26 | ZHAO Diandian, WILLIAMS Jonah M, HOU Pengkun, et al. Stabilizing mechanisms of metastable vaterite in cement systems[J]. Cement and Concrete Research, 2024, 178: 107441. |
| 27 | NAKANISHI Yurie, CHENG Bohan, RICHARDSON Joseph J, et al. Using phenolic polymers to control the size and morphology of calcium carbonate microparticles[J]. RSC Advances, 2023, 13(43): 30539-30547. |
| 28 | SONG Kyungsun, JANG Young-Nam, KIM Wonbaek, et al. Precipitation of calcium carbonate during direct aqueous carbonation of flue gas desulfurization gypsum[J]. Chemical Engineering Journal, 2012, 213: 251-258. |
| 29 | LI Qing, DING Yi, LI Fanqing, et al. Solvothermal growth of vaterite in the presence of ethylene glycol, 1, 2-propanediol and glycerin[J]. Journal of Crystal Growth, 2002, 236(1/2/3): 357-362. |
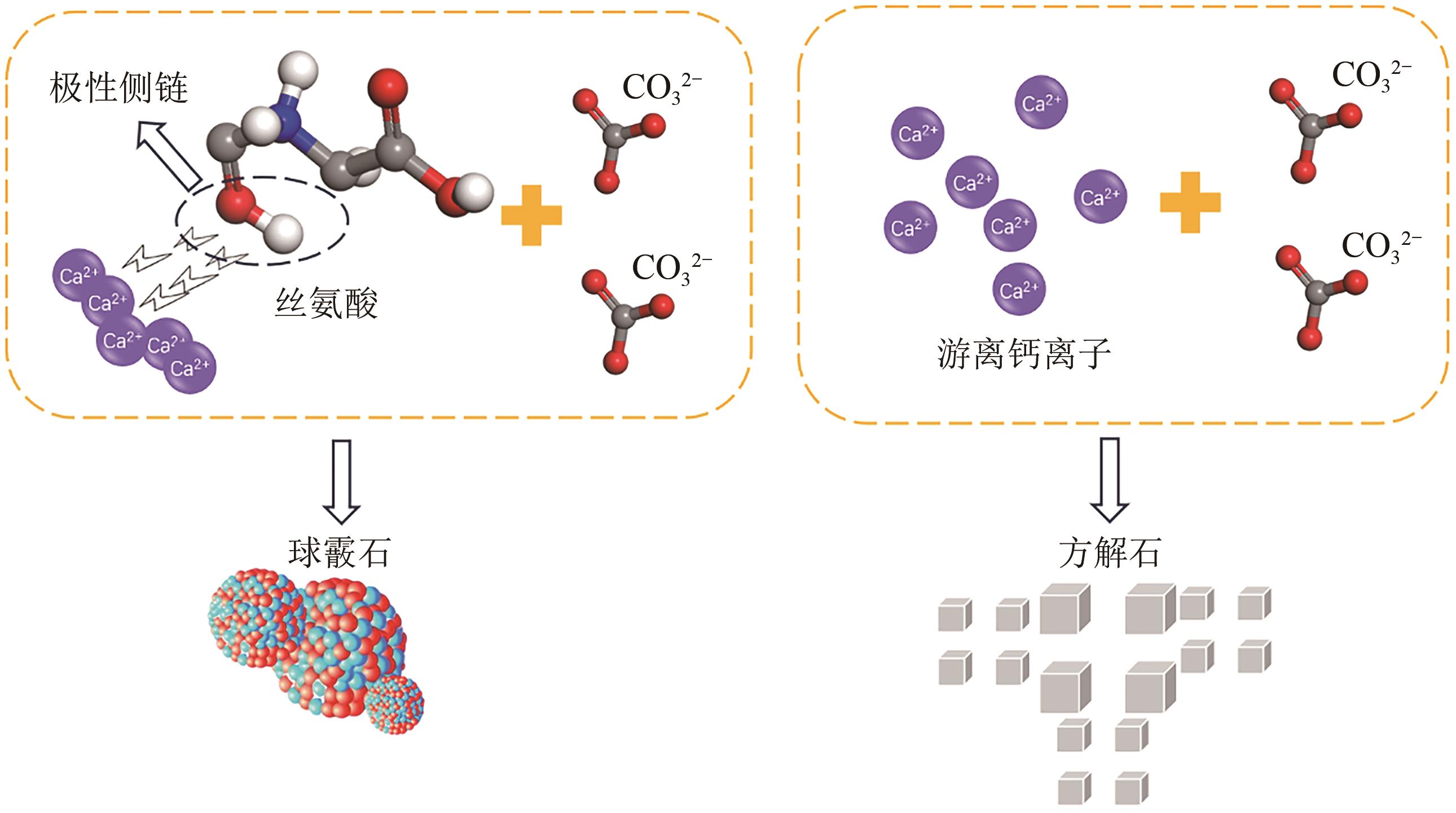
| 30 | POLAT Sevgi. Experimental investigations on the effects of asparagine and serine on the polymorphism of calcium carbonate[J]. Advanced Powder Technology, 2020, 31(10): 4282-4291. |
| 31 | MENAHEM Tali, MASTAI Yitzhak. Controlled crystallization of calcium carbonate superstructures in macroemulsions[J]. Journal of Crystal Growth, 2008, 310(15): 3552-3556. |
| 32 | XU Meng, YUAN Liang, ZHANG Shuiqin, et al. Effects of a preparation containing amino acids on pakchoi nutrient absorption, yield, and quality when grown in saline-alkali soil[J]. Agriculture, 2023, 13(4): 863. |
| 33 | ZIMMERMANN Sandra E, BENSTEIN Ruben M, María FLORES-TORNERO, et al. The phosphorylated pathway of serine biosynthesis links plant growth with nitrogen metabolism[J]. Plant Physiology, 2021, 186(3): 1487-1506. |
| [1] | WANG Ning, LU Shijian, LIU Ling, LIANG Jing, LIU Miaomiao, SUN Mengyuan, KANG Guojun. Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 445-464. |
| [2] | LI Letian, LU Shijian, LIU Hanxiao, WU Liming, LIU Ling, KANG Guojun. Progress of desorption and regeneration of organic amine-enriched liquids [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 490-499. |
| [3] | LU Shijian, ZHANG Juanjuan, YANG Fei, LIU Ling, CHEN Siming, KANG Guojun, FANG Qinqin. Research progress of amine escape control technology by chemical absorption method [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4562-4570. |
| [4] | LI Weijie, LU Leilei, LI Deke, WANG Chunhang, ZHANG Zuming, TAN Qiang. Lithium-ion battery disassembly and recycling technology and progress [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4601-4613. |
| [5] | LIU Kefeng, LIU Taoran, CAI Yong, HU Xuesheng, DONG Weigang, ZHOU Huaqun, GAO Fei. Progress in research and engineering demonstration of CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2901-2914. |
| [6] | ZHI Yuan, MA Jiliang, CHEN Xiaoping, LIU Daoyin, LIANG Cai. Decarbonization capability of supported Na-based CO2 adsorbents prepared by fluidized bed spray impregnation [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2961-2967. |
| [7] | XIAN Xuequan, DU Fangli, LIU Zhonglin, LIU Wanyu, LI Yanming, LONG Siyu, HUANG Hualin. Preparation and formation mechanism of vaterite calcium carbonate microspheres by PEG/Na2CO3 aqueous two-phase emulsion method [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3221-3231. |
| [8] | MIAO Yihe, WANG Yaozu, LIU Yuhang, ZHU Xuancan, LI Jia, YU Lijun. Research progress on the improving effect of additives on supported amine adsorbents for carbon capture [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2739-2759. |
| [9] | GAO Fanxiang, LIU Yang, ZHANG Guiquan, QIN Feng, YAO Jiantao, JIN Hui, SHI Jinwen. Research progress of wet process synergistic desulfurization and decarbonization technology for coal-fired flue gas [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2324-2342. |
| [10] | SUN Weiji, LIU Lang, FANG Zhiyu, ZHU Mengbo, XIE Geng, HE Wei, GAO Yuheng. Technique of wet carbonation of modified magnesium slag [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2161-2173. |
| [11] | XU Zewen, WANG Ming, WANG Qiang, HOU Yingfei. Recent advances in amine-rich membrane for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1374-1386. |
| [12] | CHU Zhenpu, CHEN Yumeng, LI Junguo, SUN Qingxuan, LIU Ke. Review on recycling of graphite anode from spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1524-1534. |
| [13] | QIN Xue, ZHAO Chuanwen, HUANG Pu, ZENG Pengxin, SUN Jian, GUO Yafei. Preparation and performance optimization of Na2CO3-based CO2 forming adsorbent by graphite-casting method [J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7095-7104. |
| [14] | WU Dawei, YIN Yihan, CAO Zhiyong, LIN Haizhou, FAN Yongchun, GAO Hongxia, LIANG Zhiwu. Experimental study on CO2 mass transfer performance of TETA-DEEA-TMS-H2O phase separation absorbent in hollow fiber membrane contactor [J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6039-6048. |
| [15] | WEI Shihui, ZHENG Xuan, WANG Yan, WANG Yang, JI Long, YAN Shuiping. Desorption performance of CO2-rich ammonia aqueous via CO2 mineralization using biomass ash [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5950-5957. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||