Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (9): 5377-5390.DOI: 10.16085/j.issn.1000-6613.2024-2118
• Resources and environmental engineering • Previous Articles
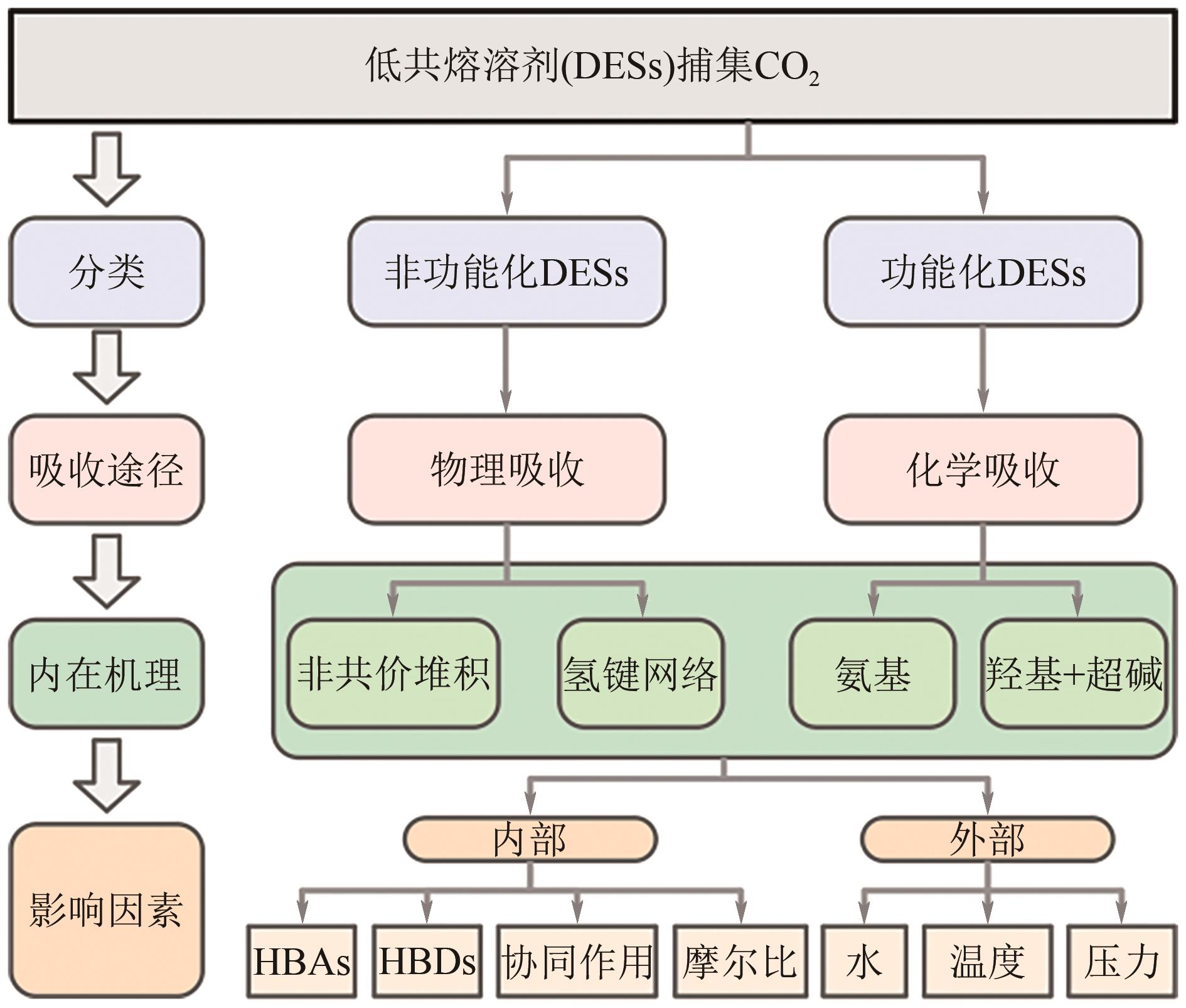
Development and application of deep eutectic solvents in carbon dioxide capture
CHEN Siming1,2,3( ), LIU Jingchao4, ZHONG Zhixuan5, ZHANG Xinzhu3, ZHU Tianhao6, PENG Yiqing6, YOU Sai6, WANG Yikai6, YUAN Jiajun6, ZHANG Yongchun7(
), LIU Jingchao4, ZHONG Zhixuan5, ZHANG Xinzhu3, ZHU Tianhao6, PENG Yiqing6, YOU Sai6, WANG Yikai6, YUAN Jiajun6, ZHANG Yongchun7( )
)
- 1.Institute of Carbon Neutralization, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
2.Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
3.School of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
4.School of Environmental Science and Engineering, Qingdao University, Qingdao 266075, Shandong, China
5.School of Resources and Geosciences, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
6.SUNYUEQI Honors College, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
7.School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China
-
Received:2024-12-30Revised:2025-05-09Online:2025-09-30Published:2025-09-25 -
Contact:CHEN Siming, ZHANG Yongchun
低共熔溶剂在二氧化碳捕集中的发展与应用
陈思铭1,2,3( ), 刘景超4, 钟志轩5, 张新柱3, 祝天浩6, 彭毅勍6, 游赛6, 王一凯6, 袁嘉骏6, 张永春7(
), 刘景超4, 钟志轩5, 张新柱3, 祝天浩6, 彭毅勍6, 游赛6, 王一凯6, 袁嘉骏6, 张永春7( )
)
- 1.中国矿业大学碳中和研究院,江苏 徐州 221116
2.中国矿业大学江苏省煤基温室气体减排与资源化利用重点实验室,江苏 徐州 221116
3.中国矿业大学化工学院,江苏 徐州 221116
4.青岛大学环境科学与工程学院,山东 青岛 2660754
5.中国矿业大学资源与地球科学学院,江苏 徐州 221116
6.中国矿业大学孙越崎学院,江苏 徐州 221116
7.大连理工大学化工学院,辽宁 大连 116024
-
通讯作者:陈思铭,张永春 -
作者简介:陈思铭(1988—),女,博士,副研究员,硕士生导师,研究方向为CO2捕集与转化。E-mail:chensiming@cumt.edu.cn。 -
基金资助:国家自然科学基金青年基金(52006112);徐州市重点研发计划社会发展项目(KC23293);中国博士后科学基金(2023M743768)
CLC Number:
Cite this article
CHEN Siming, LIU Jingchao, ZHONG Zhixuan, ZHANG Xinzhu, ZHU Tianhao, PENG Yiqing, YOU Sai, WANG Yikai, YUAN Jiajun, ZHANG Yongchun. Development and application of deep eutectic solvents in carbon dioxide capture[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5377-5390.
陈思铭, 刘景超, 钟志轩, 张新柱, 祝天浩, 彭毅勍, 游赛, 王一凯, 袁嘉骏, 张永春. 低共熔溶剂在二氧化碳捕集中的发展与应用[J]. 化工进展, 2025, 44(9): 5377-5390.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-2118
| 类型 | 通式 | 组成 |
|---|---|---|
| Ⅰ | Cat+X-zMCl x | M=Zn, Sn, Fe, Al, Ga, In等 |
| Ⅱ | Cat+X-zMCl x ·yH2O | M=Cr, Co, Cu, Ni, Fe等 |
| Ⅲ | Cat+X-zR*Z | Z=CONH2, COOH, OH |
| Ⅳ | MCl x +R*Z | M=Al, Zn等; Z=CONH2, OH |
| 类型 | 通式 | 组成 |
|---|---|---|
| Ⅰ | Cat+X-zMCl x | M=Zn, Sn, Fe, Al, Ga, In等 |
| Ⅱ | Cat+X-zMCl x ·yH2O | M=Cr, Co, Cu, Ni, Fe等 |
| Ⅲ | Cat+X-zR*Z | Z=CONH2, COOH, OH |
| Ⅳ | MCl x +R*Z | M=Al, Zn等; Z=CONH2, OH |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度/mol·kg-1 | 文献 |
|---|---|---|---|---|---|---|
| BHDE | AC | 1∶2 | 298.15 | 210~2026 | 0.064~0.84 | [ |
| BHDE | LA | 1∶2 | 298.15 | 283~2086 | 0.016~0.50 | [ |
| BTEA | AC | 1∶2 | 298.15 | 325~2054 | 0.13~0.97 | [ |
| BTMA | AC | 1∶2 | 298.15 | 219~2037 | 0.078~1.45 | [ |
| BTMA | Gly | 1∶2 | 298.15 | 394~2026 | 0.037~0.26 | [ |
| [BTPP]Br | EG | 1∶12 | 298.15 | 1000 | 0.6 | [ |
| [BTPP]Cl | Gly | 1∶12 | 298.15 | 1000 | 0.47 | [ |
| ChCl | MEA | 1∶7 | 298.15 | 182~2035 | 0.78~3.58 | [ |
| Gua | MEA | 1∶2 | 298.15 | 226~2025 | 0.31~1.66 | [ |
| MTPP | PG | 1∶4 | 298.15 | 220~2026 | 0.022~0.55 | [ |
| MTPP | AC | 1∶4 | 298.15 | 173~2014 | 0.073~3.02 | [ |
| MTPP | EG | 1∶3 | 298.15 | 192~2018 | 0.045~0.35 | [ |
| MTPP | Gly | 1∶4 | 298.15 | 161~2026 | 0.009~0.29 | [ |
| MTPP | LV | 1∶3 | 298.15 | 301~2068 | 0.024~0.69 | [ |
| MTPP | MEA | 1∶6 | 298.15 | 1000 | 1.63 | [ |
| MTPP | MEA | 1∶7 | 298.15 | 1000 | 1.46 | [ |
| MTPP | MEA | 1∶8 | 298.15 | 1000 | 1.44 | [ |
| TBAC | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.52 | [ |
| [N8881]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.041~1.31 | [ |
| [N8881]Cl | DecA | 1∶2 | 298.15~308.15 | 90~1990 | 0.045~1.35 | [ |
| [N8888]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.039~1.33 | [ |
| [N8888]Cl | DecA | 1∶1.5 | 298.15~323.15 | 90~1990 | 0.041~1.41 | [ |
| [N8888]Cl | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.41 | [ |
| TBAB | AC | 1∶2 | 298.15 | 388~2011 | 0.14~1.13 | [ |
| TBAB | DEA | 1∶6 | 298.15 | 1000 | 0.85 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 1000 | 1.34 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 351~2021 | 0.44~2.78 | [ |
| TBAB | MEA | 1∶7 | 298.15 | 381~2040 | 0.53~3.01 | [ |
| TBAB | TEA | 1∶3 | 298.15 | 1000 | 0.47 | [ |
| TBAC | AC | 1∶2 | 298.15 | 348~2002 | 0.18~1.41 | [ |
| TEAC | AC | 1∶2 | 298.15 | 281~2018 | 0.14~1.18 | [ |
| TEAC | AC | 1∶3 | 298.15 | 397~2016 | 0.13~1.23 | [ |
| TEAC | OCT | 1∶3 | 298.15 | 353~2018 | 0.16~1.39 | [ |
| TEMA | AC | 1∶2 | 298.15 | 198~1837 | 0.081~1.18 | [ |
| TEMA | EG | 1∶2 | 298.15 | 138~1345 | 0.062~0.63 | [ |
| TEMA | Gly | 1∶2 | 298.15 | 150~1648 | 0.017~0.43 | [ |
| TEMA | LA | 1∶2 | 298.15 | 143~1863 | 0.047~0.53 | [ |
| TEMA | LV | 1∶2 | 298.15 | 136~1617 | 0.057~0.61 | [ |
| TMAC | AC | 1∶4 | 298.15 | 294~2096 | 0.12~1.56 | [ |
| TPAC | AC | 1∶6 | 298.15 | 350~2030 | 0.25~1.72 | [ |
| TPAC | MEA | 1∶4 | 298.15 | 481~2009 | 0.34~1.43 | [ |
| TPAC | MEA | 1∶7 | 298.15 | 357~2019 | 1.71~3.53 | [ |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度/mol·kg-1 | 文献 |
|---|---|---|---|---|---|---|
| BHDE | AC | 1∶2 | 298.15 | 210~2026 | 0.064~0.84 | [ |
| BHDE | LA | 1∶2 | 298.15 | 283~2086 | 0.016~0.50 | [ |
| BTEA | AC | 1∶2 | 298.15 | 325~2054 | 0.13~0.97 | [ |
| BTMA | AC | 1∶2 | 298.15 | 219~2037 | 0.078~1.45 | [ |
| BTMA | Gly | 1∶2 | 298.15 | 394~2026 | 0.037~0.26 | [ |
| [BTPP]Br | EG | 1∶12 | 298.15 | 1000 | 0.6 | [ |
| [BTPP]Cl | Gly | 1∶12 | 298.15 | 1000 | 0.47 | [ |
| ChCl | MEA | 1∶7 | 298.15 | 182~2035 | 0.78~3.58 | [ |
| Gua | MEA | 1∶2 | 298.15 | 226~2025 | 0.31~1.66 | [ |
| MTPP | PG | 1∶4 | 298.15 | 220~2026 | 0.022~0.55 | [ |
| MTPP | AC | 1∶4 | 298.15 | 173~2014 | 0.073~3.02 | [ |
| MTPP | EG | 1∶3 | 298.15 | 192~2018 | 0.045~0.35 | [ |
| MTPP | Gly | 1∶4 | 298.15 | 161~2026 | 0.009~0.29 | [ |
| MTPP | LV | 1∶3 | 298.15 | 301~2068 | 0.024~0.69 | [ |
| MTPP | MEA | 1∶6 | 298.15 | 1000 | 1.63 | [ |
| MTPP | MEA | 1∶7 | 298.15 | 1000 | 1.46 | [ |
| MTPP | MEA | 1∶8 | 298.15 | 1000 | 1.44 | [ |
| TBAC | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.52 | [ |
| [N8881]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.041~1.31 | [ |
| [N8881]Cl | DecA | 1∶2 | 298.15~308.15 | 90~1990 | 0.045~1.35 | [ |
| [N8888]Br | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.039~1.33 | [ |
| [N8888]Cl | DecA | 1∶1.5 | 298.15~323.15 | 90~1990 | 0.041~1.41 | [ |
| [N8888]Cl | DecA | 1∶2 | 298.15~323.15 | 90~1990 | 0.042~1.41 | [ |
| TBAB | AC | 1∶2 | 298.15 | 388~2011 | 0.14~1.13 | [ |
| TBAB | DEA | 1∶6 | 298.15 | 1000 | 0.85 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 1000 | 1.34 | [ |
| TBAB | MEA | 1∶6 | 298.15 | 351~2021 | 0.44~2.78 | [ |
| TBAB | MEA | 1∶7 | 298.15 | 381~2040 | 0.53~3.01 | [ |
| TBAB | TEA | 1∶3 | 298.15 | 1000 | 0.47 | [ |
| TBAC | AC | 1∶2 | 298.15 | 348~2002 | 0.18~1.41 | [ |
| TEAC | AC | 1∶2 | 298.15 | 281~2018 | 0.14~1.18 | [ |
| TEAC | AC | 1∶3 | 298.15 | 397~2016 | 0.13~1.23 | [ |
| TEAC | OCT | 1∶3 | 298.15 | 353~2018 | 0.16~1.39 | [ |
| TEMA | AC | 1∶2 | 298.15 | 198~1837 | 0.081~1.18 | [ |
| TEMA | EG | 1∶2 | 298.15 | 138~1345 | 0.062~0.63 | [ |
| TEMA | Gly | 1∶2 | 298.15 | 150~1648 | 0.017~0.43 | [ |
| TEMA | LA | 1∶2 | 298.15 | 143~1863 | 0.047~0.53 | [ |
| TEMA | LV | 1∶2 | 298.15 | 136~1617 | 0.057~0.61 | [ |
| TMAC | AC | 1∶4 | 298.15 | 294~2096 | 0.12~1.56 | [ |
| TPAC | AC | 1∶6 | 298.15 | 350~2030 | 0.25~1.72 | [ |
| TPAC | MEA | 1∶4 | 298.15 | 481~2009 | 0.34~1.43 | [ |
| TPAC | MEA | 1∶7 | 298.15 | 357~2019 | 1.71~3.53 | [ |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度(摩尔分数) | 文献 |
|---|---|---|---|---|---|---|
| ChCl | EG | 1∶2 | 303.15 | 10000 | 0.046 | [ |
| ChCl | DEG | 1∶3 | 303.15 | 8200 | 0.028 | [ |
| ChCl | DEG | 1∶4 | 303.15 | 8300 | 0.028 | [ |
| TBAB | EG | 1∶2 | 303.15 | 7800 | 0.031 | [ |
| TBAB | EG | 1∶3 | 303.15 | 8800 | 0.039 | [ |
| TBAB | EG | 1∶4 | 303.15 | 8300 | 0.032 | [ |
| TBAB | DEG | 1∶2 | 303.15 | 7200 | 0.034 | [ |
| TBAB | DEG | 1∶3 | 303.15 | 6900 | 0.040 | [ |
| TBAB | DEG | 1∶4 | 303.15 | 9000 | 0.084 | [ |
| ChCl | MDEA | 1∶6 | 303.15 | 7100 | 0.160 | [ |
| ChCl | MDEA | 1∶7 | 303.15 | 5900 | 0.120 | [ |
| ChCl | DEA | 1∶6 | 303.15 | 8000 | 0.078 | [ |
| TBTA | DEA | 1∶6 | 303.15 | 6000 | 0.050 | [ |
| TBAB | MDEA | 1∶3 | 303.15 | 8100 | 0.020 | [ |
| TBAB | MDEA | 1∶4 | 303.15 | 7000 | 0.020 | [ |
| ChCl | PG | 1∶2 | 303.15 | 9000 | 0.025 | [ |
| ChCl | GLY | 1∶2 | 303.15 | 11000 | 0.030 | [ |
| ChCl | MA | 1∶1 | 303.15 | 10000 | 0.025 | [ |
| TMG | PhAc | 1∶2 | 313.15 | 400 | (38.1±1.2)mg/g | [ |
| TMG | GA | 1∶2 | 313.15 | 400 | (2.84±0.19)mg/g | [ |
| TMG | EA | 1∶2 | 313.15 | 400 | (0.52±0.04)mg/g | [ |
| HBA | HBD | HBA∶HBD | T/K | P/kPa | CO2溶解度(摩尔分数) | 文献 |
|---|---|---|---|---|---|---|
| ChCl | EG | 1∶2 | 303.15 | 10000 | 0.046 | [ |
| ChCl | DEG | 1∶3 | 303.15 | 8200 | 0.028 | [ |
| ChCl | DEG | 1∶4 | 303.15 | 8300 | 0.028 | [ |
| TBAB | EG | 1∶2 | 303.15 | 7800 | 0.031 | [ |
| TBAB | EG | 1∶3 | 303.15 | 8800 | 0.039 | [ |
| TBAB | EG | 1∶4 | 303.15 | 8300 | 0.032 | [ |
| TBAB | DEG | 1∶2 | 303.15 | 7200 | 0.034 | [ |
| TBAB | DEG | 1∶3 | 303.15 | 6900 | 0.040 | [ |
| TBAB | DEG | 1∶4 | 303.15 | 9000 | 0.084 | [ |
| ChCl | MDEA | 1∶6 | 303.15 | 7100 | 0.160 | [ |
| ChCl | MDEA | 1∶7 | 303.15 | 5900 | 0.120 | [ |
| ChCl | DEA | 1∶6 | 303.15 | 8000 | 0.078 | [ |
| TBTA | DEA | 1∶6 | 303.15 | 6000 | 0.050 | [ |
| TBAB | MDEA | 1∶3 | 303.15 | 8100 | 0.020 | [ |
| TBAB | MDEA | 1∶4 | 303.15 | 7000 | 0.020 | [ |
| ChCl | PG | 1∶2 | 303.15 | 9000 | 0.025 | [ |
| ChCl | GLY | 1∶2 | 303.15 | 11000 | 0.030 | [ |
| ChCl | MA | 1∶1 | 303.15 | 10000 | 0.025 | [ |
| TMG | PhAc | 1∶2 | 313.15 | 400 | (38.1±1.2)mg/g | [ |
| TMG | GA | 1∶2 | 313.15 | 400 | (2.84±0.19)mg/g | [ |
| TMG | EA | 1∶2 | 313.15 | 400 | (0.52±0.04)mg/g | [ |
| HBA | HBD | 助剂 | HBA∶HBD∶助剂 | T/K | P/kPa | CO2溶解度(质量分数)/% | 文献 |
|---|---|---|---|---|---|---|---|
| ChCl | Gly | DBN | 1∶2∶3 | RT③ | 101 | 9.6 | [ |
| ChCl | Gly | DBN | 1∶2∶6 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶2∶7 | RT | 101 | 10.5 | [ |
| ChCl | Gly | DBN | 1∶2∶8 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶3∶10 | RT | 101 | 10.4 | [ |
| ChCl | Gly | DBU | 1∶2∶6 | RT | 101 | 3.55 | [ |
| ChCl | Gly | MTBD | 1∶2∶6 | RT | 101 | 10 | [ |
| ChCl | MEA | 1∶5 | 303.15 | 101 | 25.23 | [ | |
| BmimCl | Im | DBN | 1∶1∶1 | 298.15 | 101 | (1.02) ① | [ |
| BmimCl | Im | DBN | 1∶1∶2 | 298.15 | 101 | (0.97) ① | [ |
| BmimCl | Im | DBN | 1∶2∶1 | 298.15 | 101 | (1.07) ① | [ |
| DBN | DMLU | 2∶1 | 318.15 | 101 | 4.27;2.47 ② | [ | |
| DBN | DMU | 2∶1 | 318.15 | 101 | 17.34;16.8 ② | [ | |
| [HDBU][Im] | EG | 7∶3 | 313.15 | 101 | 0.141 | [ | |
| [HDBU][Ind] | EG | 7∶3 | 313.15 | 101 | 0.117 | [ | |
| [HDBU][Triz] | EG | 7∶3 | 313.15 | 101 | 0.108 | [ | |
| HmimCl | AP | 1∶1 | RT | 101 | 2.0 (0.04) ① | [ | |
| HmimCl | AP | 1∶2 | RT | 101 | 9.5 (0.21) ① | [ | |
| HmimCl | AP | 1∶3 | RT | 101 | 13.9 (0.30) ① | [ | |
| HmimCl | AP | 1∶4 | RT | 101 | 19.4 (0.37) ① | [ | |
| HmimCl | PEHA | 1∶4 | RT | 101 | 8.4 (0.4) ① | [ | |
| HmimCl | DETA | 1∶4 | RT | 101 | 22.8 (0.55) ① | [ | |
| HmimCl | EDA | 1∶1 | RT | 101 | 9.0 (0.19) ① | [ | |
| HmimCl | EDA | 1∶2 | RT | 101 | 25.0 (0.45) ① | [ | |
| HmimCl | EDA | 1∶3 | RT | 101 | 26.7 (0.45) ① | [ | |
| HmimCl | EDA | 1∶4 | RT | 101 | 30.8 (0.50) ① | [ | |
| HmimCl | TEPA | 1∶4 | RT | 101 | 9.9 (0.39) ① | [ | |
| MEAC | AP | 1∶1 | RT | 101 | 15.8 (0.28) ① | [ | |
| MEAC | AP | 1∶2 | RT | 101 | 21 (0.37) ① | [ | |
| MEAC | AP | 1∶3 | RT | 101 | 24.3 (0.42) ① | [ | |
| MEAC | AP | 1∶4 | RT | 101 | 26.3 (0.46) ① | [ | |
| MEAC | DETA | 1∶4 | RT | 101 | 25.5 (0.57) ① | [ | |
| MEAC | EDA | 1∶1 | RT | 101 | 23.5 (0.38) ① | [ | |
| MEAC | EDA | 1∶2 | RT | 101 | 30.9 (0.47) ① | [ | |
| MEAC | EDA | 1∶3 | RT | 101 | 36.5 (0.54) ① | [ | |
| MEAC | EDA | 1∶3 | 303.15 | 101 | 33.7 | [ | |
| MEAC | EDA | 1∶4 | RT | 101 | 39.0 (0.57) ① | [ | |
| MEAC | PEHA | 1∶4 | RT | 101 | 12.7 (0.59) ① | [ | |
| MEAC | TEPA | 1∶4 | RT | 101 | 16.6 (0.63) ① | [ | |
| [N2222][Im] | EG | 1∶2 | 298.15 | 101 | 12.9 (0.94) ① | [ | |
| [N2222][Triz] | EG | 1∶2 | 298.15 | 101 | 12.5 (0.92) ① | [ | |
| [P2222][Im] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| [P2222][Triz] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| MEACl | EDA | 1∶3 | 303.15 | 101 | 31.5 | [ | |
| TBAB | AMP | 1∶3 | RT | 101 | 10.5 (0.35) ① | [ | |
| TBAB | AMP | 1∶4 | RT | 101 | 12.2 (0.38) ① | [ | |
| TBAB | AP | 1∶2 | RT | 101 | 11.1 (0.43) ① | [ | |
| TBAB | AP | 1∶3 | RT | 101 | 15.6 (0.49) ① | [ | |
| TBAB | AP | 1∶4 | RT | 101 | 18.1 (0.51) ① | [ | |
| TEAC | DA | 1∶3 | 303.15 | 101 | 24.2 | [ | |
| [TETA]Cl | DG | 1∶2 | 313.15 | 101 | 0.159 | [ | |
| [TEPA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.088 (1.355) ① | [ | |
| [TETA]Cl | EG | 1∶3 | 313.15 | 101 | 0.175 | [ | |
| [TETA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.09 (1.298) ① | [ | |
| UEC | EDA | 1∶3 | 303.15 | 101 | 17.8 | [ | |
| TPAC | EA | 1∶6 | 298.15 | 20.0 | 0.276 | [ | |
| TPAC | AC | 1∶4 | 298.15 | 20.0 | 0.240 | [ | |
| TPAC | AC | 1∶7 | 298.15 | 20.0 | 0.435 | [ | |
| TBAB | OCT+OA | 1∶2 | 298.15 | 16.46 | 0.285 | [ | |
| TPAC | DecA | 1∶2 | 298.15 | 19.9 | 0.299 | [ | |
| TMP | thymol | 1∶3 | 313.15 | 10.13 | 1.355 | [ |
| HBA | HBD | 助剂 | HBA∶HBD∶助剂 | T/K | P/kPa | CO2溶解度(质量分数)/% | 文献 |
|---|---|---|---|---|---|---|---|
| ChCl | Gly | DBN | 1∶2∶3 | RT③ | 101 | 9.6 | [ |
| ChCl | Gly | DBN | 1∶2∶6 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶2∶7 | RT | 101 | 10.5 | [ |
| ChCl | Gly | DBN | 1∶2∶8 | RT | 101 | 10.3 | [ |
| ChCl | Gly | DBN | 1∶3∶10 | RT | 101 | 10.4 | [ |
| ChCl | Gly | DBU | 1∶2∶6 | RT | 101 | 3.55 | [ |
| ChCl | Gly | MTBD | 1∶2∶6 | RT | 101 | 10 | [ |
| ChCl | MEA | 1∶5 | 303.15 | 101 | 25.23 | [ | |
| BmimCl | Im | DBN | 1∶1∶1 | 298.15 | 101 | (1.02) ① | [ |
| BmimCl | Im | DBN | 1∶1∶2 | 298.15 | 101 | (0.97) ① | [ |
| BmimCl | Im | DBN | 1∶2∶1 | 298.15 | 101 | (1.07) ① | [ |
| DBN | DMLU | 2∶1 | 318.15 | 101 | 4.27;2.47 ② | [ | |
| DBN | DMU | 2∶1 | 318.15 | 101 | 17.34;16.8 ② | [ | |
| [HDBU][Im] | EG | 7∶3 | 313.15 | 101 | 0.141 | [ | |
| [HDBU][Ind] | EG | 7∶3 | 313.15 | 101 | 0.117 | [ | |
| [HDBU][Triz] | EG | 7∶3 | 313.15 | 101 | 0.108 | [ | |
| HmimCl | AP | 1∶1 | RT | 101 | 2.0 (0.04) ① | [ | |
| HmimCl | AP | 1∶2 | RT | 101 | 9.5 (0.21) ① | [ | |
| HmimCl | AP | 1∶3 | RT | 101 | 13.9 (0.30) ① | [ | |
| HmimCl | AP | 1∶4 | RT | 101 | 19.4 (0.37) ① | [ | |
| HmimCl | PEHA | 1∶4 | RT | 101 | 8.4 (0.4) ① | [ | |
| HmimCl | DETA | 1∶4 | RT | 101 | 22.8 (0.55) ① | [ | |
| HmimCl | EDA | 1∶1 | RT | 101 | 9.0 (0.19) ① | [ | |
| HmimCl | EDA | 1∶2 | RT | 101 | 25.0 (0.45) ① | [ | |
| HmimCl | EDA | 1∶3 | RT | 101 | 26.7 (0.45) ① | [ | |
| HmimCl | EDA | 1∶4 | RT | 101 | 30.8 (0.50) ① | [ | |
| HmimCl | TEPA | 1∶4 | RT | 101 | 9.9 (0.39) ① | [ | |
| MEAC | AP | 1∶1 | RT | 101 | 15.8 (0.28) ① | [ | |
| MEAC | AP | 1∶2 | RT | 101 | 21 (0.37) ① | [ | |
| MEAC | AP | 1∶3 | RT | 101 | 24.3 (0.42) ① | [ | |
| MEAC | AP | 1∶4 | RT | 101 | 26.3 (0.46) ① | [ | |
| MEAC | DETA | 1∶4 | RT | 101 | 25.5 (0.57) ① | [ | |
| MEAC | EDA | 1∶1 | RT | 101 | 23.5 (0.38) ① | [ | |
| MEAC | EDA | 1∶2 | RT | 101 | 30.9 (0.47) ① | [ | |
| MEAC | EDA | 1∶3 | RT | 101 | 36.5 (0.54) ① | [ | |
| MEAC | EDA | 1∶3 | 303.15 | 101 | 33.7 | [ | |
| MEAC | EDA | 1∶4 | RT | 101 | 39.0 (0.57) ① | [ | |
| MEAC | PEHA | 1∶4 | RT | 101 | 12.7 (0.59) ① | [ | |
| MEAC | TEPA | 1∶4 | RT | 101 | 16.6 (0.63) ① | [ | |
| [N2222][Im] | EG | 1∶2 | 298.15 | 101 | 12.9 (0.94) ① | [ | |
| [N2222][Triz] | EG | 1∶2 | 298.15 | 101 | 12.5 (0.92) ① | [ | |
| [P2222][Im] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| [P2222][Triz] | EG | 1∶2 | 298.15 | 101 | 11.8 (0.91) ① | [ | |
| MEACl | EDA | 1∶3 | 303.15 | 101 | 31.5 | [ | |
| TBAB | AMP | 1∶3 | RT | 101 | 10.5 (0.35) ① | [ | |
| TBAB | AMP | 1∶4 | RT | 101 | 12.2 (0.38) ① | [ | |
| TBAB | AP | 1∶2 | RT | 101 | 11.1 (0.43) ① | [ | |
| TBAB | AP | 1∶3 | RT | 101 | 15.6 (0.49) ① | [ | |
| TBAB | AP | 1∶4 | RT | 101 | 18.1 (0.51) ① | [ | |
| TEAC | DA | 1∶3 | 303.15 | 101 | 24.2 | [ | |
| [TETA]Cl | DG | 1∶2 | 313.15 | 101 | 0.159 | [ | |
| [TEPA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.088 (1.355) ① | [ | |
| [TETA]Cl | EG | 1∶3 | 313.15 | 101 | 0.175 | [ | |
| [TETA]Cl | thymol | 1∶3 | 313.15 | 101 | 0.09 (1.298) ① | [ | |
| UEC | EDA | 1∶3 | 303.15 | 101 | 17.8 | [ | |
| TPAC | EA | 1∶6 | 298.15 | 20.0 | 0.276 | [ | |
| TPAC | AC | 1∶4 | 298.15 | 20.0 | 0.240 | [ | |
| TPAC | AC | 1∶7 | 298.15 | 20.0 | 0.435 | [ | |
| TBAB | OCT+OA | 1∶2 | 298.15 | 16.46 | 0.285 | [ | |
| TPAC | DecA | 1∶2 | 298.15 | 19.9 | 0.299 | [ | |
| TMP | thymol | 1∶3 | 313.15 | 10.13 | 1.355 | [ |
| [1] | ROGELJ Joeri, HUPPMANN Daniel, KREY Volker, et al. A new scenario logic for the Paris Agreement long-term temperature goal[J]. Nature, 2019, 573(7774): 357-363. |
| [2] | LIU Helei, TANTIKHAJORNGOSOL Puttipong, CHAN Christine, et al. Technology development and applications of artificial intelligence for post-combustion carbon dioxide capture: Critical literature review and perspectives[J]. International Journal of Greenhouse Gas Control, 2021, 108: 103307. |
| [3] | YOUNAS M, SOHAIL M, LEONG L K, et al. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems[J]. International Journal of Environmental Science and Technology, 2016, 13(7): 1839-1860. |
| [4] | CHEN Pao chi, LAI Yanlin. Optimization in the stripping process of CO2 gas using mixed amines[J]. Energies, 2019, 12(11): 2202. |
| [5] | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| [6] | WU Shu-Yii, LIU Yongfang, CHU Chen-Yeon, et al. Optimal absorbent evaluation for the CO2 separating process by absorption loading, desorption efficiency, cost, and environmental tolerance[J]. International Journal of Green Energy, 2015, 12(10): 1025-1030. |
| [7] | OLAJIRE Abass A. CO2 capture and separation technologies for end-of-pipe applications—A review[J]. Energy, 2010, 35(6): 2610-2628. |
| [8] | ZHOU Shan, WANG Shujuan, CHEN Changhe. Thermal degradation of monoethanolamine in CO2 capture with acidic impurities in flue gas[J]. Industrial & Engineering Chemistry Research, 2012, 51(6): 2539-2547. |
| [9] | Se-Young OH, BINNS Michael, CHO Habin, et al. Energy minimization of MEA-based CO2 capture process[J]. Applied Energy, 2016, 169: 353-362. |
| [10] | LEUNG Dennis Y C, CARAMANNA Giorgio, Mercedes MAROTO-VALER M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| [11] | FYTIANOS Georgios, VEVELSTAD Solrun J, KNUUTILA Hanna K. Degradation and corrosion inhibitors for MEA-based CO2 capture plants[J]. International Journal of Greenhouse Gas Control, 2016, 50: 240-247. |
| [12] | KITTEL J, IDEM R, GELOWITZ D, et al. Corrosion in MEA units for CO2 capture: Pilot plant studies[J]. Energy Procedia, 2009, 1(1): 791-797. |
| [13] | Gregorio GARCÍA, ATILHAN Mert, APARICIO Santiago. Interfacial properties of deep eutectic solvents regarding to CO2 capture[J]. The Journal of Physical Chemistry C, 2015, 119(37): 21413-21425. |
| [14] | GHAEDI Hosein, AYOUB Muhammad, SUFIAN Suriati, et al. Measurement and correlation of physicochemical properties of phosphonium-based deep eutectic solvents at several temperatures (293.15K-343.15K) for CO2 capture[J]. The Journal of Chemical Thermodynamics, 2017, 113: 41-51. |
| [15] | PAIVA Alexandre, CRAVEIRO Rita, AROSO Ivo, et al. Natural deep eutectic solvents-solvents for the 21st century[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(5): 1063-1071. |
| [16] | 白佳乐, 燕童凡, 谷嘉诚, 等. 低共熔溶剂的性质与应用研究进展[J]. 工业催化, 2024, 32(9): 26-32. |
| BAI Jiale, YAN Tongfan, GU Jiacheng, et al. Research progress on the properties and applications of deep eutectic solvents[J]. Industrial Catalysis, 2024, 32(9): 26-32. | |
| [17] | ZHANG Qinghua, DE OLIVEIRA VIGIER Karine, ROYER Sébastien, et al. Deep eutectic solvents: Syntheses, properties and applications[J]. Chemical Society Reviews, 2012, 41(21): 7108-7146. |
| [18] | FRANCISCO María, VAN DEN BRUINHORST Adriaan, ZUBEIR Lawien F, et al. A new low transition temperature mixture (LTTM) formed by choline chloride+lactic acid: Characterization as solvent for CO2 capture[J]. Fluid Phase Equilibria, 2013, 340: 77-84. |
| [19] | LERON Rhoda B, CAPARANGA Alvin, LI Menghui. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T =303.15-343.15K and moderate pressures[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(6): 879-885. |
| [20] | ZHANG Yingying, JI Xiaoyan, LU Xiaohua. Choline-based deep eutectic solvents for CO2 separation: Review and thermodynamic analysis[J]. Renewable and Sustainable Energy Reviews, 2018, 97: 436-455. |
| [21] | KUMAR K, KESHRI S, BHARTI A, et al. Solubility of gases in choline chloride-based deep eutectic solvents from molecular dynamics simulation[J]. Industrial & Engineering Chemistry Research, 2022, 61(13): 4659-4671. |
| [22] | Emad ALI, HADJ-KALI Mohamed K, MULYONO Sarwono, et al. Analysis of operating conditions for CO2 capturing process using deep eutectic solvents[J]. International Journal of Greenhouse Gas Control, 2016, 47: 342-350. |
| [23] | Emad ALI, HADJ-KALI Mohamed K, MULYONO Sarwono, et al. Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng-Robinson equation of state[J]. Chemical Engineering Research and Design, 2014, 92(10): 1898-1906. |
| [24] | SARMAD Shokat, XIE Yujiao, MIKKOLA Jyri-Pekka, et al. Screening of deep eutectic solvents (DESs) as green CO2 sorbents: From solubility to viscosity[J]. New Journal of Chemistry, 2017, 41(1): 290-301. |
| [25] | ZUBEIR Lawien F, VAN OSCH Dannie J G P, ROCHA Marisa A A, et al. Carbon dioxide solubilities in decanoic acid-based hydrophobic deep eutectic solvents[J]. Journal of Chemical and Engineering Data, 2018, 63(4): 913-919. |
| [26] | CHOI Young Hae, VAN SPRONSEN Jaap, DAI Yuntao, et al. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology?[J]. Plant Physiology, 2011, 156(4): 1701-1705. |
| [27] | LIU Yang, BRENT FRIESEN J, MCALPINE James B, et al. Natural deep eutectic solvents: Properties, applications, and perspectives[J]. Journal of Natural Products, 2018, 81(3): 679-690. |
| [28] | FLORINDO Catarina, BRANCO Prof Luís C, MARRUCHO Prof Dr Isabel M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents[J]. ChemSusChem, 2019, 12(8): 1549-1559. |
| [29] | TOMÉ Luciana I N, Vanessa BAIÃO, SILVA Wanderson DA, et al. Deep eutectic solvents for the production and application of new materials[J]. Applied Materials Today, 2018, 10: 30-50. |
| [30] | ZHANG Man, ZHANG Xingyilong, LIU Yingying, et al. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents[J]. Environmental Science and Pollution Research International, 2021, 28(27): 35537-35563. |
| [31] | PADUCH Roman, Martyna KANDEFER-SZERSZEŃ, TRYTEK Mariusz, et al. Terpenes: Substances useful in human healthcare[J]. Archivum Immunologiae et Therapiae Experimentalis, 2007, 55(5): 315-327. |
| [32] | HAIDER Mohd Belal, Divyam JHA, MARRIYAPPAN SIVAGNANAM Balathanigaimani, et al. Thermodynamic and kinetic studies of CO2 capture by glycol and amine-based deep eutectic solvents[J]. Journal of Chemical & Engineering Data, 2018, 63(8): 2671-2680. |
| [33] | ESPINO Magdalena, DE LOS ÁNGELES FERNÁNDEZ María, GOMEZ Federico J V, et al. Natural designer solvents for greening analytical chemistry[J]. TrAC Trends in Analytical Chemistry, 2016, 76: 126-136. |
| [34] | MULIA Kamarza, PUTRI Sylvania, KRISANTI Elsa, et al. Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture[C]//International Conference on Chemistry, Chemical Process and Engieering (IC3PE), 2017. |
| [35] | CRAIG Stuart AS. Betaine in human nutrition[J]. The American Journal of Clinical Nutrition, 2004, 80(3): 539-549. |
| [36] | VRANOVA Valerie, REJSEK Klement, FORMANEK Pavel. Aliphatic, cyclic, and aromatic organic acids, vitamins, and carbohydrates in soil: A review[J]. The Scientific World Journal, 2013, 2013(1): 524239. |
| [37] | SIANI Gabriella, TIECCO Matteo, DI PROFIO Pietro, et al. Physical absorption of CO2 in betaine/carboxylic acid-based Natural Deep Eutectic Solvents[J]. Journal of Molecular Liquids, 2020, 315: 113708. |
| [38] | Leonhard L SZE, PANDEY Shubha, RAVULA Sudhir, et al. Ternary deep eutectic solvents tasked for carbon dioxide capture[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(9): 2117-2123. |
| [39] | ZHANG Na, HUANG Zhaohe, ZHANG Haiming, et al. Highly efficient and reversible CO2 capture by task-specific deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13321-13329. |
| [40] | JIANG Bin, MA Jingwen, YANG Na, et al. Superbase/acylamido-based deep eutectic solvents for multiple-site efficient CO2 absorption[J]. Energy & Fuels, 2019, 33(8): 7569-7577. |
| [41] | SHUKLA Shashi Kant, MIKKOLA Jyri-Pekka. Intermolecular interactions upon carbon dioxide capture in deep-eutectic solvents[J]. Physical Chemistry Chemical Physics, 2018, 20(38): 24591-24601. |
| [42] | CUI Ge, Meng LYU, YANG Dezhong. Efficient CO2 absorption by azolide-based deep eutectic solvents[J]. Chemical Communications, 2019, 55(10): 1426-1429. |
| [43] | TRIVEDI Tushar J, LEE Ji Hoon, LEE Hyeon Jeong, et al. Deep eutectic solvents as attractive media for CO2 capture[J]. Green Chemistry, 2016, 18(9): 2834-2842. |
| [44] | ZHANG Kai, HOU Yucui, WANG Yiming, et al. Efficient and reversible absorption of CO2 by functional deep eutectic solvents[J]. Energy & Fuels, 2018, 32(7): 7727-7733. |
| [45] | GU Yanxue, HOU Yucui, REN Shuhang, et al. Hydrophobic functional deep eutectic solvents used for efficient and reversible capture of CO2 [J]. ACS Omega, 2020, 5(12): 6809-6816. |
| [46] | HAIDER Mohd Belal, Divyam JHA, KUMAR Rakesh, et al. Ternary hydrophobic deep eutectic solvents for carbon dioxide absorption[J]. International Journal of Greenhouse Gas Control, 2020, 92: 102839. |
| [47] | CAO Lingdi, HUANG Junhua, ZHANG Xiangping, et al. Imidazole tailored deep eutectic solvents for CO2 capture enhanced by hydrogen bonds[J]. Physical Chemistry Chemical Physics, 2015, 17(41): 27306-27316. |
| [48] | LI Zhuo, WANG Lili, LI Changping, et al. Absorption of carbon dioxide using ethanolamine-based deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10403-10414. |
| [49] | GURKAN B, GOODRICH B F, MINDRUP E M, et al. Molecular design of high capacity, low viscosity, chemically tunable ionic liquids for CO2 capture[J]. The Journal of Physical Chemistry Letters, 2010, 1(24): 3494-3499. |
| [50] | LIU Fan, SHEN Yao, SHEN Li, et al. Novel amino-functionalized ionic liquid/organic solvent with low viscosity for CO2 capture[J]. Environmental Science & Technology, 2020, 54(6): 3520-3529. |
| [51] | LEE Yunyang, PENLEY Drace, KLEMM Aidan, et al. Deep eutectic solvent formed by imidazolium cyanopyrrolide and ethylene glycol for reactive CO2 separations[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(3): 1090-1098. |
| [52] | Ahmad AL-BODOUR, ALOMARI Noor, Alberto GUTIÉRREZ, et al. High-pressure carbon dioxide solubility in terpene based deep eutectic solvents[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108237. |
| [53] | SONG Xueyi, YUAN Junjie, YANG Chen, et al. Carbon dioxide separation performance evaluation of amine-based versus choline-based deep eutectic solvents[J]. Renewable and Sustainable Energy Reviews, 2023, 184: 113499. |
| [54] | XIN Kun, VAN SINT ANNALAND Martin. Diffusivities and solubilities of carbon dioxide in deep eutectic solvents[J]. Separation and Purification Technology, 2023, 307: 122779. |
| [55] | DENG Dongshun, JIANG Yaotai, LIU Xiaobang, et al. Investigation of solubilities of carbon dioxide in five levulinic acid-based deep eutectic solvents and their thermodynamic properties[J]. The Journal of Chemical Thermodynamics, 2016, 103: 212-217. |
| [56] | Iwona CICHOWSKA-KOPCZYŃSKA, NOWOSIELSKI Bartosz, Dorota WARMIŃSKA. Deep eutectic solvents: Properties and applications in CO2 separation[J]. Molecules, 2023, 28(14): 5293. |
| [57] | FAN Jing, ZHANG Xin, HE Nan, et al. Physical absorption and thermodynamic modeling of CO2 in new deep eutectic solvents[J]. Journal of Molecular Liquids, 2024, 402: 124752. |
| [58] | LI Xiaoyong, HOU Minqiang, HAN Buxing, et al. Solubility of CO2 in a choline chloride + urea eutectic mixture[J]. Journal of Chemical & Engineering Data, 2008, 53(2): 548-550. |
| [59] | LI Ziliang, ZHONG Fuyu, HUANG Jiyong, et al. Sugar-based natural deep eutectic solvents as potential absorbents for NH3 capture at elevated temperatures and reduced pressures[J]. Journal of Molecular Liquids, 2020, 317: 113992. |
| [60] | ADEYEMI Idowu, ABU-ZAHRA Mohammad R M, ALNASHEF Inas. Experimental study of the solubility of CO2 in novel amine based deep eutectic solvents[J]. Energy Procedia, 2017, 105: 1394-1400. |
| [61] | SHUKLA Shashi Kant, NIKJOO Dariush, MIKKOLA Jyri-Pekka. Is basicity the sole criterion for attaining high carbon dioxide capture in deep-eutectic solvents?[J]. Physical Chemistry Chemical Physics, 2020, 22(3): 966-970. |
| [62] | QIAN Wenbin, HAO Jin, ZHU Mingjian, et al. Development of green solvents for efficient post-combustion CO2 capture with good regeneration performance[J]. Journal of CO2 Utilization, 2022, 59: 101955. |
| [63] | CHEN Yanfei, AI Ning, LI Guihua, et al. Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols[J]. Journal of Chemical & Engineering Data, 2014, 59(4): 1247-1253. |
| [64] | LU Meizhen, HAN Guoqiang, JIANG Yaotai, et al. Solubilities of carbon dioxide in the eutectic mixture of levulinic acid (or furfuryl alcohol) and choline chloride[J]. The Journal of Chemical Thermodynamics, 2015, 88: 72-77. |
| [65] | CHEN Mingzhe, XU Jinming. CO2 capture mechanism by deep eutectic solvents formed by choline prolinate and ethylene glycol[J]. Molecules, 2023, 28(14): 5461. |
| [66] | ZHANG Kaiqing, WANG Rui. A critical review on new and efficient adsorbents for CO2 capture[J]. Chemical Engineering Journal, 2024, 485: 149495. |
| [67] | RUAN Jiawei, CHEN Lifang, QI Zhiwen. Deep eutectic solvents as a versatile platform toward CO2 capture and utilization[J]. Green Chemistry, 2023, 25(21): 8328-8348. |
| [68] | WANG Ze, WANG Zonghua, CHEN Jie, et al. The influence of hydrogen bond donors on the CO2 absorption mechanism by the bio-phenol-based deep eutectic solvents[J]. Molecules, 2021, 26(23): 7167. |
| [69] | PISHRO Khatereh ALI, MURSHID Ghulam, MJALLI Farouq Sabri, et al. Investigation of CO2 solubility in monoethanolamine hydrochloride based deep eutectic solvents and physical properties measurements[J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2848-2856. |
| [70] | RUAN Jiawei, YE Xiangzhu, WANG Ruizhuan, et al. Experimental and theoretical study on efficient CO2 absorption coordinated by molecules and ions of DBN and 1,2,4-triazole formed deep eutectic solvents[J]. Fuel, 2023, 334: 126709. |
| [71] | FOORGINEZHAD Sahar, JI Xiaoyan. Development of monoethanolamine chloride-ethylene diamine deep eutectic solvent for ffficient carbon dioxide capture[J]. Separation and Purification Technology, 2024, 347: 127593. |
| [72] | ZHEKENOV Temirlan, TOKSANBAYEV Nursultan, KAZAKBAYEVA Zhanna, et al. Formation of type Ⅲ Deep Eutectic Solvents and effect of water on their intermolecular interactions[J]. Fluid Phase Equilibria, 2017, 441: 43-48. |
| [73] | ZHANG Yue, HAN Rui, ZHOU Shujun, et al. Amine-based deep eutectic solvents for CO2 capture: Experiments and molecular thermodynamics[J]. Separation and Purification Technology, 2025, 359: 130559. |
| [74] | LI Guihua, DENG Dongshun, CHEN Yanfei, et al. Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents[J]. The Journal of Chemical Thermodynamics, 2014, 75: 58-62. |
| [75] | SU Wen cheng, WONG David Shan Hill, LI Meng hui. Effect of water on solubility of carbon dioxide in (aminomethanamide + 2-hydroxy-N,N,N-trimethylethanaminium chloride)[J]. Journal of Chemical & Engineering Data, 2009, 54(6): 1951-1955. |
| [76] | XIE Yujiao, DONG Haifeng, ZHANG Suojiang, et al. Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea[J]. Journal of Chemical & Engineering Data, 2014, 59(11): 3344-3352. |
| [77] | FETISOV Evgenii O, HARWOOD David B, KUO I-Feng William, et al. First-principles molecular dynamics study of a deep eutectic solvent: Choline chloride/urea and its mixture with water[J]. The Journal of Physical Chemistry B, 2018, 122(3): 1245-1254. |
| [78] | SHAH Dhawal, MJALLI Farouq S. Effect of water on the thermo-physical properties of reline: An experimental and molecular simulation based approach[J]. Physical Chemistry Chemical Physics, 2014, 16(43): 23900-23907. |
| [79] | Alberto GUTIÉRREZ, ATILHAN Mert, APARICIO Santiago. Molecular dynamics study on water confinement in deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 339: 116758. |
| [80] | Kai TÖPFER, PASTI Andrea, Anuradha DAS, et al. Structure, organization, and heterogeneity of water-containing deep eutectic solvents[J]. Journal of the American Chemical Society, 2022, 144(31): 14170-14180. |
| [81] | WENG Lindong, TONER Mehmet. Janus-faced role of water in defining nanostructure of choline chloride/glycerol deep eutectic solvent[J]. Physical Chemistry Chemical Physics, 2018, 20(35): 22455-22462. |
| [82] | ABDRABOU Hossam K, ALNASHEF Inas, ZAHRA Mohammad ABU, et al. Experimental investigation of novel ternary amine-based deep eutectic solvents for CO2 capture[J]. PLoS One, 2023, 18(6): e0286960. |
| [83] | HUAN Qun, ZHANG Yan, WIBOWO Haryo, et al. Study on regeneration characteristics of choline chloride-monoethanolamine deep eutectic solvent after capturing CO2 from biogas[J]. Separation and Purification Technology, 2022, 302: 122064. |
| [84] | LIU Xiangwei, AO Qian, SHI Shengyou, et al. CO2 capture by alcohol ammonia based deep eutectic solvents with different water content[J]. Materials Research Express, 2022, 9(1): 015504. |
| [85] | GUTIÉRREZ María C, FERRER María L, REYES MATEO C, et al. Freeze-drying of aqueous solutions of deep eutectic solvents: A suitable approach to deep eutectic suspensions of self-assembled structures[J]. Langmuir, 2009, 25(10): 5509-5515. |
| [86] | ZHANG Yuqi, ZHU Chunying, FU Taotao, et al. CO2 absorption performance of ChCl-MEA deep eutectic solvent in microchannel[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108792. |
| [87] | AL-DAWSARI Jiyad N, Abdelbasset BESSADOK-JEMAI, WAZEER Irfan, et al. Fitting of experimental viscosity to temperature data for deep eutectic solvents[J]. Journal of Molecular Liquids, 2020, 310: 113127. |
| [88] | ALIZADEH Vahideh, ESSER Lars, KIRCHNER Barbara. How is CO2 absorbed into a deep eutectic solvent?[J]. The Journal of Chemical Physics, 2021, 154(9): 094503. |
| [89] | YAN Mi, HUAN Qun, ZHANG Yan, et al. Effect of operating parameters on CO2 capture from biogas with choline chloride-monoethanolamine deep eutectic solvent and its aqueous solution[J]. Biomass Conversion and Biorefinery, 2024, 14(1): 283-297. |
| [1] | ZHANG Wenjing, HUANG Zhixin, LI Shiteng, DENG Shuai, LI Shuangjun. Biomass carbon aerogels for CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5018-5032. |
| [2] | QI Yan, CHANG Hao, ZHANG Lei. Structural product formulation design method based on molecular dynamics simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4341-4351. |
| [3] | HUANG Ke’er, LIU Jiahao, LI Haoming, ZHOU Tianhang, GAO Jinsen, LAN Xingying. Self-diffusion coefficients in the process of carbon capture by amine solvents based on molecular dynamics simulation [J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4352-4364. |
| [4] | FU Zijun, SONG Xuehang, SHEN Qun, WANG Xiaobo, GU Jiaming, WANG Danfeng, WEI Wei, SUN Nannan. Carbon footprint analysis of integrated CO2 capture and methanation technology based on life cycle assessment [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2879-2887. |
| [5] | DOU Yu, WANG Wenxuan, FAN Chunlei, MA Jiliang, LIANG Cai, CHEN Xiaoping. Preparation of vaterite CaCO3 by mineralizing CO2 from desulfurized gypsum [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2328-2337. |
| [6] | LI Letian, LU Shijian, LIU Hanxiao, WU Liming, LIU Ling, KANG Guojun. Progress of desorption and regeneration of organic amine-enriched liquids [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 490-499. |
| [7] | WANG Ning, LU Shijian, LIU Ling, LIANG Jing, LIU Miaomiao, SUN Mengyuan, KANG Guojun. Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 445-464. |
| [8] | LU Shijian, ZHANG Juanjuan, YANG Fei, LIU Ling, CHEN Siming, KANG Guojun, FANG Qinqin. Research progress of amine escape control technology by chemical absorption method [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4562-4570. |
| [9] | ZHI Yuan, MA Jiliang, CHEN Xiaoping, LIU Daoyin, LIANG Cai. Decarbonization capability of supported Na-based CO2 adsorbents prepared by fluidized bed spray impregnation [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2961-2967. |
| [10] | LIU Kefeng, LIU Taoran, CAI Yong, HU Xuesheng, DONG Weigang, ZHOU Huaqun, GAO Fei. Progress in research and engineering demonstration of CO2 capture technology [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 2901-2914. |
| [11] | GAO Fanxiang, LIU Yang, ZHANG Guiquan, QIN Feng, YAO Jiantao, JIN Hui, SHI Jinwen. Research progress of wet process synergistic desulfurization and decarbonization technology for coal-fired flue gas [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2324-2342. |
| [12] | MIAO Yihe, WANG Yaozu, LIU Yuhang, ZHU Xuancan, LI Jia, YU Lijun. Research progress on the improving effect of additives on supported amine adsorbents for carbon capture [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2739-2759. |
| [13] | SUN Weiji, LIU Lang, FANG Zhiyu, ZHU Mengbo, XIE Geng, HE Wei, GAO Yuheng. Technique of wet carbonation of modified magnesium slag [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2161-2173. |
| [14] | HE Jin, LAI Yuwen, LI Yanchun, ZHOU Shilin, ZHOU Yong, GAO Congjie. DES changed the diffusion rate of amine monomer to prepare high-performance composite reverse osmosis membrane [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1972-1980. |
| [15] | XU Zewen, WANG Ming, WANG Qiang, HOU Yingfei. Recent advances in amine-rich membrane for CO2 separation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1374-1386. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||