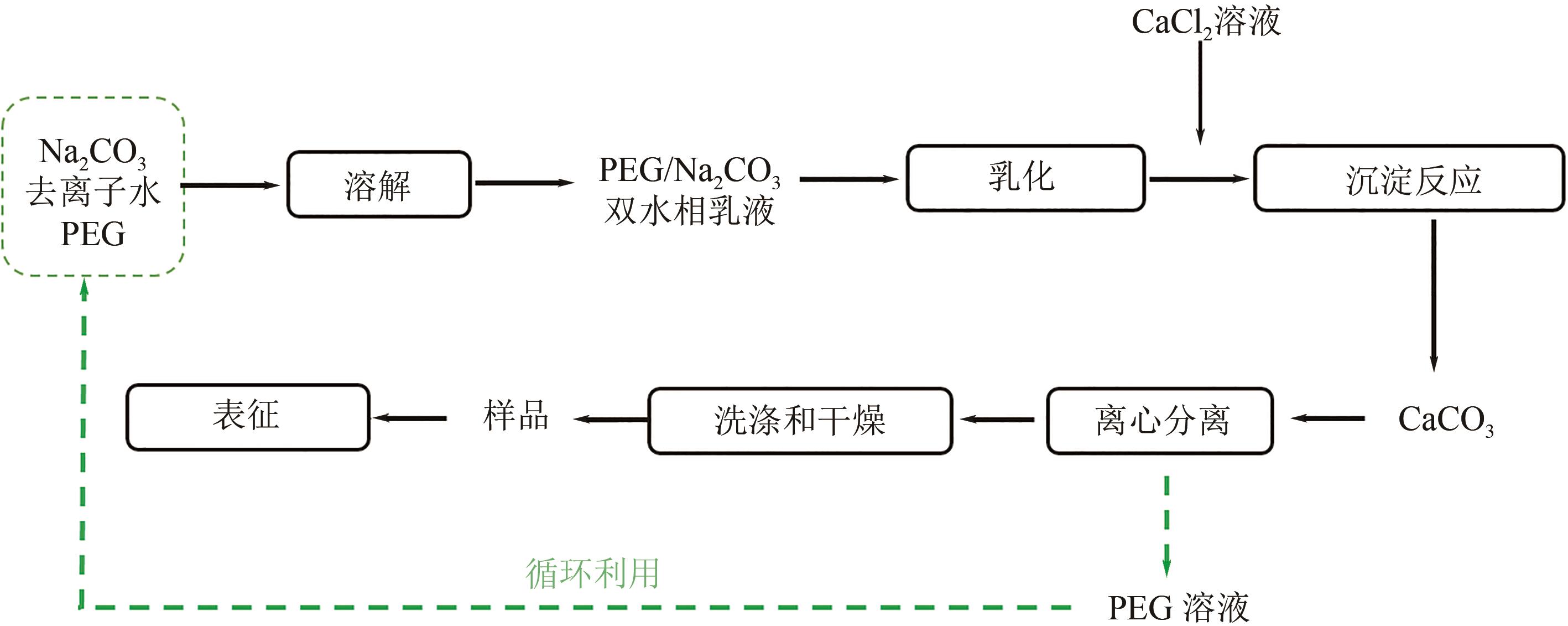
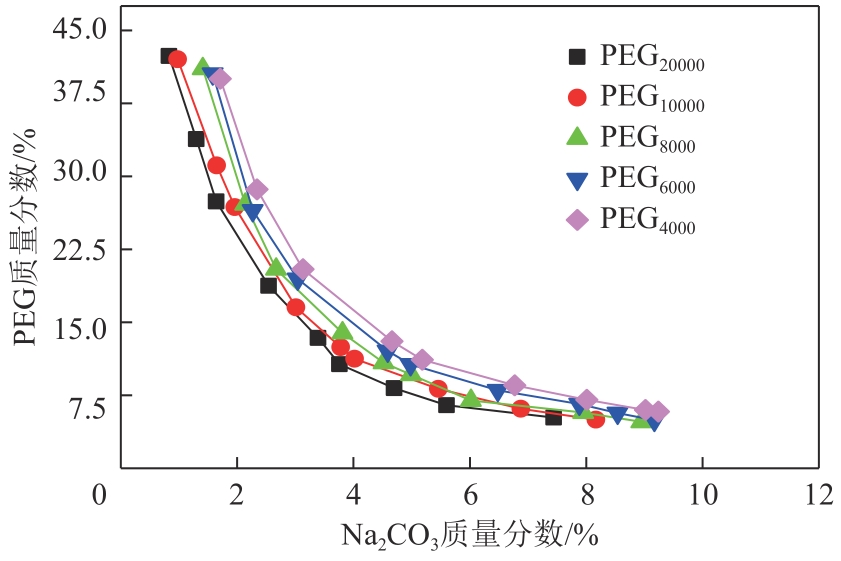
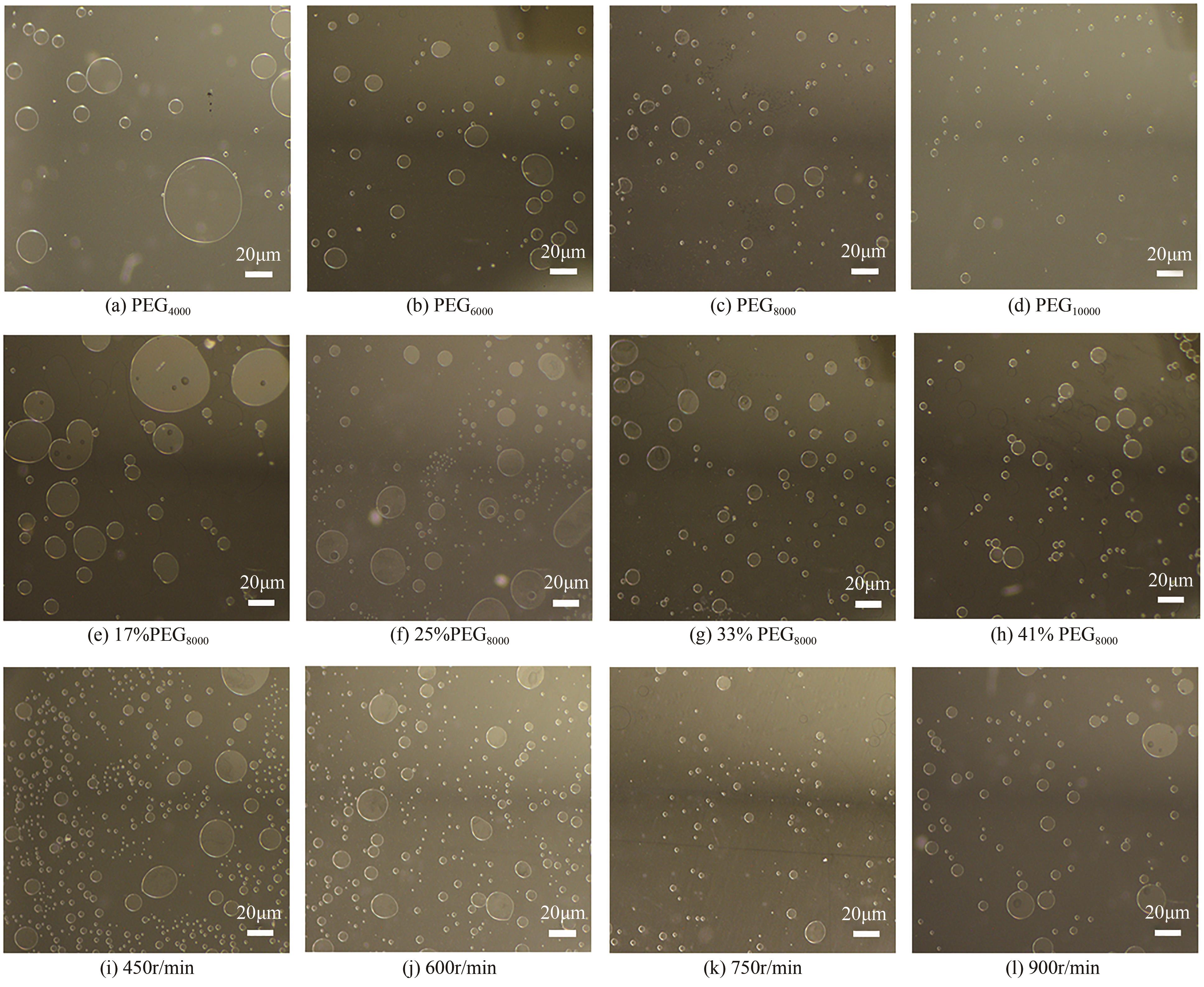
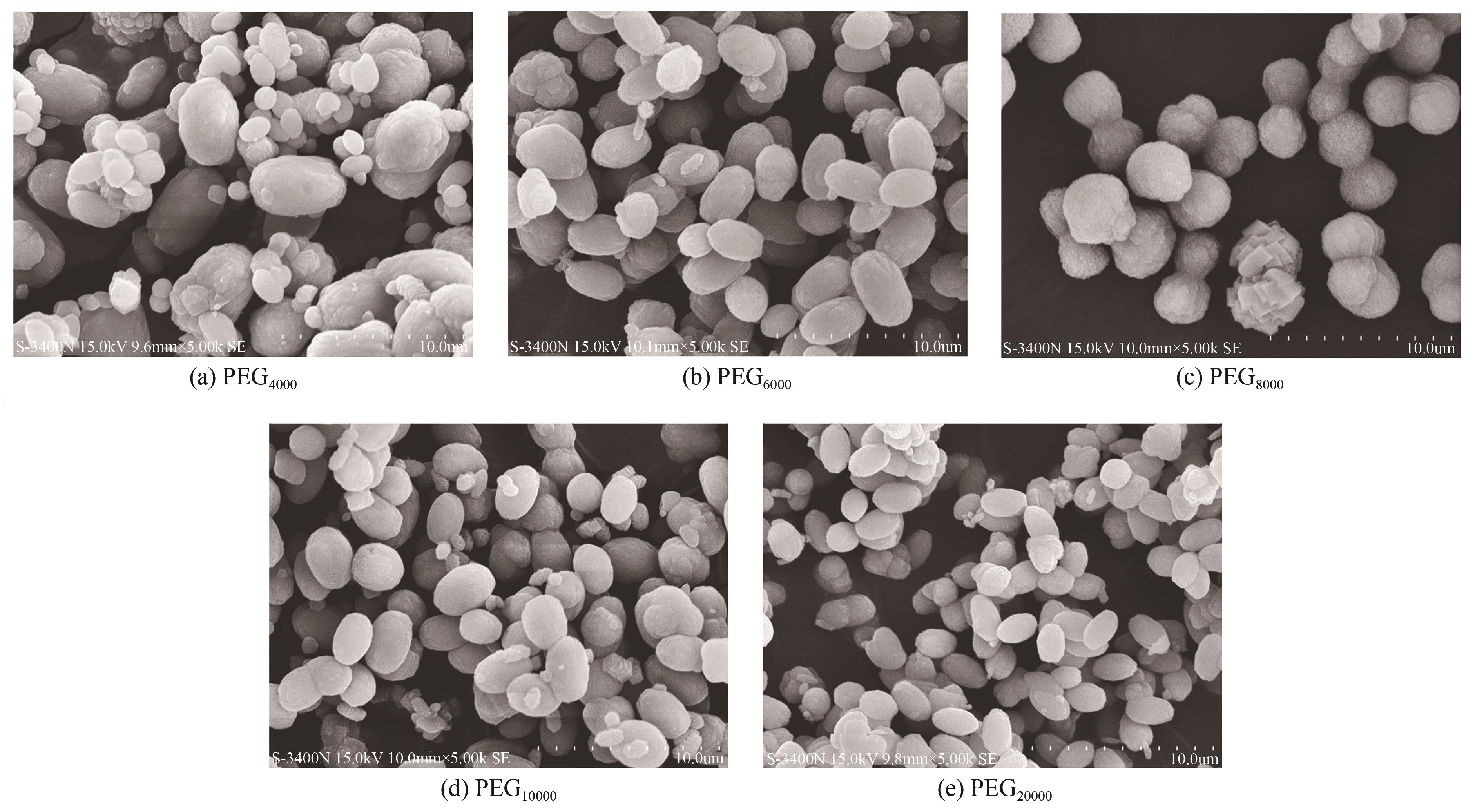
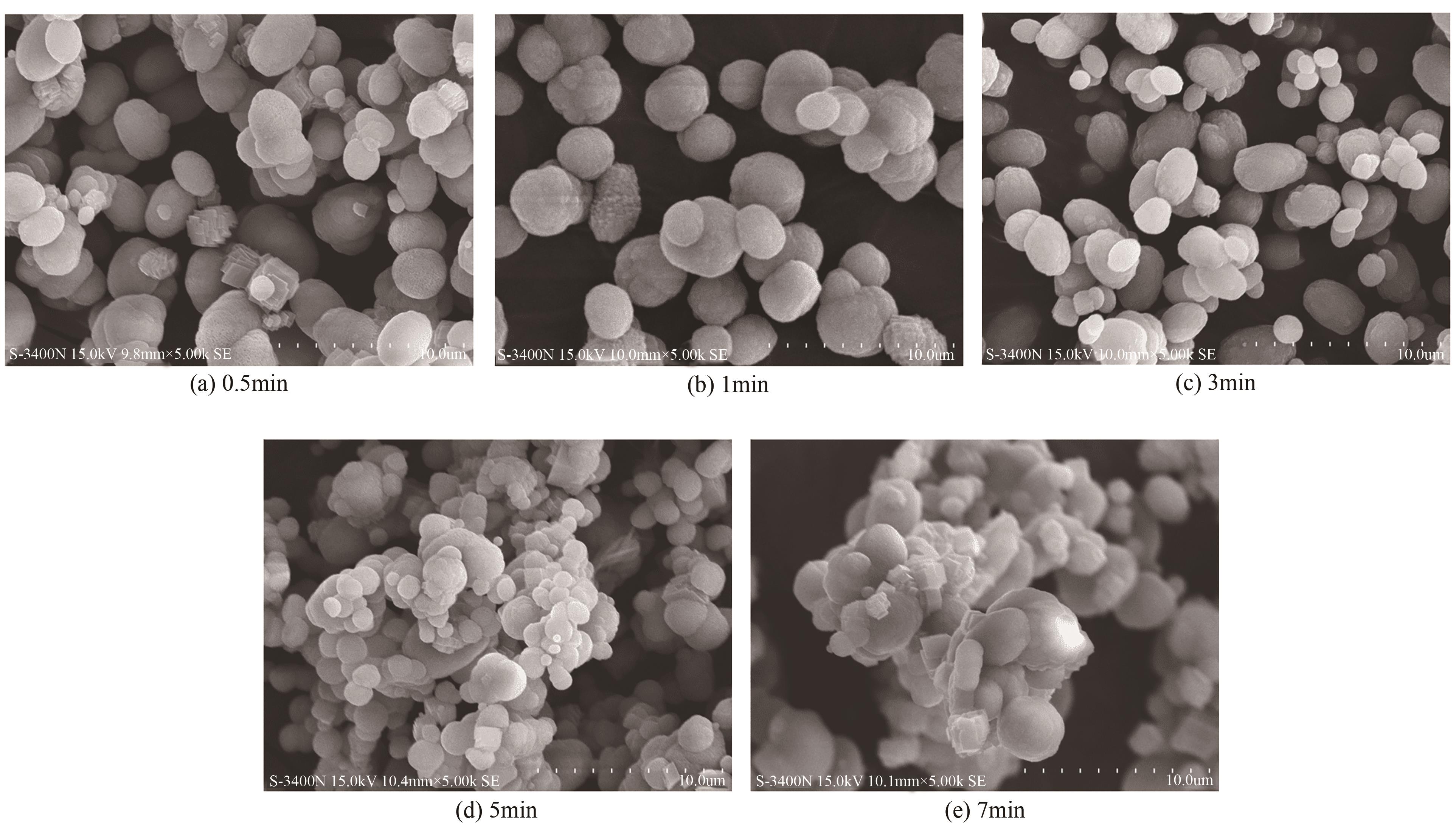
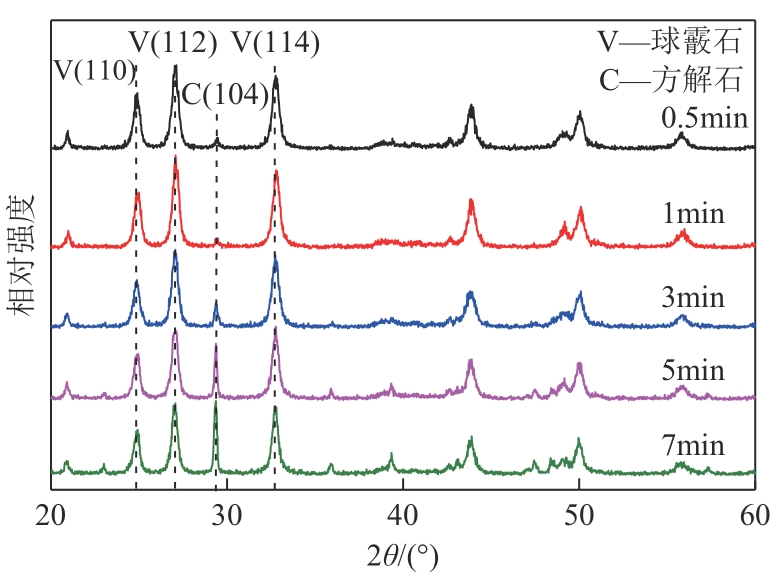
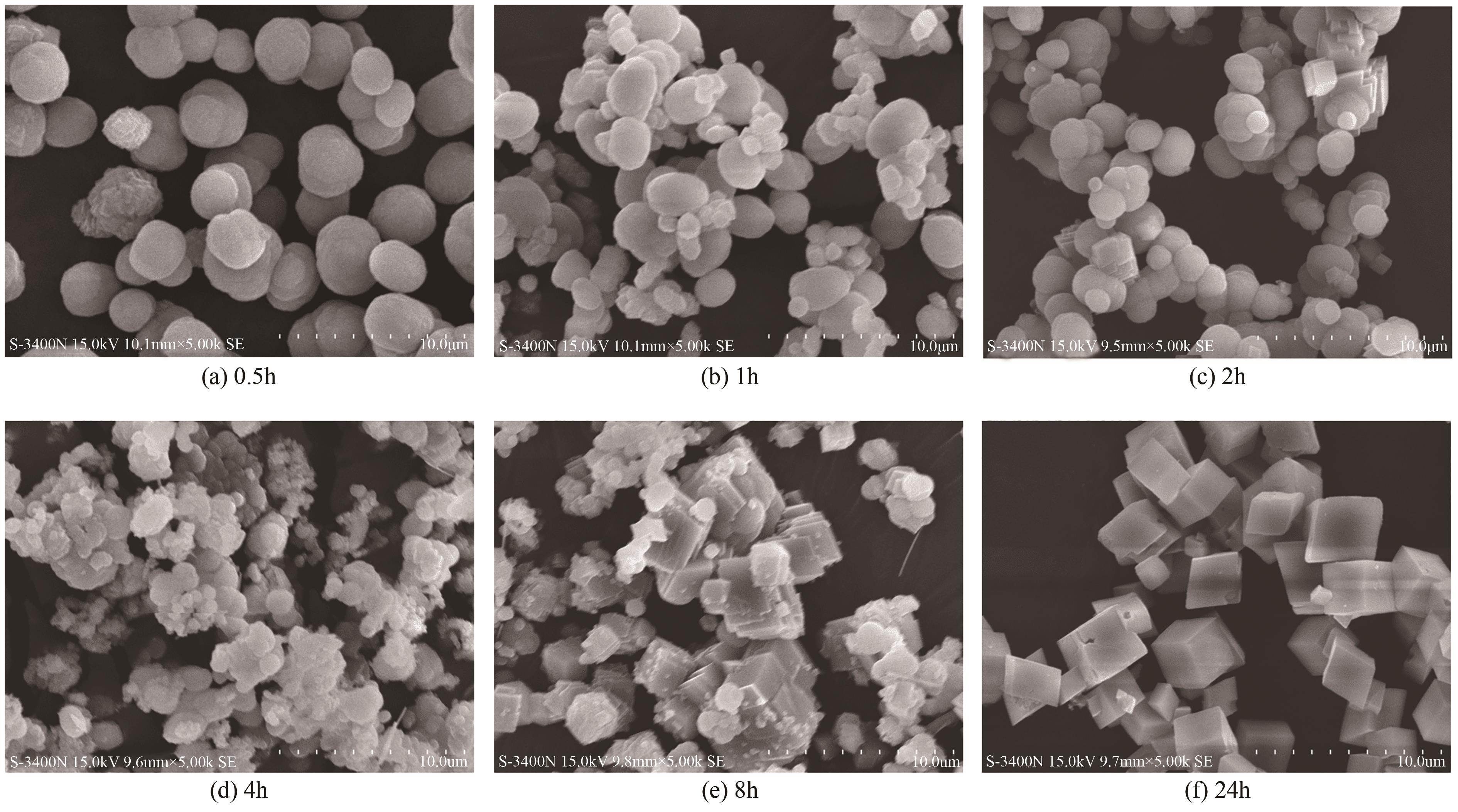
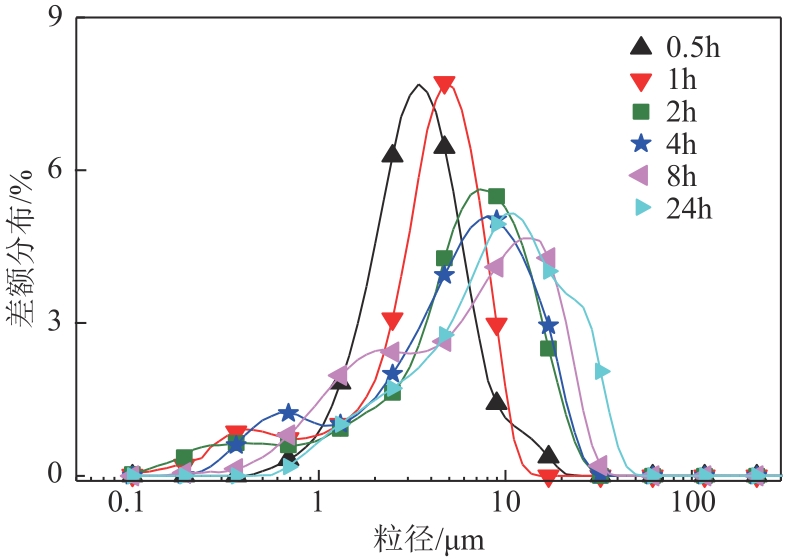
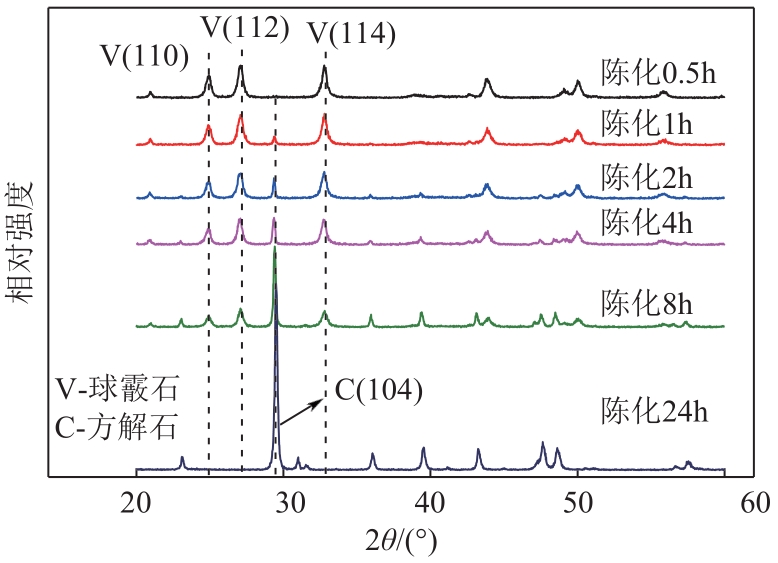
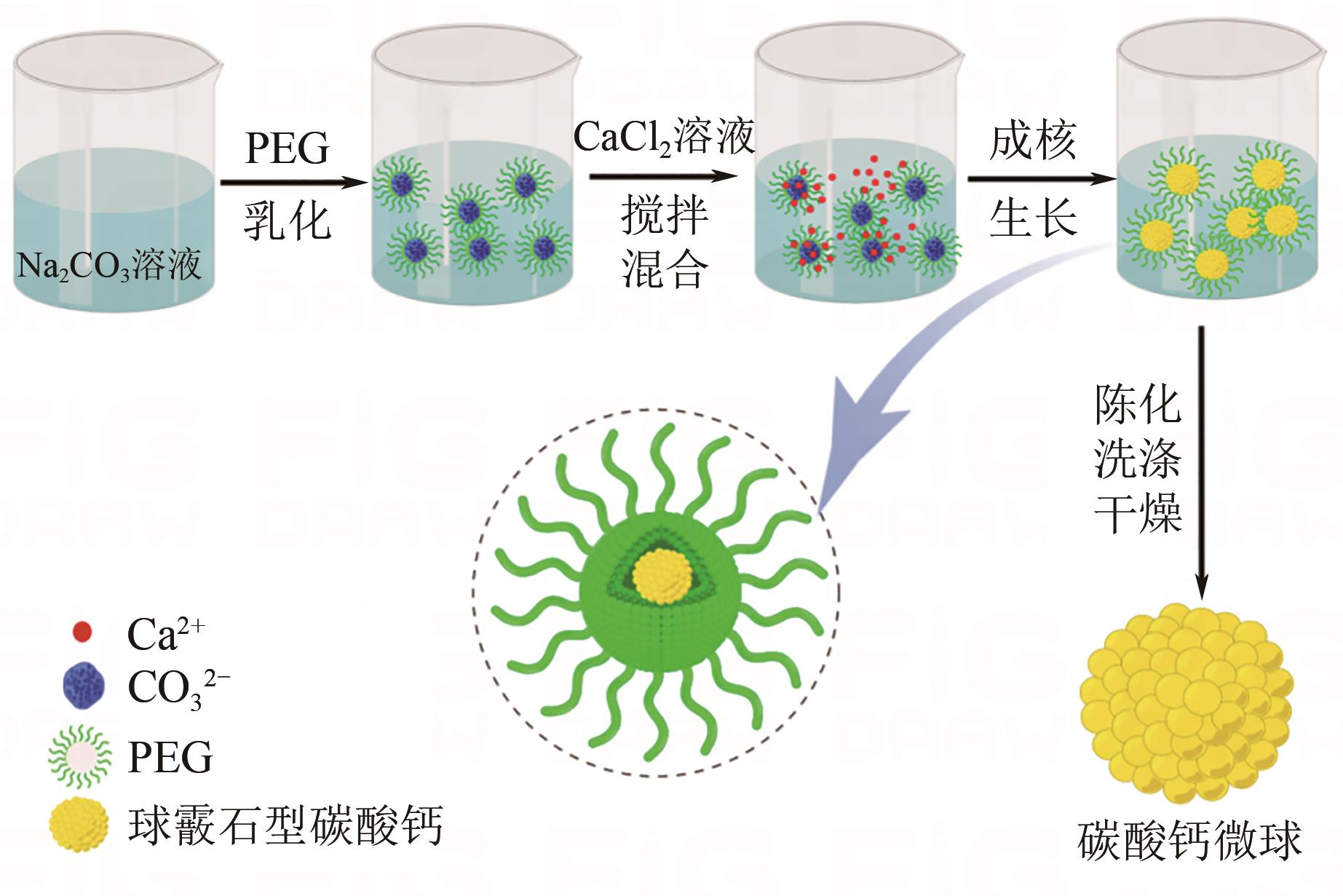
| 1 |
LIU Yating, SHEN Wen, CUI Hua. Combined transition-metal/enzyme dual catalytic system for highly intensive glow-type chemiluminescence-functionalized CaCO3 microspheres[J]. Analytical Chemistry, 2019, 91(16): 10614-10621.
|
| 2 |
ABEBE Mihret, HEDIN Niklas, BACSIK Zoltán. Spherical and porous particles of calcium carbonate synthesized with food friendly polymer additives[J]. Crystal Growth & Design, 2015, 15(8): 3609-3616.
|
| 3 |
LUO Jia, KONG Fantao, MA Xinsheng. Role of aspartic acid in the synthesis of spherical vaterite by the Ca(OH)2-CO2 reaction[J]. Crystal Growth & Design, 2016, 16(2): 728-736.
|
| 4 |
赵历, 卓民权, 龚福忠, 等. 碳化法制备球霰石碳酸钙微球及形成机理[J]. 无机盐工业, 2021, 53(3): 38-43.
|
|
ZHAO Li, ZHUO Minquan, GONG Fuzhong, et al. Synthesis of vaterite CaCO3 microspheres by carbonization method and its formation mechanism[J]. Inorganic Chemicals Industry, 2021, 53(3): 38-43.
|
| 5 |
郑天文, 陈雪梅. 球霰石碳酸钙微球的合成及其机理[J]. 材料科学与工程学报, 2018, 36(3): 358-364.
|
|
ZHENG Tianwen, CHEN Xuemei. Synthesis of vaterite calcium carbonate microspheres and the crystal growth mechanism[J]. Journal of Materials Science and Engineering, 2018, 36(3): 358-364.
|
| 6 |
刘晨民, 刘曦曦, 陈小鹏, 等. 超重力反应结晶碳化法制备球形碳酸钙[J]. 化工进展, 2021, 40(11): 6323-6331.
|
|
LIU Chenmin, LIU Xixi, CHEN Xiaopeng, et al. Preparation of spherical calcium carbonate by high-gravity reaction crystallization carbonization[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6323-6331.
|
| 7 |
SHUM Ho Cheung, BANDYOPADHYAY Amit, BOSE Susmita, et al. Double emulsion droplets as microreactors for synthesis of mesoporous hydroxyapatite[J]. Chemistry of Materials, 2009, 21(22): 5548-5555.
|
| 8 |
DEMELLO Andrew J. Control and detection of chemical reactions in microfluidic systems[J]. Nature, 2006, 442(7101): 394-402.
|
| 9 |
Elke Scholten, VISSER Jendo E, SAGIS Leonard M C, et al. Ultralow interfacial tensions in an aqueous phase-separated gelatin/dextran and gelatin/gum Arabic system: A comparison[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2004, 20(6): 2292-2297.
|
| 10 |
CHAO Youchuang, SHUM Ho Cheung. Emerging aqueous two-phase systems: From fundamentals of interfaces to biomedical applications[J]. Chemical Society Reviews, 2020, 49(1): 114-142.
|
| 11 |
CACACE David N, ROWLAND Andrew T, STAPLETON Joshua J, et al. Aqueous emulsion droplets stabilized by lipid vesicles as microcompartments for biomimetic mineralization[J]. Langmuir, 2015, 31(41): 11329-11338.
|
| 12 |
ZHAO Dazhou, JIANG Jinhua, XU Jianing, et al. Synthesis of template-free hollow vaterite CaCO3 microspheres in the H2O/EG system[J]. Materials Letters, 2013, 104: 28-30.
|
| 13 |
LI Bingzheng, XIAN Xuequan, WANG Yong, et al. Production of recrystallized starch microspheres using water-in-water emulsion and multiple recycling of polyethylene glycol solution[J]. LWT, 2018, 97: 76-82.
|
| 14 |
HADIKO Gunawan, HAN Yong sheng, FUJI Masayoshi, et al. Synthesis of hollow calcium carbonate particles by the bubble templating method[J]. Materials Letters, 2005, 59(19/20): 2519-2522.
|
| 15 |
刘梅芳, 陈素芬, 刘一杨, 等. 厚壁空心微球的球形度和壁厚均匀性的表征研究[J]. 强激光与粒子束, 2014, 26(2): 159-163.
|
|
LIU Meifang, CHEN Sufen, LIU Yiyang, et al. Characterization of sphericity and wall thickness uniformity of thick-walled hollow microspheres[J]. High Power Laser and Particle Beams, 2014, 26(2): 159-163.
|
| 16 |
HAMTA Afshin, DEHGHANI Mohammad Reza, GHOLAMI Mahsa. Novel experimental data on aqueous two-phase system containing PEG-6000 and Na2CO3 at T=(293.15, 303.15 and 313.15)K[J]. Journal of Molecular Liquids, 2017, 241: 144-149.
|
| 17 |
王佳, 刘金彦, 高军, 等. 季膦盐离子液体/表面活性剂/盐双水相的性质及萃取效果研究[J]. 无机盐工业, 2021, 53(4): 43-47.
|
|
WANG Jia, LIU Jinyan, GAO Jun, et al. Study on properties and extraction efficiency of quaternary phosphonium salt ionic liquid/Surfactant/salt aqueous two phase systems[J]. Inorganic Chemicals Industry, 2021, 53(4): 43-47.
|
| 18 |
LI Yan, LI Ximei, CAO Zhinan, et al. Fabrication of uniform casein/CaCO3 vaterite microspheres and investigation of its formation mechanism[J]. Crystal Growth & Design, 2017, 17(12): 6178-6188.
|
| 19 |
费贵强, 安静, 肖文娟, 等. 聚乙二醇对醇酸树脂乳液及漆膜性能的影响[J]. 涂料工业, 2022, 52(8): 14-19, 28.
|
|
FEI Guiqiang, AN Jing, XIAO Wenjuan, et al. Effect of polyethylene glycol on properties of VOC free alkyd resin lotion and paint coating[J]. Paint & Coatings Industry, 2022, 52(8): 14-19, 28.
|
| 20 |
HAN Yongsheng, HADIKO Gunawan, FUJI Masayoshi, et al. Factors affecting the phase and morphology of CaCO3 prepared by a bubbling method[J]. Journal of the European Ceramic Society, 2006, 26(4/5): 843-847.
|
| 21 |
WU Zhigang, GUO Yang, WANG Jian, et al. Preparation of vaterite CaCO3 microspheres by fast precipitation method[J]. International Journal of Materials Research, 2017, 108(3): 245-248.
|
| 22 |
张唯一, 肖洋, 马长健, 等. 水流近壁面水力剪切力对滴灌系统碳酸钙污垢的影响[J]. 农业工程学报, 2023, 39(7): 137-144.
|
|
ZHANG Weiyi, XIAO Yang, MA Changjian, et al. Effects of hydraulic shear on calcium carbonate fouling in drip irrigation systems near the wall of water flow[J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(7): 137-144.
|
| 23 |
贾露凡, 王艺颖, 董钰漫, 等. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246, 1420.
|
|
JIA Lufan, WANG Yiying, DONG Yuman, et al. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction[J]. CIESC Journal, 2023, 74(3): 1239-1246, 1420.
|
| 24 |
JIANG Jiuxin, WU Yue, HE Yao, et al. Progress in tuning of metastable vaterite calcium carbonate[J]. Journal of Inorganic Materials, 2017, 32(7): 681.
|
| 25 |
陈佳琳. 双水相乳液体系构建载酶碳酸钙微球强化级联酶促反应研究[D]. 成都: 西南交通大学, 2021.
|
|
CHEN Jialin. Study on fabrication of compartmentalized CaCO3 microspheres via biomimetic mineralization in aqueous two phase systems emulsion for the enhancement of cascaded enzyme reaction[D]. Chengdu: Southwest Jiaotong University, 2021.
|
| 26 |
CARTWRIGHT Julyan H E, CHECA Antonio G, GALE Julian D, et al. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there?[J]. Angewandte Chemie International Edition, 2012, 51(48): 11960-11970.
|
 ), DU Fangli, LIU Zhonglin, LIU Wanyu, LI Yanming, LONG Siyu, HUANG Hualin(
), DU Fangli, LIU Zhonglin, LIU Wanyu, LI Yanming, LONG Siyu, HUANG Hualin( )
)
 ), 杜芳黎, 刘忠林, 刘婉玉, 黎演明, 龙思宇, 黄华林(
), 杜芳黎, 刘忠林, 刘婉玉, 黎演明, 龙思宇, 黄华林( )
)