Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 5969-5980.DOI: 10.16085/j.issn.1000-6613.2022-2376
• Resources and environmental engineering • Previous Articles
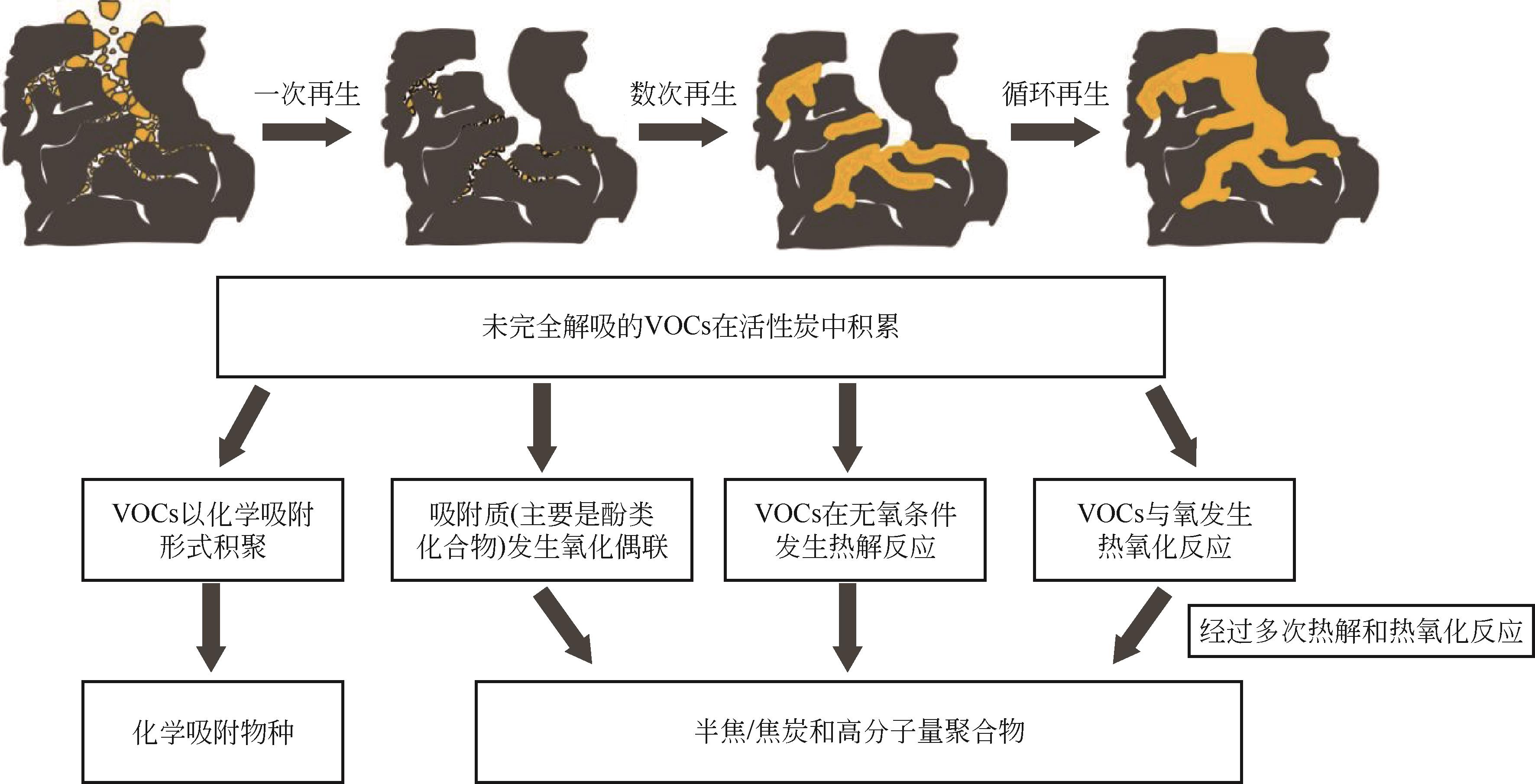
Heel of VOCs in activated carbon: Formation mechanism and influencing factors
ZHOU Hongyang1( ), ZHOU Yihuan1, ZHANG Lianxiu2, LIANG Dingcheng1, XIE Qiang1(
), ZHOU Yihuan1, ZHANG Lianxiu2, LIANG Dingcheng1, XIE Qiang1( )
)
- 1.School of Chemical and Environmental Engineering, China University of Mining and Technology(Beijing), Beijing 100083, China
2.Institute for Artificial Environment Industry, Shandong Grad Group, Dezhou 253000, Shandong, China
-
Received:2022-12-28Revised:2023-03-01Online:2023-12-15Published:2023-11-20 -
Contact:XIE Qiang
VOCs在活性炭中的堆积:形成机制及影响因素
周红阳1( ), 周逸寰1, 张连秀2, 梁鼎成1, 解强1(
), 周逸寰1, 张连秀2, 梁鼎成1, 解强1( )
)
- 1.中国矿业大学(北京)化学与环境工程学院,北京 100083
2.山东格瑞德集团人工环境产业设计研究院,山东 德州 253000
-
通讯作者:解强 -
作者简介:周红阳(1994—),女,博士研究生,研究方向为多孔炭材料。E-mail:hongyang_zhou@163.com。 -
基金资助:国家重点研发计划(2022YFC3701901)
CLC Number:
Cite this article
ZHOU Hongyang, ZHOU Yihuan, ZHANG Lianxiu, LIANG Dingcheng, XIE Qiang. Heel of VOCs in activated carbon: Formation mechanism and influencing factors[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5969-5980.
周红阳, 周逸寰, 张连秀, 梁鼎成, 解强. VOCs在活性炭中的堆积:形成机制及影响因素[J]. 化工进展, 2023, 42(11): 5969-5980.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2376
| 吸附质 | 吸附剂 平均孔径或微孔率 | 结论 | 参考 文献 |
|---|---|---|---|
| 苯酚、邻甲酚 | GAC 2.48nm ACF 1.76nm/2.10nm | 吸附质的低聚反应随着孔径增加而增加,多聚体的形成与吸附剂的孔径有关 | [ |
邻甲酚、2-乙基苯酚 (单一吸附质及二元吸附质) | GAC 1~50nm ACF 1.92nm/2.1nm/2.38nm/2.51nm | 对于单一吸附质,ACF的孔径越小,吸附能力更高,而且不易发生低聚反应;对于二元吸附质,竞争吸附会导致ACF吸附位点减少,任一吸附质低聚反应均减少 | [ |
苯酚、2-甲基苯酚、2-乙基苯酚 (单一吸附质、二元及三元吸附质) | GAC 0.8~80nm ACF 0.8nm/1.28nm | 对于三元吸附质,尽管单溶质和二元溶质系统会在ACF上发生低聚,但由于孔径较窄(12.8Å临界孔径,1Å=0.1nm),阻碍三元溶质系统发生低聚反应 | [ |
| 2-甲基苯酚、2-硝基苯酚、2-氯苯酚(二元及三元吸附质) | GAC 0.4nm~80nm ACF 0.8nm/1.28nm | 孔径范围较窄,临界孔径较大能有效阻碍低聚反应,但是低聚反应还与吸附剂的竞争吸附有关 | [ |
| DBT | PACS微孔率36.9%/30.2%/39.8%/31.3% | PACS的窄微孔有较高的吸附潜力,在解吸过程中,随着温度升高,DBT中的硫会与活性炭表面化学键结合,而且DBT会发生聚合反应形成聚合物难以从PACS中去除 | [ |
| 苯酚、2-甲基苯酚、2-乙基苯酚 | 未经碱活化AC微孔率60.7% 碱活化AC微孔率52.8%/55.0%/64.9%/71.6%/72.4% | 以烟煤为原料,KOH活化制备的微孔率较高,酸性官能团更多的活性炭能有效地阻止酚类化合物发生低聚 | [ |
| TMB | ACFC 0.6nm/0.7nm/0.86nm | 孔径较小的ACFC 10经过再生循环后吸附容量降低83%,而孔径较大的ACFC 15和ACFC 20的吸附容量分别只有9%和5%的变化 | [ |
| 苯、甲苯 | AC 微孔率54%/74%/79%/80%/86% | 窄微孔数量越多会降低活性炭循环再生效率 | [ |
| 9种含有不同有机基团的化合物 | AC 微孔率30%/43%/78%/88%/78% | 孔隙堆积的形成与活性炭微孔体积成线性关系,即微孔体积越大,活性炭孔隙堆积量越多 | [ |
| TMB | AC 微孔率37.1%/85.5%/97.2% | 微孔率最高的活性炭脱附过程产生的堆积量最多,而每个样品的中、大孔体积几乎保持不变 | [ |
| 吸附质 | 吸附剂 平均孔径或微孔率 | 结论 | 参考 文献 |
|---|---|---|---|
| 苯酚、邻甲酚 | GAC 2.48nm ACF 1.76nm/2.10nm | 吸附质的低聚反应随着孔径增加而增加,多聚体的形成与吸附剂的孔径有关 | [ |
邻甲酚、2-乙基苯酚 (单一吸附质及二元吸附质) | GAC 1~50nm ACF 1.92nm/2.1nm/2.38nm/2.51nm | 对于单一吸附质,ACF的孔径越小,吸附能力更高,而且不易发生低聚反应;对于二元吸附质,竞争吸附会导致ACF吸附位点减少,任一吸附质低聚反应均减少 | [ |
苯酚、2-甲基苯酚、2-乙基苯酚 (单一吸附质、二元及三元吸附质) | GAC 0.8~80nm ACF 0.8nm/1.28nm | 对于三元吸附质,尽管单溶质和二元溶质系统会在ACF上发生低聚,但由于孔径较窄(12.8Å临界孔径,1Å=0.1nm),阻碍三元溶质系统发生低聚反应 | [ |
| 2-甲基苯酚、2-硝基苯酚、2-氯苯酚(二元及三元吸附质) | GAC 0.4nm~80nm ACF 0.8nm/1.28nm | 孔径范围较窄,临界孔径较大能有效阻碍低聚反应,但是低聚反应还与吸附剂的竞争吸附有关 | [ |
| DBT | PACS微孔率36.9%/30.2%/39.8%/31.3% | PACS的窄微孔有较高的吸附潜力,在解吸过程中,随着温度升高,DBT中的硫会与活性炭表面化学键结合,而且DBT会发生聚合反应形成聚合物难以从PACS中去除 | [ |
| 苯酚、2-甲基苯酚、2-乙基苯酚 | 未经碱活化AC微孔率60.7% 碱活化AC微孔率52.8%/55.0%/64.9%/71.6%/72.4% | 以烟煤为原料,KOH活化制备的微孔率较高,酸性官能团更多的活性炭能有效地阻止酚类化合物发生低聚 | [ |
| TMB | ACFC 0.6nm/0.7nm/0.86nm | 孔径较小的ACFC 10经过再生循环后吸附容量降低83%,而孔径较大的ACFC 15和ACFC 20的吸附容量分别只有9%和5%的变化 | [ |
| 苯、甲苯 | AC 微孔率54%/74%/79%/80%/86% | 窄微孔数量越多会降低活性炭循环再生效率 | [ |
| 9种含有不同有机基团的化合物 | AC 微孔率30%/43%/78%/88%/78% | 孔隙堆积的形成与活性炭微孔体积成线性关系,即微孔体积越大,活性炭孔隙堆积量越多 | [ |
| TMB | AC 微孔率37.1%/85.5%/97.2% | 微孔率最高的活性炭脱附过程产生的堆积量最多,而每个样品的中、大孔体积几乎保持不变 | [ |
| 样品 | 吸附质 | 再生方法 | 结论 | 参考文献 |
|---|---|---|---|---|
| AC | 正十二烷 | 微波加热 | 微波加热对吸附剂完全再生时所需的最小能量是传导加热再生所需能量的6% | [ |
| AC | 甲基乙基酮 | 微波加热 | 吸附甲基乙基酮的活性炭经微波再生基本恢复吸附能力。在20次吸附/再生循环后,吸附容量从13.5g甲乙酮(MEK)/100g GAC降至12.5g MEK/100g GAC | [ |
| AC | 甲基乙基酮和甲苯 | 微波加热 | 在微波功率为600W的条件下,微波再生可在8min内去除大部分吸附质,去除率高达93.03% | [ |
| SiC-AC | 甲苯 | 微波加热 | SiC-AC中掺杂了高导热材料碳化硅,提高了活性炭的导热性能,再生时可以均匀地分散微波辐射过程中的热量,甲苯可以快速解吸,减少堆积,节约能耗 | [ |
| PAC | CAP | 超声 | 超声辅助解吸方法有效地恢复了中孔和大孔的体积和比表面积;超声过程中产生的HO·自由基也可能在PAC再生中发挥重要作用,但是再生PAC对吸附质分子的选择性显著降低 | [ |
| ACF | 正己烷、甲基乙基酮和甲苯 | 超临界CO2 | 在超临界CO2再生实验中,较高压力更有利于再生,但在每个压力条件下的最佳操作温度为318K | [ |
| 样品 | 吸附质 | 再生方法 | 结论 | 参考文献 |
|---|---|---|---|---|
| AC | 正十二烷 | 微波加热 | 微波加热对吸附剂完全再生时所需的最小能量是传导加热再生所需能量的6% | [ |
| AC | 甲基乙基酮 | 微波加热 | 吸附甲基乙基酮的活性炭经微波再生基本恢复吸附能力。在20次吸附/再生循环后,吸附容量从13.5g甲乙酮(MEK)/100g GAC降至12.5g MEK/100g GAC | [ |
| AC | 甲基乙基酮和甲苯 | 微波加热 | 在微波功率为600W的条件下,微波再生可在8min内去除大部分吸附质,去除率高达93.03% | [ |
| SiC-AC | 甲苯 | 微波加热 | SiC-AC中掺杂了高导热材料碳化硅,提高了活性炭的导热性能,再生时可以均匀地分散微波辐射过程中的热量,甲苯可以快速解吸,减少堆积,节约能耗 | [ |
| PAC | CAP | 超声 | 超声辅助解吸方法有效地恢复了中孔和大孔的体积和比表面积;超声过程中产生的HO·自由基也可能在PAC再生中发挥重要作用,但是再生PAC对吸附质分子的选择性显著降低 | [ |
| ACF | 正己烷、甲基乙基酮和甲苯 | 超临界CO2 | 在超临界CO2再生实验中,较高压力更有利于再生,但在每个压力条件下的最佳操作温度为318K | [ |
| 样品 | 改性剂 | 浓度 | —COOH/mmol·g-1 | —COOR/mmol·g-1 | —OH/mmol·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|
| AC | HNO3 (体积分数) | 0 | 0.025 | 0.099 | 0.249 | [ |
| 20% | 0.275 | 0.276 | 0.299 | |||
| 40% | 0.703 | 0.743 | 0.614 | |||
| 80% | 0.131 | 0.651 | 0.050 | |||
| AC | HNO3(mol/L) | 0 | 0.25 | 0.6 | 0.45 | [ |
| 7 | 1.5 | 4.37 | 8.37 | |||
| AC1 | HNO3(体积分数) | 0 | 0.5036 | 0.0454 | 0.1037 | [ |
| 32.5% | 1.5151 | 0.4265 | 0.5146 | |||
| AC2 | 0 | 0.5097 | 0.1459 | 0.2144 | ||
| 32.5% | 1.7144 | 0.4406 | 0.5496 | |||
| AC | HNO3(体积分数) | 0 | 0.08 | 0.29 | — | [ |
| 35% | 0.47 | 0.09 | 0.02 | |||
| 65% | 0.58 | 0.18 | 0.02 |
| 样品 | 改性剂 | 浓度 | —COOH/mmol·g-1 | —COOR/mmol·g-1 | —OH/mmol·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|
| AC | HNO3 (体积分数) | 0 | 0.025 | 0.099 | 0.249 | [ |
| 20% | 0.275 | 0.276 | 0.299 | |||
| 40% | 0.703 | 0.743 | 0.614 | |||
| 80% | 0.131 | 0.651 | 0.050 | |||
| AC | HNO3(mol/L) | 0 | 0.25 | 0.6 | 0.45 | [ |
| 7 | 1.5 | 4.37 | 8.37 | |||
| AC1 | HNO3(体积分数) | 0 | 0.5036 | 0.0454 | 0.1037 | [ |
| 32.5% | 1.5151 | 0.4265 | 0.5146 | |||
| AC2 | 0 | 0.5097 | 0.1459 | 0.2144 | ||
| 32.5% | 1.7144 | 0.4406 | 0.5496 | |||
| AC | HNO3(体积分数) | 0 | 0.08 | 0.29 | — | [ |
| 35% | 0.47 | 0.09 | 0.02 | |||
| 65% | 0.58 | 0.18 | 0.02 |
| 样品 | 改性方法 | 改性结果 | 参考文献 |
|---|---|---|---|
| AC | 氟气 | 在活性炭表面引入C—F键;微孔体积的减少 | [ |
| ACF | 氟气和氧气 | 断裂C—C键,引入C—O和 C—F键;随着氧氟化时间的增加,孔体积有所减少,因为新形成的C—F和C—O键从一定程度上堵塞活性碳纤维表面微孔 | [ |
| ACF | 氟气和氧气 | 0.6~1.0nm孔径范围内的孔体积下降;微孔壁被破坏形成更大的孔 | [ |
| ACF | 氟气和氮气 | 微孔宽度显著减小;氟气比越高,引入的C—F键越不稳定;含氧基团的存在也可能影响C—F键的性质和稳定性 | [ |
| 样品 | 改性方法 | 改性结果 | 参考文献 |
|---|---|---|---|
| AC | 氟气 | 在活性炭表面引入C—F键;微孔体积的减少 | [ |
| ACF | 氟气和氧气 | 断裂C—C键,引入C—O和 C—F键;随着氧氟化时间的增加,孔体积有所减少,因为新形成的C—F和C—O键从一定程度上堵塞活性碳纤维表面微孔 | [ |
| ACF | 氟气和氧气 | 0.6~1.0nm孔径范围内的孔体积下降;微孔壁被破坏形成更大的孔 | [ |
| ACF | 氟气和氮气 | 微孔宽度显著减小;氟气比越高,引入的C—F键越不稳定;含氧基团的存在也可能影响C—F键的性质和稳定性 | [ |
| 1 | NAIR Abhilash T, SENTHILNATHAN Jaganathan, S M Shiva NAGENDRA. Emerging perspectives on VOC emissions from landfill sites: Impact on tropospheric chemistry and local air quality[J]. Process Safety and Environmental Protection, 2019, 121: 143-154. |
| 2 | Xiaopu LYU, GUO Hai, WANG Yu, et al. Hazardous volatile organic compounds in ambient air of China[J]. Chemosphere, 2020, 246: 125731. |
| 3 | YANG Cuiting, MIAO Guang, PI Yunhong, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. |
| 4 | SHARMA Himanshu, DHIR Amit. Capture of carbon dioxide using solid carbonaceous and non-carbonaceous adsorbents: A review[J].Environmental Chemistry Letters, 2021, 19(2): 851-873. |
| 5 | GONG Huijuan, ZHOU Shuyu, CHEN Zezhi, et al. Effect of volatile organic compounds on carbon dioxide adsorption performance via pressure swing adsorption for landfill gas upgrading[J]. Renewable Energy, 2019, 135: 811-818. |
| 6 | BHAT Adarsh, VENKAT Maithri, CHEN Xiaoyin, et al. Chemical surface modification of beaded activated carbon: A strategy to inhibit heel accumulation from VOC[J]. Journal of Industrial and Engineering Chemistry, 2021, 103: 205-215. |
| 7 | BREITENBACH J W, PREUSSLER H. Influence of activated carbon on styrene polymerization[J]. Journal of Polymer Science, 1949, 4(6): 751-754. |
| 8 | SADAKNE George S, WHITE James L. An experimental study of adsorption of polymers on activated carbon: Butadiene—styrene polymers and poly(methyl methacrylate)[J]. Journal of Applied Polymer Science, 1973, 17(2): 453-469. |
| 9 | BOKI K, TANADA S, KITA T. Adsorption of styrene monomer on activated carbon[J]. Bulletin of Environmental Contamination and Toxicology, 1980, 24(1): 185-189. |
| 10 | TANADA Seiki, BOKI Keito, TAKAHASHI Hitoshi, et al. Adsorption of styrene on activated carbon and regeneration of spent activated carbon[J]. Chemical and Pharmaceutical Bulletin, 1980, 28(12): 3681-3685. |
| 11 | OLIVIER Marie-Georges, BERLIER Karl, BOUGARD Jacques. Adsorption of 2-methylpropene and 1,3-butadiene on activated carbon[J]. Journal of Chemical & Engineering Data, 1994, 39(4): 774-776. |
| 12 | OLIVIER Marie-Georges, BERLIER Karl, JADOT Roger. Adsorption of butane, 2-methylpropane, and 1-butene on activated carbon[J]. Journal of Chemical & Engineering Data, 1994, 39(4): 770-773. |
| 13 | MATUSIK Jakub, Anna KOTEJA-KUNECKA, MAZIARZ Paulina, et al. Styrene removal by surfactant-modified smectite group minerals: Efficiency and factors affecting adsorption/desorption[J]. Chemical Engineering Journal, 2022, 428: 130848. |
| 14 | SUZUKI Motoyuki, MISIC Dragoslav M, KOYAMA Osamu, et al. Study of thermal regeneration of spent activated carbons: Thermogravimetric measurement of various single component organics loaded on activated carbons[J]. Chemical Engineering Science, 1978, 33(3): 271-279. |
| 15 | GRANT Terry M, Judson KING C. Mechanism of irreversible adsorption of phenolic compounds by activated carbons[J]. Industrial & Engineering Chemistry Research, 1990, 29(2): 264-271. |
| 16 | CHATZOPOULOS Dimitrios, VARMA Arvind. Aqueous-phase adsorption and desorption of toluene in activated carbon fixed beds: Experiments and model[J]. Chemical Engineering Science, 1995, 50(1): 127-141. |
| 17 | DĄBROWSKI A, PODKOŚCIELNY P, HUBICKI Z, et al. Adsorption of phenolic compounds by activated carbon—A critical review[J]. Chemosphere, 2005, 58(8): 1049-1070. |
| 18 | MASOUD Jahandar Lashaki, MOHAMMADREZA Fayaz, HAIYAN Helena Wang, et al. Effect of adsorption and regeneration temperature on irreversible adsorption of organic vapors on beaded activated carbon[J]. Environmental Science & Technology, 2012, 46(7): 4083-4090. |
| 19 | Özgür AKTAŞ, Ferhan ÇEÇEN. Bioregeneration of activated carbon: A review[J]. International Biodeterioration & Biodegradation, 2007, 59(4): 257-272. |
| 20 | KHAN Faisal I, GHOSHAL Aloke KR. Removal of volatile organic compounds from polluted air[J]. Journal of Loss Prevention in the Process Industries, 2000, 13(6): 527-545. |
| 21 | MOJTABA HASHEMI Seyed, JAHANDAR LASHAKI Masoud, HASHISHO Zaher, et al. Oxygen impurity in nitrogen desorption purge gas can increase heel buildup on activated carbon[J]. Separation and Purification Technology, 2019, 210: 497-503. |
| 22 | ANIA C O, MENÉNDEZ J A, PARRA J B, et al. Microwave-induced regeneration of activated carbons polluted with phenol. A comparison with conventional thermal regeneration[J]. Carbon, 2004, 42(7): 1383-1387. |
| 23 | WANG Jiacheng, KASKEL Stefan. KOH activation of carbon-based materials for energy storage[J]. Journal of Materials Chemistry, 2012, 22(45): 23710-23725. |
| 24 | NIKNADDAF Saeid, ATKINSON John D, SHARIATY Pooya, et al. Heel formation during volatile organic compound desorption from activated carbon fiber cloth[J]. Carbon, 2016, 96: 131-138. |
| 25 | JAHANDAR LASHAKI Masoud, HASHISHO Zaher, PHILLIPS John H, et al. Mechanisms of heel buildup during cyclic adsorption-desorption of volatile organic compounds in a full-scale adsorber-desorber[J]. Chemical Engineering Journal, 2020, 400: 124937. |
| 26 | IWANAGA Masaru, AMANO Yoshimasa, AIKAWA Masami, et al. Oxidative coupling and dechlorination of aromatic compounds on modified activated carbon[J]. Carbon, 2013, 60: 566. |
| 27 | COONEY David O, XI Zhenpeng. Activated carbon catalyzes reactions of phenolics during liquid-phase adsorption[J]. AIChE Journal, 1994, 40(2): 361-364. |
| 28 | VIDIC Radisav D, SUIDAN Makram T, BRENNER Richard C. Oxidative coupling of phenols on activated carbon: Impact on adsorption equilibrium[J]. Environmental Science & Technology, 1993, 27(10): 2079-2085. |
| 29 | LIU Pin, LIU Xiongmin, SABURI Tei, et al. Thermal characteristics and hazard of 1,3-butadiene (BD) polymerization and oxidation[J]. Thermochimica Acta, 2020, 691: 178713. |
| 30 | TERZYK Artur P. The impact of carbon surface chemical composition on the adsorption of phenol determined at the real oxic and anoxic conditions[J]. Applied Surface Science, 2007, 253(13): 5752-5755. |
| 31 | TERZYK Artur P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption[J]. Journal of Colloid and Interface Science, 2003, 268(2): 301-329. |
| 32 | VIDIC Radisav D, SUIDAN Makram T. Role of dissolved oxygen on the adsorptive capacity of activated carbon for synthetic and natural organic matter[J]. Environmental Science & Technology, 1991, 25(9): 1612-1618. |
| 33 | LU Qiuli, SORIAL George A. The effect of functional groups on oligomerization of phenolics on activated carbon[J]. Journal of Hazardous Materials, 2007, 148(1/2): 436-445. |
| 34 | TENG Hsisheng, HSIEH Chien-To. Activation energy for oxygen chemisorption on carbon at low temperatures[J]. Industrial & Engineering Chemistry Research, 1999, 38(1): 292-297. |
| 35 | MAGNE P, WALKER P L. Phenol adsorption on activated carbons: Application to the regeneration of activated carbons polluted with phenol[J]. Carbon, 1986, 24(2): 101-107. |
| 36 | NAKHLA G, ABUZAID N, FAROOQ S. Activated carbon adsorption of phenolics in oxic systems: Effect of pH and temperature variations[J]. Water Environment Research, 1994, 66(6): 842-850. |
| 37 | ÇALıŞKAN E, BERMÚDEZ J M, PARRA J B, et al. Low temperature regeneration of activated carbons using microwaves: Revising conventional wisdom[J]. Journal of Environmental Management, 2012, 102: 134-140. |
| 38 | MOHAMMADREZA Fayaz, POOYA Shariaty, ATKINSON John D, et al. Using microwave heating to improve the desorption efficiency of high molecular weight VOC from beaded activated carbon[J]. Environmental Science & Technology, 2015, 49(7): 4536-4542. |
| 39 | FERRO-GARCIA M A, JOLY J P, RIVERA-UTRILLA J, et al. Thermal desorption of chlorophenols from activated carbons with different porosity[J]. Langmuir, 1995, 11(7): 2648-2651. |
| 40 | NIKNADDAF Saeid, ATKINSON John D, GHOLIDOUST Abedeh, et al. Influence of purge gas flow and heating rates on volatile organic compound decomposition during regeneration of an activated carbon fiber cloth[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3521-3530. |
| 41 | FAYAZ M. Adsorption and microwave regeneration for controlling volatile organic compounds emissions from automotive paint booths[D]. Edmonton: University of Alberta, 2016. |
| 42 | JAHANDAR LASHAKI Masoud, ATKINSON John D, HASHISHO Zaher, et al. Effect of desorption purge gas oxygen impurity on irreversible adsorption of organic vapors[J]. Carbon, 2016, 99: 310-317. |
| 43 | FEIZBAKHSHAN Mohammad, HASHISHO Zaher, CROMPTON David, et al. Effect of activated carbon’s pore size distribution on oxygen induced heel build-up[J]. Journal of Hazardous Materials, 2021: 126905. |
| 44 | KAMRAVAEI Samineh, SHARIATY Pooya, JAHANDAR Lashaki Masoud, et al. Effect of beaded activated carbon fluidization on adsorption of volatile organic compounds[J]. Industrial & Engineering Chemistry Research, 2017, 56(5): 1297-1305. |
| 45 | LIU Paul K T, FELTCH Steve M, WAGNER Norman J. Thermal desorption behavior of aliphatic and aromatic hydrocarbons loaded on activated carbon[J]. Industrial & Engineering Chemistry Research, 1987, 26(8): 1540-1545. |
| 46 | WANG Haiyan, MASOUD Jahandar Lashaki, MOHAMMADREZA Fayaz, et al. Adsorption and desorption of mixtures of organic vapors on beaded activated carbon[J]. Environmental Science & Technology, 2012, 46(15): 8341-8350. |
| 47 | ÁLVAREZ P M, BELTRÁN F J, GÓMEZ-SERRANO V, et al. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol[J]. Water Research, 2004, 38(8): 2155-2165. |
| 48 | JAHANDAR Lashaki Masoud, ATKINSON John D, HASHISHO Zaher, et al. The role of beaded activated carbon’s pore size distribution on heel formation during cyclic adsorption/desorption of organic vapors[J]. Journal of Hazardous Materials, 2016, 315: 42-51. |
| 49 | Özgür AKTAŞ, Ferhan ÇEÇEN. Effect of type of carbon activation on adsorption and its reversibility[J]. Journal of Chemical Technology & Biotechnology, 2006, 81(1): 94-101. |
| 50 | LU Q, SORIAL G. Impact of pore size on competitive adsorption of phenolic compounds[J]. Water Supply, 2004, 4(5/6): 1-7. |
| 51 | LU Qiuli, SORIAL George A. Adsorption of phenolics on activated carbon impact of pore size and molecular oxygen[J]. Chemosphere, 2004, 55(5): 671-679. |
| 52 | LU Qiuli, SORIAL George A. The role of adsorbent pore size distribution in multicomponent adsorption on activated carbon[J]. Carbon, 2004, 42(15): 3133-3142. |
| 53 | LU Qiuli, SORIAL George A. A comparative study of multicomponent adsorption of phenolic compounds on GAC and ACFs[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 89-96. |
| 54 | WANG Qin, LIANG Xiaoyi, ZHANG Rui, et al. Preparation of polystyrene-based activated carbon spheres and their adsorption of dibenzothiophene[J]. New Carbon Materials, 2009, 24(1): 55-60. |
| 55 | YAN Liang, SORIAL George A. Chemical activation of bituminous coal for hampering oligomerization of organic contaminants[J]. Journal of Hazardous Materials, 2011, 197: 311-319. |
| 56 | CARRATALÁ-ABRIL J, LILLO-RÓDENAS M A, LINARES-SOLANO A, et al. Regeneration of activated carbons saturated with benzene or toluene using an oxygen-containing atmosphere[J]. Chemical Engineering Science, 2010, 65(6): 2190-2198. |
| 57 | SONG Xue, WANG Li'ao, GONG Jian, et al. Exploring a new method to study the effects of surface functional groups on adsorption of CO2 and CH4 on activated carbons[J]. Langmuir, 2020, 36(14): 3862-3870. |
| 58 | POPESCU M, JOLY J P, CARRÉ J, et al. Dynamical adsorption and temperature-programmed desorption of VOCs (toluene, butyl acetate and butanol) on activated carbons[J]. Carbon, 2003, 41(4): 739-748. |
| 59 | VIDIC R D, TESSMER C H, URANOWSKI L J. Impact of surface properties of activated carbons on oxidative coupling of phenolic compounds[J]. Carbon, 1997, 35(9): 1349-1359. |
| 60 | AHMARUZZAMAN Md. Adsorption of phenolic compounds on low-cost adsorbents: A review[J]. Advances in Colloid and Interface Science, 2008, 143(1/2): 48-67. |
| 61 | COUGHLIN Robert W, EZRA Fouad S. Role of surface acidity in the adsorption of organic pollutants on the surface of carbon[J]. Environmental Science & Technology, 1968, 2(4): 291-297. |
| 62 | TESSMER Charles H, VIDIC Radisav D, URANOWSKI Lois J. Impact of oxygen-containing surface functional groups on activated carbon adsorption of phenols[J]. Environmental Science & Technology, 1997, 31(7): 1872-1878. |
| 63 | LENG C-C, PINTO N G. Effects of surface properties of activated carbons on adsorption behavior of selected aromatics[J]. Carbon, 1997, 35(9): 1375-1385. |
| 64 | YONGE D R, KEINATH T M, POZNANSKA K, et al. Single-solute irreversible adsorption on granular activated carbon[J]. Environmental Science & Technology, 1985, 19(8): 690-694. |
| 65 | JAHANDAR Lashaki Masoud, ATKINSON John D, HASHISHO Zaher, et al. The role of beaded activated carbon’s surface oxygen groups on irreversible adsorption of organic vapors[J]. Journal of Hazardous Materials, 2016, 317: 284-294. |
| 66 | NEZAMABAD N M. Effect of surface oxygen groups on irreversible adsorption of volatile organic compounds on beaded activated carbon[D]. Edmonton: University of Alberta, 2017. |
| 67 | SORIAL George A, SUIDAN Makram T, VIDIC Radisav D, et al. Effect of GAC characteristics on adsorption of organic pollutants[J]. Water Environment Research, 1993, 65(1): 53-57. |
| 68 | MAHAJAN O P, MORENO-CASTILLA C, WALKER P L. Surface-treated activated carbon for removal of phenol from water[J]. Separation Science and Technology, 1980, 15(10): 1733-1752. |
| 69 | HASHEMI Seyed Mojtaba, FEIZBAKHSHAN Mohammad, HASHISHO Zaher, et al. Heel buildup during thermal desorption of volatile organic compounds off beaded activated carbon in the presence of oxygen impurity[J]. Industrial & Engineering Chemistry Research, 2022, 61(3): 1475-1485. |
| 70 | FEIZBAKHSHAN Mohammad, AMDEBRHAN Biniyam, HASHISHO Zaher, et al. Effects of oxygen impurity and desorption temperature on heel build-up in activated carbon[J]. Chemical Engineering Journal, 2021, 409: 128232. |
| 71 | RAHMANI Keivan, MAMAGHANI Alireza Haghighat, HASHISHO Zaher, et al. Prediction of heel build-up on activated carbon using machine learning[J]. Journal of Hazardous Materials, 2022, 433: 128747. |
| 72 | CHEN Yuting, HUANG Yingpin, WANG Can, et al. Comprehending adsorption of methylethylketone and toluene and microwave regeneration effectiveness for beaded activated carbon derived from recycled waste bamboo tar[J]. Journal of the Air & Waste Management Association, 2020, 70(6): 616-628. |
| 73 | Chang Yul CHA, CARLISLE Charlie T. Microwave process for volatile organic compound abatement[J]. Journal of the Air & Waste Management Association, 2001, 51(12): 1628-1641. |
| 74 | GU Xuexian, SU Zhanjun, XI Hongxia. New activated carbon with high thermal conductivity and its microwave regeneration performance[J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2016, 31(2): 328-333. |
| 75 | ZHANG Tingting, YANG Yanling, LI Xing, et al. Adsorption characteristics of chloramphenicol onto powdered activated carbon and its desorption performance by ultrasound[J]. Environmental Technology, 2021, 42(4): 571-583. |
| 76 | Young-Ki RYU, KIM Kyung-Lim, LEE Changha. Adsorption and desorption of n-hexane, methyl ethyl ketone, and toluene on an activated carbon fiber from supercritical carbon dioxide[J]. Industrial & Engineering Chemistry Research, 2000, 39(7): 2510-2518. |
| 77 | MOURA Flávia C C, RIOS Regiane D F, GALVÃO Breno R L. Emerging contaminants removal by granular activated carbon obtained from residual Macauba biomass[J]. Environmental Science and Pollution Research, 2018, 25(26): 26482-26492. |
| 78 | Ramiro RUIZ-ROSAS, GARCÍA-MATEOS Francisco J, GUTIÉRREZ María Del Carmen, et al. About the role of porosity and surface chemistry of phosphorus-containing activated carbons in the removal of micropollutants[J]. Frontiers in Materials, 2019, 6: 134. |
| 79 | Arash ARAMI-NIYA, DAUD Wan Mohd Ashri Wan, MJALLI Farouq S. Using granular activated carbon prepared from oil palm shell by ZnCl2 and physical activation for methane adsorption[J]. Journal of Analytical and Applied Pyrolysis, 2010, 89(2): 197-203. |
| 80 | LI Jinjun, LU Renjie, DOU Baojuan, et al. Porous graphitized carbon for adsorptive removal of benzene and the electrothermal regeneration[J]. Environmental Science & Technology, 2012, 46(22): 12648-12654. |
| 81 | PEGO Matheus, CARVALHO Janaína, GUEDES David. Surface modifications of activated carbon and its impact on application[J]. Surface Review and Letters, 2019, 26(1): 1830006. |
| 82 | REHMAN Adeela, PARK Mira, PARK Soo-Jin. Current progress on the surface chemical modification of carbonaceous materials[J]. Coatings, 2019, 9(2): 103. |
| 83 | GOKCE Yavuz, AKTAS Zeki. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol[J]. Applied Surface Science, 2014, 313: 352-359. |
| 84 | LIU S X, CHEN X, CHEN X Y, et al. Activated carbon with excellent chromium(Ⅵ) adsorption performance prepared by acid-base surface modification[J]. Journal of Hazardous Materials, 2007, 141(1): 315-319. |
| 85 | LI Kunquan, JIANG Yuan, WANG Xiaohua, et al. Effect of nitric acid modification on the lead(Ⅱ) adsorption of mesoporous biochars with different mesopore size distributions[J]. Clean Technologies and Environmental Policy, 2016, 18(3): 797-805. |
| 86 | QIU Jianghua, WANG Guanghui, BAO Yuncheng, et al. Effect of oxidative modification of coal tar pitch-based mesoporous activated carbon on the adsorption of benzothiophene and dibenzothiophene[J]. Fuel Processing Technology, 2015, 129: 85-90. |
| 87 | TRESSAUD Alain, DURAND Etienne, Christine LABRUGÈRE. Surface modification of several carbon-based materials: Comparison between CF4 RF plasma and direct F2-gas fluorination routes[J]. Journal of Fluorine Chemistry, 2004, 125(11): 1639-1648. |
| 88 | KIM Kyung Hoon, KANG Da Hee, KIM Min Ji, et al. Effect of CF bonds introduced by fluorination on the desalination properties of activated carbon as the cathode for capacitive deionization[J]. Desalination, 2019, 457: 1-7. |
| 89 | PARK Soo-Jin, KIM Byung-Joo. Ammonia removal of activated carbon fibers produced by oxyfluorination[J]. Journal of Colloid and Interface Science, 2005, 291(2): 597-599. |
| 90 | JUNG Min-Jung, KIM Ju Wan, Ji Sun IM, et al. Nitrogen and hydrogen adsorption of activated carbon fibers modified by fluorination[J]. Journal of Industrial and Engineering Chemistry, 2009, 15(3): 410-414. |
| 91 | VELASCO Leticia F, KYUNG Hoon Kim, Lee YOUNG-SEAK, et al. Influence of fluorine doping of activated carbon fibers on their water vapor adsorption characteristics[J]. Frontiers in Chemistry, 2021, 8: 593756. |
| [1] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [2] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [3] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [4] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [5] | XU Zhongshuo, ZHOU Panpan, WANG Yuhui, HUANG Wei, SONG Xinshan. Advances in sulfur iron ore mediated autotrophic denitrification [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4863-4871. |
| [6] | SONG Weitao, SONG Huiping, FAN Zhenlian, FAN Biao, XUE Fangbin. Research progress of fly ash in anti-corrosion coatings [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4894-4904. |
| [7] | LI Xin, YANG Zao, ZHONG Xinru, HAN Haoxuan, ZHUANG Xuning, BAI Jianfeng, DONG Bin, XU Zuxin. Binding mechanism of Pb2+ onto humic acids from sludge hyper-thermophilic composting [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4957-4966. |
| [8] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [9] | YANG Han, ZHANG Yibo, LI Qi, ZHANG Jun, TAO Ying, YANG Quanhong. Practical carbon anodes for sodium-ion batteries: progress and challenge [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4029-4042. |
| [10] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [11] | WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413. |
| [12] | OUYANG Sufang, ZHOU Daowei, HUANG Wei, JIA Feng. Research progress on novel anti-migration rubber antioxidants [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3708-3719. |
| [13] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [14] | WANG Xue, XU Qiyong, ZHANG Chao. Hydrothermal carbonization of the lignocellulosic biomass and application of the hydro-char [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2536-2545. |
| [15] | SI Yinfang, HU Yujie, ZHANG Fan, DONG Hao, SHE Yuehui. Biosynthesis of zinc oxide nanoparticles and its application to antibacterial [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2013-2023. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||