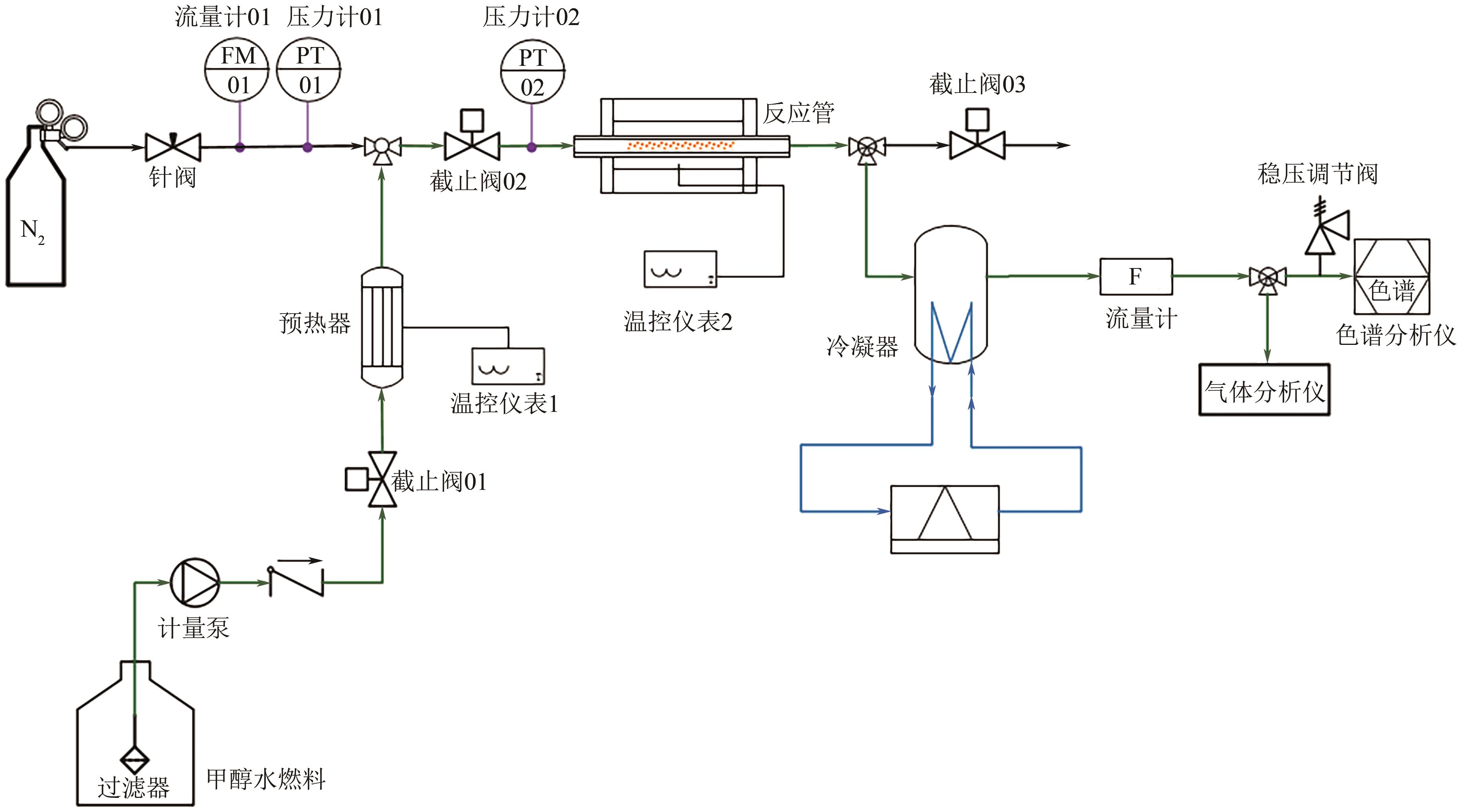
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (3): 1338-1346.DOI: 10.16085/j.issn.1000-6613.2024-0446
• Industrial catalysis • Previous Articles Next Articles
Durability testing and life prediction of methanol reforming catalysts for hydrogen production
ZHU Guoyu( ), GE Qi, FU Mingli(
), GE Qi, FU Mingli( )
)
- School of Environment and Energy, Huanan University of Science and Technology, Guangzhou 510655, Guangdong, China
-
Received:2024-03-18Revised:2024-04-11Online:2025-04-16Published:2025-03-25 -
Contact:FU Mingli
甲醇重整制氢催化剂耐久性评价和寿命预测方法
- 华南理工大学环境与能源学院,广东 广州 510655
-
通讯作者:付名利 -
作者简介:朱国瑜(1977—),男,高级工程师,博士研究生,研究方向为氢能。E-mail:wanke_gz@126.com。 -
基金资助:广东省发展和改革委员会基本建设项目(2020-440112-38-03-033897)
CLC Number:
Cite this article
ZHU Guoyu, GE Qi, FU Mingli. Durability testing and life prediction of methanol reforming catalysts for hydrogen production[J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1338-1346.
朱国瑜, 葛棋, 付名利. 甲醇重整制氢催化剂耐久性评价和寿命预测方法[J]. 化工进展, 2025, 44(3): 1338-1346.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0446
| 试剂/仪器 | 型号/规格 | 品牌/生产厂家 |
|---|---|---|
| 甲醇 | 分析纯 | 阿拉丁试剂有限公司 |
| 氮气 | 高纯氮99.99% | 液化空气工业气体有限公司 |
| 甲醇重整催化剂 | Cu/ZnO/Al2O3 | 四川蜀泰化工科技有限公司 |
| 气体质量流量控制器 | N2-5L/min | 北京七星华创流量计有限公司 |
| 流量计 | 0~100L/h | 重庆三协益新仪表有限公司 |
| 压力传感器 | 0~50kPa | 北京华控兴业仪表有限公司 |
| 温控仪表 | DSN-800D | 东莞市德欣电子科技有限公司 |
| 针阀 | 0~3mm可调节 | 世伟洛克流体科技有限公司 |
| 冷凝器 | DLSB-20L-10 | 郑州世联良工仪器设备有限公司 |
| 电磁阀 | VXE2350L-02-5DL1 | SMC中国有限公司 |
| 气体分析仪 | Gas-3800P | 武汉锐意自控系统有限公司 |
| 气相色谱仪 | GC-9790PNS | 浙江福立分析仪器股份有限公司 |
| 微型进液计量泵 | AP0010 | 上海三为科学仪器有限公司 |
| 试剂/仪器 | 型号/规格 | 品牌/生产厂家 |
|---|---|---|
| 甲醇 | 分析纯 | 阿拉丁试剂有限公司 |
| 氮气 | 高纯氮99.99% | 液化空气工业气体有限公司 |
| 甲醇重整催化剂 | Cu/ZnO/Al2O3 | 四川蜀泰化工科技有限公司 |
| 气体质量流量控制器 | N2-5L/min | 北京七星华创流量计有限公司 |
| 流量计 | 0~100L/h | 重庆三协益新仪表有限公司 |
| 压力传感器 | 0~50kPa | 北京华控兴业仪表有限公司 |
| 温控仪表 | DSN-800D | 东莞市德欣电子科技有限公司 |
| 针阀 | 0~3mm可调节 | 世伟洛克流体科技有限公司 |
| 冷凝器 | DLSB-20L-10 | 郑州世联良工仪器设备有限公司 |
| 电磁阀 | VXE2350L-02-5DL1 | SMC中国有限公司 |
| 气体分析仪 | Gas-3800P | 武汉锐意自控系统有限公司 |
| 气相色谱仪 | GC-9790PNS | 浙江福立分析仪器股份有限公司 |
| 微型进液计量泵 | AP0010 | 上海三为科学仪器有限公司 |
| 物性 | Cu/ZnO/Al2O3 |
|---|---|
| 形状 | 黑色圆柱体 |
| 粒径大小 | Φ3mm×(2.5~3)mm |
| 堆密度 | 1.5kg/L |
| 压力 | 常压约3MPa |
| 温度 | 160~280℃ |
| 甲醇LHSV | <1.5h-1 |
| 物性 | Cu/ZnO/Al2O3 |
|---|---|
| 形状 | 黑色圆柱体 |
| 粒径大小 | Φ3mm×(2.5~3)mm |
| 堆密度 | 1.5kg/L |
| 压力 | 常压约3MPa |
| 温度 | 160~280℃ |
| 甲醇LHSV | <1.5h-1 |
| 催化剂反应温度/℃ | 催化剂反应时间/h | LHSV/h-1 | 压力/kPa | 甲醇水流量/g·min-1 | 密度/g·cm-3 |
|---|---|---|---|---|---|
| 250 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 450 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 催化剂反应温度/℃ | 催化剂反应时间/h | LHSV/h-1 | 压力/kPa | 甲醇水流量/g·min-1 | 密度/g·cm-3 |
|---|---|---|---|---|---|
| 250 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 450 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 催化剂反应温度/℃ | 催化剂反应时间/h | LHSV/h-1 | 压力/kPa | 甲醇水流量/g·min-1 | 密度/g·cm-3 |
|---|---|---|---|---|---|
| 350 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 100 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 200 | 1.5 | 50 | 0.75 | 0.915 |
| 催化剂反应温度/℃ | 催化剂反应时间/h | LHSV/h-1 | 压力/kPa | 甲醇水流量/g·min-1 | 密度/g·cm-3 |
|---|---|---|---|---|---|
| 350 | 10 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 100 | 1.5 | 50 | 0.75 | 0.915 |
| 350 | 200 | 1.5 | 50 | 0.75 | 0.915 |
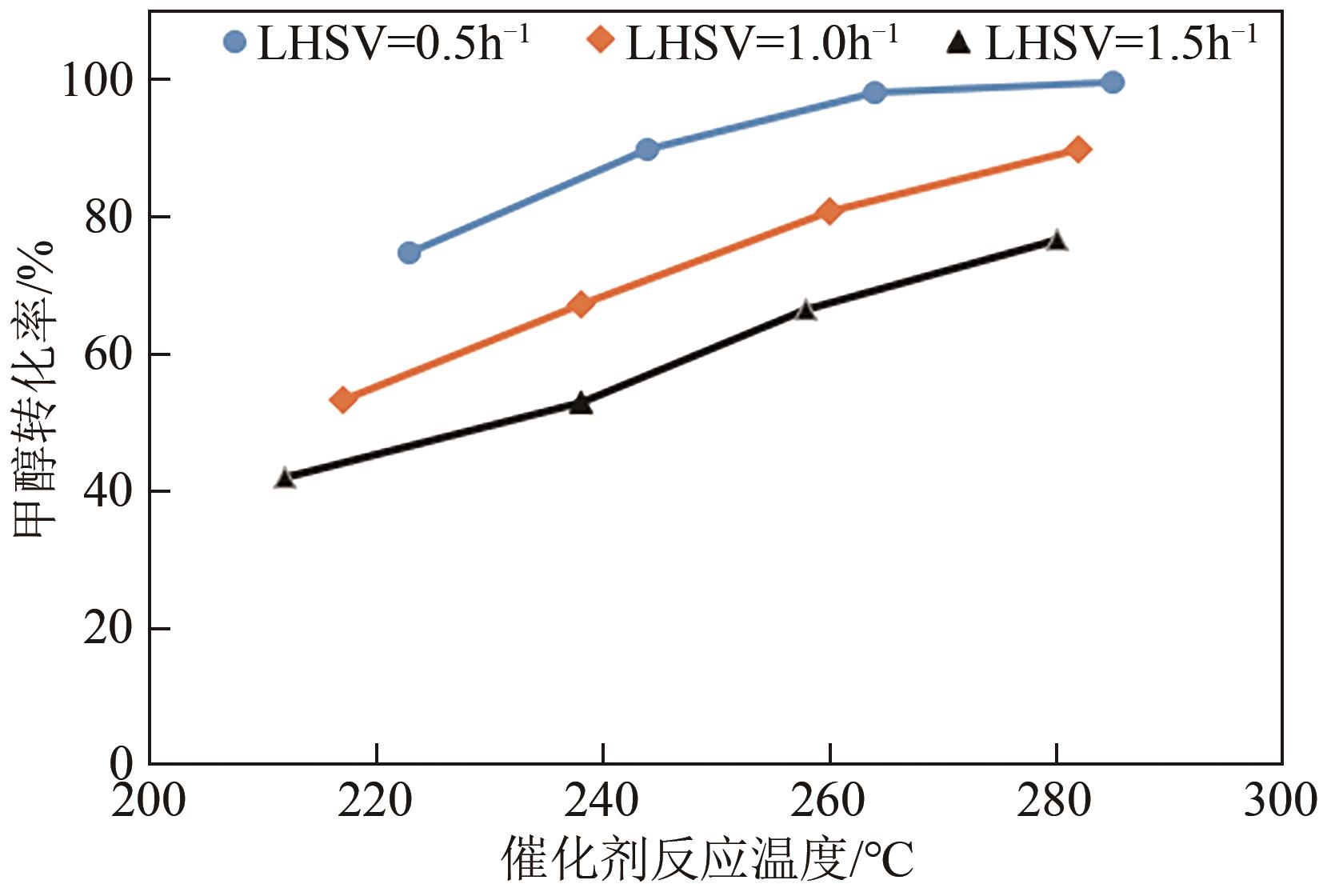
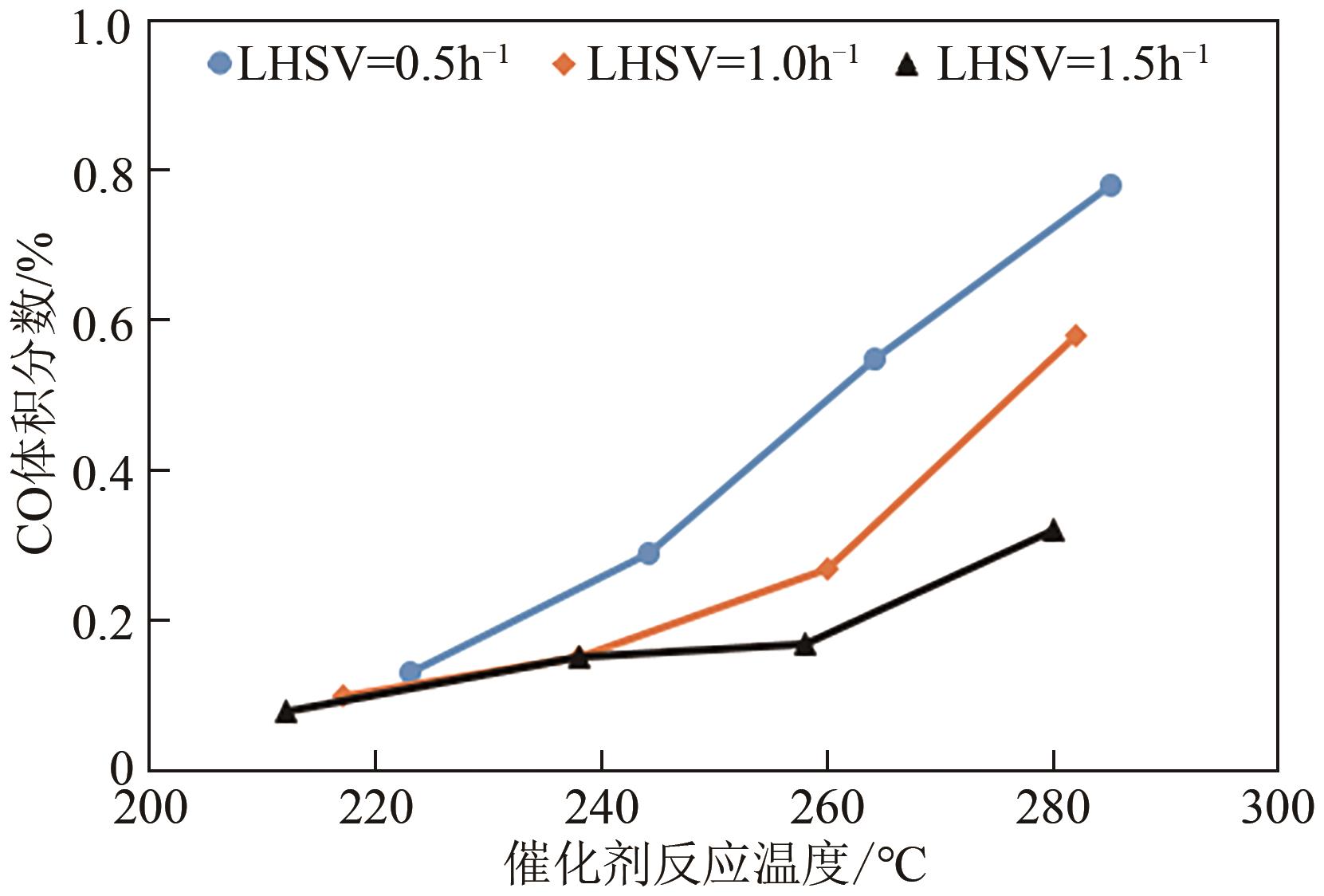
LHSV /h-1 | 催化床层温度 /℃ | 气体成分(体积分数)/% | 重整气体流量 /L·h-1 | H2 收率/% | CH3OH 转化率/% | |||
|---|---|---|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | |||||
| 0.5 | 223 | 62.54 | 0.09 | 0.13 | 18.65 | 20.82 | 82.67 | 74.84 |
| 0.5 | 244 | 62.17 | 0.07 | 0.29 | 19.45 | 23.82 | 94.02 | 89.89 |
| 0.5 | 264 | 62.43 | 0.08 | 0.55 | 20.17 | 24.79 | 98.26 | 98.22 |
| 0.5 | 285 | 62.53 | 0.08 | 0.78 | 20.22 | 24.87 | 98.74 | 99.87 |
| 1.0 | 217 | 71.53 | 0.13 | 0.10 | 20.08 | 27.6 | 62.67 | 53.39 |
| 1.0 | 238 | 69.62 | 0.14 | 0.15 | 21.67 | 32.16 | 71.08 | 67.26 |
| 1.0 | 260 | 69.56 | 0.14 | 0.27 | 22.45 | 37.2 | 82.15 | 80.99 |
| 1.0 | 282 | 69.84 | 0.15 | 0.58 | 22.8 | 40.2 | 89.13 | 90.09 |
| 1.5 | 212 | 68.15 | 0.07 | 0.08 | 21.57 | 30.42 | 43.88 | 41.95 |
| 1.5 | 238 | 68.63 | 0.07 | 0.15 | 21.87 | 37.68 | 54.73 | 52.85 |
| 1.5 | 258 | 69.88 | 0.07 | 0.17 | 22.32 | 46.5 | 68.77 | 66.61 |
| 1.5 | 280 | 70.77 | 0.07 | 0.32 | 22.66 | 52.38 | 78.45 | 76.66 |
LHSV /h-1 | 催化床层温度 /℃ | 气体成分(体积分数)/% | 重整气体流量 /L·h-1 | H2 收率/% | CH3OH 转化率/% | |||
|---|---|---|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | |||||
| 0.5 | 223 | 62.54 | 0.09 | 0.13 | 18.65 | 20.82 | 82.67 | 74.84 |
| 0.5 | 244 | 62.17 | 0.07 | 0.29 | 19.45 | 23.82 | 94.02 | 89.89 |
| 0.5 | 264 | 62.43 | 0.08 | 0.55 | 20.17 | 24.79 | 98.26 | 98.22 |
| 0.5 | 285 | 62.53 | 0.08 | 0.78 | 20.22 | 24.87 | 98.74 | 99.87 |
| 1.0 | 217 | 71.53 | 0.13 | 0.10 | 20.08 | 27.6 | 62.67 | 53.39 |
| 1.0 | 238 | 69.62 | 0.14 | 0.15 | 21.67 | 32.16 | 71.08 | 67.26 |
| 1.0 | 260 | 69.56 | 0.14 | 0.27 | 22.45 | 37.2 | 82.15 | 80.99 |
| 1.0 | 282 | 69.84 | 0.15 | 0.58 | 22.8 | 40.2 | 89.13 | 90.09 |
| 1.5 | 212 | 68.15 | 0.07 | 0.08 | 21.57 | 30.42 | 43.88 | 41.95 |
| 1.5 | 238 | 68.63 | 0.07 | 0.15 | 21.87 | 37.68 | 54.73 | 52.85 |
| 1.5 | 258 | 69.88 | 0.07 | 0.17 | 22.32 | 46.5 | 68.77 | 66.61 |
| 1.5 | 280 | 70.77 | 0.07 | 0.32 | 22.66 | 52.38 | 78.45 | 76.66 |
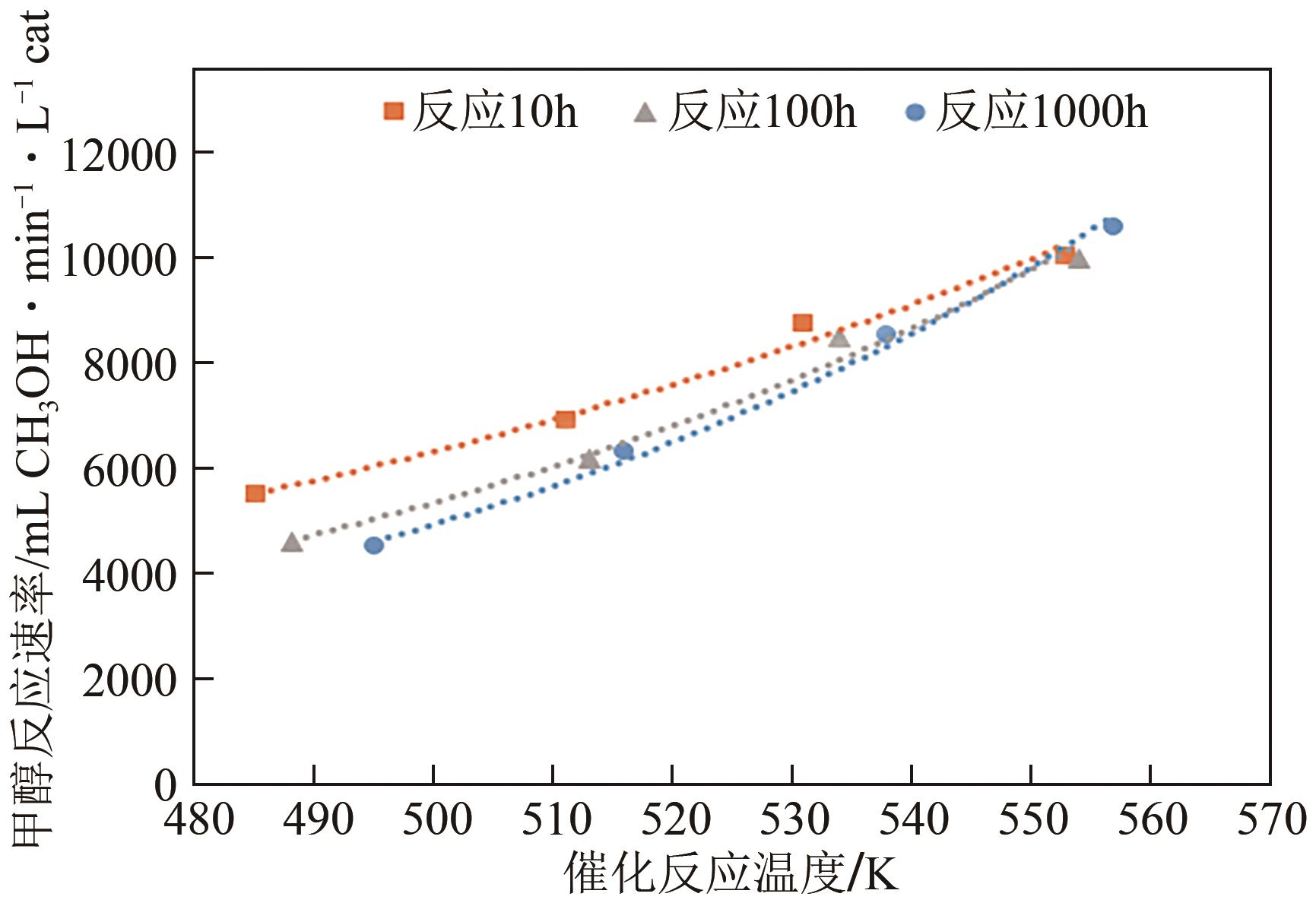
反应时间 /h | 催化床层 温度/℃ | 气体成分(体积分数)/% | 富氢气体流量 /L·h-1 | CH3OH 转化率/% | 甲醇反应速率 /mL CH3OH·min-1·L-1 cat | |||
|---|---|---|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | |||||
| 10 | 212 | 68.15 | 0.07 | 0.08 | 21.57 | 30.42 | 41.95 | 5505.94 |
| 10 | 238 | 68.63 | 0.07 | 0.15 | 21.87 | 37.68 | 52.85 | 6936.56 |
| 10 | 258 | 69.88 | 0.07 | 0.17 | 22.32 | 46.5 | 66.61 | 8742.56 |
| 10 | 280 | 70.77 | 0.07 | 0.32 | 22.66 | 52.38 | 76.66 | 10061.63 |
| 100 | 215 | 72.77 | 0.07 | 0.09 | 22.72 | 24.23 | 35.20 | 4620.00 |
| 100 | 240 | 72.86 | 0.08 | 0.13 | 22.81 | 32.17 | 47.02 | 6171.38 |
| 100 | 261 | 72.92 | 0.07 | 0.15 | 22.58 | 44.72 | 64.74 | 8497.13 |
| 100 | 281 | 73.01 | 0.07 | 0.29 | 22.66 | 52.04 | 76.06 | 9982.88 |
| 1000 | 222 | 73.21 | 0.08 | 0.10 | 24.06 | 22.32 | 34.35 | 4508.44 |
| 1000 | 243 | 74.28 | 0.08 | 0.12 | 24.31 | 31.02 | 48.27 | 6335.44 |
| 1000 | 265 | 75.18 | 0.07 | 0.15 | 24.58 | 41.28 | 65.00 | 8531.25 |
| 1000 | 284 | 75.15 | 0.07 | 0.28 | 24.47 | 51.18 | 80.65 | 10585.31 |
反应时间 /h | 催化床层 温度/℃ | 气体成分(体积分数)/% | 富氢气体流量 /L·h-1 | CH3OH 转化率/% | 甲醇反应速率 /mL CH3OH·min-1·L-1 cat | |||
|---|---|---|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | |||||
| 10 | 212 | 68.15 | 0.07 | 0.08 | 21.57 | 30.42 | 41.95 | 5505.94 |
| 10 | 238 | 68.63 | 0.07 | 0.15 | 21.87 | 37.68 | 52.85 | 6936.56 |
| 10 | 258 | 69.88 | 0.07 | 0.17 | 22.32 | 46.5 | 66.61 | 8742.56 |
| 10 | 280 | 70.77 | 0.07 | 0.32 | 22.66 | 52.38 | 76.66 | 10061.63 |
| 100 | 215 | 72.77 | 0.07 | 0.09 | 22.72 | 24.23 | 35.20 | 4620.00 |
| 100 | 240 | 72.86 | 0.08 | 0.13 | 22.81 | 32.17 | 47.02 | 6171.38 |
| 100 | 261 | 72.92 | 0.07 | 0.15 | 22.58 | 44.72 | 64.74 | 8497.13 |
| 100 | 281 | 73.01 | 0.07 | 0.29 | 22.66 | 52.04 | 76.06 | 9982.88 |
| 1000 | 222 | 73.21 | 0.08 | 0.10 | 24.06 | 22.32 | 34.35 | 4508.44 |
| 1000 | 243 | 74.28 | 0.08 | 0.12 | 24.31 | 31.02 | 48.27 | 6335.44 |
| 1000 | 265 | 75.18 | 0.07 | 0.15 | 24.58 | 41.28 | 65.00 | 8531.25 |
| 1000 | 284 | 75.15 | 0.07 | 0.28 | 24.47 | 51.18 | 80.65 | 10585.31 |
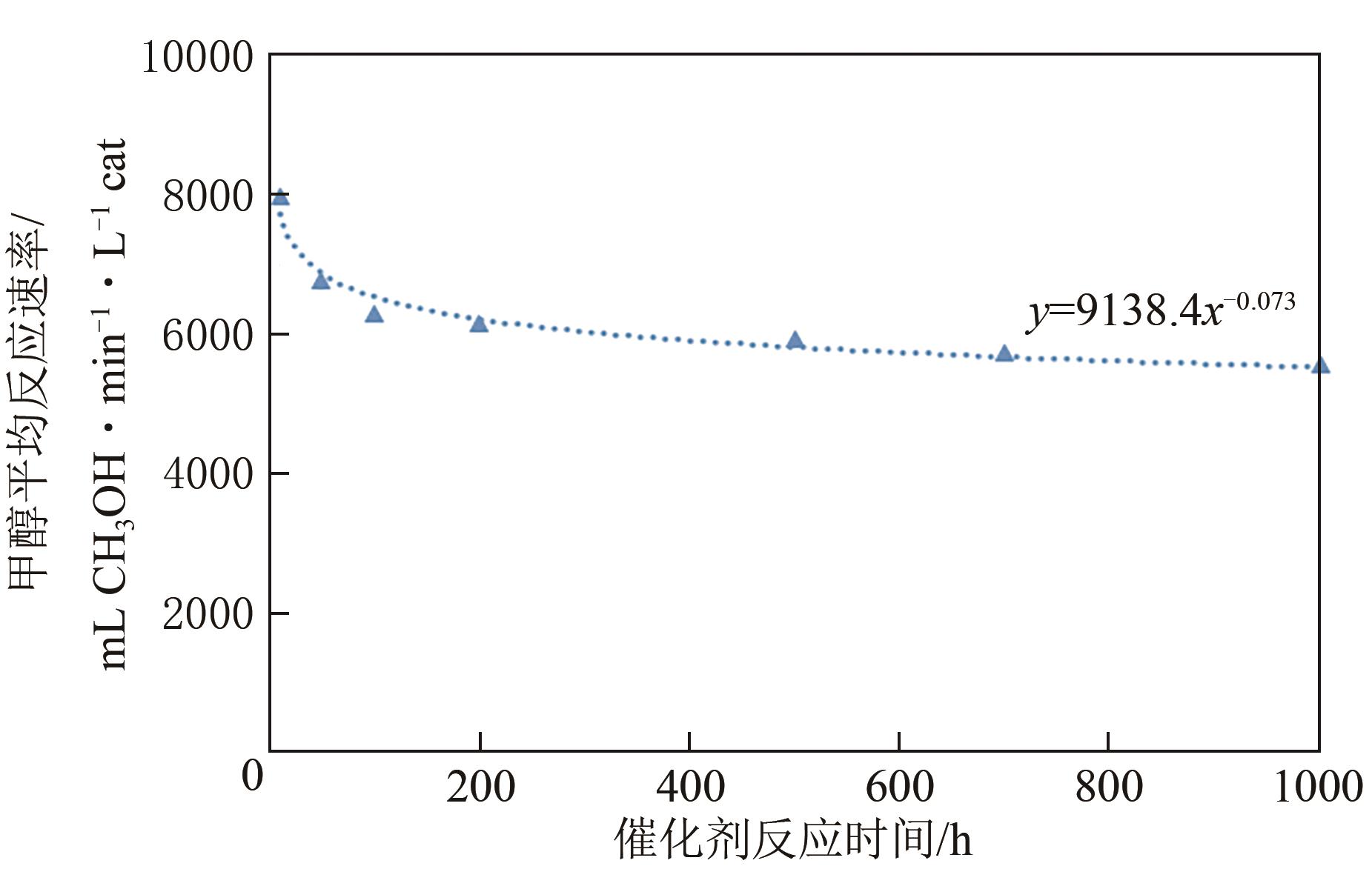
| 项目 | 甲醇平均反应速率/mL CH3OH·min-1·L-1 cat |
|---|---|
| 反应10h(BOL) | 7974.14 |
| 反应100h | 6287.35 |
| 反应1000h | 5566.87 |
| 评估20000h(EOL) | 4434.82 |
| 性能衰减(EOL/BOL) | 0.56 |
| 项目 | 甲醇平均反应速率/mL CH3OH·min-1·L-1 cat |
|---|---|
| 反应10h(BOL) | 7974.14 |
| 反应100h | 6287.35 |
| 反应1000h | 5566.87 |
| 评估20000h(EOL) | 4434.82 |
| 性能衰减(EOL/BOL) | 0.56 |
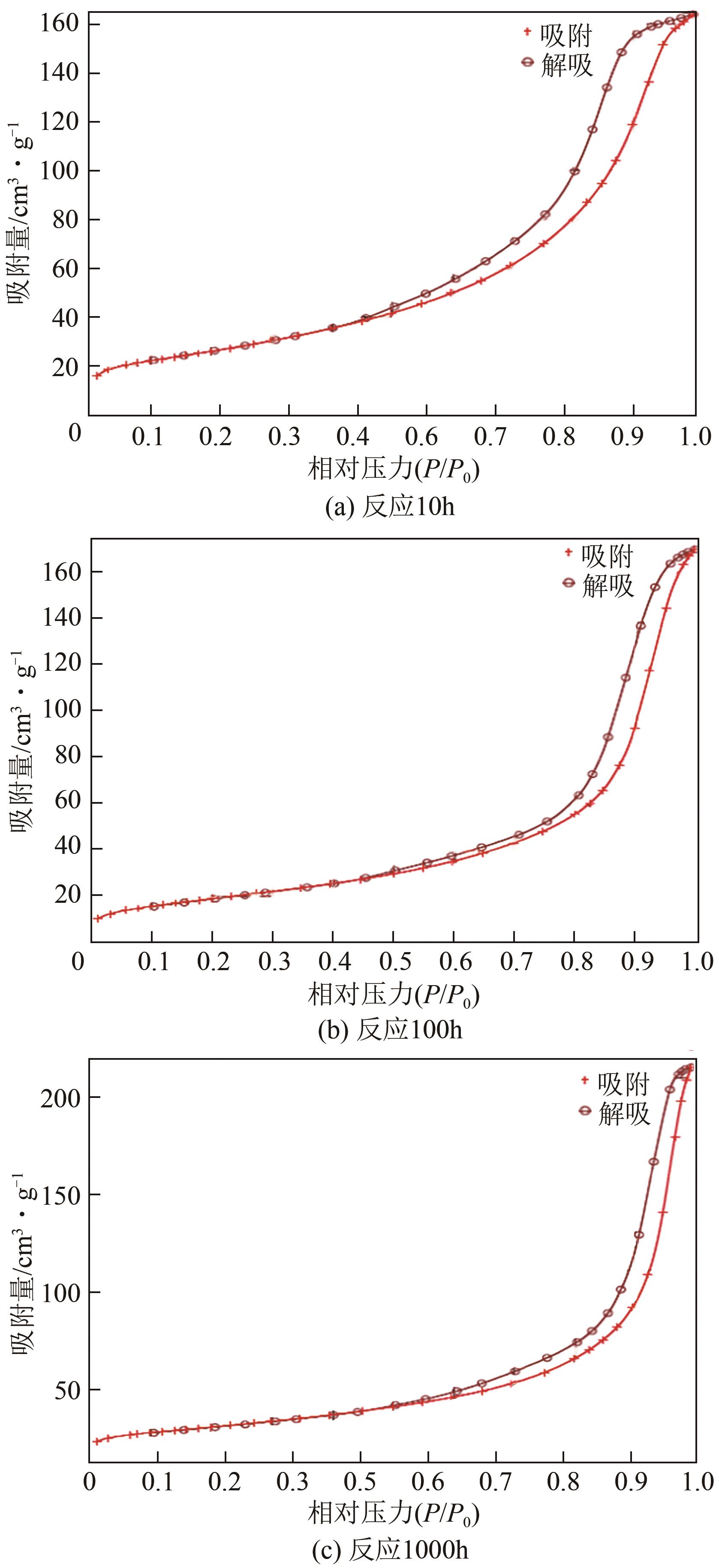
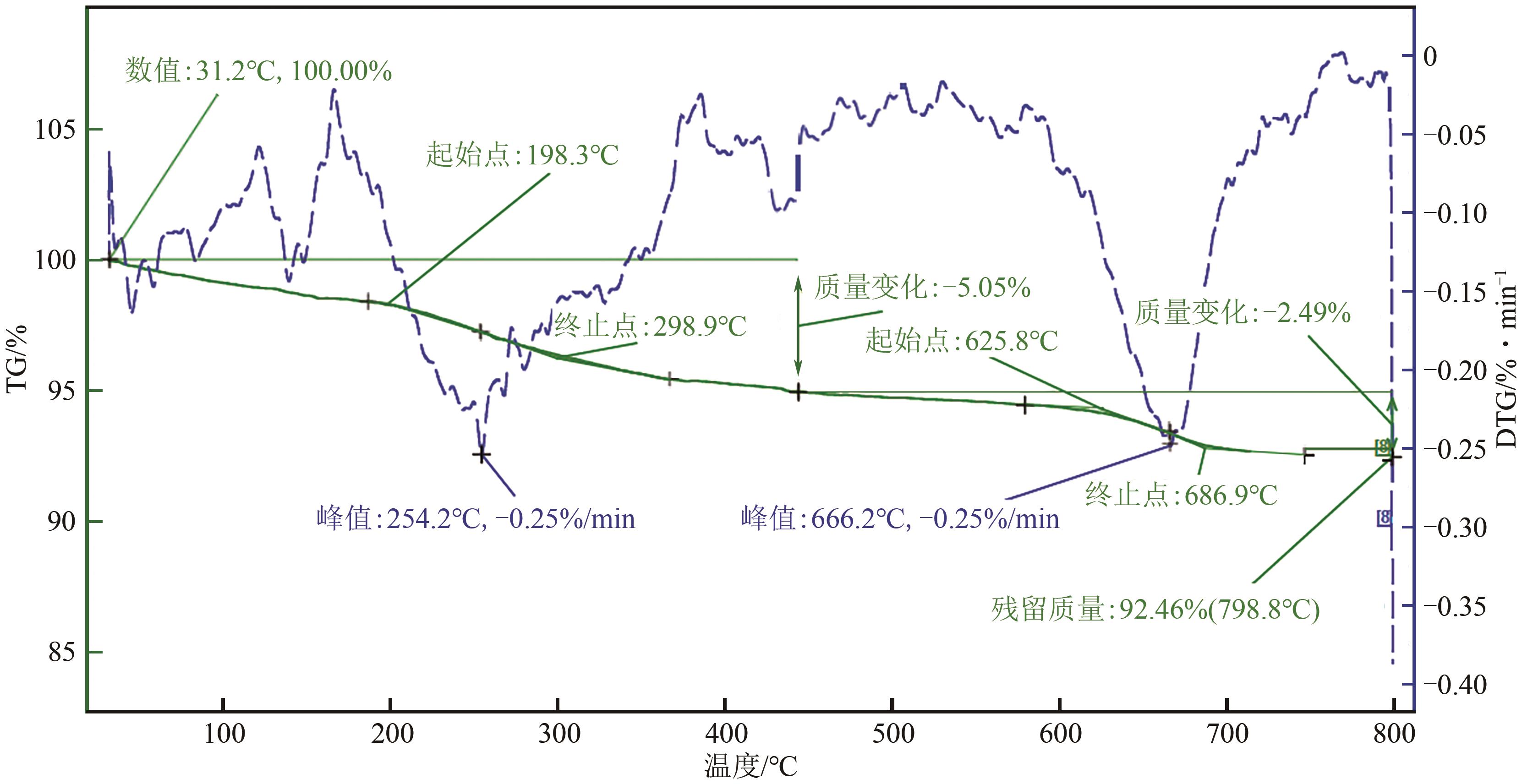
| 催化剂 | 比表面积(BET)/m2·g-1 |
|---|---|
| 未使用过的催化剂(fresh) | 94.21 |
| 反应10h(BOL) | 92.73 |
| 反应100h | 70.27 |
| 反应1000h | 66.84 |
| 催化剂 | 比表面积(BET)/m2·g-1 |
|---|---|
| 未使用过的催化剂(fresh) | 94.21 |
| 反应10h(BOL) | 92.73 |
| 反应100h | 70.27 |
| 反应1000h | 66.84 |
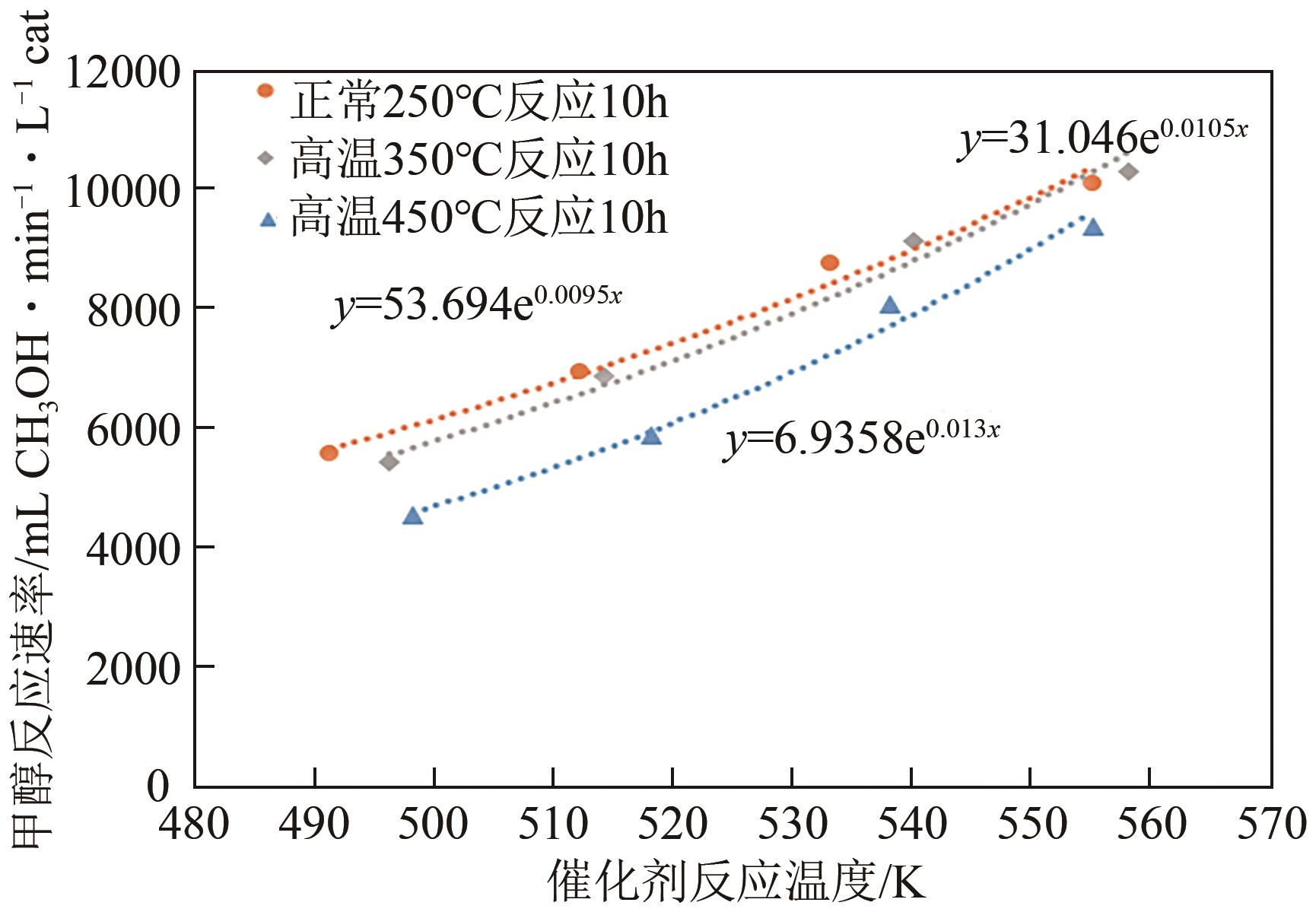
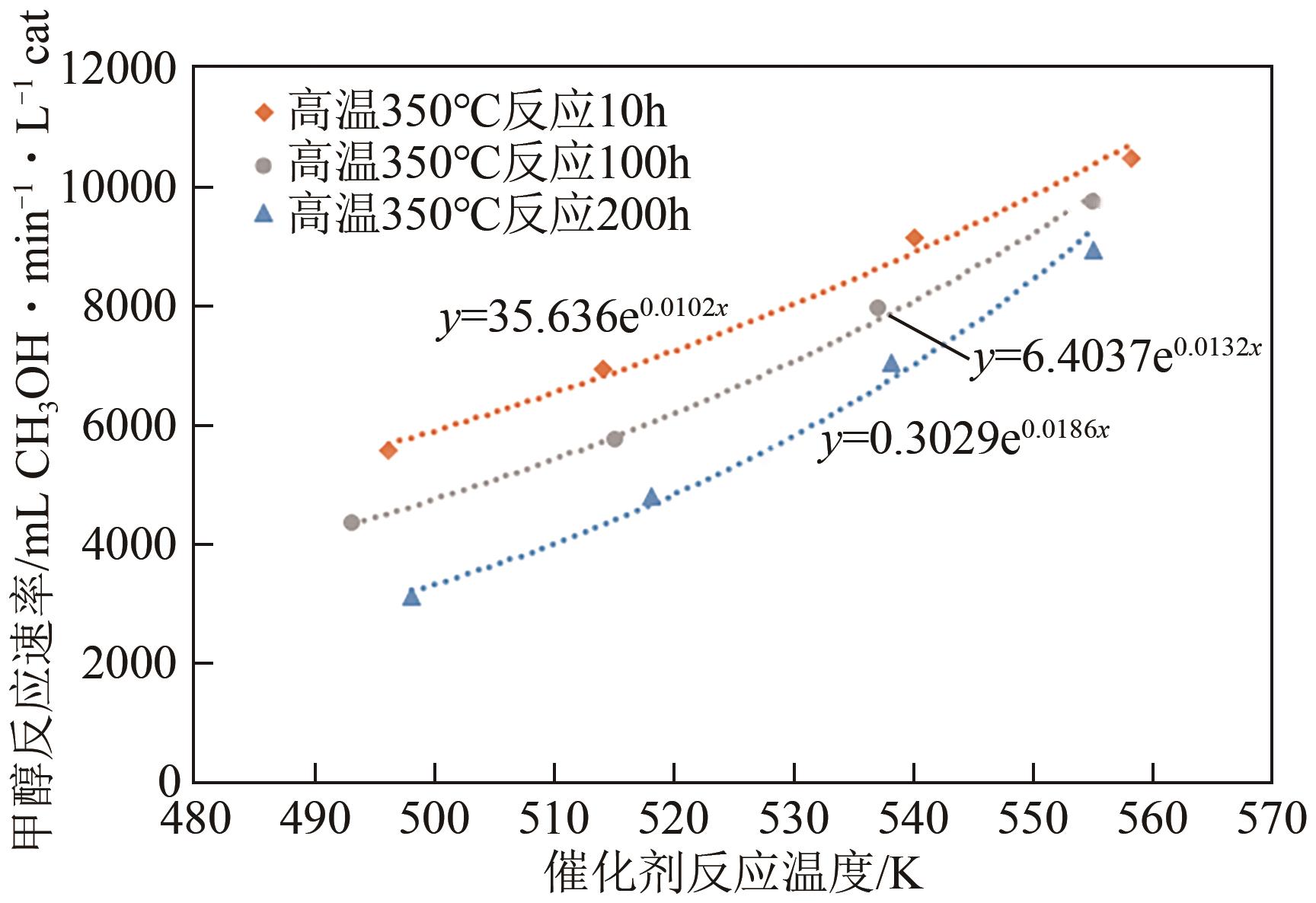
| 催化剂 | 比表面积(BET)/m2·g-1 |
|---|---|
| 未使用过的催化剂(fresh) | 94.21 |
| 250℃反应10h | 92.73 |
| 350℃反应10h | 87.86 |
| 350℃反应200h | 46.35 |
| 450℃反应10h | 54.13 |
| 催化剂 | 比表面积(BET)/m2·g-1 |
|---|---|
| 未使用过的催化剂(fresh) | 94.21 |
| 250℃反应10h | 92.73 |
| 350℃反应10h | 87.86 |
| 350℃反应200h | 46.35 |
| 450℃反应10h | 54.13 |
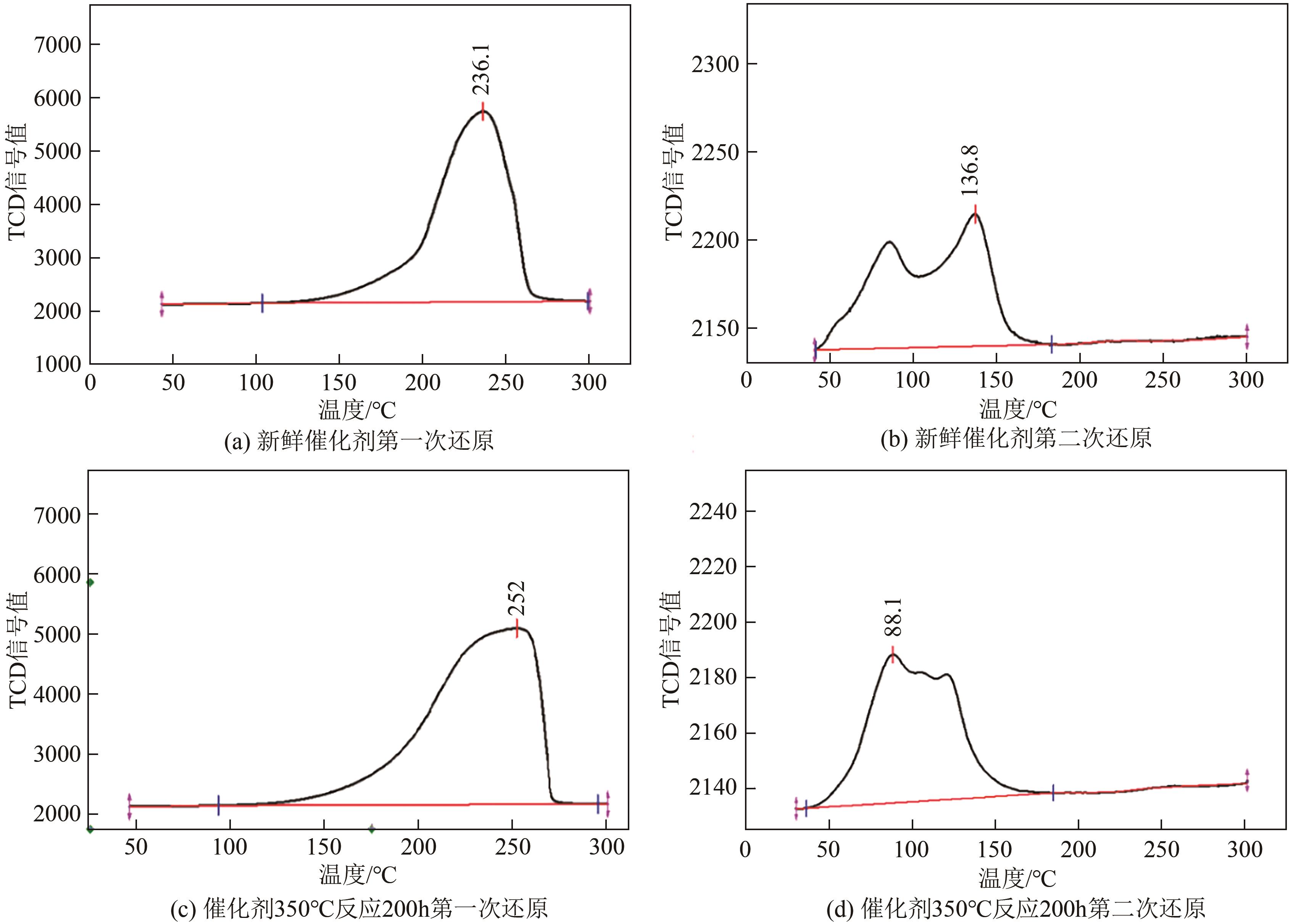
| 催化剂 | Cu晶粒比表面积/m2·g-1 | Cu晶粒的分散度 |
|---|---|---|
| 未使用过的催化剂(fresh) | 15.60 | 5.1 |
| 350℃反应 | 9.99 | 3.3 |
| 催化剂 | Cu晶粒比表面积/m2·g-1 | Cu晶粒的分散度 |
|---|---|---|
| 未使用过的催化剂(fresh) | 15.60 | 5.1 |
| 350℃反应 | 9.99 | 3.3 |
| 1 | SAAFI Mohamed ALI, Shiqi OU, JIANG Yilan, et al. Exploring the potential of hydrogen in decarbonizing China’s light-duty vehicle market[J]. International Journal of Hydrogen Energy, 2022, 47(86): 36355-36371. |
| 2 | LI Yanfei, SHI Xunpeng, PHOUMIN Han. A strategic roadmap for large-scale green hydrogen demonstration and commercialisation in China: A review and survey analysis[J]. International Journal of Hydrogen Energy, 2022, 47(58): 24592-24609. |
| 3 | 许家珩, 李永胜, 罗春欢, 等. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| XU Jiaheng, LI Yongsheng, LUO Chunhuan, et al. Optimization of methanol steam reforming process[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. | |
| 4 | FAYE Omar, SZPUNAR Jerzy, EDUOK Ubong. A critical review on the current technologies for the generation, storage, and transportation of hydrogen[J]. International Journal of Hydrogen Energy, 2022, 47(29): 13771-13802. |
| 5 | 潘伟滔, 周阳, 袁逸军, 等. 氢气储存技术发展现状分析及展望[J]. 城市燃气, 2022(8): 26-31. |
| PAN Weitao, ZHOU Yang, YUAN Yijun, et al. Analysis and prospect of hydrogen storage technology development[J]. Urban Gas, 2022(8): 26-31. | |
| 6 | 李林, 刘彤宇, 李爽, 等. 甲醇重整制氢燃料电池发电研究进展[J]. 发电技术, 2022, 43(1): 44-53. |
| LI Lin, LIU Tongyu, LI Shuang, et al. Research progress of hydrogen production by methanol reforming for fuel cell power generation[J]. Power Generation Technology, 2022, 43(1): 44-53. | |
| 7 | LI Haozhen, MA Chao, ZOU Xinyao, et al. On-board methanol catalytic reforming for hydrogen production—A review[J]. International Journal of Hydrogen Energy, 2021, 46(43): 22303-22327. |
| 8 | 庄晓如, 徐心海, 夏鑫, 等. 甲醇蒸汽重整制氢反应动力学研究进展[J]. 化工进展, 2020, 39(1): 152-165. |
| ZHUANG Xiaoru, XU Xinhai, XIA Xin, et al. Review of reaction kinetics of methanol steam reforming for hydrogen production[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 152-165. | |
| 9 | 吕祎壮, 张冰姿, 许征兵, 等. 甲醇水蒸气重整制氢CuO-ZnO-Al2O3催化剂的制备与性能[J]. 工业催化, 2022, 30(1): 56-62. |
| Yizhuang LYU, ZHANG Bingzi, XU Zhengbing, et al. Preparation and properties of CuO-ZnO-Al2O3 catalyst for hydrogen production by methanol steam reforming[J]. Industrial Catalysis, 2022, 30(1): 56-62. | |
| 10 | 孙朝, 孙志强. 应用于燃料电池的甲醇重整制氢研究综述[J].中南大学学报, 2020, 27(4): 1074-1103. |
| SUN Zhao, SUN Zhiqiang. Hydrogen generation from methanol reforming for fuel cell applications: A review[J]. Journal of Central South University, 2020, 27(4): 1074-1103. | |
| 11 | 闫月君, 刘启斌, 隋军, 等. 甲醇水蒸气催化重整制氢技术研究进展[J]. 化工进展, 2012, 31(7): 1468-1476. |
| YAN Yuejun, LIU Qibin, SUI Jun, et al. Research progress of hydrogen production with methanol steam reforming[J]. Chemical Industry and Engineering Progress, 2012, 31(7): 1468-1476. | |
| 12 | YU Hangyu, LI Yuanzhi, XU Chao, et al. Distinct facets to enhance the process of hydrogen production via methanol steam reforming—A review[J]. Energy Storage and Saving, 2022, 1(1): 53-69. |
| 13 | 黄媛媛, 巢磊, 李工, 等. Cu-ZrO2-CeO2/γ-Al2O3催化甲醇水蒸气重整制氢反应的性能[J]. 化工进展, 2017, 36(1): 216-223. |
| HUANG Yuanyuan, CHAO Lei, LI Gong, et al. Performance of Cu-ZrO2-CeO2/γ-Al2O3 catalysts for hydrogen production from steam reforming of methanol[J]. Chemical Industry and Engineering Progress, 2017, 36(1): 216-223. | |
| 14 | MEI Deqing, QIU Xingye, LIU Haiyu, et al. Progress on methanol reforming technologies for highly efficient hydrogen production and applications[J]. International Journal of Hydrogen Energy, 2022, 47(84): 35757-35777. |
| 15 | 莫帆, 冯品铭, 陶祖珊, 等. 甲醇水蒸气重整制氢催化剂的研究[J]. 电源技术, 2022, 46(8): 838-841. |
| MO Fan, FENG Pinmimg, TAO Zushan, et al. Study on catalysts for hydrogen production from methanol steam reforming[J]. Chinese Journal of Power Sources, 2022, 46(8): 838-841. | |
| 16 | MATSUMURA Yasuyuki. Durable Cu composite catalyst for hydrogen production by high temperature methanol steam reforming[J]. Journal of Power Sources, 2014, 272: 961-969. |
| 17 | 周文强, 程载哲, 蓝国钧, 等. 甲醇蒸汽重整制氢催化剂的研究进展(下)[J]. 石油化工, 2022, 51(2): 199-205. |
| ZHOU Wenqiang, CHENG Zaizhe, LAN Guojun, et al. Research progress in catalysts for methanol steam reforming to hydrogen[J]. Petrochemical Technology, 2022, 51(2): 199-205. |
| [1] | ZHANG Xin’er, PEI Liujun, ZHOU Yudie, JIN Kaili, WANG Jiping. Progress of TiO2-based photocatalysts for hydrogen production by water splitting with solar energy [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1298-1308. |
| [2] | LIU Junjie, WU Jianmin, SUN Qiwen, WANG Jiancheng, SUN Yan. Research of metallocene catalysts for linear α-olefins polymerization to obtain high molecular weight products [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1309-1322. |
| [3] | HAN Yingna, LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao. Separation of methanol-acetonitrile azeotrope by ionic liquid extractive distillation [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 660-668. |
| [4] | HU Yang, HAN Chuanjun, HU Qiang, LI Wenying, AN Quancheng, SU Yang, WU Hongsong, YUAN Guo. Research progress on methanol steam reforming reactors for SOFC [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 169-183. |
| [5] | QIN Tingting, NIU Qiang. Research progress on Fe-based catalysts for CO2 hydrogenation to higher alcohols [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 253-265. |
| [6] | ZHOU Yu, TANG Tian, XIONG Ziyou, WEI Qi. Methanol to olefin wastewater treatment based on a two-stage microchannel separation process [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 100-108. |
| [7] | WANG Yue, ZHANG Xuerui, SONG Xiwen, CHEN Boyan, LI Qingxun, ZHONG Haijun, HU Xiaowei, HE Shuai. Overview and prospect of ammonia synthesis with hydrogen produced via water electrolysis [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 180-188. |
| [8] | LI Shuaizhe, NIE Yichen, PHIDJAVARD Keomeesay, GU Wen, ZHANG Wei, LIU Na, XU Gaoxiang, LIU Ying, LI Xingyong, CHEN Yubao. Research progress on non-precious metal-catalyzed hydrogenation and deoxygenation of biomass to produce hydrocarbon-based biofuels [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 225-242. |
| [9] | HAN Hongjing, CHE Yu, TIAN Yuxuan, WANG Haiying, ZHANG Yanan, CHEN Yanguang. Advances on catalysts and solvents for catalytic hydrogenolysis of lignin [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 315-324. |
| [10] | ZHOU Yu, XIA Taiyang, WEI Qi, TANG Tian, TIAN Lei. Optimization of micro-channel coupled reverse osmosis membrane series treatment of methanol to olefin wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 43-51. |
| [11] | LIU Zhentao, MEI Jinlin, WANG Chunya, DUAN Aijun, GONG Yanjun, XU Chunming, WANG Xilong. Development in catalysts for one-step hydrogenation of bio-jet fuels [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4909-4924. |
| [12] | LONG Tao, ZHOU Feng, ZHANG Wei, WU Hong, WANG Jian, CHEN Lin. Synthesis and modification of deuterated methanol catalyst used in CO-CO2 system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4411-4420. |
| [13] | HE Yixue, QIN Xianchao, MA Weifang. Research progress on in situ remediation of halogenated hydrocarbon contamination in groundwater by persulfate-based advanced oxidation process [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4072-4088. |
| [14] | GONG Decheng, SHEN Qian, ZHU Xianqing, HUANG Yun, XIA Ao, ZHANG Jingmiao, ZHU Xun, LIAO Qiang. Recent progress in the production of hydrogen-rich syngas via supercritical water gasification of microalgae [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3709-3728. |
| [15] | GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||