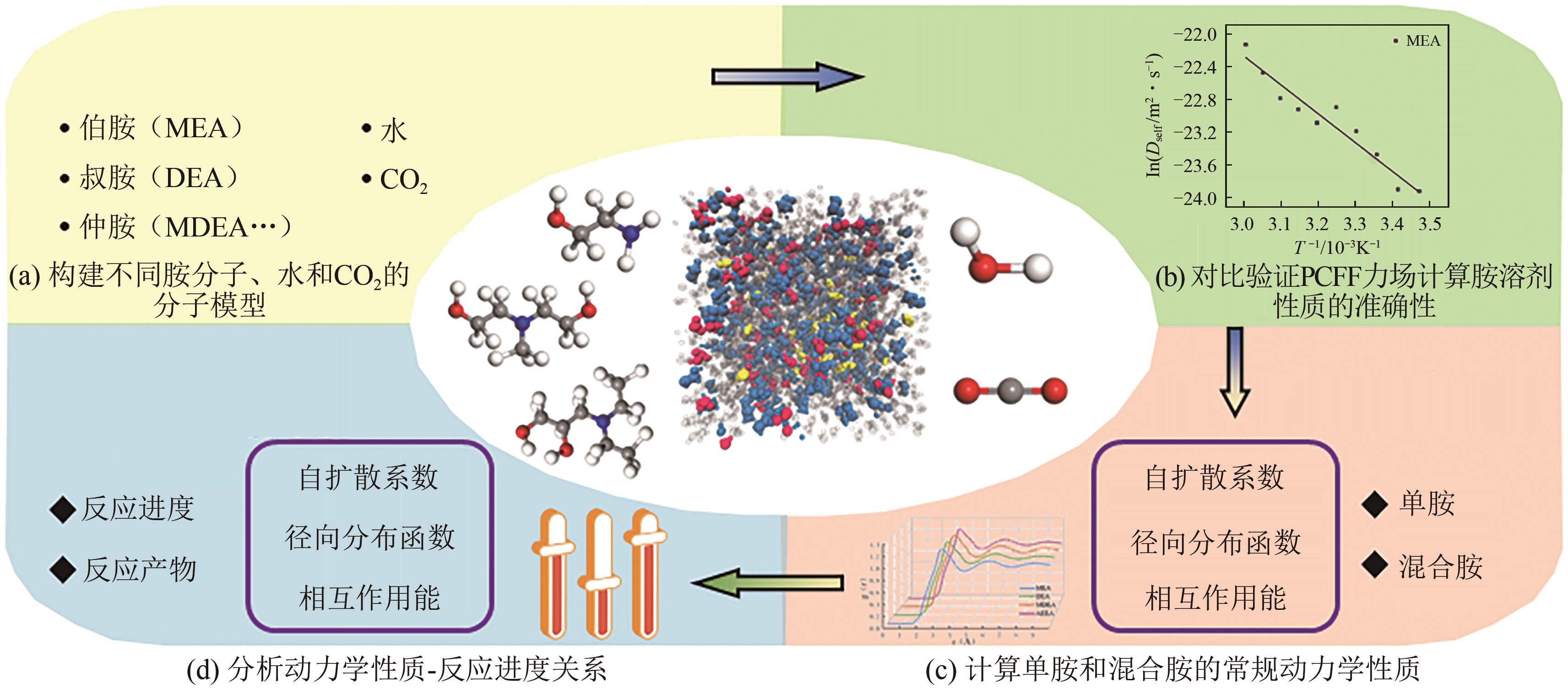
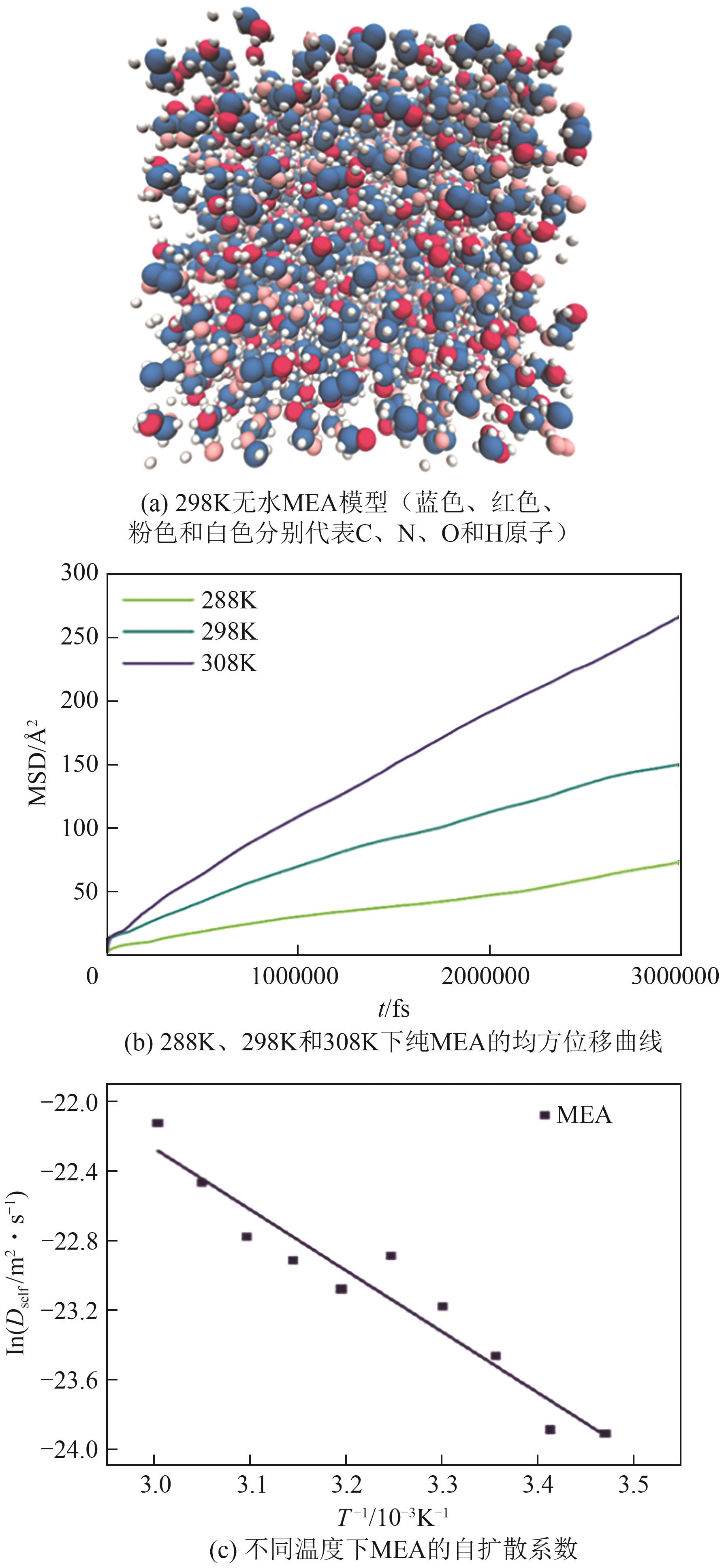
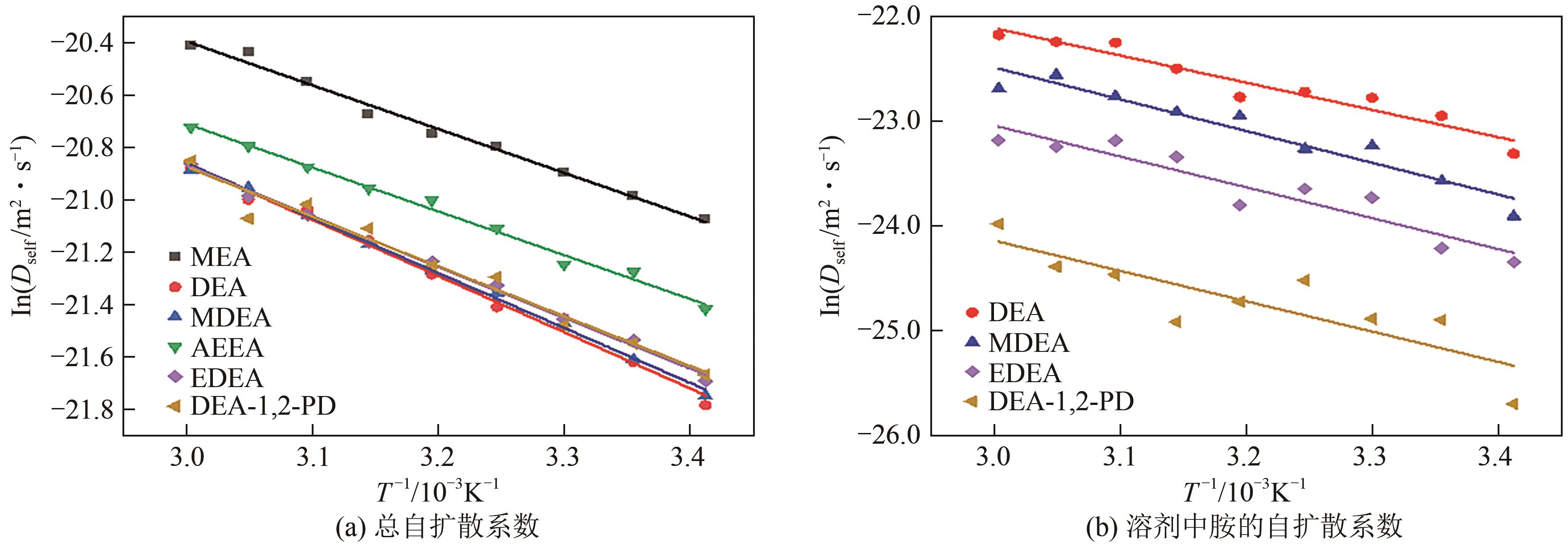
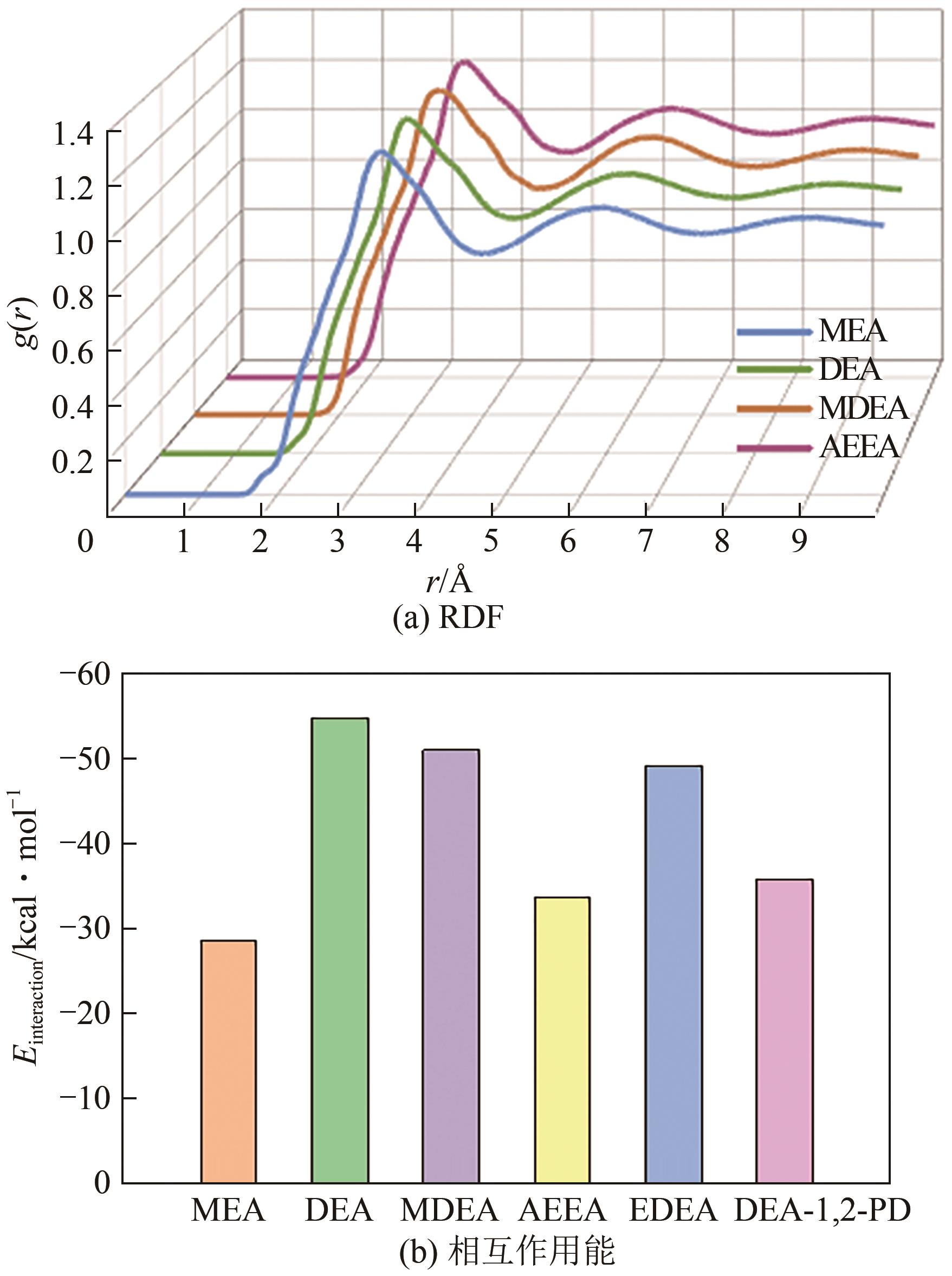
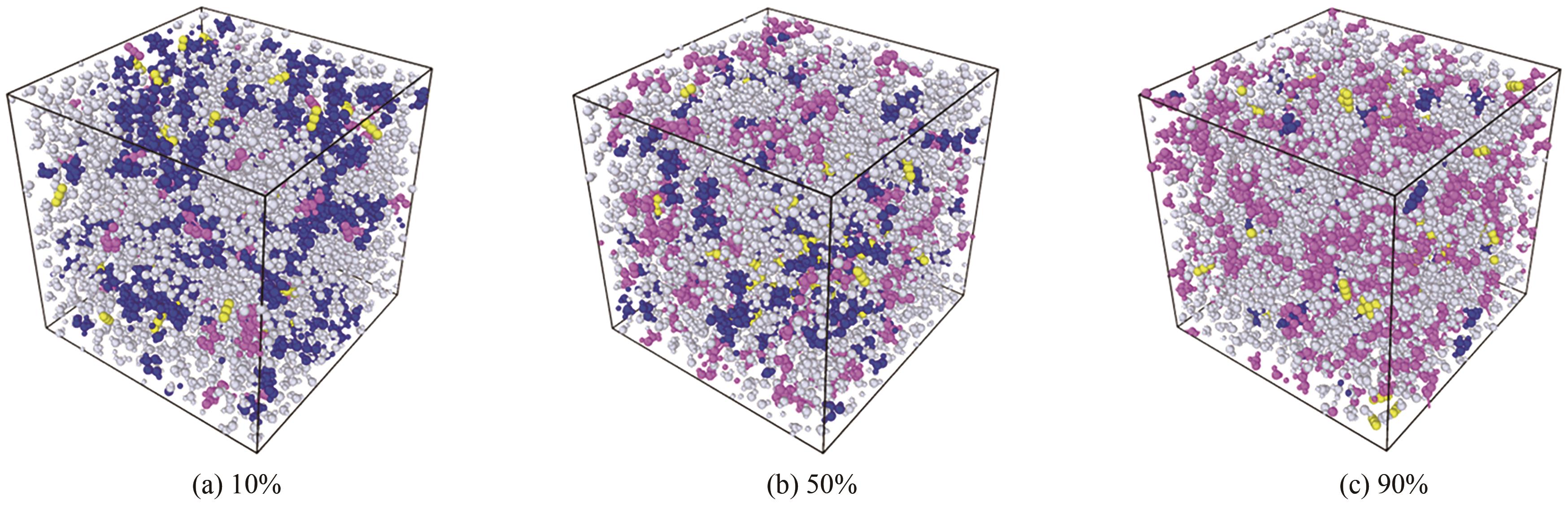
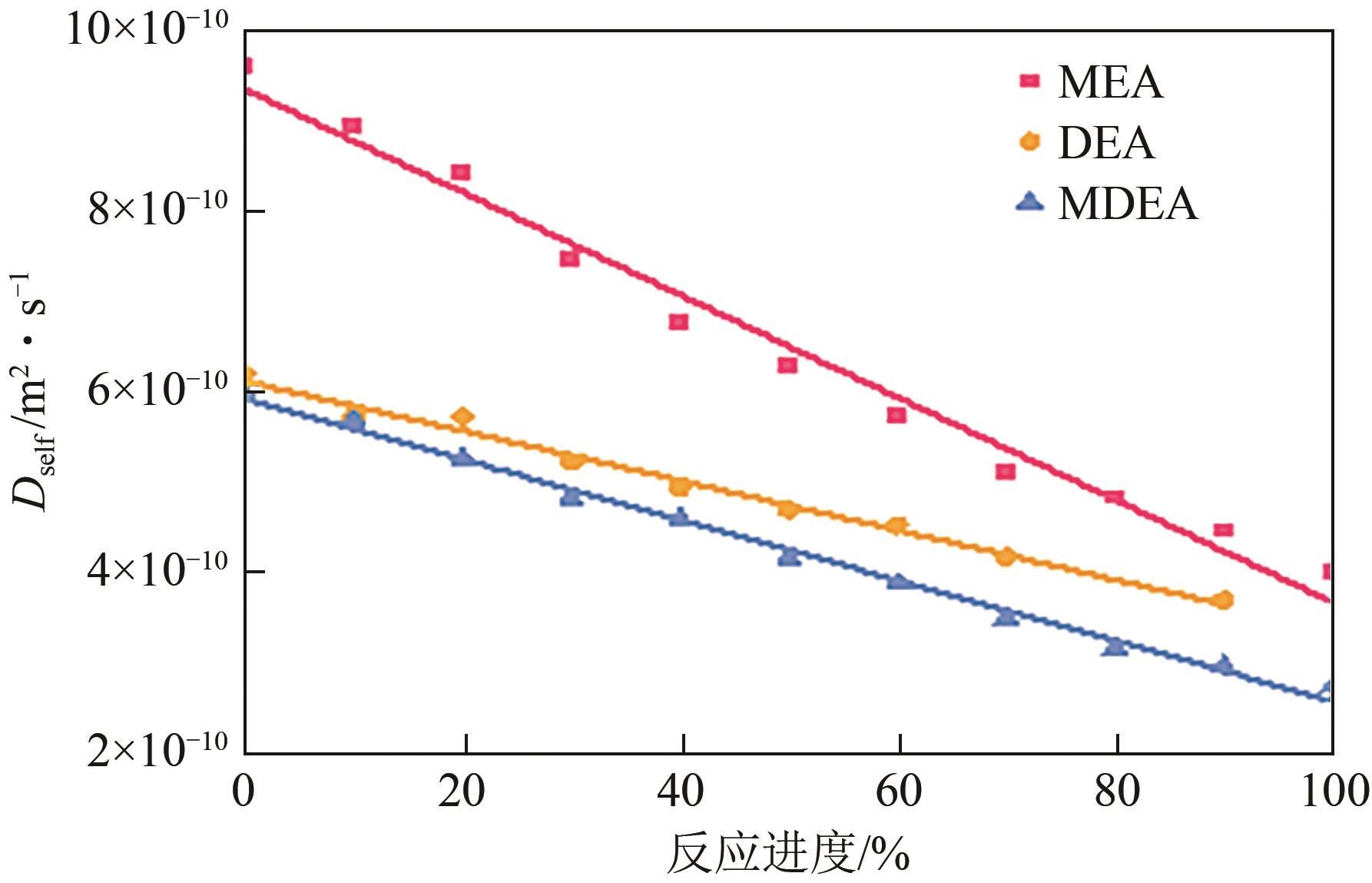
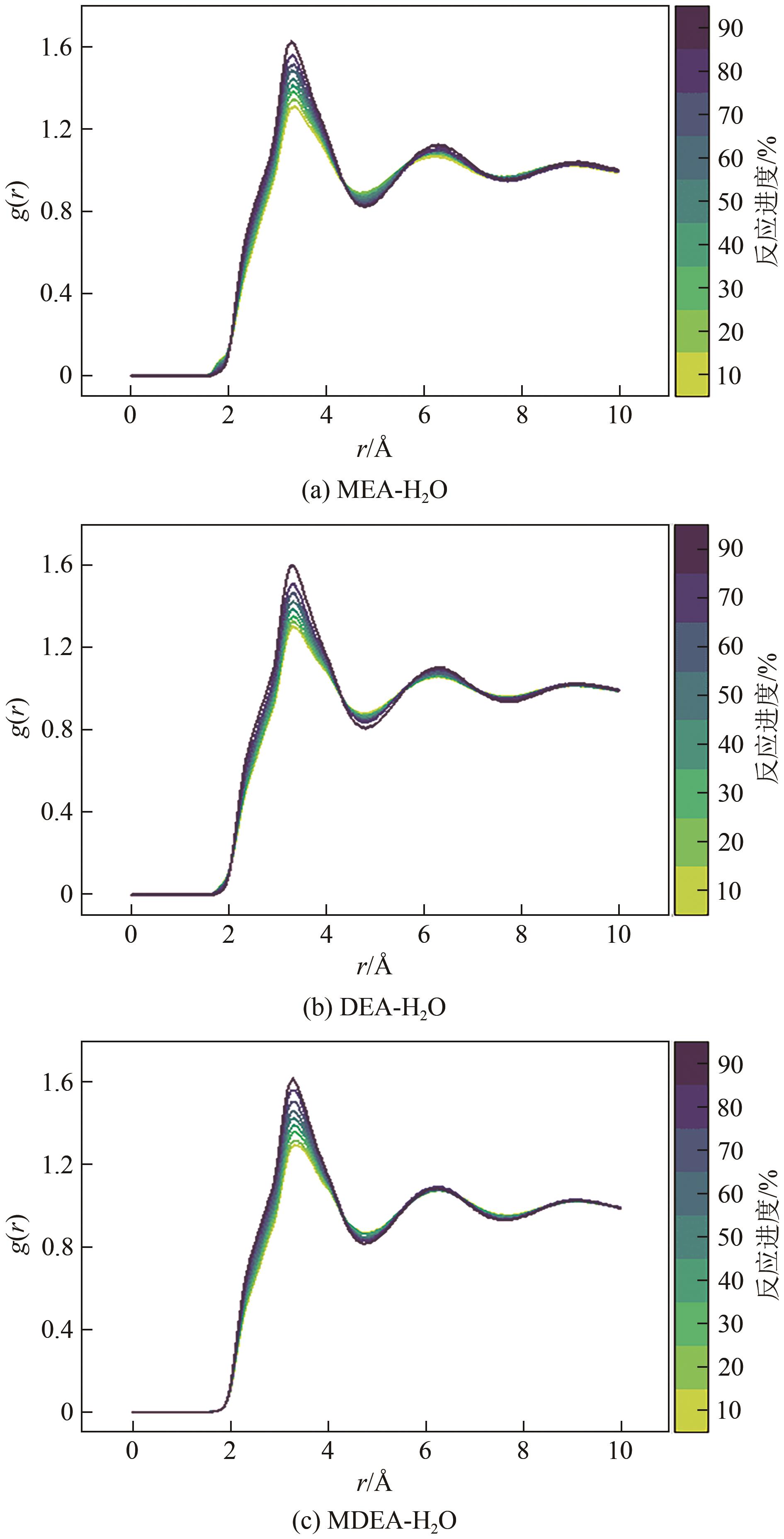
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (8): 4352-4364.DOI: 10.16085/j.issn.1000-6613.2025-0068
• Micro-mesoscale process and material modeling and simulation • Previous Articles
Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation
HUANG Ke’er( ), LIU Jiahao, LI Haoming, ZHOU Tianhang(
), LIU Jiahao, LI Haoming, ZHOU Tianhang( ), GAO Jinsen, LAN Xingying(
), GAO Jinsen, LAN Xingying( )
)
- State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing), Beijing 102249, China
-
Received:2025-01-10Revised:2025-03-28Online:2025-09-08Published:2025-08-25 -
Contact:ZHOU Tianhang, LAN Xingying
基于分子动力学模拟的胺溶剂碳捕集过程自扩散系数
黄可儿( ), 刘佳豪, 李昊明, 周天航(
), 刘佳豪, 李昊明, 周天航( ), 高金森, 蓝兴英(
), 高金森, 蓝兴英( )
)
- 中国石油大学(北京)重质油全国重点实验室,北京 102249
-
通讯作者:周天航,蓝兴英 -
作者简介:黄可儿(2000—),女,博士研究生,研究方向为人工智能及化学产品设计。E-mail:huangkeer0728@163.com。 -
基金资助:国家自然科学基金(U22B20149)
CLC Number:
Cite this article
HUANG Ke’er, LIU Jiahao, LI Haoming, ZHOU Tianhang, GAO Jinsen, LAN Xingying. Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4352-4364.
黄可儿, 刘佳豪, 李昊明, 周天航, 高金森, 蓝兴英. 基于分子动力学模拟的胺溶剂碳捕集过程自扩散系数[J]. 化工进展, 2025, 44(8): 4352-4364.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2025-0068
| 项目 | 288K | 298K | 308K |
|---|---|---|---|
| 未修正力场结果/10-10m2·s-1 | 11.55 | 12.84 | 14.75 |
| 修正力场结果/10-10m2·s-1 | 0.41 | 0.64 | 1.15 |
| 阿伦尼乌斯方程拟合结果/10-10m2·s-1 | 0.41 | 0.61 | 0.89 |
| 实验结果/10-10m2·s-1 | 0.4 | 0.55 | 0.93 |
| 项目 | 288K | 298K | 308K |
|---|---|---|---|
| 未修正力场结果/10-10m2·s-1 | 11.55 | 12.84 | 14.75 |
| 修正力场结果/10-10m2·s-1 | 0.41 | 0.64 | 1.15 |
| 阿伦尼乌斯方程拟合结果/10-10m2·s-1 | 0.41 | 0.61 | 0.89 |
| 实验结果/10-10m2·s-1 | 0.4 | 0.55 | 0.93 |
| 分子 | 原子 | ϵ/kcal·mol-1 | σ/Å |
|---|---|---|---|
| 反应物 | N | 0.325(修正值) | 4.070 |
| H(—CH2—、—NH2) | 0.013 | 1.098 | |
| C | 0.054 | 4.010 | |
| O | 1.200(修正值) | 3.535 | |
| H(—OH) | 0.020 | 2.955 | |
| H2O | H | 0.013 | 1.098 |
| O | 0.9864(修正值) | 3.608 | |
| CO2 | C | 0.064 | 4.010 |
| O | 0.060 | 3.535 | |
| 生成物 | N | 0.325(修正值) | 3.262 |
| H | 0.013 | 1.098 | |
| C | 0.120 | 3.908 | |
| O(—NHCOO—中第一个O) | 0.167 | 3.596 | |
| O(—NHCOO—中第二个O) | 1.200(修正值) | 3.535 |
| 分子 | 原子 | ϵ/kcal·mol-1 | σ/Å |
|---|---|---|---|
| 反应物 | N | 0.325(修正值) | 4.070 |
| H(—CH2—、—NH2) | 0.013 | 1.098 | |
| C | 0.054 | 4.010 | |
| O | 1.200(修正值) | 3.535 | |
| H(—OH) | 0.020 | 2.955 | |
| H2O | H | 0.013 | 1.098 |
| O | 0.9864(修正值) | 3.608 | |
| CO2 | C | 0.064 | 4.010 |
| O | 0.060 | 3.535 | |
| 生成物 | N | 0.325(修正值) | 3.262 |
| H | 0.013 | 1.098 | |
| C | 0.120 | 3.908 | |
| O(—NHCOO—中第一个O) | 0.167 | 3.596 | |
| O(—NHCOO—中第二个O) | 1.200(修正值) | 3.535 |
| 结果 | 303K | 313K | 323K |
|---|---|---|---|
| 模拟值/10-10m2·s-1 | 4.76 | 5.81 | 7.12 |
| 实验值/10-10m2·s-1 | 4.49 | 5.76 | 7.27 |
| 结果 | 303K | 313K | 323K |
|---|---|---|---|
| 模拟值/10-10m2·s-1 | 4.76 | 5.81 | 7.12 |
| 实验值/10-10m2·s-1 | 4.49 | 5.76 | 7.27 |
| 英文缩写 | 中文名称 | 分子式 | 结构式 | 分子量 |
|---|---|---|---|---|
| MEA | 单乙醇胺 | C2H7NO | NH2CH2CH2OH | 61.083 |
| DEA | 二乙醇胺 | C4H11NO2 | NH(CH2CH2OH)2 | 105.136 |
| AEEA | 羟乙基乙二胺 | C4H12N2O | NH2CH2CH2NHCH2CH2OH | 104.15 |
| MDEA | 甲基二乙醇胺 | C5H13NO2 | CH3N(CH2CH2OH)2 | 119.16 |
| EDEA | N-乙基二乙醇胺 | C6H15NO2 | CH3CH2N(CH2CH2OH)2 | 133.1888 |
| DEA-1,2-PD | 3-二乙氨基-1,2-丙二醇 | C7H17NO2 | (CH3CH2)2NCH2(CHOH)CH2OH | 147.215 |
| 英文缩写 | 中文名称 | 分子式 | 结构式 | 分子量 |
|---|---|---|---|---|
| MEA | 单乙醇胺 | C2H7NO | NH2CH2CH2OH | 61.083 |
| DEA | 二乙醇胺 | C4H11NO2 | NH(CH2CH2OH)2 | 105.136 |
| AEEA | 羟乙基乙二胺 | C4H12N2O | NH2CH2CH2NHCH2CH2OH | 104.15 |
| MDEA | 甲基二乙醇胺 | C5H13NO2 | CH3N(CH2CH2OH)2 | 119.16 |
| EDEA | N-乙基二乙醇胺 | C6H15NO2 | CH3CH2N(CH2CH2OH)2 | 133.1888 |
| DEA-1,2-PD | 3-二乙氨基-1,2-丙二醇 | C7H17NO2 | (CH3CH2)2NCH2(CHOH)CH2OH | 147.215 |
| 不同单胺溶剂 | 自扩散系数公式 |
|---|---|
| MEA | |
| DEA | |
| MDEA | |
| AEEA | |
| EDEA | |
| DEA-1,2-PD |
| 不同单胺溶剂 | 自扩散系数公式 |
|---|---|
| MEA | |
| DEA | |
| MDEA | |
| AEEA | |
| EDEA | |
| DEA-1,2-PD |
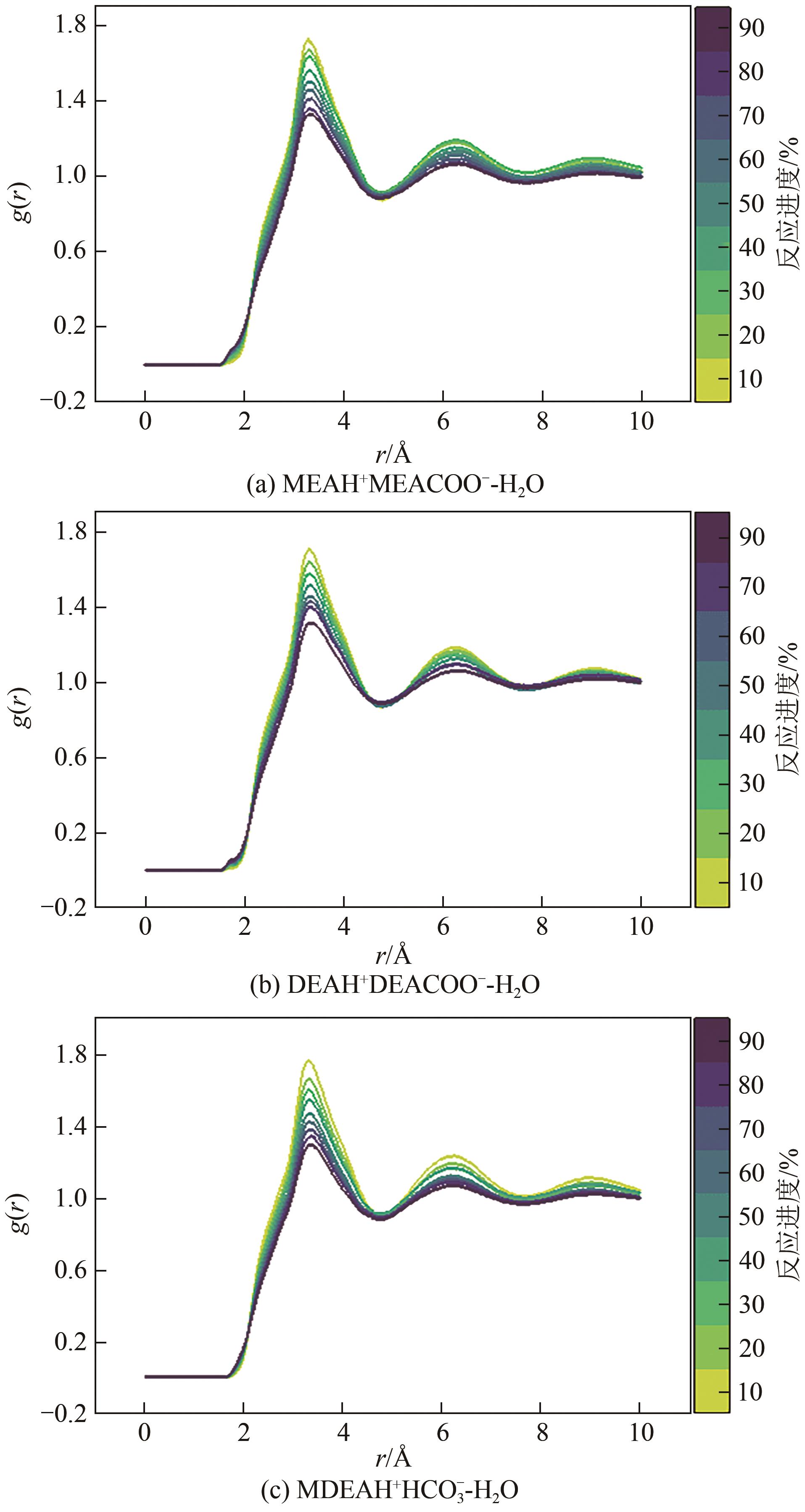
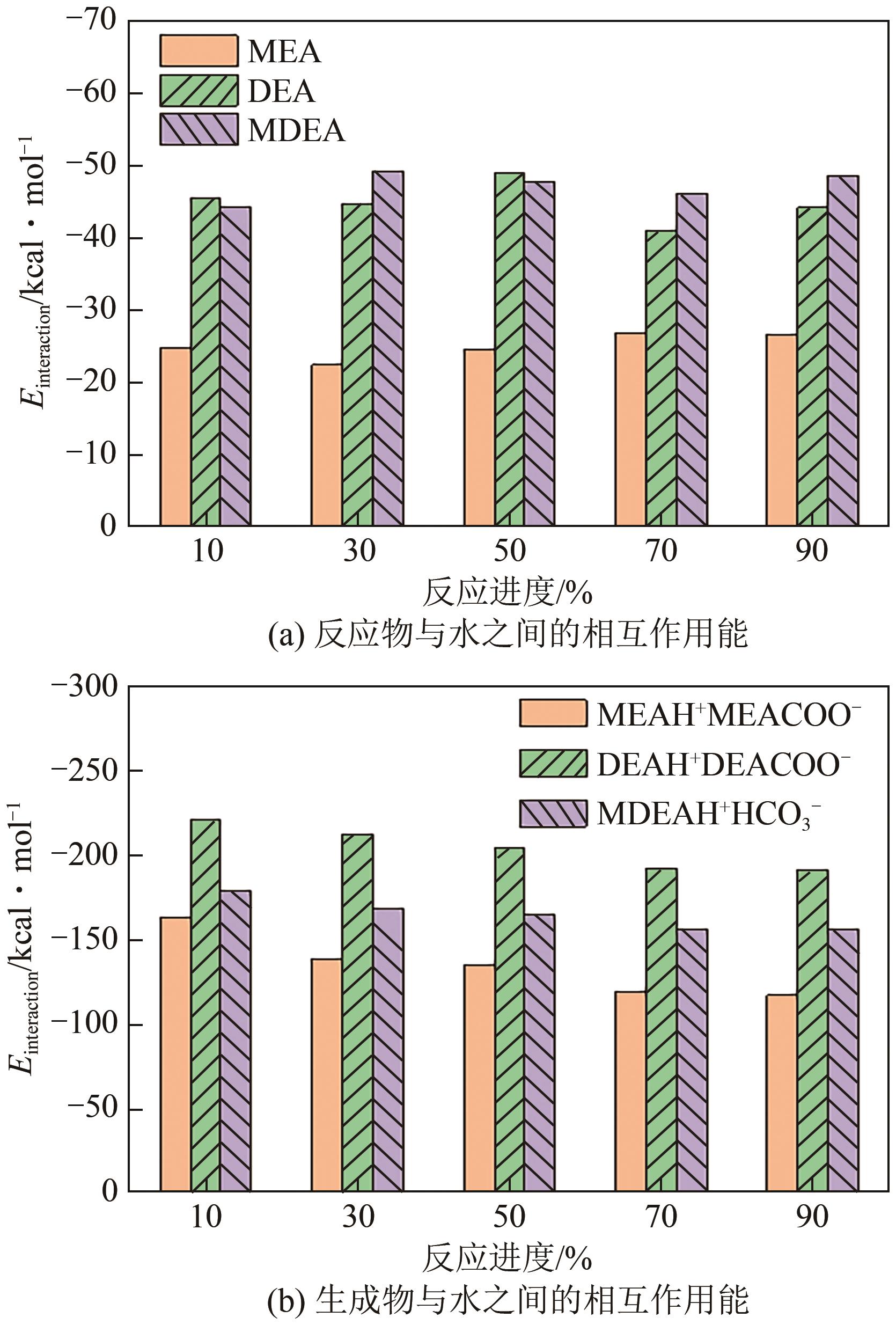
| 反应进度/% | 分子个数 | |||||
|---|---|---|---|---|---|---|
| MEA | DEA | MDEA | MEAH+MEACOO- | DEAH+DEACOO- | MDEAH+HCO | |
| 0 | 253 | 147 | 130 | 0 | 0 | 0 |
| 10 | 227 | 133 | 117 | 13 | 7 | 13 |
| 20 | 203 | 117 | 104 | 25 | 15 | 26 |
| 30 | 177 | 103 | 91 | 38 | 22 | 39 |
| 40 | 151 | 89 | 78 | 51 | 29 | 52 |
| 50 | 127 | 73 | 65 | 63 | 37 | 65 |
| 60 | 101 | 59 | 52 | 76 | 44 | 78 |
| 70 | 77 | 45 | 39 | 88 | 51 | 91 |
| 80 | 51 | 29 | 26 | 101 | 59 | 104 |
| 90 | 25 | 15 | 13 | 114 | 66 | 117 |
| 100 | 0 | 0 | 0 | 126 | 74 | 130 |
| 反应进度/% | 分子个数 | |||||
|---|---|---|---|---|---|---|
| MEA | DEA | MDEA | MEAH+MEACOO- | DEAH+DEACOO- | MDEAH+HCO | |
| 0 | 253 | 147 | 130 | 0 | 0 | 0 |
| 10 | 227 | 133 | 117 | 13 | 7 | 13 |
| 20 | 203 | 117 | 104 | 25 | 15 | 26 |
| 30 | 177 | 103 | 91 | 38 | 22 | 39 |
| 40 | 151 | 89 | 78 | 51 | 29 | 52 |
| 50 | 127 | 73 | 65 | 63 | 37 | 65 |
| 60 | 101 | 59 | 52 | 76 | 44 | 78 |
| 70 | 77 | 45 | 39 | 88 | 51 | 91 |
| 80 | 51 | 29 | 26 | 101 | 59 | 104 |
| 90 | 25 | 15 | 13 | 114 | 66 | 117 |
| 100 | 0 | 0 | 0 | 126 | 74 | 130 |
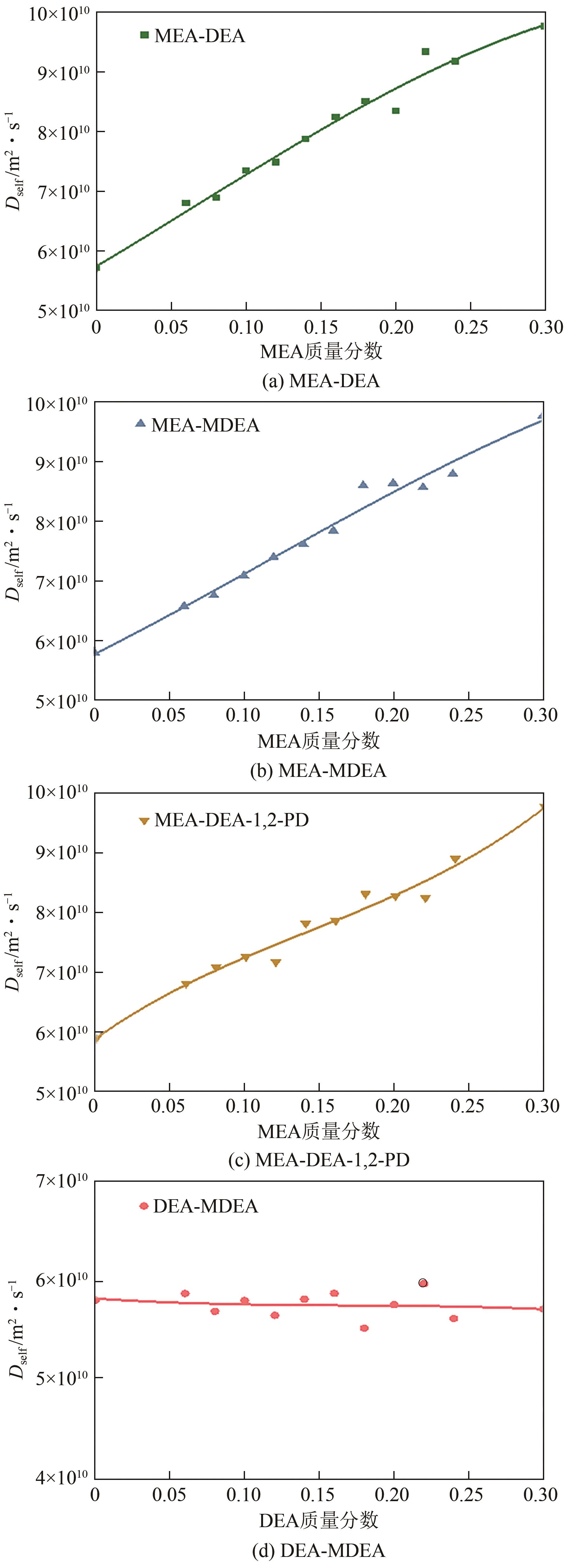
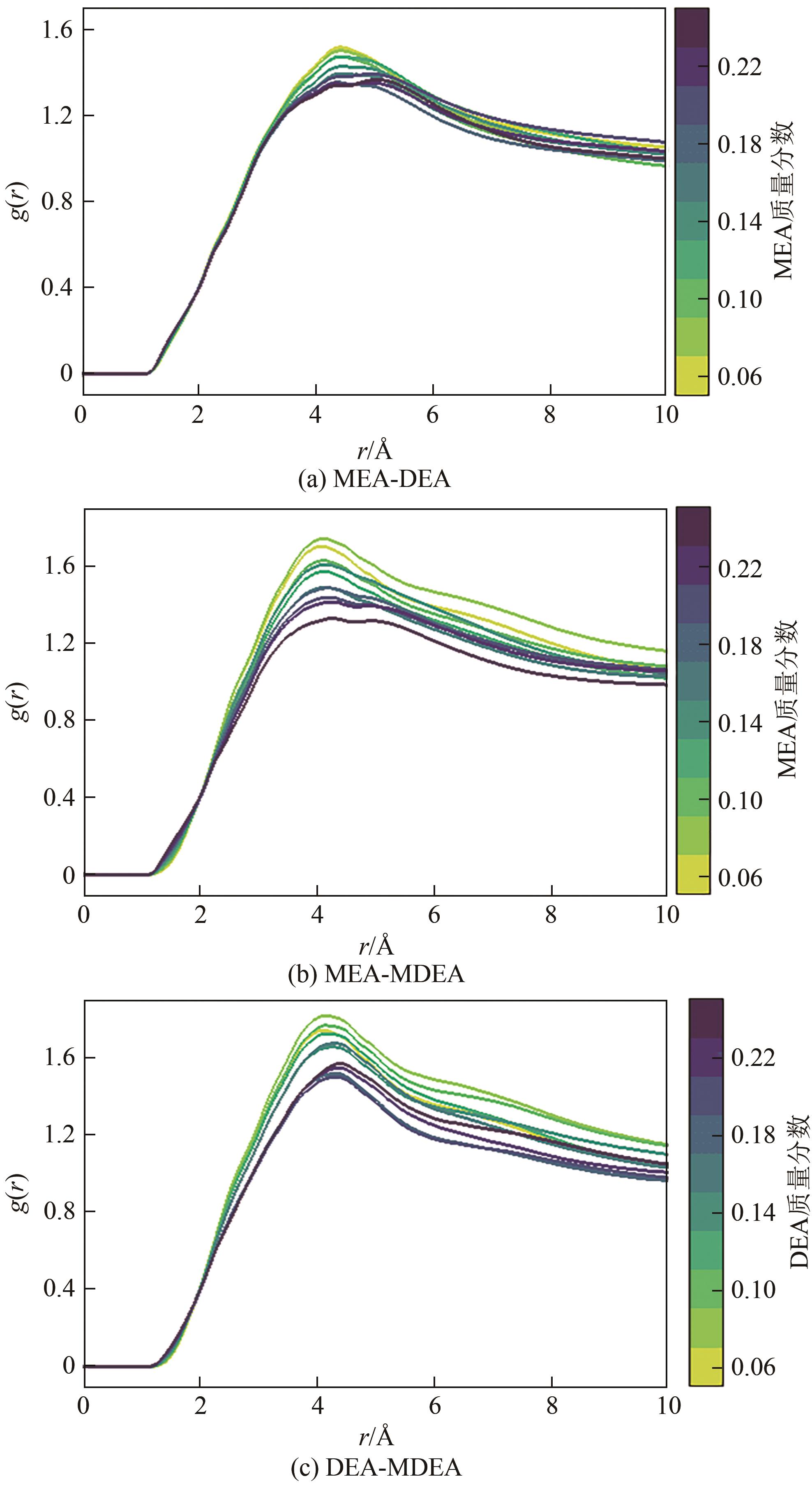
| 质量分数 | 分子个数 | |||
|---|---|---|---|---|
| MEA | DEA | MDEA | DEA-1,2-PD | |
| 0.06 | 50 | 29 | 26 | 21 |
| 0.08 | 67 | 39 | 35 | 28 |
| 0.10 | 84 | 49 | 43 | 35 |
| 0.12 | 101 | 59 | 52 | 42 |
| 0.14 | 118 | 69 | 60 | 49 |
| 0.16 | 135 | 78 | 69 | 56 |
| 0.18 | 152 | 88 | 78 | 63 |
| 0.20 | 169 | 98 | 86 | 70 |
| 0.22 | 185 | 108 | 95 | 77 |
| 0.24 | 202 | 117 | 104 | 84 |
| 质量分数 | 分子个数 | |||
|---|---|---|---|---|
| MEA | DEA | MDEA | DEA-1,2-PD | |
| 0.06 | 50 | 29 | 26 | 21 |
| 0.08 | 67 | 39 | 35 | 28 |
| 0.10 | 84 | 49 | 43 | 35 |
| 0.12 | 101 | 59 | 52 | 42 |
| 0.14 | 118 | 69 | 60 | 49 |
| 0.16 | 135 | 78 | 69 | 56 |
| 0.18 | 152 | 88 | 78 | 63 |
| 0.20 | 169 | 98 | 86 | 70 |
| 0.22 | 185 | 108 | 95 | 77 |
| 0.24 | 202 | 117 | 104 | 84 |
| [1] | D’ALESSANDRO Deanna M, SMIT Berend, LONG Jeffrey R. Carbon dioxide capture: Prospects for new materials[J]. Angewandte Chemie International Edition, 2010, 49(35): 6058-6082. |
| [2] | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| [3] | ZHAO Xiaomeng, LI Xingyu, LU Houfang, et al. Predicting phase-splitting behaviors of an amine-organic solvent-water system for CO2 absorption: A new model developed by density functional theory and statistical and experimental methods[J]. Chemical Engineering Journal, 2021, 422: 130389. |
| [4] | Mai BUI, ADJIMAN Claire S, BARDOW André, et al. Carbon capture and storage (CCS): The way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| [5] | HEPBURN Cameron, ADLEN Ella, BEDDINGTON John, et al. The technological and economic prospects for CO2 utilization and removal[J]. Nature, 2019, 575(7781): 87-97. |
| [6] | MAKUL Natt. Towards computational CO2 capture and storage models[J]. The Global Environmental Engineers, 2021, 8: 55-69. |
| [7] | ROCHELLE Gary T. Amine scrubbing for CO2 capture[J]. Science, 2009, 325(5948): 1652-1654. |
| [8] | ZHANG Qi, BAHAMON Daniel, ALKHATIB Ismail I I, et al. Molecular insights into the CO2 absorption mechanism by superbase protic ionic liquids by a combined density functional theory and molecular dynamics approach[J]. Journal of Molecular Liquids, 2024, 394: 123683. |
| [9] | DUTCHER Bryce, FAN Maohong, RUSSELL Armistead G. Amine-based CO2 capture technology development from the beginning of 2013—A review[J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. |
| [10] | OSMAN Ahmed I, HEFNY Mahmoud, ABDEL MAKSOUD M I A, et al. Recent advances in carbon capture storage and utilisation technologies: A review[J]. Environmental Chemistry Letters, 2021, 19(2): 797-849. |
| [11] | SMIT Berend. Carbon capture and storage: Introductory lecture[J]. Faraday Discussions, 2016, 192: 9-25. |
| [12] | Alicia GARCÍA-ABUÍN, Diego GÓMEZ-DÍAZ, NAVAZA José M, et al. Carbon dioxide capture with tertiary amines. Absorption rate and reaction mechanism[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 356-362. |
| [13] | WANG M, LAWAL A, STEPHENSON P, et al. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review[J]. Chemical Engineering Research and Design, 2011, 89(9): 1609-1624. |
| [14] | YU Y S, LU H F, WANG G X, et al. Characterizing the transport properties of multiamine solutions for CO2 capture by molecular dynamics simulation[J]. Journal of Chemical & Engineering Data, 2013, 58(6): 1429-1439. |
| [15] | CASTRO-ANAYA Luis E, OROZCO Gustavo A. Self-diffusion coefficients of amines, a molecular dynamics study[J]. Fluid Phase Equilibria, 2022, 553: 113301. |
| [16] | FENG Huajie, LIU Xin, GAO Wei, et al. Evolution of self-diffusion and local structure in some amines over a wide temperature range at high pressures: A molecular dynamics simulation study[J]. Physical Chemistry Chemical Physics, 2010, 12(45): 15007-15017. |
| [17] | SHARIF Maimoona, WU Xiaomei, YU Yunsong, et al. Estimation of diffusivity and intermolecular interaction strength of secondary and tertiary amine for CO2 absorption process by molecular dynamic simulation[J]. Molecular Simulation, 2022, 48(6): 484-494. |
| [18] | MELNIKOV Sergey M, STEIN Matthias. The effect of CO2 loading on alkanolamine absorbents in aqueous solutions[J]. Physical Chemistry Chemical Physics, 2019, 21(33): 18386-18392. |
| [19] | SNIJDER Erwin D, RIELE Marcel J M TE, VERSTEEG Geert F, et al. Diffusion coefficients of several aqueous alkanolamine solutions[J]. Journal of Chemical & Engineering Data, 1993, 38(3): 475-480. |
| [20] | BABA Hiromi, URANO Ryo, NAGAI Tetsuro, et al. Prediction of self-diffusion coefficients of chemically diverse pure liquids by all-atom molecular dynamics simulations[J]. Journal of Computational Chemistry, 2022, 43(28): 1892-1900. |
| [21] | HARUN N, MASIREN E E. Molecular dynamic simulation of amine-CO2 absorption process[J]. Indian Journal of Science and Technology, 2017, 10(2): 110382. |
| [22] | LIN Po-Hsun, Chih-Chiang KO, LI Menghui. Ternary diffusion coefficients of diethanolamine and N-methyldiethanolamine in aqueous solutions containing diethanolamine and N-methyldiethanolamine[J]. Fluid Phase Equilibria, 2009, 276(1): 69-74. |
| [23] | KIM Sunkyung, SHI Hu, LEE Jin Yong. CO2 absorption mechanism in amine solvents and enhancement of CO2 capture capability in blended amine solvent[J]. International Journal of Greenhouse Gas Control, 2016, 45: 181-188. |
| [24] | YIANNOURAKOU M, UNGERER P, LEBLANC B, et al. Molecular simulation of adsorption in microporous materials[J]. Oil & Gas Science and Technology-Revue d’IFP Energies Nouvelles, 2013, 68(6): 977-994. |
| [25] | MOOSAVI Fatemeh, ABDOLLAHI Farkhondeh, RAZMKHAH Mohammad. Carbon dioxide in monoethanolamine: Interaction and its effect on structural and dynamic properties by molecular dynamics simulation[J]. International Journal of Greenhouse Gas Control, 2015, 37: 158-169. |
| [26] | RODNIKOVA M N, SAMIGULLIN F M, SOLONINA I A, et al. Molecular mobility and the structure of polar liquids[J]. Journal of Structural Chemistry, 2014, 55(2): 256-262. |
| [27] | Chih-Chiang KO, CHANG Wen-Haur, LI Menghui. Ternary diffusion coefficients of monoethanolamine and N-methyldiethanolamine in aqueous solutions[J]. Journal of the Chinese Institute of Chemical Engineers, 2008, 39(6): 645-651. |
| [28] | ORLOV Alexey Alam, VALTZ Alain, COQUELET Christophe, et al. Computational screening methodology identifies effective solvents for CO2 capture[J]. Communications Chemistry, 2022, 5(1): 37. |
| [29] | ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6): 3660-3668. |
| [30] | CHOWDHURY Firoz A, YAMADA Hidetaka, HIGASHII Takayuki, et al. CO2 capture by tertiary amine absorbents: A performance comparison study[J]. Industrial & Engineering Chemistry Research, 2013, 52(24): 8323-8331. |
| [1] | LIU Yanyan, LI Feiquan, LIU Dong, WANG Juntao, LUO Xue. Molecular simulation study on the interfacial properties of recycled asphalt-aggregate at the nanoscale [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4302-4310. |
| [2] | LI Yanping, YANG Tao, WANG Hongxun, ZHANG Cheng, WEN Guosheng, HAN Zhicheng, LAN Gongjia, YAN Dazhou. Reaction molecular dynamics simulation of the thermal decomposition and reduction system of trichlorosilane in a hydrogen atmosphere [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4322-4330. |
| [3] | LIU Lihan, WANG Qijun, WANG Xuan, PENG Yangfeng, XU Xiaofei. All-atom molecular dynamics simulation on stress softening of styrene-butadiene rubber [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4331-4340. |
| [4] | QI Yan, CHANG Hao, ZHANG Lei. Structural product formulation design method based on molecular dynamics simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4341-4351. |
| [5] | SUN Jinlei, LIAO Dankui, CHEN Xiaopeng, TONG Zhangfa. Preparation of spheroidal nano-calcium carbonate via high gravity-microinterface method [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3757-3769. |
| [6] | ZHENG Huizhe, WANG Haoze, JIANG Jie, ZHAO Ling, XI Zhenhao. Modeling and simulation of PCTG copolymer rotating disc reactor based on the coupling of reaction and mass transfer [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3372-3381. |
| [7] | FU Zijun, SONG Xuehang, SHEN Qun, WANG Xiaobo, GU Jiaming, WANG Danfeng, WEI Wei, SUN Nannan. Carbon footprint analysis of integrated CO2 capture and methanation technology based on life cycle assessment [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2879-2887. |
| [8] | MA Zixuan, SHI Ruichen, LIU Mingjie, YANG Yingjie, SONG Ziyu, MEI Xiaopeng, GAO Xiaofeng, HONG Longcheng, YAO Siyu, ZHANG Zhiguo, REN Qilong. Design and performance optimization of reactors for catalytic hydrogen production from cycloalkanes: Frontline progress and challenges [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2919-2937. |
| [9] | CHEN Aohui, SONG Yanfang, CHEN Wei, WEI Wei. Self-supported porous electrodes for efficient electrocatalytic CO2 reduction [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2806-2810. |
| [10] | DAI Yueming, ZHOU Meifang, SHEN Jianhua, JIANG Haibo, LI Chunzhong. Molecular dynamics simulation of sintering mechanism of TiO2 nanoparticles [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2202-2214. |
| [11] | DOU Yu, WANG Wenxuan, FAN Chunlei, MA Jiliang, LIANG Cai, CHEN Xiaoping. Preparation of vaterite CaCO3 by mineralizing CO2 from desulfurized gypsum [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2328-2337. |
| [12] | DING Hongbing, CHAI Xutian, WANG Shiwei, SONG Xinyu, SUN Hongjun. Experimental investigation on single and successive droplet impacts on flowing liquid film [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1888-1897. |
| [13] | WANG Jiaqi, LIU Jiaxing, WEI Haoqi, ZHOU Xinlin, CHENG Chuanxiao, GE Kun. Rhamnolipid-enhanced CO2 hydrate production [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 1998-2007. |
| [14] | SHE Yonglu, XU Qiang, LUO Xinyi, NIE Tengfei, GUO Liejin. Effect of reaction temperature on bubble dynamics and mass transfer characteristics on photoanode surface [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1243-1252. |
| [15] | FENG Peng, XU Donghai, HE Bing, LIU Huanteng, YANG Lijie, WANG Pan, LIU Qingshan. Dissolution characteristics and mechanisms of typical sulphates Na2SO4 and K2SO4 in sub-/supercritical water [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1706-1715. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||