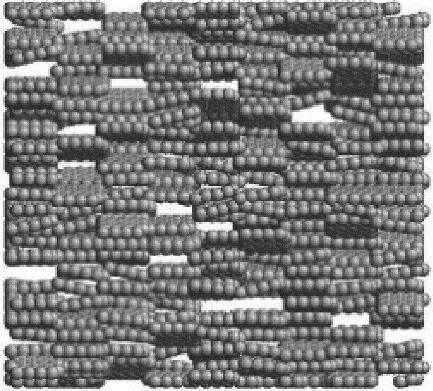
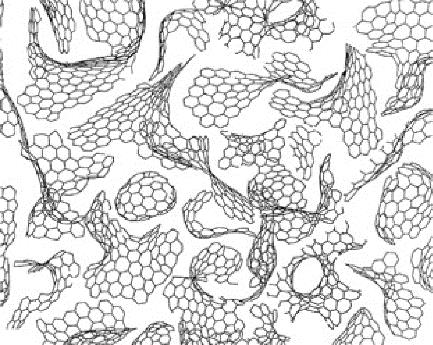
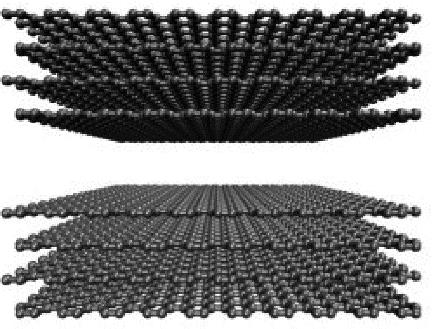
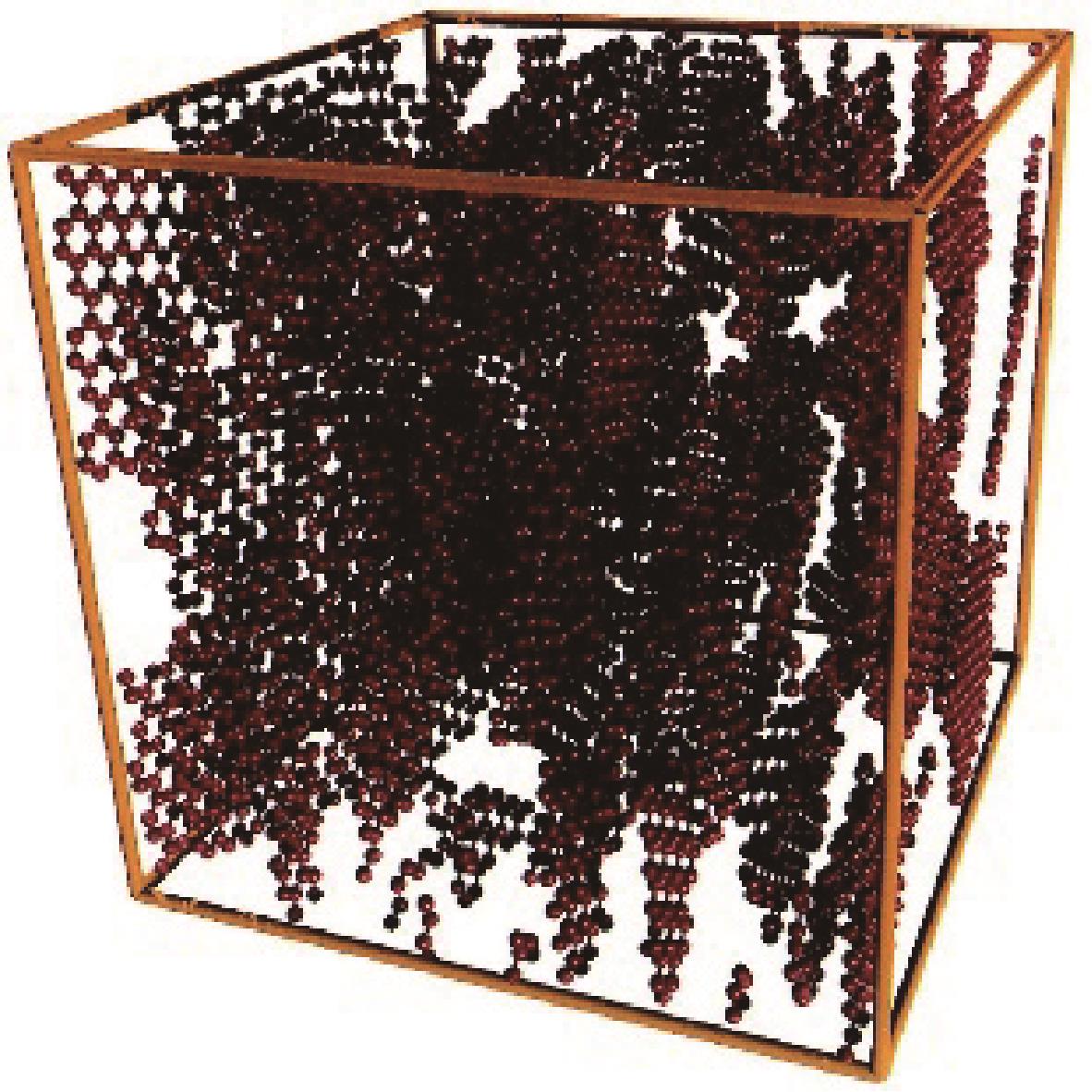
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (3): 1535-1551.DOI: 10.16085/j.issn.1000-6613.2023-0485
• Resources and environmental engineering • Previous Articles
Modeling of porous carbon materials based on molecular simulation: State-of-the art
ZHOU Yihuan( ), XIE Qiang(
), XIE Qiang( ), ZHOU Hongyang, LIANG Dingcheng, LIU Jinchang
), ZHOU Hongyang, LIANG Dingcheng, LIU Jinchang
- School of Chemical and Environmental Engineering, China University of Mining and Technology(Beijing), Beijing 100083, China
-
Received:2023-03-29Revised:2023-05-21Online:2024-04-11Published:2024-03-10 -
Contact:XIE Qiang
基于分子模拟的多孔炭材料结构模型构建方法研究进展
- 中国矿业大学(北京)化学与环境工程学院,北京 100083
-
通讯作者:解强 -
作者简介:周逸寰(1995—),男,博士研究生,研究方向为多孔炭材料。E-mail:yihuanzhou@student.cumtb.edu.cn。 -
基金资助:国家重点研发计划(2022YFC3701901)
CLC Number:
Cite this article
ZHOU Yihuan, XIE Qiang, ZHOU Hongyang, LIANG Dingcheng, LIU Jinchang. Modeling of porous carbon materials based on molecular simulation: State-of-the art[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1535-1551.
周逸寰, 解强, 周红阳, 梁鼎成, 刘金昌. 基于分子模拟的多孔炭材料结构模型构建方法研究进展[J]. 化工进展, 2024, 43(3): 1535-1551.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0485
| 模型 | 描述 | 密度/g·cm-3 | 约束方式 | 实验数据拟合 | 参考文献 |
|---|---|---|---|---|---|
| 四面体无定形炭(ta-C) | 由80%以上sp3杂化碳组成 | 2.9~2.98 | 几何约束 | ND | [ |
| 玻璃炭(V10,V25) | 由sp2杂化碳组成的石墨层组成,在1000℃和2500℃的热处理下形成 | 2.27 | 几何约束 | ED | [ |
| 多孔炭(CS400,CS1000,CS2800) | N2气氛围下蔗糖在400℃、1000℃、2800℃热解 | 1.275、1.584、1.57 | 几何约束 | XRD;SAXS | [ |
| 纳米多孔炭 | TiC的高温氯化反应 | 1.40 | 几何约束 | ND | [ |
| 多孔炭(CS1000a) | CS1000在CO2气氛中的活化 | 0.722 | 几何约束 | XRD;SAXS | [ |
| 工业炭(industrial char)X | 致密石墨晶格 | 几何约束;能量约束 | ED | [ | |
| 中间相炭微球(a-MCMB) | KOH活化的中间相炭微球 | 0.97 | 几何约束 | SAXS;SANS | [ |
| 模型 | 描述 | 密度/g·cm-3 | 约束方式 | 实验数据拟合 | 参考文献 |
|---|---|---|---|---|---|
| 四面体无定形炭(ta-C) | 由80%以上sp3杂化碳组成 | 2.9~2.98 | 几何约束 | ND | [ |
| 玻璃炭(V10,V25) | 由sp2杂化碳组成的石墨层组成,在1000℃和2500℃的热处理下形成 | 2.27 | 几何约束 | ED | [ |
| 多孔炭(CS400,CS1000,CS2800) | N2气氛围下蔗糖在400℃、1000℃、2800℃热解 | 1.275、1.584、1.57 | 几何约束 | XRD;SAXS | [ |
| 纳米多孔炭 | TiC的高温氯化反应 | 1.40 | 几何约束 | ND | [ |
| 多孔炭(CS1000a) | CS1000在CO2气氛中的活化 | 0.722 | 几何约束 | XRD;SAXS | [ |
| 工业炭(industrial char)X | 致密石墨晶格 | 几何约束;能量约束 | ED | [ | |
| 中间相炭微球(a-MCMB) | KOH活化的中间相炭微球 | 0.97 | 几何约束 | SAXS;SANS | [ |
| 模型特点描述 | 研究内容 | 研究成果 | 应用 | 参考文献 |
|---|---|---|---|---|
| 孔径分布范围逐渐减少的S0~S35微孔模型 | 研究模型的密度、孔径分布,并模拟了所有结构对Ar(87K)的吸附 | 吸附等温线模拟与BG法[Bhattacharya and Gubbins (BG) method]计算的孔径具有良好一致性 | 模型开发、吸附性能研究 | [ |
| S0和S35 | 模拟了稀有气体(从Ne到Xe)、四氯化碳和苯的吸附等温线 | 模型模拟结果很好地再现了活性炭的实验行为 | 吸附性能研究 | [ |
| 在S0模型中引入不同比例的氧原子 | 研究炭材料表面氧化对模拟Ar、N2、CO2等温线测定的孔径分布曲线的影响 | 炭结构的轻度氧化不会对炭的绝对孔隙率产生影响;无表面氧化炭结构,随着所研究分子的四极矩的增加,孔径分布范围更广 | 模型验证、多孔炭表面化学及构效关系研究 | [ |
| S系列的10个孔隙率不同的模型(S0,S4,S8,S12,S16,S20,S24,S28,S32,S35) | 研究CF4的吸附模拟 | 吸附模拟结果与文献中实验数据有很好的一致性 | 吸附性能研究 | [ |
| Terzyk构建的S系列模型 | 选用孔隙形态不同(狭缝、纳米管、随机多孔结构和泡沫状结构)的模型研究炭材料储氢能力和从甲烷和氢气混合物中吸附甲烷的选择性 | 炭材料的孔隙结构和孔径分布对 CH4/H2混合物中甲烷吸附选择性有重大影响,随机填充模型的吸附选择性为4.2~5.3 | 孔结构及构效关系研究 | [ |
| 具有羟基官能团的类富勒烯片段作为基本单元构建模型,再通过随机去除一些片段拟合Maxsorb MSC-30的性质构建模型 | 研究类富勒烯片段的尺寸、表面积、表面官能团、电荷模型、几何形状对吸附性质的影响,并进行CO2与CH4吸附模拟 | 开发了比表面积、孔隙体积、碳/氧比与Maxsorb活性炭一致的预测模型,在一定条件下可以准确再现CO2和CH4的吸附等温线 | 模型开发、吸附性能研究 | [ |
| Maxsorb MSC-30结构模型 | 研究高表面积活性炭在二氧化碳捕获、分离中作为吸附剂的行为,研究多组分混合物在燃烧过程中的分离 | 验证了在高压下模型对CO2的吸附容量,表明该材料碳捕获能力强 | 吸附性能(选择性)研究 | [ |
| 活性炭BAM-P109的微孔和微孔-中孔模型 | 研究不同孔径分布模型的结构特征和吸附行为 | 模型正确预测了全氟己烷吸附密度的趋势,但该模型低估了平均20%的吸附密度 | 吸附性能研究 | [ |
| 基于不同环数、缺陷和极性含氧位点的多环芳烃分子单元的多孔炭模型 | 研究了5种药物分子(布洛芬、双氯芬酸、萘普生、扑热息痛和阿莫西林)在水中的吸附 | 5种药物在液体环境中的吸附与实验吸附数据吻合较好 | 液体环境吸附性能研究 | [ |
| 将—H、—OH、—NH2和—COOH 4种官能团分别添加到类石墨烯片段边缘构建模型 | 采用密度泛函理论(DFT)和GCMC模拟相结合的方法,研究了边缘官能团对CO2/CH4二元混合物竞争吸附的影响 | 在低压条件下,边缘官能团对CO2单组分吸附的影响大于CH4,从而显著提高了CO2对CH4的选择性 | 改性材料模型构建及吸附性能研究 | [ |
| —COOH、—OH、—NH2、—SO3H 4种官能团改性的多孔炭模型 | 研究表面官能团改性活性炭对甲醇-丙酮的捕获和分离性质 | 在较低的压力范围内,具有强给电子或接受电子能力的表面官能团(—NH2、—OH和—SO3H)是甲醇-丙酮捕获和分离性能的关键因素 | 表面改性材料模型及构效关系研究 | [ |
| 4种不同结构的活性炭模型(3个微孔结构以及1个微孔-中孔模型) | 研究了苯在273.15K、288.15K、303.15K和318.15K温度下在不同结构模型上的吸附 | 在低温条件下,微孔和中孔较大的活性炭有利于苯的吸附;对于高温条件,微孔较小的活性炭有利于苯的吸附 | 孔结构与吸附性能关系研究 | [ |
| 4种不同孔径分布的活性炭模型 | 研究了303.15K下苯和甲苯的共吸附过程 | 小于1.3nm的小微孔和1.3~2.4nm的较大微孔、较小的中孔有利于苯吸附和甲苯吸附,吸附量随着AC结构中氧化程度的增加而增加 | 孔结构与VOCs吸附竞争性研究 | [ |
| 通过在两个随机填充多环芳烃片段的结构中插入2~4nm的空区域作为中孔结构构建模型 | 研究苯、甲苯、对二甲苯在活性炭模型上的吸附特性 | 吸附质分子直径和中孔尺寸对吸附行为有显著影响,随着吸附质分子直径的增加,饱和吸附压力降低 | 孔结构与吸附竞争性研究 | [ |
| —OH、—COOH、 | 系统性地研究不同官能团以及不同湿度条件下丙酮的吸附性能 | 在相同条件下,不同官能团对丙酮吸附容量的非静电贡献和静电贡献不同。含氧官能团的静电贡献优于含氮官能团,含有氧和氢的官能团优于仅含有氧的官能团。湿度导致非静电对所有官能团的贡献显著降低,静电贡献受湿度的影响较小 | 表面化学与VOCs吸附性能关系研究 | [ |
| 不同孔径(0.6~8.0nm)氮掺杂活性炭模型 | 研究高效吸附、分离甲醇-丙酮混合蒸气的最佳氮官能团和最合适的孔径 | AC-吡啶具有最高的甲醇-丙酮吸附能力,2.5~3nm的中孔适用于甲醇-丙酮的吸附分离 | 氮掺杂炭模型构建与吸附性能研究 | [ |
| 3种不同大小的石墨微元(7环、19环、37环)构建三类密度为0.5g/cm3的活性炭模型 | 研究甲烷和甲苯在活性炭中的吸附和扩散特性 | 石墨微元大小对多孔炭材料吸附甲烷和甲苯有一定影响,37个碳环构成的多孔炭材料是最佳的吸附结构 | 孔结构与吸附性能关系研究 | [ |
| 3种不同大小的石墨微元构建的高比表面积活性炭模型 | 研究在NaCl颗粒存在的情况下活性炭模型对甲苯的吸附 | 在NaCl的存在下,甲苯在活性炭中的扩散系数降低了27%,甲苯的吸收量降低了20.8%~28.6% | 盐对活性炭吸附性能的影响研究 | [ |
| 使用具有典型官能团(羧基、羰基、苯酚、吡啶N)的9种不同碳片构建活性炭模型 | 研究孔结构和表面官能团对甲醛吸附性能影响 | 活性炭有效吸附孔径范围窄是导致甲醛吸附能力低的原因之一;孔径为0.4~0.7nm的微孔只能容纳一种甲醛,在甲醛的吸附中起着主导作用;尽管较大的微孔可以同时容纳多个甲醛分子,但吸附的甲醛分子之间存在强烈的相互作用,导致吸附的甲醛发生脱附 | 孔结构-表面化学-吸附性能关系研究 | [ |
| 模型特点描述 | 研究内容 | 研究成果 | 应用 | 参考文献 |
|---|---|---|---|---|
| 孔径分布范围逐渐减少的S0~S35微孔模型 | 研究模型的密度、孔径分布,并模拟了所有结构对Ar(87K)的吸附 | 吸附等温线模拟与BG法[Bhattacharya and Gubbins (BG) method]计算的孔径具有良好一致性 | 模型开发、吸附性能研究 | [ |
| S0和S35 | 模拟了稀有气体(从Ne到Xe)、四氯化碳和苯的吸附等温线 | 模型模拟结果很好地再现了活性炭的实验行为 | 吸附性能研究 | [ |
| 在S0模型中引入不同比例的氧原子 | 研究炭材料表面氧化对模拟Ar、N2、CO2等温线测定的孔径分布曲线的影响 | 炭结构的轻度氧化不会对炭的绝对孔隙率产生影响;无表面氧化炭结构,随着所研究分子的四极矩的增加,孔径分布范围更广 | 模型验证、多孔炭表面化学及构效关系研究 | [ |
| S系列的10个孔隙率不同的模型(S0,S4,S8,S12,S16,S20,S24,S28,S32,S35) | 研究CF4的吸附模拟 | 吸附模拟结果与文献中实验数据有很好的一致性 | 吸附性能研究 | [ |
| Terzyk构建的S系列模型 | 选用孔隙形态不同(狭缝、纳米管、随机多孔结构和泡沫状结构)的模型研究炭材料储氢能力和从甲烷和氢气混合物中吸附甲烷的选择性 | 炭材料的孔隙结构和孔径分布对 CH4/H2混合物中甲烷吸附选择性有重大影响,随机填充模型的吸附选择性为4.2~5.3 | 孔结构及构效关系研究 | [ |
| 具有羟基官能团的类富勒烯片段作为基本单元构建模型,再通过随机去除一些片段拟合Maxsorb MSC-30的性质构建模型 | 研究类富勒烯片段的尺寸、表面积、表面官能团、电荷模型、几何形状对吸附性质的影响,并进行CO2与CH4吸附模拟 | 开发了比表面积、孔隙体积、碳/氧比与Maxsorb活性炭一致的预测模型,在一定条件下可以准确再现CO2和CH4的吸附等温线 | 模型开发、吸附性能研究 | [ |
| Maxsorb MSC-30结构模型 | 研究高表面积活性炭在二氧化碳捕获、分离中作为吸附剂的行为,研究多组分混合物在燃烧过程中的分离 | 验证了在高压下模型对CO2的吸附容量,表明该材料碳捕获能力强 | 吸附性能(选择性)研究 | [ |
| 活性炭BAM-P109的微孔和微孔-中孔模型 | 研究不同孔径分布模型的结构特征和吸附行为 | 模型正确预测了全氟己烷吸附密度的趋势,但该模型低估了平均20%的吸附密度 | 吸附性能研究 | [ |
| 基于不同环数、缺陷和极性含氧位点的多环芳烃分子单元的多孔炭模型 | 研究了5种药物分子(布洛芬、双氯芬酸、萘普生、扑热息痛和阿莫西林)在水中的吸附 | 5种药物在液体环境中的吸附与实验吸附数据吻合较好 | 液体环境吸附性能研究 | [ |
| 将—H、—OH、—NH2和—COOH 4种官能团分别添加到类石墨烯片段边缘构建模型 | 采用密度泛函理论(DFT)和GCMC模拟相结合的方法,研究了边缘官能团对CO2/CH4二元混合物竞争吸附的影响 | 在低压条件下,边缘官能团对CO2单组分吸附的影响大于CH4,从而显著提高了CO2对CH4的选择性 | 改性材料模型构建及吸附性能研究 | [ |
| —COOH、—OH、—NH2、—SO3H 4种官能团改性的多孔炭模型 | 研究表面官能团改性活性炭对甲醇-丙酮的捕获和分离性质 | 在较低的压力范围内,具有强给电子或接受电子能力的表面官能团(—NH2、—OH和—SO3H)是甲醇-丙酮捕获和分离性能的关键因素 | 表面改性材料模型及构效关系研究 | [ |
| 4种不同结构的活性炭模型(3个微孔结构以及1个微孔-中孔模型) | 研究了苯在273.15K、288.15K、303.15K和318.15K温度下在不同结构模型上的吸附 | 在低温条件下,微孔和中孔较大的活性炭有利于苯的吸附;对于高温条件,微孔较小的活性炭有利于苯的吸附 | 孔结构与吸附性能关系研究 | [ |
| 4种不同孔径分布的活性炭模型 | 研究了303.15K下苯和甲苯的共吸附过程 | 小于1.3nm的小微孔和1.3~2.4nm的较大微孔、较小的中孔有利于苯吸附和甲苯吸附,吸附量随着AC结构中氧化程度的增加而增加 | 孔结构与VOCs吸附竞争性研究 | [ |
| 通过在两个随机填充多环芳烃片段的结构中插入2~4nm的空区域作为中孔结构构建模型 | 研究苯、甲苯、对二甲苯在活性炭模型上的吸附特性 | 吸附质分子直径和中孔尺寸对吸附行为有显著影响,随着吸附质分子直径的增加,饱和吸附压力降低 | 孔结构与吸附竞争性研究 | [ |
| —OH、—COOH、 | 系统性地研究不同官能团以及不同湿度条件下丙酮的吸附性能 | 在相同条件下,不同官能团对丙酮吸附容量的非静电贡献和静电贡献不同。含氧官能团的静电贡献优于含氮官能团,含有氧和氢的官能团优于仅含有氧的官能团。湿度导致非静电对所有官能团的贡献显著降低,静电贡献受湿度的影响较小 | 表面化学与VOCs吸附性能关系研究 | [ |
| 不同孔径(0.6~8.0nm)氮掺杂活性炭模型 | 研究高效吸附、分离甲醇-丙酮混合蒸气的最佳氮官能团和最合适的孔径 | AC-吡啶具有最高的甲醇-丙酮吸附能力,2.5~3nm的中孔适用于甲醇-丙酮的吸附分离 | 氮掺杂炭模型构建与吸附性能研究 | [ |
| 3种不同大小的石墨微元(7环、19环、37环)构建三类密度为0.5g/cm3的活性炭模型 | 研究甲烷和甲苯在活性炭中的吸附和扩散特性 | 石墨微元大小对多孔炭材料吸附甲烷和甲苯有一定影响,37个碳环构成的多孔炭材料是最佳的吸附结构 | 孔结构与吸附性能关系研究 | [ |
| 3种不同大小的石墨微元构建的高比表面积活性炭模型 | 研究在NaCl颗粒存在的情况下活性炭模型对甲苯的吸附 | 在NaCl的存在下,甲苯在活性炭中的扩散系数降低了27%,甲苯的吸收量降低了20.8%~28.6% | 盐对活性炭吸附性能的影响研究 | [ |
| 使用具有典型官能团(羧基、羰基、苯酚、吡啶N)的9种不同碳片构建活性炭模型 | 研究孔结构和表面官能团对甲醛吸附性能影响 | 活性炭有效吸附孔径范围窄是导致甲醛吸附能力低的原因之一;孔径为0.4~0.7nm的微孔只能容纳一种甲醛,在甲醛的吸附中起着主导作用;尽管较大的微孔可以同时容纳多个甲醛分子,但吸附的甲醛分子之间存在强烈的相互作用,导致吸附的甲醛发生脱附 | 孔结构-表面化学-吸附性能关系研究 | [ |
| 构建方法 | 描述 | 优点 | 缺点 | 适合材料 | 应用 | 参考文献 |
|---|---|---|---|---|---|---|
| 结构重建法 | ||||||
| 炭狭缝孔 | 多孔炭内局部狭缝孔结构作为多孔炭的近似模型 | 结构简单、易于构建;相对较低的计算费用 | 不能捕捉到多孔炭的重要特性,忽略了边缘效应、孔隙连通性和孔隙形状等 | 活性炭、活性碳纤维 | 吸附模拟研究 | [ |
| 逆蒙特卡洛 | 将炭材料的各种结构元素进行排列组合以匹配核磁共振、X射线衍射等实验数据 | 对多种炭体系进行建模;准确地反映内部结构 | 产生结构非唯一,会产生不符合实际的键角特征;并不能表征出中孔结构;模型构建效率低 | 玻璃炭、活性炭、煤焦、中间相炭微球 | 材料基础性质、吸附模拟研究 | [ |
| 混合逆蒙特卡洛 | 在逆蒙特卡洛的基础上加入几何和能量约束 | 对多种炭体系进行建模;准确反映孔隙形态 | 可以合理表征微孔结构,但是并不能表征出中孔结构;模型构建效率低 | 活性炭、活性碳纤维 | 材料基础性质、吸附模拟研究 | [ |
| HRTEM主导的原子重建法 | 对HRTEM图像进行图像处理提取信息构建二维模型,随后转化为三维模型 | 模型构建过程高效便捷 | Fringe3D和Vol3D是未公布脚本;图像处理过程烦琐 | 煤、烟黑、煤焦、活性炭 | 材料基础性质、吸附模拟研究 | [ |
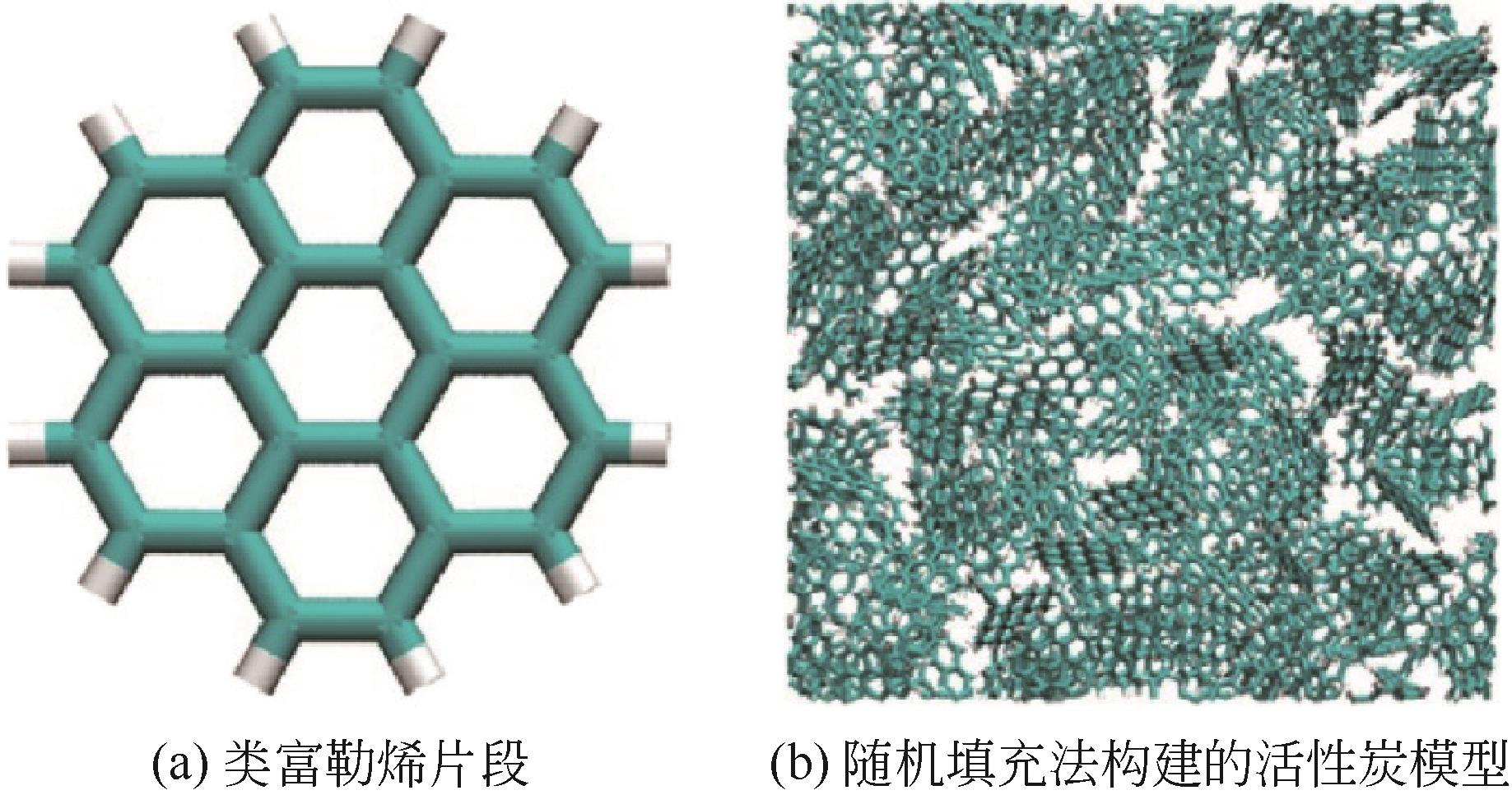
| 随机填充法 | 将一种或多种多环芳烃分子随机插入周期性边界中 | 构建方法简单,构建效率高;可针对性地调控孔结构和官能团 | 模型构建过程随机性强,对于匹配实际材料需要调整模型 | 活性炭 | 材料基础性质、吸附模拟研究 | [ |
| 仿真过程法 | ||||||
| 动力学仿真法 | 先构建材料初始模型,通过淬火和退火分子动力学使模型微观结构进行演变 | 预测实验的条件,深入观测和研究材料在微小时间尺度下基本的物理和化学变化;原材料的结构信息不需要作为输入 | 化学反应速率时间尺度上与实际不匹配 | 活性炭、碳化物衍生炭、无序石墨烯网络、碳纤维 | 材料力学性能和结构特征研究、反应速率研究 | [ |
| kMC-MD联用法 | 大量阶梯单元通过蒙特卡洛和分子动力学联用模拟炭化过程微观结构演变 | 只需稳定的前体和化学反应速率作为输入,深入探究微观结构演变 | 模型处于研究初期,模型应用方面还需验证 | 活性碳纤维 | 材料力学性能和结构特征研究 | [ |
| 构建方法 | 描述 | 优点 | 缺点 | 适合材料 | 应用 | 参考文献 |
|---|---|---|---|---|---|---|
| 结构重建法 | ||||||
| 炭狭缝孔 | 多孔炭内局部狭缝孔结构作为多孔炭的近似模型 | 结构简单、易于构建;相对较低的计算费用 | 不能捕捉到多孔炭的重要特性,忽略了边缘效应、孔隙连通性和孔隙形状等 | 活性炭、活性碳纤维 | 吸附模拟研究 | [ |
| 逆蒙特卡洛 | 将炭材料的各种结构元素进行排列组合以匹配核磁共振、X射线衍射等实验数据 | 对多种炭体系进行建模;准确地反映内部结构 | 产生结构非唯一,会产生不符合实际的键角特征;并不能表征出中孔结构;模型构建效率低 | 玻璃炭、活性炭、煤焦、中间相炭微球 | 材料基础性质、吸附模拟研究 | [ |
| 混合逆蒙特卡洛 | 在逆蒙特卡洛的基础上加入几何和能量约束 | 对多种炭体系进行建模;准确反映孔隙形态 | 可以合理表征微孔结构,但是并不能表征出中孔结构;模型构建效率低 | 活性炭、活性碳纤维 | 材料基础性质、吸附模拟研究 | [ |
| HRTEM主导的原子重建法 | 对HRTEM图像进行图像处理提取信息构建二维模型,随后转化为三维模型 | 模型构建过程高效便捷 | Fringe3D和Vol3D是未公布脚本;图像处理过程烦琐 | 煤、烟黑、煤焦、活性炭 | 材料基础性质、吸附模拟研究 | [ |
| 随机填充法 | 将一种或多种多环芳烃分子随机插入周期性边界中 | 构建方法简单,构建效率高;可针对性地调控孔结构和官能团 | 模型构建过程随机性强,对于匹配实际材料需要调整模型 | 活性炭 | 材料基础性质、吸附模拟研究 | [ |
| 仿真过程法 | ||||||
| 动力学仿真法 | 先构建材料初始模型,通过淬火和退火分子动力学使模型微观结构进行演变 | 预测实验的条件,深入观测和研究材料在微小时间尺度下基本的物理和化学变化;原材料的结构信息不需要作为输入 | 化学反应速率时间尺度上与实际不匹配 | 活性炭、碳化物衍生炭、无序石墨烯网络、碳纤维 | 材料力学性能和结构特征研究、反应速率研究 | [ |
| kMC-MD联用法 | 大量阶梯单元通过蒙特卡洛和分子动力学联用模拟炭化过程微观结构演变 | 只需稳定的前体和化学反应速率作为输入,深入探究微观结构演变 | 模型处于研究初期,模型应用方面还需验证 | 活性碳纤维 | 材料力学性能和结构特征研究 | [ |
| 1 | PENG Xuan, VICENT-LUNA J M, JIN Qibing. Separation of CF4/N2, C2F6/N2, and SF6/N2 mixtures in amorphous activated carbons using molecular simulations[J]. ACS Applied Materials & Interfaces, 2020, 12(17): 20044-20055. |
| 2 | 姚晨牧, 冯丽, 安亚雄, 等. 碳材料的分子模型及模拟方法在吸附中应用的研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(S1): 1-9. |
| YAO Chenmu, FENG Li, AN Yaxiong, et al. Research progress of molecular model and simulation method of carbon materials in adsorption application[J]. Natural Gas Chemical Industry, 2021, 46(S1): 1-9. | |
| 3 | MERLET C, ROTENBERG B, MADDEN P A, et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes[J]. Nature Materials, 2012, 11(4): 306-310. |
| 4 | 解强, 张香兰, 梁鼎成, 等. 煤基活性炭定向制备: 原理·方法·应用[J]. 煤炭科学技术, 2021, 49(1): 100-127. |
| XIE Qiang, ZHANG Xianglan, LIANG Dingcheng, et al. Directional preparation of coal-based activated carbon: Principles, approaches and applications[J]. Coal Science and Technology, 2021, 49(1): 100-127. | |
| 5 | 李辉, 王登辉, 惠世恩. 煤化工VOCs治理技术应用现状及展望[J]. 洁净煤技术, 2021, 27(1): 144-154. |
| LI Hui, WANG Denghui, HUI Shi’en. Application status and prospects of coal chemical VOCs treatment technology[J]. Clean Coal Technology, 2021, 27(1): 144-154. | |
| 6 | BHATIA S K. Characterizing structural complexity in disordered carbons: From the slit pore to atomistic models[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2017, 33(4): 831-847. |
| 7 | 杨忠霖, 解强, 郝郑平, 等. VOCs治理工程安全评价体系研究与构建[J]. 洁净煤技术, 2022, 28(2): 77-85. |
| YANG Zhonglin, XIE Qiang, HAO Zhengping, et al. Research and construction of safety assessment methodology for VOCs treatment projects[J]. Clean Coal Technology, 2022, 28(2): 77-85. | |
| 8 | PIKUNIC J, CLINARD C, COHAUT N, et al. Reconstruction method for the characterization of porous carbons[J]. Studies in Surface Science and Catalysis, 2002, 144: 19-26. |
| 9 | FRANKLIN R E. Crystallite growth in graphitizing and non-graphitizing carbons[J]. Proceedings of the Royal Society of London, Series A, Mathematical and Physical Sciences, 1951, 209(1097): 196-218. |
| 10 | OBERLIN A, VILLEY M, COMBAZ A. Influence of elemental composition on carbonization[J]. Carbon, 1980, 18(5): 347-353. |
| 11 | SEGARRA E I, GLANDT E D. Model microporous carbons: Microstructure, surface polarity and gas adsorption[J]. Chemical Engineering Science, 1994, 49(17): 2953-2965. |
| 12 | LIU Jinchen, MONSON P A. Molecular modeling of adsorption in activated carbon: Comparison of Monte Carlo simulations with experiment[J]. Adsorption, 2005, 11(1): 5-13. |
| 13 | LIU Jinchen, MONSON P A. Monte Carlo simulation study of water adsorption in activated carbon[J]. Industrial & Engineering Chemistry Research, 2006, 45(16): 5649-5656. |
| 14 | BIGGS M, AGARWAL P. Mass diffusion of atomic fluids in random micropore spaces using equilibrium molecular dynamics[J]. Physical Review A, 1992, 46(6): 3312-3318. |
| 15 | BIGGS M J, BUTS A. Virtual porous carbons: What they are and what they can be used for[J]. Molecular Simulation, 2006, 32(7): 579-593. |
| 16 | HARRIS P J F, TSANG S C. High-resolution electron microscopy studies of non-graphitizing carbons[J]. Philosophical Magazine A, 1997, 76(3): 667-677. |
| 17 | HARRIS P J F, BURIAN A, DUBER S. High-resolution electron microscopy of a microporous carbon[J]. Philosophical Magazine Letters, 2000, 80(6): 381-386. |
| 18 | HARRIS P J F. New perspectives on the structure of graphitic carbons[J]. Critical Reviews in Solid State and Materials Sciences, 2005, 30(4): 235-253. |
| 19 | EVERETT D H, POWL J C. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1976, 72: 619-636. |
| 20 | PALMER J C, GUBBINS K E. Atomistic models for disordered nanoporous carbons using reactive force fields[J]. Microporous and Mesoporous Materials, 2012, 154: 24-37. |
| 21 | KUMAR A, LOBO R F, WAGNER Norman J. Porous amorphous carbon models from periodic Gaussian chains of amorphous polymers[J]. Carbon, 2005, 43(15): 3099-3111. |
| 22 | BAHAMON D, CARRO L, GURI S, et al. Computational study of ibuprofen removal from water by adsorption in realistic activated carbons[J]. Journal of Colloid and Interface Science, 2017, 498: 323-334. |
| 23 | CHEN M Y, COASNE B, GUYER R, et al. Molecular simulation of sorption-induced deformation in atomistic nanoporous materials[J]. Langmuir, 2019, 35(24): 7751-7758. |
| 24 | CHEN Gaofei, AN Yaxiong, SHEN Yuanhui, et al. Effect of pore size on CH4/N2 separation using activated carbon[J]. Chinese Journal of Chemical Engineering, 2020, 28(4): 1062-1068. |
| 25 | BEDNÁREK J, MATĚJOVÁ L, JANKOVSKÁ Z, et al. The influence of structural properties on the adsorption capacities of microwave-assisted biochars for metazachlor removal from aqueous solutions[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 108003. |
| 26 | 安亚雄, 付强, 刘冰, 等. 不同孔径活性炭吸附挥发性有机物的分子模拟[J]. 化工进展, 2019, 38(11): 5136-5141. |
| AN Yaxiong, FU Qiang, LIU Bing, et al. Molecular simulation of adsorption of volatile organic compounds by activated carbon with different pore sizes[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 5136-5141. | |
| 27 | BHATIA S K. Density functional theory analysis of the influence of pore wall heterogeneity on adsorption in carbons[J]. Langmuir, 2002, 18(18): 6845-6856. |
| 28 | ALEKSEY V, PIOTROVSKAYA E M, BRODSKAYA E N. Capillary condensation and melting/freezing transitions for methane in slit coal pores[J]. Adsorption, 1998, 4(3): 207-224. |
| 29 | SHEVADE A V, JIANG S Y, GUBBINS K E. Molecular simulation study of water-methanol mixtures in activated carbon pores[J]. The Journal of Chemical Physics, 2000, 113(16): 6933-6942. |
| 30 | JIN Wenzheng, WANG Wenchuan. Computer simulation of adsorption of a Stockmayer molecule chlorodifluoromethane in activated carbon slit pores[J]. The Journal of Chemical Physics, 2001, 114(22): 10163-10169. |
| 31 | JORGE M, SCHUMACHER C, SEATON N A. Simulation study of the effect of the chemical heterogeneity of activated carbon on water adsorption[J]. Langmuir, 2002, 18(24): 9296-9306. |
| 32 | THOMSON K T, GUBBINS K E. Modeling structural morphology of microporous carbons by reverse Monte Carlo[J]. Langmuir, 2000, 16(13): 5761-5773. |
| 33 | JAIN S K, FUHR J, J-M PELLENQ R, et al. Stability of porous carbon structures obtained from reverse Monte Carlo using tight binding and bond order Hamiltonians[M]//Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 2007: 169-176. |
| 34 | OPLETAL G, PETERSEN T, O'MALLEY B, et al. Hybrid approach for generating realistic amorphous carbon structure using metropolis and reverse Monte Carlo[J]. Molecular Simulation, 2002, 28(10/11): 927-938. |
| 35 | O’MALLEY B, SNOOK I, MCCULLOCH D. Reverse Monte Carlo analysis of the structure of glassy carbon using electron-microscopy data[J]. Physical Review B, 1998, 57(22): 14148-14157. |
| 36 | PIKUNIC J, J-M PELLENQ R, THOMSON K T, et al. Improved molecular models for porous carbons[M]//Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 2001: 647-652. |
| 37 | ZETTERSTRÖM P, URBONAITE S, LINDBERG F, et al. Reverse Monte Carlo studies of nanoporous carbon from TiC[J]. Journal of Physics: Condensed Matter, 2005, 17(23): 3509-3524. |
| 38 | JAIN S K, PIKUNIC J P, J-M PELLENQ R, et al. Effects of activation on the structure and adsorption properties of a nanoporous carbon using molecular simulation[J]. Adsorption, 2005, 11(1): 355-360. |
| 39 | PETERSEN T, YAROVSKY I, SNOOK I, et al. Microstructure of an industrial char by diffraction techniques and reverse Monte Carlo modelling[J]. Carbon, 2004, 42(12/13): 2457-2469. |
| 40 | BRENNAN J K, THOMSON K T, GUBBINS K E. Adsorption of water in activated carbons: Effects of pore blocking and connectivity[J]. Langmuir, 2002, 18(14): 5438-5447. |
| 41 | PETERSEN T, YAROVSKY I, SNOOK I, et al. Structural analysis of carbonaceous solids using an adapted reverse Monte Carlo algorithm[J]. Carbon, 2003, 41(12): 2403-2411. |
| 42 | OPLETAL G, PETERSEN T C, O'MALLEY B, et al. HRMC: Hybrid reverse Monte Carlo method with silicon and carbon potentials[J]. Computer Physics Communications, 2008, 178(10): 777-787. |
| 43 | OPLETAL G, PETERSEN T C, O'MALLEY B, et al. HRMC1.1: Hybrid reverse Monte Carlo method with silicon and carbon potentials[J]. Computer Physics Communications, 2011, 182(2): 542. |
| 44 | OPLETAL G, PETERSEN T C, SNOOK I K, et al. HRMC2.0: Hybrid reverse Monte Carlo method with silicon, carbon and germanium potentials[J]. Computer Physics Communications, 2013, 184(8): 1946-1957. |
| 45 | OPLETAL G, PETERSEN T C, RUSSO S P. HRMC2.1: Hybrid reverse Monte Carlo method with silicon, carbon, germanium and silicon carbide potentials[J]. Computer Physics Communications, 2014, 185(6): 1854-1855. |
| 46 | JAIN S K, J-M PELLENQ R, PIKUNIC J P, et al. Molecular modeling of porous carbons using the hybrid reverse Monte Carlo method[J]. Langmuir, 2006, 22(24): 9942-9948. |
| 47 | PIKUNIC J, CLINARD C, COHAUT N, et al. Structural modeling of porous carbons: Constrained reverse Monte Carlo method[J]. Langmuir, 2003, 19(20): 8565-8582. |
| 48 | NGUYEN T X, BHATIA S K, JAIN S K, et al. Structure of saccharose-based carbon and transport of confined fluids: Hybrid reverse Monte Carlo reconstruction and simulation studies[J]. Molecular Simulation, 2006, 32(7): 567-577. |
| 49 | PIKUNIC J, LLEWELLYN P, PELLENQ R, et al. Argon and nitrogen adsorption in disordered nanoporous carbons: Simulation and experiment[J]. Langmuir, 2005, 21(10): 4431-4440. |
| 50 | NGUYEN T X, COHAUT N, Jun-Seok BAE, et al. New method for atomistic modeling of the microstructure of activated carbons using hybrid reverse Monte Carlo simulation[J]. Langmuir, 2008, 24(15): 7912-7922. |
| 51 | LIU Lang, BHATIA S K. Influence of morphology on transport properties and interfacial resistance in nanoporous carbons[J]. The Journal of Physical Chemistry C, 2019, 123(34): 21050-21058. |
| 52 | NGUYEN T X, BHATIA S K. How water adsorbs in hydrophobic nanospaces[J]. The Journal of Physical Chemistry C, 2011, 115(33): 16606-16612. |
| 53 | KOWALCZYK P, GAUDEN P A, WIŚNIEWSKI M, et al. Atomic-scale molecular models of oxidized activated carbon fibre nanoregions: Examining the effects of oxygen functionalities on wet formaldehyde adsorption[J]. Carbon, 2020, 165: 67-81. |
| 54 | MARKS N A. Generalizing the environment-dependent interaction potential for carbon[J]. Physical Review B, 2000, 63(3): 035401. |
| 55 | NI B, LEE Ki-Ho, SINNOTT S B. A reactive empirical bond order (REBO) potential for hydrocarbon-oxygen interactions[J]. Journal of Physics: Condensed Matter, 2004, 16(41): 7261-7275. |
| 56 | 黄杨. 活性炭分子结构模型的构建及其吸附行为的模拟研究[D]. 重庆: 重庆大学, 2016. |
| HUANG Yang. Construction of activated carbon efficient atomistic model, and molecular simulation insight into the behavior of sorption[D]. Chongqing: Chongqing University, 2016. | |
| 57 | FERNANDEZ-ALOS V, WATSON J K, VANDER W R, et al. Soot and char molecular representations generated directly from HRTEM lattice fringe images using Fringe3D[J]. Combustion and Flame, 2011, 158(9): 1807-1813. |
| 58 | WANG Changan, WATSON J K, LOUW E, et al. Construction strategy for atomistic models of coal chars capturing stacking diversity and pore size distribution[J]. Energy & Fuels, 2015, 29(8): 4814-4826. |
| 59 | J-M LEYSSALE, COSTA J-P DA, GERMAIN C, et al. An image-guided atomistic reconstruction of pyrolytic carbons[J]. Applied Physics Letters, 2009, 95(23): 231912. |
| 60 | J-M LEYSSALE, COSTA J-P DA, GERMAIN C, et al. Structural features of pyrocarbon atomistic models constructed from transmission electron microscopy images[J]. Carbon, 2012, 50(12): 4388-4400. |
| 61 | CASTRO-MARCANO F, LOBODIN V V, RODGERS R P, et al. A molecular model for Illinois No.6 Argonne Premium coal: Moving toward capturing the continuum structure[J]. Fuel, 2012, 95: 35-49. |
| 62 | CASTRO-MARCANO F, WINANS R E, CHUPAS Peter, et al. Fine structure evaluation of the pair distribution function with molecular models of the Argonne Premium coals[J]. Energy & Fuels, 2012, 26(7): 4336-4345. |
| 63 | SONG Yu, JIANG Bo, LI Ming, et al. Macromolecular transformations for tectonically-deformed high volatile bituminous via HRTEM and XRD analyses[J]. Fuel, 2020, 263: 116756. |
| 64 | HUANG Yang, CANNON F S, WATSON J K, et al. Activated carbon efficient atomistic model construction that depicts experimentally-determined characteristics[J]. Carbon, 2015, 83: 1-14. |
| 65 | HUANG Yang, CANNON F S, GUO Jinsong, et al. Atomistic modelling insight into the structure of lignite-based activated carbon and benzene sorption behavior[J]. RSC Advances, 2016, 6(61): 56623-56637. |
| 66 | TERZYK A P, FURMANIAK S, GAUDEN P A, et al. Hyper-parallel tempering Monte Carlo simulations of Ar adsorption in new models of microporous non-graphitizing activated carbon: Effect of microporosity[J]. Journal of Physics: Condensed Matter, 2007, 19(40): 406208. |
| 67 | TERZYK A P, FURMANIAK S, HARRIS P J F, et al. How realistic is the pore size distribution calculated from adsorption isotherms if activated carbon is composed of fullerene-like fragments?[J]. Physical Chemistry Chemical Physics, 2007, 9(44): 5919-5927. |
| 68 | FURMANIAK S, TERZYK A P, GAUDEN P A, et al. Can carbon surface oxidation shift the pore size distribution curve calculated from Ar, N2 and CO2 adsorption isotherms? Simulation results for a realistic carbon model[J]. Journal of Physics: Condensed Matter, 2009, 21(31): 315005. |
| 69 | ALBESA A G, RAFTI M, VICENTE J L, et al. Adsorption of CO2/CH4 mixtures in a molecular model of activated carbon through Monte Carlo simulations[J]. Adsorption Science & Technology, 2012, 30(8): 669-689. |
| 70 | KUMAR K V, RODRÍGUEZ-REINOSO F. Co-adsorption of N2 in the presence of CH4 within carbon nanospaces: Evidence from molecular simulations[J]. Nanotechnology, 2013, 24(3): 035401. |
| 71 | DI BIASE E, SARKISOV L. Systematic development of predictive molecular models of high surface area activated carbons for adsorption applications[J]. Carbon, 2013, 64: 262-280. |
| 72 | SARKISOV L. Molecular simulation of perfluorohexane adsorption in BAM-P109 activated carbon[J]. Adsorption Science & Technology, 2016, 34(1): 42-63. |
| 73 | LI Shi, SONG Kunli, ZHAO Dongfeng, et al. Molecular simulation of benzene adsorption on different activated carbon under different temperatures[J]. Microporous and Mesoporous Materials, 2020, 302: 110220. |
| 74 | GU Zekai, ZHU Chuanyong, HUANG Zhaoqin, et al. Numerical simulations on the adsorption characteristics of aromatics on activated carbon by the GCMC method[J]. Case Studies in Thermal Engineering, 2021, 26: 101116. |
| 75 | 李小斐, 宋坤莉, 赵东风, 等. 活性炭表面官能团对苯吸附性能影响的分子模拟[J]. 石油学报(石油加工), 2021, 37(5): 1078-1085. |
| LI Xiaofei, SONG Kunli, ZHAO Dongfeng, et al. Molecular simulations on the effects of surface functional groups of activated carbon on the benzene adsorption performance[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(5): 1078-1085. | |
| 76 | GUO Yang, ZENG Zheng, LI Liqing, et al. Competitive adsorption of methanol-acetone on surface functionalization (—COOH, —OH, —NH2, and —SO3H): Grand canonical Monte Carlo and density functional theory simulations[J]. ACS Applied Materials & Interfaces, 2019, 11(37): 34241-34250. |
| 77 | TERZYK A P, FURMANIAK S, GAUDEN P A, et al. Testing isotherm models and recovering empirical relationships for adsorption in microporous carbons using virtual carbon models and grand canonical Monte Carlo simulations[J]. Journal of Physics: Condensed Matter, 2008, 20(38): 385212. |
| 78 | FURMANIAK S, TERZYK A P, GAUDEN P A, et al. Applicability of molecular simulations for modelling the adsorption of the greenhouse gas CF4 on carbons[J]. Journal of Physics: Condensed Matter, 2013, 25(1): 015004. |
| 79 | KUMAR K V, SALIH A, LU Linghong, et al. Molecular simulation of hydrogen physisorption and chemisorption in nanoporous carbon structures[J]. Adsorption Science & Technology, 2011, 29(8): 799-817. |
| 80 | KUMAR K V, MÜLLER E A, RODRÍGUEZ-REINOSO F. Effect of pore morphology on the adsorption of methane/hydrogen mixtures on carbon micropores[J]. The Journal of Physical Chemistry C, 2012, 116(21): 11820-11829. |
| 81 | DI BIASE E, SARKISOV L. Molecular simulation of multi-component adsorption processes related to carbon capture in a high surface area, disordered activated carbon[J]. Carbon, 2015, 94: 27-40. |
| 82 | BAHAMON D, VEGA L F. Pharmaceutical removal from water effluents by adsorption on activated carbons: A Monte Carlo simulation study[J]. Langmuir, 2017, 33(42): 11146-11155. |
| 83 | LI Shi, SONG Kunli, YU Lan, et al. Study on co-adsorption mechanisms of benzene and toluene on activated carbon via molecular simulation[J]. Materials Letters, 2020, 280: 128554. |
| 84 | SU Zhibin, ZHANG Yingying, HUANG Liangliang, et al. Acetone adsorption on activated carbons: Roles of functional groups and humidity[J]. Fluid Phase Equilibria, 2020, 521: 112645. |
| 85 | YU Lingyun, GUO Yang, CHEN Hongyu, et al. Influence of functional groups and pore sizes in porous carbon for methanol acetone adsorptive separation based on molecular simulation[J]. Journal of Materials Science, 2021, 56: 18550-18565. |
| 86 | 周日峰, 石基弘, 刘全祯, 等. 活性炭吸附甲烷和甲苯的分子模拟研究[J]. 过程工程学报, 2018, 18(S1): 97-102. |
| ZHOU Rifeng, SHI Jihong, LIU Quanzhen, et al. Molecular simulation on adsorption of methane and toluene by activated carbon[J]. The Chinese Journal of Process Engineering, 2018, 18(S1): 97-102. | |
| 87 | WANG Mingyang, SHENG Ying. Molecular simulation to analyze the influence of ultrafine particles on activated carbon adsorbing low concentration toluene[J]. Building and Environment, 2022, 213: 108875. |
| 88 | AN Kaibo, WANG Zhonghua, YANG Xue, et al. Reasons of low formaldehyde adsorption capacity on activated carbon: Multi-scale simulation of dynamic interaction between pore size and functional groups[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108723. |
| 89 | GELB L D, GUBBINS K E. Characterization of porous glasses: Simulation models, adsorption isotherms, and the Brunauer-Emmett-Teller analysis method[J]. Langmuir, 1998, 14(8): 2097-2111. |
| 90 | SHI Yunfeng. A mimetic porous carbon model by quench molecular dynamics simulation[J]. The Journal of Chemical Physics, 2008, 128(23): 234707. |
| 91 | MI Xi, SHI Yunfeng. Elastic properties of mimetically synthesized model nanoporous carbon[J]. MRS Online Proceedings Library, 2010, 1224(1): 1010. |
| 92 | MI Xi, SHI Yunfeng. Topological defects in nanoporous carbon[J]. Carbon, 2013, 60: 202-214. |
| 93 | PALMER J C, LLOBET A, S-H YEON, et al. Modeling the structural evolution of carbide-derived carbons using quenched molecular dynamics[J]. Carbon, 2010, 48(4): 1116-1123. |
| 94 | PALMER J C, MOORE J D, BRENNAN J K, et al. Adsorption and diffusion of argon in disordered nanoporous carbons[J]. Adsorption, 2011, 17(1): 189-199. |
| 95 | THOMPSON M, DYATKIN B, WANG Hsiu-Wen, et al. An atomistic carbide-derived carbon model generated using ReaxFF-based quenched molecular dynamics[J]. C, 2017, 3(4): 32. |
| 96 | LI Longqiu, XU Ming, SONG Wenping, et al. The effect of empirical potential functions on modeling of amorphous carbon using molecular dynamics method[J]. Applied Surface Science, 2013, 286: 287-297. |
| 97 | LIU Qingkang, LI Longqiu, JENG Yeau-Ren, et al. Effect of interatomic potentials on modeling the nanostructure of amorphous carbon by liquid quenching method[J]. Computational Materials Science, 2020, 184: 109939. |
| 98 | LÓPEZ M J, CABRIA I, ALONSO J A. Simulated porosity and electronic structure of nanoporous carbons[J]. The Journal of Chemical Physics, 2011, 135(10): 104706. |
| 99 | NORDLUND K, KEINONEN J, MATTILA T. Formation of ion irradiation induced small-scale defects on graphite surfaces[J]. Physical Review Letters, 1996, 77(4): 699-702. |
| 100 | DE TOMAS C, SUAREZ-MARTINEZ I, VALLEJOS-BURGOS F, et al. Structural prediction of graphitization and porosity in carbide-derived carbons[J]. Carbon, 2017, 119: 1-9. |
| 101 | DE TOMAS C, SUAREZ-MARTINEZ I, MARKS N A. Carbide-derived carbons for dense and tunable 3D graphene networks[J]. Applied Physics Letters, 2018, 112(25): 251907. |
| 102 | WANG Yongchao, ZHU Yinbo, WU Hengan. Porous characteristics of three-dimensional disordered graphene networks[J]. Crystals, 2021, 11(2): 127. |
| 103 | WANG Yongchao, ZHU Yinbo, WU Hengan. Formation and topological structure of three-dimensional disordered graphene networks[J]. Physical Chemistry Chemical Physics, 2021, 23(17): 10290-10302. |
| 104 | CORRENTE N J, HINKS E L, KASERA A, et al. Modeling adsorption of simple fluids and hydrocarbons on nanoporous carbons[J]. Carbon, 2022, 197: 526-533. |
| 105 | RANGANATHAN R, ROKKAM S, DESAI T, et al. Generation of amorphous carbon models using liquid quench method: A reactive molecular dynamics study[J]. Carbon, 2017, 113: 87-99. |
| 106 | LUO Zhifen, BURROWS S A, FAN Xiaoli, et al. Virtual voids method to generate low-density microporous carbon structures using quenched molecular dynamics simulation[J]. Carbon, 2021, 183: 438-448. |
| 107 | NEWCOMB B A. Processing, structure, and properties of carbon fibers[J]. Composites Part A: Applied Science and Manufacturing, 2016, 91: 262-282. |
| 108 | SAHA B, SCHATZ G C. Carbonization in polyacrylonitrile (PAN) based carbon fibers studied by ReaxFF molecular dynamics simulations[J]. The Journal of Physical Chemistry B, 2012, 116(15): 4684-4692. |
| 109 | PENEV E S, ARTYUKHOV V I, YAKOBSON B I. Basic structural units in carbon fibers: Atomistic models and tensile behavior[J]. Carbon, 2015, 85: 72-78. |
| 110 | JOSHI K, AREFEV M I, ZHIGILEI L V. Generation and characterization of carbon fiber microstructures by atomistic simulations[J]. Carbon, 2019, 152: 396-408. |
| 111 | DESAI S, LI Chunyu, SHEN Tongtong, et al. Molecular modeling of the microstructure evolution during carbon fiber processing[J]. The Journal of Chemical Physics, 2017, 147(22): 224705. |
| 112 | SHI L Y, SESSIM M, TONKS M R, et al. Generation and characterization of an improved carbon fiber model by molecular dynamics[J]. Carbon, 2021, 173: 232-244. |
| 113 | SHI Linyuan, SESSIM M, TONKS M R, et al. High-temperature oxidation of carbon fiber and char by molecular dynamics simulation[J]. Carbon, 2021, 185: 449-463. |
| 114 | SHEN V K, SIDERIUS D W, MAHYNSKI N A. Molecular simulation of capillary phase transitions in flexible porous materials[J]. The Journal of Chemical Physics, 2018, 148(12): 124115. |
| [1] | GUO Yingchun, LIANG Xiaoyi. Effect of citric acid modification on the spherical activated carbon's ammonia adsorption performance [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1082-1088. |
| [2] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [3] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [4] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [5] | OUYANG Sufang, ZHOU Daowei, HUANG Wei, JIA Feng. Research progress on novel anti-migration rubber antioxidants [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3708-3719. |
| [6] | ZHAO Yi, YANG Zhen, ZHANG Xinwei, WANG Gang, YANG Xuan. Molecular simulation of self-healing behavior of asphalt under different crack damage and healing temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3147-3156. |
| [7] | YANG Farong, GU Lili, LIU Yang, LI Weixue, CAI Jieyun, WANG Huiping. Preparation and application of molecularly imprinted polymers of terbutylazine assisted by computer simulation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3157-3166. |
| [8] | LI Ruidong, HUANG Hui, TONG Guohu, WANG Yueshe. Hygroscopic properties and corrosion behavior of ammonium salt in a crude oil distillation column [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2809-2818. |
| [9] | XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539. |
| [10] | ZHOU Hongyang, ZHOU Yihuan, ZHANG Lianxiu, LIANG Dingcheng, XIE Qiang. Heel of VOCs in activated carbon: Formation mechanism and influencing factors [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5969-5980. |
| [11] | LI Zhi, PEI Jialing, LI Nan, KAN Jingyu, GUO Xuqiang, LIU Bei, CHEN Guangjin. Simulation on the micro formation process of methane hydrate in the fixed bed filled with wet materials [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5689-5699. |
| [12] | LIU Yajuan. Research status of membrane fouling mitigation by PAC in submerged PAC-AMBRs [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 457-468. |
| [13] | LIU Nan, HU Yiming, YANG Ying, LI Hongjin, GAO Zhuqing, HAO Xiuli. Microwave assisted co-pyrolysis of waste polypropylene /activated carbon to produce combustible pyrolysis gas and light pyrolysis oil [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 150-159. |
| [14] | CHEN Xiaoyun, GUO Yadong, DI Lu, BI Dongmei, LI Kaikai, LIN Xiaona. Catalytic co-pyrolysis of biomass and plastic for aromatics production with boron doped activated carbon catalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 199-209. |
| [15] | SONG Chao, YE Xuemin, LI Chunxi. Molecular dynamics study on the influence of self-assembly behaviors of nanoparticles and surfactants on the properties of silicone oil/water interface [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 366-375. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||