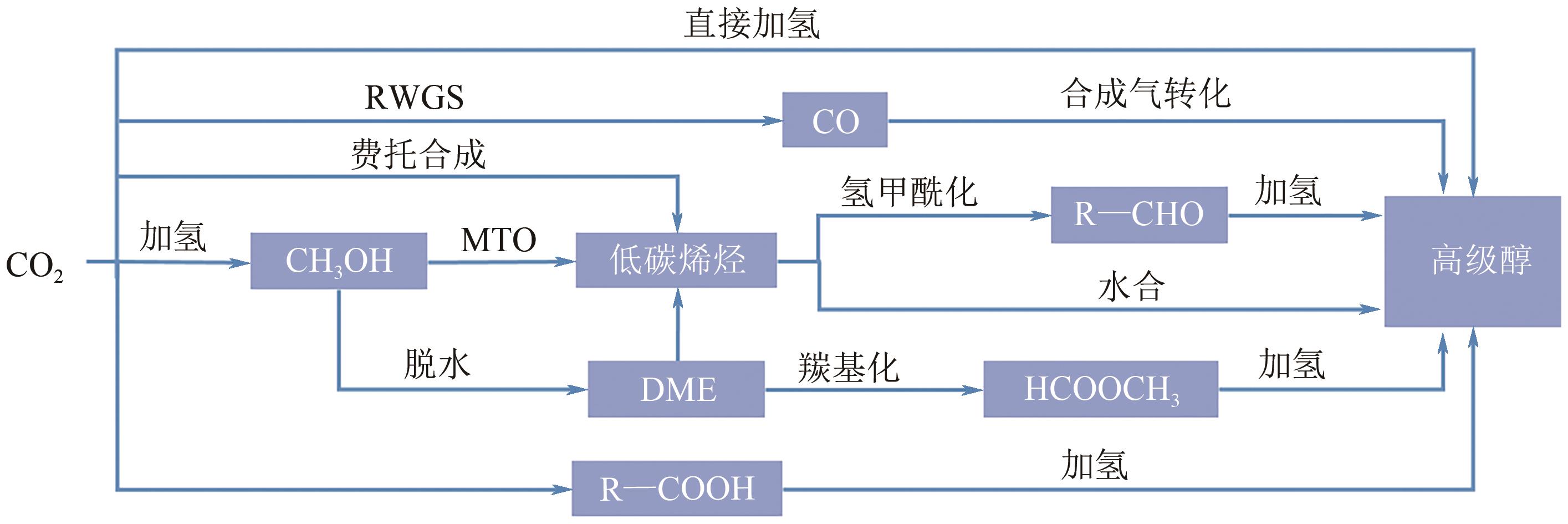
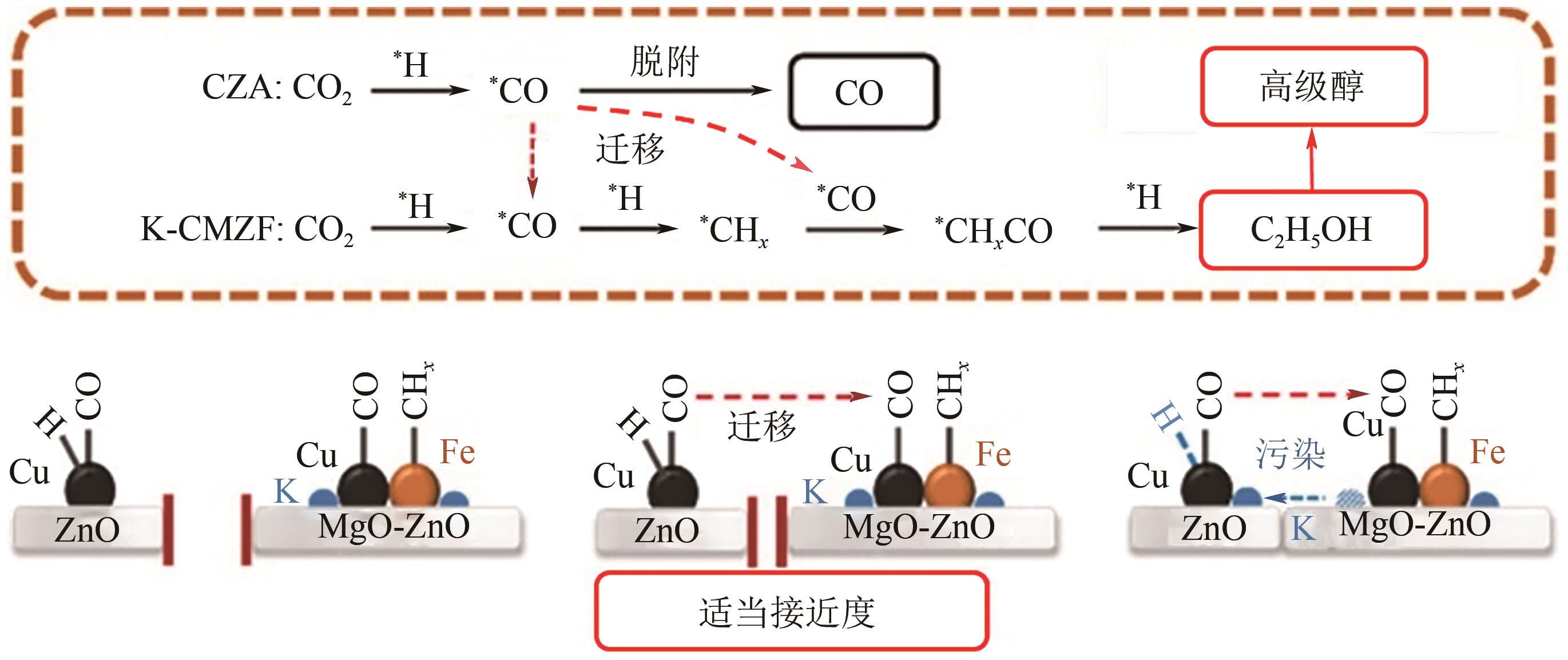
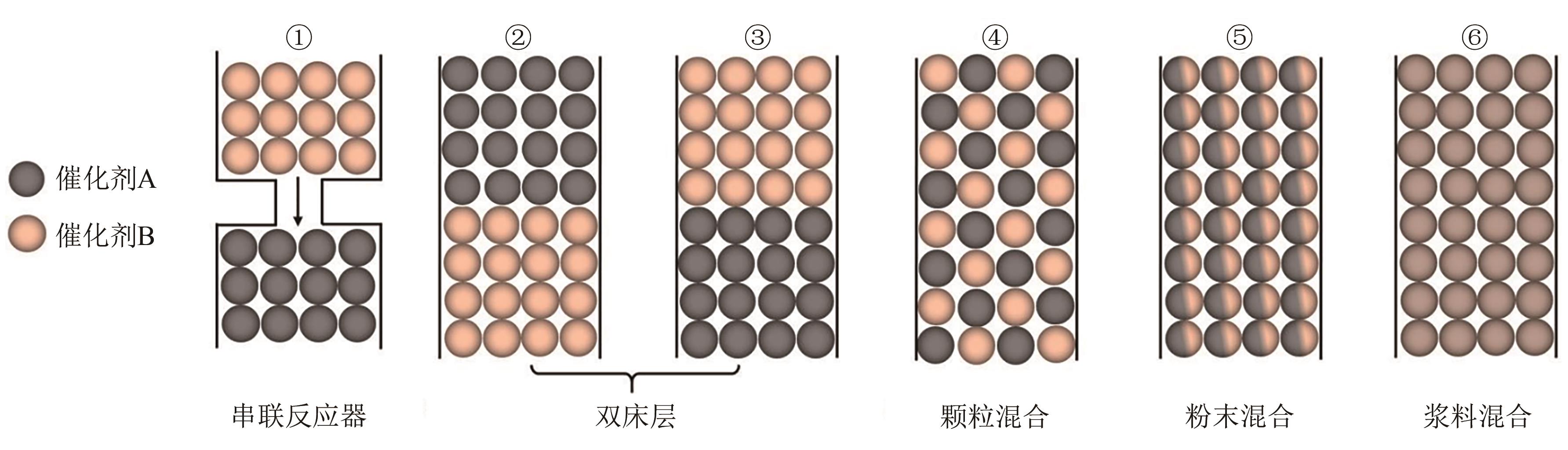
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (1): 253-265.DOI: 10.16085/j.issn.1000-6613.2024-0066
• Industrial catalysis • Previous Articles Next Articles
Research progress on Fe-based catalysts for CO2 hydrogenation to higher alcohols
- Inner Mongolia Erdos Electric Power and Metallurgy Group Co. , Ltd. , Ordos 016064, Inner Mongolia, China
-
Received:2024-01-09Revised:2024-06-11Online:2025-02-13Published:2025-01-15 -
Contact:NIU Qiang
二氧化碳加氢制高级醇Fe基催化剂研究进展
- 内蒙古鄂尔多斯电力冶金集团股份有限公司,内蒙古 鄂尔多斯 016064
-
通讯作者:牛强 -
作者简介:秦婷婷(1993—),女,博士,工程师,研究方向为C1催化转化、水处理技术开发。E-mail:qintingting1@chinaerdos.com。 -
基金资助:内蒙古自治区科技重大专项(2021ZD0042);内蒙古自治区科技计划(2023YFHH0043)
CLC Number:
Cite this article
QIN Tingting, NIU Qiang. Research progress on Fe-based catalysts for CO2 hydrogenation to higher alcohols[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 253-265.
秦婷婷, 牛强. 二氧化碳加氢制高级醇Fe基催化剂研究进展[J]. 化工进展, 2025, 44(1): 253-265.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0066
| 序号 | 反应方程 | ΔG298K/kJ·mol-1 | ΔH298K/kJ·mol-1 | K298K |
|---|---|---|---|---|
| (1) | CO2+H2 | 28.6 | 41.1 | 9.67×10-6 |
| (2) | CO+3H2 | -141.9 | -206.0 | — |
| (3) | CO2+4H2 | -113.5 | -165.0 | 7.79×1019 |
| (4) | CO+2H2 | — | -90.4 | — |
| (5) | CO2+3H2 | 3.5 | -49.3 | 2.45×10-1 |
| (6) | 2CH3OH | — | -24.52 | — |
| (7) | CH3OCH3+CO | — | — | — |
| (8) | CH3COOCH3+2H2 | — | — | — |
| (9) | CH3OH+CO+2H2 | -97.0 | -165.1 | — |
| (10) | 2CH4+H2O | — | — | — |
| (11) | C2H5OH→CH3CHO+H2 | — | — | — |
| (12) | 2CO+4H2 | -221.1 | -253.6 | — |
| (13) | 2CO2+6H2 | -32.4 | -86.7 | 4.70×105 |
| (14) | C2H5OH+3H2O | — | — | — |
| (15) | 2CO2+7H2 | -78.7 | -132.1 | 6.26×1013 |
| (16) | 3CO2+10H2 | -70.9 | -125.0 | 2.64×1012 |
| (17) | CH4 | — | 74.9 | — |
| (18) | 2CO | — | -172.5 | — |
| 序号 | 反应方程 | ΔG298K/kJ·mol-1 | ΔH298K/kJ·mol-1 | K298K |
|---|---|---|---|---|
| (1) | CO2+H2 | 28.6 | 41.1 | 9.67×10-6 |
| (2) | CO+3H2 | -141.9 | -206.0 | — |
| (3) | CO2+4H2 | -113.5 | -165.0 | 7.79×1019 |
| (4) | CO+2H2 | — | -90.4 | — |
| (5) | CO2+3H2 | 3.5 | -49.3 | 2.45×10-1 |
| (6) | 2CH3OH | — | -24.52 | — |
| (7) | CH3OCH3+CO | — | — | — |
| (8) | CH3COOCH3+2H2 | — | — | — |
| (9) | CH3OH+CO+2H2 | -97.0 | -165.1 | — |
| (10) | 2CH4+H2O | — | — | — |
| (11) | C2H5OH→CH3CHO+H2 | — | — | — |
| (12) | 2CO+4H2 | -221.1 | -253.6 | — |
| (13) | 2CO2+6H2 | -32.4 | -86.7 | 4.70×105 |
| (14) | C2H5OH+3H2O | — | — | — |
| (15) | 2CO2+7H2 | -78.7 | -132.1 | 6.26×1013 |
| (16) | 3CO2+10H2 | -70.9 | -125.0 | 2.64×1012 |
| (17) | CH4 | — | 74.9 | — |
| (18) | 2CO | — | -172.5 | — |
| 序号 | 催化剂 | 制备/混合方法 | 温度/℃ | 压力/MPa | H2/CO2 | GHSV | CO2转化率/% | C2+OH选择性/% | C2+OH收率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 30.4 | 15.8 | 70.6mg/(g·h) | [ |
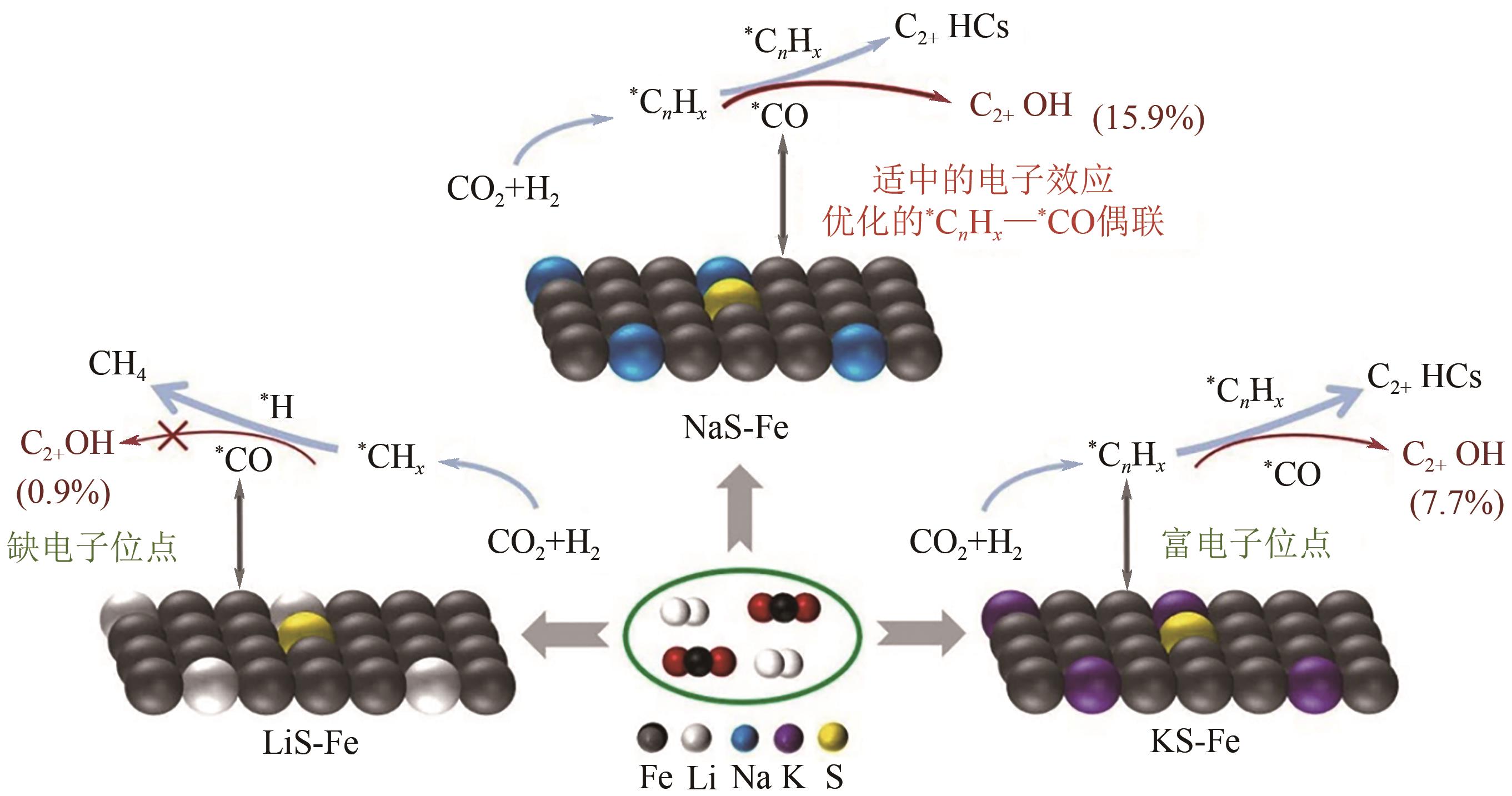
| 2 | K/S-CuFeZn | 共沉淀+浸渍 | 320 | 5 | 3 | 3000mL/(g·h) | 36.1 | 20.2 | 50.7mg/(g·h) | [ |
| 3 | 10Mn1K-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.5 | 10.7 | 4.3%① | [ |
| 4 | MnCuK-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.8 | 13.5 | 5.6%① | [ |
| 5 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 28.1 | 约15.7 | 69.6mg/(g·h) | [ |
| 6 | CuZnFe0.5K0.15 | 共沉淀+浸渍 | 300 | 6 | 3 | 5000mL/(g·h) | 42.3 | 36.7 | 170mg/(mL·h) | [ |
| 7 | K/Cu-Zn-Fe | 共沉淀 | 300 | 7 | 3 | 5000h-1 | 44.2 | 26.9 | 11.9%① | [ |
| 8 | K/Cu-Zn-Fe | 共沉淀+浸渍 | 300 | 7 | 3 | 5000mL/(g·h) | 45.0 | 19.0 | 8.6%① | [ |
| 9 | PDA/CuFeZn-N450 | 共沉淀 | 320 | 4 | 3 | 7200mL/(g·h) | 约7.0 | 41.5 | 55.9mg/(mL·h) | [ |
| 10 | CuFeZn-0.7PDA | 共沉淀 | 310 | 4 | 3 | 7200mL/(g·h) | 10.6 | 33.1 | 58.2mg/(mL·h) | [ |
| 11 | KFeCu/a-ZrO2 | 分步沉淀+浸渍 | 320 | 4 | 3 | 12000mL/(g·h) | 25.7 | 26.1 | 125.0mg/(g·h) | [ |
| 12 | K-0.82-FeIn/Ce-ZrO2 | 浸渍 | 300 | 10 | 3 | 4500mL/(g·h) | 29.6 | 28.7 | 8.5%① | [ |
| 13 | (K2O)5%/CuZnFeZrO2 | 共沉淀+浸渍 | 320 | 3 | 3 | 3600mL/(g·h) | 25.5 | 11.0 | 2.55mmol/(mL·h) | [ |
| 14 | Fe-Cu-Al-K | 均匀凝胶法 | 330 | 8 | 3 | 20000h-1 | 41.4 | 11.4 | 4.7%① | [ |
| 15 | sp-CuNaFe | 物理溅射 | 310 | 3 | 3 | 28800mL/(g·h) | 32.3 | 约10.0 | 153.0mg/(g·h) | [ |
| 16 | Na-Fe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 25.5 | 12.0(乙醇) | 52.3mg/(g·h) | [ |
| 17 | Na-ZnFe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 38.4 | 20.3(乙醇) | 158.1mg/(g·h) | [ |
| 18 | 2%Na-Fe@C | 水热+热解+浸渍 | 320 | 5 | 3 | 9000mL/(g·h) | 32.8 | 16.3 | 5.3%① | [ |
| 19 | Cr(1%)-CuFe | 溶胶-凝胶法 | 320 | 4 | 3 | 6000mL/(g·h) | 38.4 | 29.2 | 104.1mg/(g·h) | [ |
| 20 | 1%Na-50Co50Fe | 草酸盐分解 | 270 | 0.92 | 2.5 | 2.0nL/(g·h) | 22.7 | 5.3 | 1.2%① | [ |
| 21 | 90Fe10Co1.0K | 草酸盐分解 | 240 | 1.2 | 3 | 1.5nL/(g·h) | 14.5 | 5.9 | 0.85%① | [ |
| 22 | Cs-Cu0.8Fe1.0Zn1.0 | 共沉淀 | 330 | 5 | 3 | 4500h-1 | 36.6 | 19.8 | 73.4mg/(g·h) | [ |
| 23 | FeNaS-0.6 | 沉淀 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 16.1 | 78.5mg/(g·h) | [ |
| 24 | NaS-Fe | 沉淀/洗涤 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 15.9 | 78.5mg/(g·h) | [ |
| 25 | Fe/Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 22.6 | 3.3 | 0.7%① | [ |
| 26 | Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 34.4 | 27.3 | 9.4%① | [ |
| 27 | In_2_Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 36.7 | 42.0 | 15.4%① | [ |
| 28 | CuZnFe1.0K/ATP-CZO | 浸渍 | 320 | 6 | 3 | 5000mL/(g·h) | 14.3 | 35.1(混合醇ROH) | 5.0%① | [ |
| 29 | Rh-Fe/SiO2 | 浸渍 | 260 | 5 | 3 | 6000mL/(g·h) | 26.7 | 16.0 | 4.3%① | [ |
| 30 | 2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 2.7 | 2.8 | 0.07%① | [ |
| 31 | 2%Rh-2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 9.2 | 6.4 | 0.6%① | [ |
| 32 | 2.5%RhFeLi/TiO2 | 浸渍 | 250 | 3 | 3 | 6000h-1 | 15.7 | 31.4 | 4.9%① | [ |
| 33 | 2.0K20Fe5Rh-SiO2 | 浸渍 | 250 | 5 | 3 | 7000mL/(g·h) | 18.4 | 15.9 | 2.9%① | [ |
| 34 | 0.1Pd/Fe3O4 | 浸渍 | 300 | 0.1 | 4 | 60000mL/(g·h) | 0.3 | 97.5 | 0.41mmol/(g·h) | [ |
| 35 | PdFe | 水热+浸渍 | 300 | 5 | 3 | 6000mL/(g·h) | 33.3 | 19.1 | 86.5mg/(g·h) | [ |
| 36 | 0.3K-1Pd/Fe2O3 | 浸渍 | 320 | 4 | 3 | 6000mL/(g·h) | 30.0 | 13.4 | 52.2mg/(g·h) | [ |
| 37 | Cu25Fe22Co3K3 | 浸渍 | 350 | 6 | 3 | 5000h-1 | 20.0 | 6.4(混合醇ROH) | 1.3%① | [ |
| 38 | CuZn1.0K0.15/Cu25Fe22Co3K3 | 共沉淀+浸渍 | 350 | 6 | 3 | 5000h-1 | 32.4 | 11.8(混合醇ROH) | 3.8%① | [ |
| 39 | CuZnAl/K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 42.3 | 17.4 | 106.5mg/(g·h) | [ |
| 40 | 4.7KCuFeZn/CuZnAlZr | 共沉淀+粉末混合 | 300 | 5 | 3 | 3000mL/(g·h) | 27.0 | 24.6 | 42.0mg/(g·h) | [ |
| 41 | 0.6S-KCFZ+CuZnAlZr | 共沉淀+浸渍+粉末混合 | 320 | 5 | 3 | 12000mL/(g·h) | 36.6 | 18.2 | 173.9mg/(g·h) | [ |
| 42 | Na-Fe@C/K-CuZnAl | 水热+热解+ 浸渍+共沉淀 | 320 | 5 | 3 | 4500mL/(g·h) | 39.2 | 35.0 | 12.4%① | [ |
| 43 | FeCuAlK+CuZnAlK | 均匀凝胶法+物理混合 | 330 | 8 | 3 | 20000h-1 | 39.5 | 15.8 | 6.2%① | [ |
| 44 | MnCuK-FeC(1)/CuZnAlZr(1) | 热分解+共沉淀 | 300 | 3 | 3 | 6000mL/(g·h) | 42.1 | 15.5 | 6.5%① | [ |
| 45 | ZnFe2O4/Fe-Zn-Na# | 有机物燃烧+共沉淀+浸渍 | 320 | 5 | 3 | 2000mL/(g·h) | 38.8 | 30.0 | 55.5mg/(g·h) | [ |
| 序号 | 催化剂 | 制备/混合方法 | 温度/℃ | 压力/MPa | H2/CO2 | GHSV | CO2转化率/% | C2+OH选择性/% | C2+OH收率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 30.4 | 15.8 | 70.6mg/(g·h) | [ |
| 2 | K/S-CuFeZn | 共沉淀+浸渍 | 320 | 5 | 3 | 3000mL/(g·h) | 36.1 | 20.2 | 50.7mg/(g·h) | [ |
| 3 | 10Mn1K-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.5 | 10.7 | 4.3%① | [ |
| 4 | MnCuK-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.8 | 13.5 | 5.6%① | [ |
| 5 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 28.1 | 约15.7 | 69.6mg/(g·h) | [ |
| 6 | CuZnFe0.5K0.15 | 共沉淀+浸渍 | 300 | 6 | 3 | 5000mL/(g·h) | 42.3 | 36.7 | 170mg/(mL·h) | [ |
| 7 | K/Cu-Zn-Fe | 共沉淀 | 300 | 7 | 3 | 5000h-1 | 44.2 | 26.9 | 11.9%① | [ |
| 8 | K/Cu-Zn-Fe | 共沉淀+浸渍 | 300 | 7 | 3 | 5000mL/(g·h) | 45.0 | 19.0 | 8.6%① | [ |
| 9 | PDA/CuFeZn-N450 | 共沉淀 | 320 | 4 | 3 | 7200mL/(g·h) | 约7.0 | 41.5 | 55.9mg/(mL·h) | [ |
| 10 | CuFeZn-0.7PDA | 共沉淀 | 310 | 4 | 3 | 7200mL/(g·h) | 10.6 | 33.1 | 58.2mg/(mL·h) | [ |
| 11 | KFeCu/a-ZrO2 | 分步沉淀+浸渍 | 320 | 4 | 3 | 12000mL/(g·h) | 25.7 | 26.1 | 125.0mg/(g·h) | [ |
| 12 | K-0.82-FeIn/Ce-ZrO2 | 浸渍 | 300 | 10 | 3 | 4500mL/(g·h) | 29.6 | 28.7 | 8.5%① | [ |
| 13 | (K2O)5%/CuZnFeZrO2 | 共沉淀+浸渍 | 320 | 3 | 3 | 3600mL/(g·h) | 25.5 | 11.0 | 2.55mmol/(mL·h) | [ |
| 14 | Fe-Cu-Al-K | 均匀凝胶法 | 330 | 8 | 3 | 20000h-1 | 41.4 | 11.4 | 4.7%① | [ |
| 15 | sp-CuNaFe | 物理溅射 | 310 | 3 | 3 | 28800mL/(g·h) | 32.3 | 约10.0 | 153.0mg/(g·h) | [ |
| 16 | Na-Fe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 25.5 | 12.0(乙醇) | 52.3mg/(g·h) | [ |
| 17 | Na-ZnFe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 38.4 | 20.3(乙醇) | 158.1mg/(g·h) | [ |
| 18 | 2%Na-Fe@C | 水热+热解+浸渍 | 320 | 5 | 3 | 9000mL/(g·h) | 32.8 | 16.3 | 5.3%① | [ |
| 19 | Cr(1%)-CuFe | 溶胶-凝胶法 | 320 | 4 | 3 | 6000mL/(g·h) | 38.4 | 29.2 | 104.1mg/(g·h) | [ |
| 20 | 1%Na-50Co50Fe | 草酸盐分解 | 270 | 0.92 | 2.5 | 2.0nL/(g·h) | 22.7 | 5.3 | 1.2%① | [ |
| 21 | 90Fe10Co1.0K | 草酸盐分解 | 240 | 1.2 | 3 | 1.5nL/(g·h) | 14.5 | 5.9 | 0.85%① | [ |
| 22 | Cs-Cu0.8Fe1.0Zn1.0 | 共沉淀 | 330 | 5 | 3 | 4500h-1 | 36.6 | 19.8 | 73.4mg/(g·h) | [ |
| 23 | FeNaS-0.6 | 沉淀 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 16.1 | 78.5mg/(g·h) | [ |
| 24 | NaS-Fe | 沉淀/洗涤 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 15.9 | 78.5mg/(g·h) | [ |
| 25 | Fe/Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 22.6 | 3.3 | 0.7%① | [ |
| 26 | Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 34.4 | 27.3 | 9.4%① | [ |
| 27 | In_2_Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 36.7 | 42.0 | 15.4%① | [ |
| 28 | CuZnFe1.0K/ATP-CZO | 浸渍 | 320 | 6 | 3 | 5000mL/(g·h) | 14.3 | 35.1(混合醇ROH) | 5.0%① | [ |
| 29 | Rh-Fe/SiO2 | 浸渍 | 260 | 5 | 3 | 6000mL/(g·h) | 26.7 | 16.0 | 4.3%① | [ |
| 30 | 2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 2.7 | 2.8 | 0.07%① | [ |
| 31 | 2%Rh-2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 9.2 | 6.4 | 0.6%① | [ |
| 32 | 2.5%RhFeLi/TiO2 | 浸渍 | 250 | 3 | 3 | 6000h-1 | 15.7 | 31.4 | 4.9%① | [ |
| 33 | 2.0K20Fe5Rh-SiO2 | 浸渍 | 250 | 5 | 3 | 7000mL/(g·h) | 18.4 | 15.9 | 2.9%① | [ |
| 34 | 0.1Pd/Fe3O4 | 浸渍 | 300 | 0.1 | 4 | 60000mL/(g·h) | 0.3 | 97.5 | 0.41mmol/(g·h) | [ |
| 35 | PdFe | 水热+浸渍 | 300 | 5 | 3 | 6000mL/(g·h) | 33.3 | 19.1 | 86.5mg/(g·h) | [ |
| 36 | 0.3K-1Pd/Fe2O3 | 浸渍 | 320 | 4 | 3 | 6000mL/(g·h) | 30.0 | 13.4 | 52.2mg/(g·h) | [ |
| 37 | Cu25Fe22Co3K3 | 浸渍 | 350 | 6 | 3 | 5000h-1 | 20.0 | 6.4(混合醇ROH) | 1.3%① | [ |
| 38 | CuZn1.0K0.15/Cu25Fe22Co3K3 | 共沉淀+浸渍 | 350 | 6 | 3 | 5000h-1 | 32.4 | 11.8(混合醇ROH) | 3.8%① | [ |
| 39 | CuZnAl/K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 42.3 | 17.4 | 106.5mg/(g·h) | [ |
| 40 | 4.7KCuFeZn/CuZnAlZr | 共沉淀+粉末混合 | 300 | 5 | 3 | 3000mL/(g·h) | 27.0 | 24.6 | 42.0mg/(g·h) | [ |
| 41 | 0.6S-KCFZ+CuZnAlZr | 共沉淀+浸渍+粉末混合 | 320 | 5 | 3 | 12000mL/(g·h) | 36.6 | 18.2 | 173.9mg/(g·h) | [ |
| 42 | Na-Fe@C/K-CuZnAl | 水热+热解+ 浸渍+共沉淀 | 320 | 5 | 3 | 4500mL/(g·h) | 39.2 | 35.0 | 12.4%① | [ |
| 43 | FeCuAlK+CuZnAlK | 均匀凝胶法+物理混合 | 330 | 8 | 3 | 20000h-1 | 39.5 | 15.8 | 6.2%① | [ |
| 44 | MnCuK-FeC(1)/CuZnAlZr(1) | 热分解+共沉淀 | 300 | 3 | 3 | 6000mL/(g·h) | 42.1 | 15.5 | 6.5%① | [ |
| 45 | ZnFe2O4/Fe-Zn-Na# | 有机物燃烧+共沉淀+浸渍 | 320 | 5 | 3 | 2000mL/(g·h) | 38.8 | 30.0 | 55.5mg/(g·h) | [ |
| 1 | PETER Sebastian C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis[J]. ACS Energy Letters, 2018, 3(7): 1557-1561. |
| 2 | GAO Peng, ZHANG Lina, LI Shenggang, et al. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Central Science, 2020, 6(10): 1657-1670. |
| 3 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 4 | YE Runping, DING Jie, GONG Weibo, et al. CO2 hydrogenation to high-value products via heterogeneous catalysis[J]. Nature Communications, 2019, 10(1): 5698. |
| 5 | ZHANG Shunan, WU Zhaoxuan, LIU Xiufang, et al. A short review of recent advances in direct CO2 hydrogenation to alcohols[J]. Topics in Catalysis, 2021, 64(5): 371-394. |
| 6 | MURTHY Pradeep S, LIANG Weibin, JIANG Yijiao, et al. Cu-based nanocatalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2021, 35(10): 8558-8584. |
| 7 | BOWKER Michael. Methanol synthesis from CO2 hydrogenation[J]. ChemCatChem, 2019, 11(17): 4238-4246. |
| 8 | LI Zelong, WANG Jijie, QU Yuanzhi, et al. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catalysis, 2017, 7(12): 8544-8548. |
| 9 | LI Zelong, QU Yuanzhi, WANG Jijie, et al. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts[J]. Joule, 2019, 3(2): 570-583. |
| 10 | CUI Xu, GAO Peng, LI Shenggang, et al. Selective production of aromatics directly from carbon dioxide hydrogenation[J]. ACS Catalysis, 2019, 9(5): 3866-3876. |
| 11 | NI Youming, CHEN Zhiyang, FU Yi, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nature Communications, 2018, 9(1): 3457. |
| 12 | WEI Jian, GE Qingjie, YAO Ruwei, et al. Directly converting CO2 into a gasoline fuel[J]. Nature Communications, 2017, 8: 15174. |
| 13 | PRIETO Gonzalo. Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: Thermodynamic and kinetic bounds and progress with heterogeneous and homogeneous catalysis[J]. ChemSusChem, 2017, 10(6): 1056-1070. |
| 14 | GAO Peng, LI Shenggang, BU Xianni, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nature Chemistry, 2017, 9(10): 1019-1024. |
| 15 | ZENG Feng, MEBRAHTU Chalachew, XI Xiaoying, et al. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives[J]. Applied Catalysis B: Environmental, 2021, 291: 120073. |
| 16 | LATSIOU Angeliki I, CHARISIOU Nikolaos D, FRONTISTIS Zacharias, et al. CO2 hydrogenation for the production of higher alcohols: Trends in catalyst developments, challenges and opportunities[J]. Catalysis Today, 2023, 420: 114179. |
| 17 | XU Di, WANG Yanqiu, DING Mingyue, et al. Advances in higher alcohol synthesis from CO2 hydrogenation[J]. Chem, 2021, 7(4): 849-881. |
| 18 | LI Jiachen, WANG Liguo, CAO Yan, et al. Recent advances on the reduction of CO2 to important C2+ oxygenated chemicals and fuels[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2266-2279. |
| 19 | DELUGA G A, SALGE J R, SCHMIDT L D, et al. Renewable hydrogen from ethanol by autothermal reforming[J]. Science, 2004, 303(5660): 993-997. |
| 20 | LATIF Mohd Nor, WAN ISAHAK Wan Nor Roslam, SAMSURI Alinda, et al. Recent advances in the technologies and catalytic processes of ethanol production[J]. Catalysts, 2023, 13(7): 1093. |
| 21 | LAN Ethan I, LIAO James C. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources[J]. Bioresource Technology, 2013, 135: 339-349. |
| 22 | BAI F W, ANDERSON W A, MOO-YOUNG M. Ethanol fermentation technologies from sugar and starch feedstocks[J]. Biotechnology Advances, 2008, 26(1): 89-105. |
| 23 | HE Xinyi. CO2 hydrogenation for ethanol production: A thermodynamic analysis[J]. International Journal of Oil, Gas and Coal Engineering, 2017, 5(6): 145-152. |
| 24 | AZIZ M A A, JALIL A A, TRIWAHYONO S, et al. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects[J]. Green Chemistry, 2015, 17(5): 2647-2663. |
| 25 | JIANG Yongjun, WANG Kangzhou, WANG Yuan, et al. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway[J]. Journal of CO2 Utilization, 2023, 67: 102321. |
| 26 | NIE Xiaowa, LI Wenhui, JIANG Xiao, et al. Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons[M]// Advances in Catalysis. Amsterdam: Elsevier, 2019, 65: 121-233. |
| 27 | LI Xiaopeng, KE Jucang, LI Rui, et al. Research progress of hydrogenation of carbon dioxide to ethanol[J]. Chemical Engineering Science, 2023, 282: 119226. |
| 28 | STANGELAND Kristian, LI Hailong, YU Zhixin. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols[J]. Industrial & Engineering Chemistry Research, 2018, 57(11): 4081-4094. |
| 29 | KANGVANSURA Praewpilin, CHEW Ly May, SAENGSUI Worasarit, et al. Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N-functionalized carbon nanotubes[J]. Catalysis Today, 2016, 275: 59-65. |
| 30 | TAKAGAWA Makoto, OKAMOTO Atsushi, FUJIMURA Hiromitsu, et al. Ethanol synthesis from carbon dioxide and hydrogen[J]. Studies in Surface Science and Catalysis, 1998, 114: 525-528. |
| 31 | INUI Tomoyuki, YAMAMOTO Tetsuo, INOUE Masahito, et al. Highly effective synthesis of ethanol by CO2-hydrogenation on well balanced multi-functional FT-type composite catalysts[J]. Applied Catalysis A: General, 1999, 186(1/2): 395-406. |
| 32 | HE Yiming, MÜLLER Fabian H, PALKOVITS Regina, et al. Tandem catalysis for CO2 conversion to higher alcohols: A review[J]. Applied Catalysis B: Environment and Energy, 2024, 345: 123663. |
| 33 | KUSAMA Hitoshi, OKABE Kiyomi, SAYAMA Kazuhiro, et al. Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-Fe/SiO2 catalysts[J]. Energy, 1997, 22(2/3): 343-348. |
| 34 | YANG Chengsheng, MU Rentao, WANG Guishuo, et al. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation[J]. Chemical Science, 2019, 10(11): 3161-3167. |
| 35 | GORYACHEV Andrey, PUSTOVARENKO Alexey, SHTERK Genrikh, et al. A multi-parametric catalyst screening for CO2 hydrogenation to ethanol[J]. ChemCatChem, 2021, 13(14): 3324-3332. |
| 36 | GOGATE Makarand R, DAVIS Robert J. Comparative study of CO and CO2 hydrogenation over supported Rh-Fe catalysts[J]. Catalysis Communications, 2010, 11(10): 901-906. |
| 37 | CHEN Yuan, CHOI Saemin, THOMPSON Levi T. Low temperature CO2 hydrogenation to alcohols and hydrocarbons over Mo2C supported metal catalysts[J]. Journal of Catalysis, 2016, 343: 147-156. |
| 38 | CAPARRÓS Francisco J, SOLER Lluís, ROSSELL Marta D, et al. Remarkable carbon dioxide hydrogenation to ethanol on a palladium/iron oxide single-atom catalyst[J]. ChemCatChem, 2018, 10(11): 2365-2369. |
| 39 | LI Shanggui, GUO Haijun, LUO Cairong, et al. Effect of iron promoter on structure and performance of K/Cu-Zn catalyst for higher alcohols synthesis from CO2 hydrogenation[J]. Catalysis Letters, 2013, 143(4): 345-355. |
| 40 | GNANAMANI Muthu Kumaran, JACOBS Gary, HAMDEH Hussein H, et al. Hydrogenation of carbon dioxide over Co-Fe bimetallic catalysts[J]. ACS Catalysis, 2016, 6(2): 913-927. |
| 41 | GNANAMANI Muthu Kumaran, HAMDEH Hussein H, JACOBS Gary, et al. Hydrogenation of carbon dioxide over K-promoted FeCo bimetallic catalysts prepared from mixed metal oxalates[J]. ChemCatChem, 2017, 9(7): 1303-1312. |
| 42 | DING Fanshu, ZHANG Anfeng, LIU Min, et al. CO2 hydrogenation to hydrocarbons over iron-based catalyst: Effects of physicochemical properties of Al2O3 supports[J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17563-17569. |
| 43 | WANG Yang, WANG Wenhang, HE Ruosong, et al. Carbon-based electron buffer layer on ZnO x -Fe5C2-Fe3O4 boosts ethanol synthesis from CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2023, 62(46): e202311786. |
| 44 | LU Fangxu, CHEN Xin, WANG Wen, et al. Adjusting the CO2 hydrogenation pathway via the synergic effects of iron carbides and iron oxides[J]. Catalysis Science & Technology, 2021, 11(23): 7694-7703. |
| 45 | VISCONTI Carlo Giorgio, MARTINELLI Michela, FALBO Leonardo, et al. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst[J]. Applied Catalysis B: Environmental, 2017, 200: 530-542. |
| 46 | LIU Junhui, ZHANG Guanghui, JIANG Xiao, et al. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons[J]. Catalysis Today, 2021, 371: 162-170. |
| 47 | DE SMIT Emiel, CINQUINI Fabrizio, BEALE Andrew M., et al. Stability and reactivity of ε-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: Controlling μC [J]. Journal of the American Chemical Society, 2010, 132(42): 14928-14941. |
| 48 | XU Di, YANG Hengquan, HONG Xinlin, et al. Tandem catalysis of direct CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2021, 11(15): 8978-8984. |
| 49 | WANG Yanqiu, ZHANG Xinxin, HONG Xinlin, et al. Sulfate-promoted higher alcohol synthesis from CO2 hydrogenation[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(27): 8980-8987. |
| 50 | HUANG Jiamin, ZHANG Guanghui, ZHU Jie, et al. Boosting the production of higher alcohols from CO2 and H2 over Mn- and K-modified iron carbide[J]. Industrial & Engineering Chemistry Research, 2022, 61(21): 7266-7274. |
| 51 | HUANG Jiamin, ZHANG Guanghui, WANG Mingrui, et al. The synthesis of higher alcohols from CO2 hydrogenation over Mn-Cu-K modified Fe5C2 and CuZnAlZr tandem catalysts[J]. Frontiers in Energy Research, 2023, 10: 995800. |
| 52 | XU Di, DING Mingyue, HONG Xinlin, et al. Mechanistic aspects of the role of K promotion on Cu-Fe-based catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. ACS Catalysis, 2020, 10(24): 14516-14526. |
| 53 | HIGUCHI Katsumi, HANEDA Yoko, TABATA Kenji, et al. A study for the durability of catalysts in ethanol synthesis by hydrogenation of carbon dioxide[J]. Studies in Surface Science and Catalysis, 1998, 114: 517-520. |
| 54 | JIA Yazhen, WANG Bin, WEN Yueli, et al. Mechanism of stability and deactivation of N-doped CuFeZn catalysts for C2+ alcohols synthesis by hydrogenation of CO2 [J]. Fuel Processing Technology, 2023, 250: 107901. |
| 55 | YANG Chen, WANG Bin, WEN Yueli, et al. Composition control of CuFeZn catalyst derived by PDA and its effect on synthesis of C2+ alcohols from CO2 [J]. Fuel, 2022, 327: 125055. |
| 56 | LIU Tangkang, XU Di, SONG Mengyang, et al. K-ZrO2 interfaces boost CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2023, 13(7): 4667-4674. |
| 57 | XI Xiaoying, ZENG Feng, ZHANG Heng, et al. CO2 hydrogenation to higher alcohols over K-promoted bimetallic Fe-In catalysts on a Ce-ZrO2 support[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(18): 6235-6249. |
| 58 | GUO Wei, GAO Wengui, WANG Hua, et al. Higher alcohols synthesis from CO2 hydrogenation over K2O-modified CuZnFeZrO2 catalysts[J]. Advanced Materials Research, 2013, 827: 20-24. |
| 59 | SI Zhiyan, WANG Linkai, HAN Yu, et al. Synthesis of alkene and ethanol in CO2 hydrogenation on a highly active sputtering CuNaFe catalyst[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(45): 14972-14979. |
| 60 | WANG Yang, WANG Kangzhou, ZHANG Baizhang, et al. Direct conversion of CO2 to ethanol boosted by intimacy-sensitive multifunctional catalysts[J]. ACS Catalysis, 2021, 11(18): 11742-11753. |
| 61 | ZHANG Qian, WANG Sen, GENG Rui, et al. Hydrogenation of CO2 to higher alcohols on an efficient Cr-modified CuFe catalyst[J]. Applied Catalysis B: Environmental, 2023, 337: 123013. |
| 62 | XU Di, DING Mingyue, HONG Xinlin, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catalysis, 2020, 10(9): 5250-5260. |
| 63 | YAO Ruwei, WEI Jian, GE Qingjie, et al. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation[J]. Applied Catalysis B: Environmental, 2021, 298: 120556. |
| 64 | YAO Ruwei, WU Bin, YU Yang, et al. Regulating the electronic property of iron catalysts for higher alcohols synthesis from CO2 hydrogenation[J]. Applied Catalysis B: Environment and Energy, 2024, 355: 124159. |
| 65 | GOUD Devender, CHURIPARD Sathyapal R, BAGCHI Debabrata, et al. Strain-enhanced phase transformation of iron oxide for higher alcohol production from CO2 [J]. ACS Catalysis, 2022, 12(18): 11118-11128. |
| 66 | GUO Haijun, DING Shuai, ZHANG Hairong, et al. Promotion effect of iron addition on the structure and CO2 hydrogenation performance of attapulgite/Ce0.75Zr0.25O2 nanocomposite supported Cu-ZnO based catalyst[J]. Molecular Catalysis, 2021, 513: 111820. |
| 67 | WANG Yanqiu, ZHOU Ying, ZHANG Xinxin, et al. PdFe alloy-Fe5C2 interfaces for efficient CO2 hydrogenation to higher alcohols[J]. Applied Catalysis B: Environmental and Energy, 2024, 345: 123691. |
| 68 | ZHOU Ying, WANG Yanqiu, LU Hanjun, et al. Highly dispersed K- and Pd-comodified Fe catalyst for CO2 hydrogenation to higher alcohols[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(8): 3322-3330. |
| 69 | GUO Haijun, LI Shanggui, PENG Fen, et al. Roles investigation of promoters in K/Cu-Zn catalyst and higher alcohols synthesis from CO2 hydrogenation over a novel two-stage bed catalyst combination system[J]. Catalysis Letters, 2014, 145(2): 620-630. |
| 70 | WANG Yanqiu, XU Di, ZHANG Xinxin, et al. Selective C2+ alcohol synthesis by CO2 hydrogenation via a reaction-coupling strategy[J]. Catalysis Science & Technology, 2022, 12(5): 1539-1550. |
| 71 | YANG Haiyan, WEI Zhangqian, ZHANG Jian, et al. Tuning the selectivity of CO2 hydrogenation to alcohols by crystal structure engineering[J]. Chem, 2024, 10: 2245-2265. |
| 72 | KIM Jun-Sik, LEE Sunmook, LEE Sang-Bong, et al. Performance of catalytic reactors for the hydrogenation of CO2 to hydrocarbons[J]. Catalysis Today, 2006, 115(1/2/3/4): 228-234. |
| 73 | DING Mingyue, TU Junling, QIU Minghuang, et al. Impact of potassium promoter on Cu-Fe based mixed alcohols synthesis catalyst[J]. Applied Energy, 2015, 138: 584-589. |
| 74 | SHOLEHA Novia Amalia, HOLILAH Holilah, BAHRUJI Hasliza, et al. Recent trend of metal promoter role for CO2 hydrogenation to C1 and C2+ products[J]. South African Journal of Chemical Engineering, 2023, 44: 14-30. |
| 75 | GNANAMANI Muthu Kumaran, JACOBS Gary, KEOGH Robert A, et al. Fischer-Tropsch synthesis: Effect of pretreatment conditions of cobalt on activity and selectivity for hydrogenation of carbon dioxide[J]. Applied Catalysis A: General, 2015, 499: 39-46. |
| 76 | INUI Tomoyuki, YAMAMOTO Tetsuo. Effective synthesis of ethanol from CO2 on polyfunctional composite catalysts[J]. Catalysis Today, 1998, 45(1/2/3/4): 209-214. |
| 77 | ZHANG Shunan, HUANG Chaojie, SHAO Zilong, et al. Revealing and regulating the complex reaction mechanism of CO2 hydrogenation to higher alcohols on multifunctional tandem catalysts[J]. ACS Catalysis, 2023, 13(5): 3055-3065. |
| 78 | BEDIAKO Bernard Baffour Asare, QIAN Qingli, HAN Buxin. Synthesis of C2+ chemicals from CO2 and H2 via C—C bond formation[J]. Accounts of Chemical Research, 2021, 54(10): 2467-2476. |
| 79 | 潞安集团CO2重整利用工业尾气生产20万吨乙醇项目预计 9月试车[EB/OL]. . |
| 80 | “ 5万吨/年乙烯多相氢甲酰化及其加氢制正丙醇工业化技术”通过科技成果鉴定[EB/OL]. . |
| 81 | JI Lei, LI Lei, JI Xuqiang, et al. Highly selective electrochemical reduction of CO2 to alcohols on an FeP nanoarray[J]. Angewandte Chemie International Edition, 2020, 59(2): 758-762. |
| 82 | JI Lei, CHANG Le, ZHANG Ya, et al. Electrocatalytic CO2 reduction to alcohols with high selectivity over a two-dimensional Fe2P2S6 nanosheet[J]. ACS Catalysis, 2019, 9(11): 9721-9725. |
| 83 | AMPELLI C, GENOVESE C, PASSALACQUA R, et al. The use of a solar photoelectrochemical reactor for sustainable production of energy[J]. Theoretical Foundations of Chemical Engineering, 2012, 46(6): 651-657. |
| 84 | ARRIGO Rosa, SCHUSTER Manfred E, WRABETZ Sabine, et al. New insights from microcalorimetry on the FeO x /CNT-based electrocatalysts active in the conversion of CO2 to fuels[J]. ChemSusChem, 2012, 5(3): 577-586. |
| 85 | KALIYAPERUMAL Alamelu, GUPTA Pooja, PRASAD Yadavalli Satya Sivaram, et al. Recent progress and perspective of the electrochemical conversion of carbon dioxide to alcohols[J]. ACS Engineering Au, 2023, 3(6): 403-425. |
| 86 | LU Peng, CHEN Qingjun, YANG Guohui, et al. Space-confined self-regulation mechanism from a capsule catalyst to realize an ethanol direct synthesis strategy[J]. ACS Catalysis, 2020, 10(2): 1366-1374. |
| 87 | CHEN Jie, ZHA Yajun, LIU Bing, et al. Rationally designed water enriched nano reactor for stable CO2 hydrogenation with near 100% ethanol selectivity over diatomic palladium active sites[J]. ACS Catalysis, 2023, 13(10): 7110-7121. |
| [1] | DONG Jiatong, SHAN Mengqing, WANG Hua. Improved electrocatalytic CO2 reduction to ethanol by Au-CuO/Cu2O catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 277-285. |
| [2] | ZENG Zhuang, LI Kezhi, YUAN Zhiwei, DU Jintao, LI Zhuoshi, WANG Yue. Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3061-3079. |
| [3] | ZHOU Qiuming, NIU Congcong, LYU Shuaishuai, LI Hongwei, WEN Fuli, XU Run, LI Mingfeng. Promoting CO2 hydrogenation to methanol through product transformation and separation [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2776-2785. |
| [4] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [5] | TIAN Yuan, LOU Shujie, MENG Shanru, YAN Jingru, XIAO Haicheng. Recent progress of Co-based catalysts for higher alcohols synthesis form syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1869-1876. |
| [6] | XUE Machen, YANG Bolun, XIA Chungu, ZHU Gangli. Progress in heterogeneous catalyst for ethanol upgrading to higher (C6+) alcohols [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 194-203. |
| [7] | WANG Jijie, HAN Zhe, CHEN Siyu, TANG Chizhou, SHA Feng, TANG Shan, YAO Tingting, LI Can. Liquid sunshine methanol [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1309-1317. |
| [8] | LIU Chang, LIU Zhongwen. Perspective on the one-step CO2 hydrogenation to dimethyl ether [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1115-1120. |
| [9] | LIU Ruiqin, MENG Fanhui, WANG Liyan, ZHANG Peng, ZHANG Junfeng, TAN Yisheng, LI Zhong. Preparation of ordered mesoporous CuCoZr catalyst and its catalytic performance for syngas to ethanol and higher alcohols [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5870-5878. |
| [10] | JIAN Yating, YU Qiang, CHEN Xiaoyan, WANG Fan, WANG Zhongming, YUAN Zhenhong. Progress in the preparation of liquid biofuels from lignin [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 109-116. |
| [11] | ZONG Hongyuan, MA Yuchun, LIU Zhongneng. Research progress of higher alcohols synthesis from syngas [J]. Chemical Industry and Engineering Progree, 2015, 34(05): 1269-1276. |
| [12] | GUO Xiaoming,MAO Dongsen,LU Guanzhong,WANG Song. Progress in catalysts for methanol synthesis from CO2 hydrogenation [J]. Chemical Industry and Engineering Progree, 2012, 31(03): 477-488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||