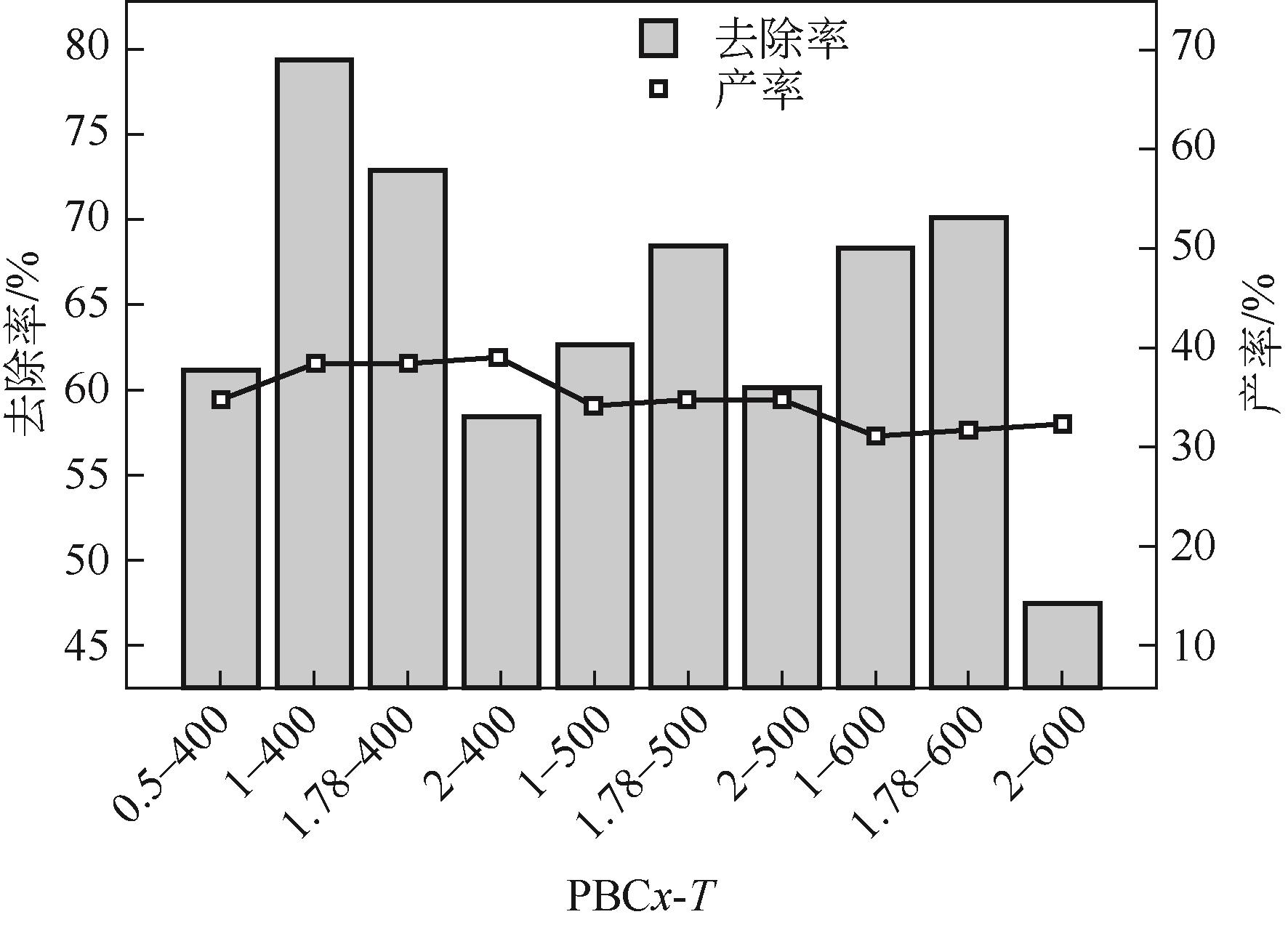
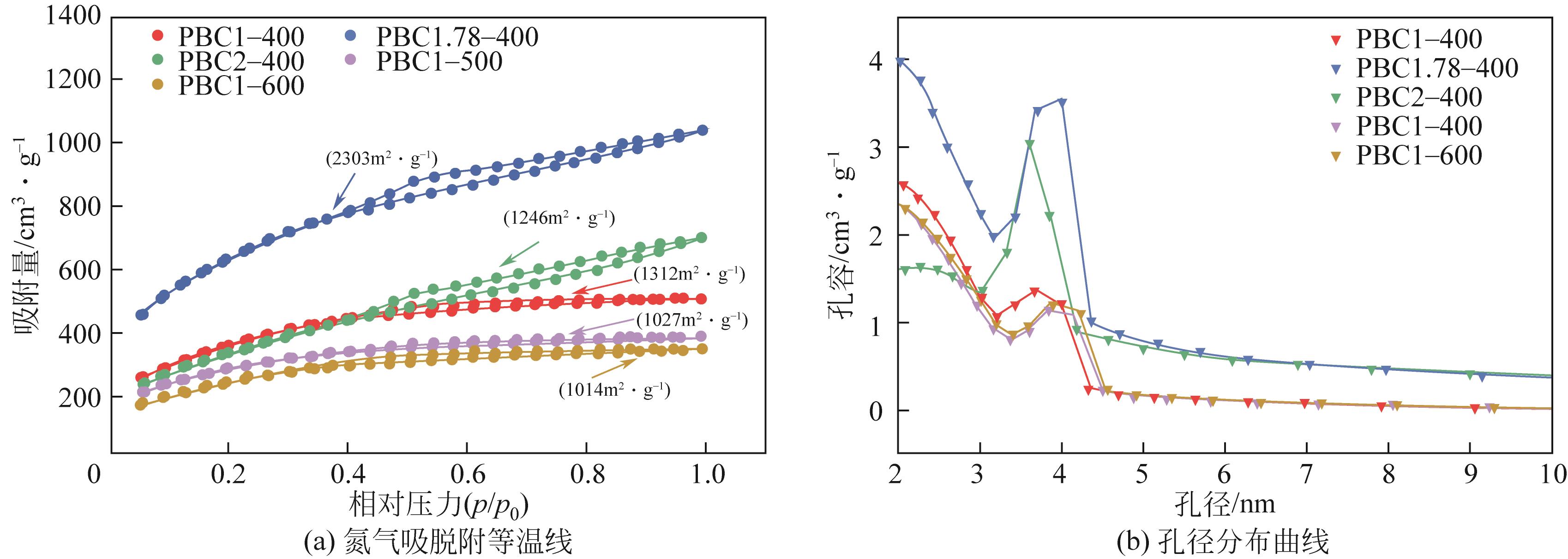
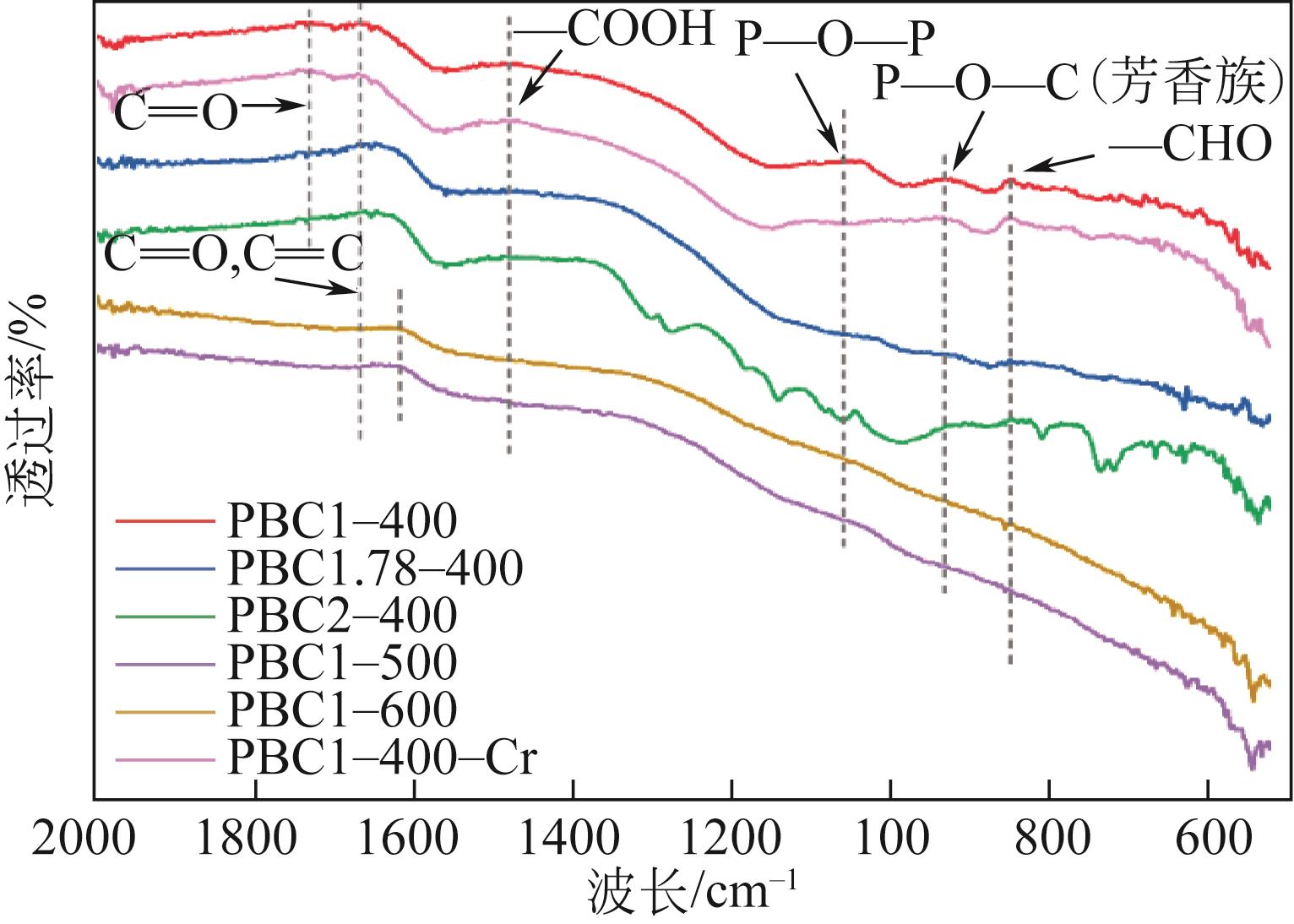
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (3): 1527-1539.DOI: 10.16085/j.issn.1000-6613.2022-0851
• Materials science and technology • Previous Articles Next Articles
Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption
XING Xianjun1,2,3,4( ), LUO Tian1, BU Yuzheng1, MA Peiyong4
), LUO Tian1, BU Yuzheng1, MA Peiyong4
- 1.College of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
2.Advanced Energy Technology and Equipment Research Institute, Hefei University of Technology, Hefei 230009, Anhui, China
3.National New Energy Vehicle Energy Storage and Supply Product Industry Measurement and Testing Center, Hefei 230051, Anhui, China
4.College of Mechanical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
-
Received:2022-05-09Revised:2022-07-03Online:2023-04-10Published:2023-03-15 -
Contact:XING Xianjun
H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用
- 1.合肥工业大学化学与化工学院,安徽 合肥 230009
2.合肥工业大学先进能源技术与装备研究院,安徽 合肥 230009
3.国家新能源汽车储供能产品产业计量测试中心,安徽 合肥 230051
4.合肥工业大学机械工程学院,安徽 合肥 230009
-
通讯作者:邢献军 -
作者简介:邢献军(1964—),男,博士,教授,博士生导师,研究方向为生物质能转化。E-mail:xxianjun@hfut.edu.cn。 -
基金资助:安徽省科技重大专项(2021e03020003)
CLC Number:
Cite this article
XING Xianjun, LUO Tian, BU Yuzheng, MA Peiyong. Preparation of biochar from walnut shells activated by H3PO4 and its application in Cr(Ⅵ) adsorption[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1527-1539.
邢献军, 罗甜, 卜玉蒸, 马培勇. H3PO4活化核桃壳制备活性炭及在Cr(Ⅵ)吸附中的应用[J]. 化工进展, 2023, 42(3): 1527-1539.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0851
| 样品名 | 总比表面积 /m2·g-1 | 总孔容 /cm3·g-1 | 微孔孔容 /cm3·g-1 | 介孔孔容 /cm3·g-1 |
|---|---|---|---|---|
| PBC1-400 | 1312 | 1.04 | 0.09 | 0.95 |
| PBC1.78-400 | 2303 | 1.64 | 0.02 | 1.01 |
| PBC2-400 | 1246 | 0.98 | 0.06 | 0.9 |
| PBC1-500 | 1027 | 0.74 | 0.03 | 0.71 |
| PBC1-600 | 1014 | 0.73 | 0.04 | 0.69 |
| 样品名 | 总比表面积 /m2·g-1 | 总孔容 /cm3·g-1 | 微孔孔容 /cm3·g-1 | 介孔孔容 /cm3·g-1 |
|---|---|---|---|---|
| PBC1-400 | 1312 | 1.04 | 0.09 | 0.95 |
| PBC1.78-400 | 2303 | 1.64 | 0.02 | 1.01 |
| PBC2-400 | 1246 | 0.98 | 0.06 | 0.9 |
| PBC1-500 | 1027 | 0.74 | 0.03 | 0.71 |
| PBC1-600 | 1014 | 0.73 | 0.04 | 0.69 |
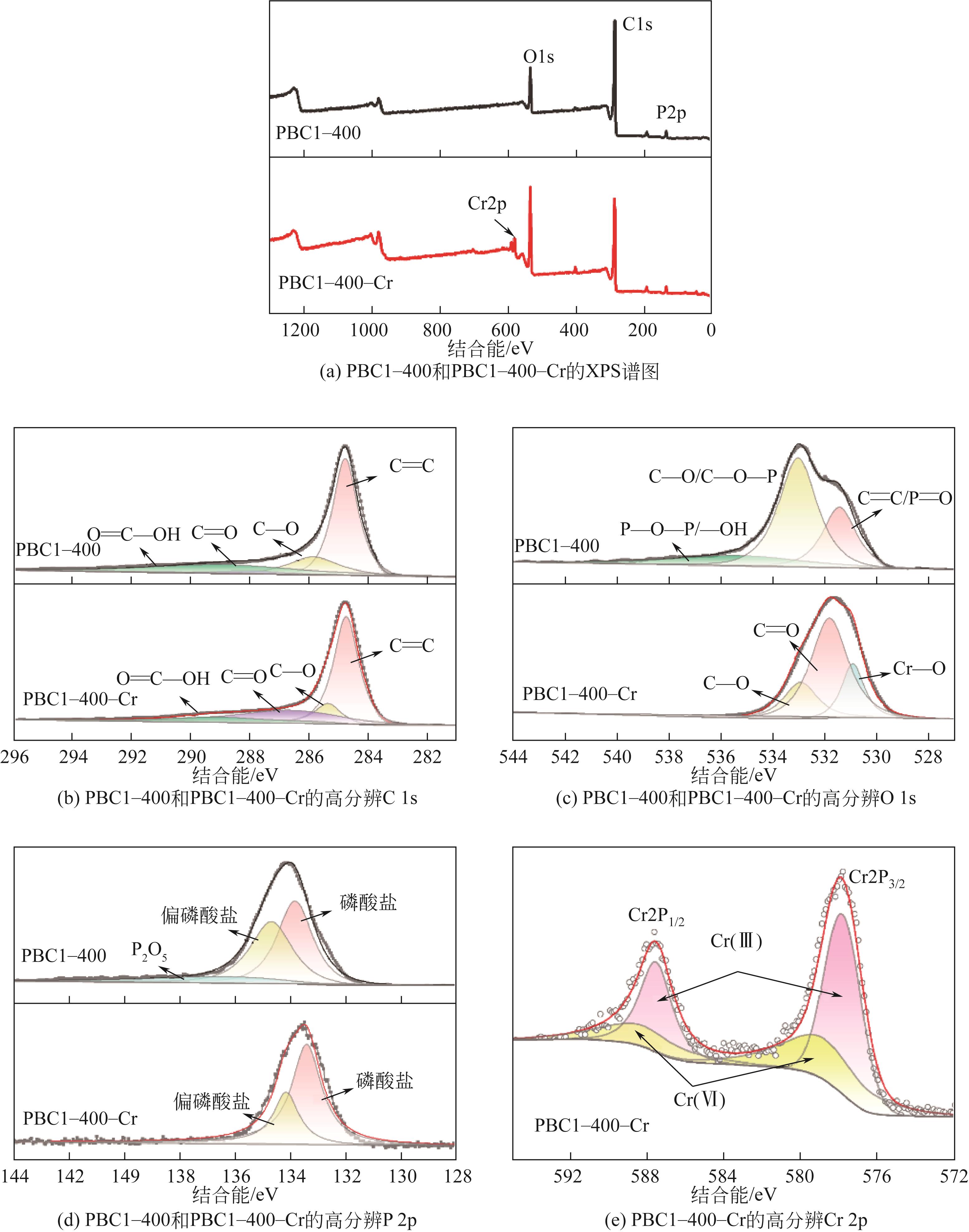
| 元素 | PBC1-400 | PBC1-400-Cr | |||
|---|---|---|---|---|---|
结合能 /eV | 相对原子分数 /% | 结合能 /eV | 相对原子分数 /% | ||
| C 1s | |||||
| C | 284.8 | 53.7 | 284.7 | 64.7 | |
| C—O | 285.8 | 16.7 | 285.4 | 11.3 | |
| C | 288 | 4.8 | 286.6 | 27.3 | |
| O | 288.9 | 24.8 | 289.2 | 6.8 | |
| O 1s | |||||
| C | 531.4 | 27.1 | 531.8 | 60.6 | |
| C—O/C—O—P | 533.4 | 55.7 | 532.9 | 19.9 | |
| P—O—P/—OH | 535.3 | 17.2 | — | — | |
| Cr—O | — | — | 530.9 | 19.5 | |
| P 2p | |||||
| 磷酸盐 | 133.8 | 47.5 | 133.3 | 70 | |
| 偏磷酸盐 | 134.6 | 41.4 | 134.1 | 30 | |
| P2O5 | 136.5 | 11.1 | — | — | |
| Cr 2p 1/2 | |||||
| Cr(Ⅲ) | — | — | 587.5 | 62.9 | |
| Cr(Ⅵ) | — | — | 588.28 | 37.1 | |
| Cr 2p 3/2 | |||||
| Cr(Ⅲ) | — | — | 577.8 | 63.7 | |
| Cr(Ⅵ) | — | — | 578.85 | 36.3 | |
| 元素 | PBC1-400 | PBC1-400-Cr | |||
|---|---|---|---|---|---|
结合能 /eV | 相对原子分数 /% | 结合能 /eV | 相对原子分数 /% | ||
| C 1s | |||||
| C | 284.8 | 53.7 | 284.7 | 64.7 | |
| C—O | 285.8 | 16.7 | 285.4 | 11.3 | |
| C | 288 | 4.8 | 286.6 | 27.3 | |
| O | 288.9 | 24.8 | 289.2 | 6.8 | |
| O 1s | |||||
| C | 531.4 | 27.1 | 531.8 | 60.6 | |
| C—O/C—O—P | 533.4 | 55.7 | 532.9 | 19.9 | |
| P—O—P/—OH | 535.3 | 17.2 | — | — | |
| Cr—O | — | — | 530.9 | 19.5 | |
| P 2p | |||||
| 磷酸盐 | 133.8 | 47.5 | 133.3 | 70 | |
| 偏磷酸盐 | 134.6 | 41.4 | 134.1 | 30 | |
| P2O5 | 136.5 | 11.1 | — | — | |
| Cr 2p 1/2 | |||||
| Cr(Ⅲ) | — | — | 587.5 | 62.9 | |
| Cr(Ⅵ) | — | — | 588.28 | 37.1 | |
| Cr 2p 3/2 | |||||
| Cr(Ⅲ) | — | — | 577.8 | 63.7 | |
| Cr(Ⅵ) | — | — | 578.85 | 36.3 | |
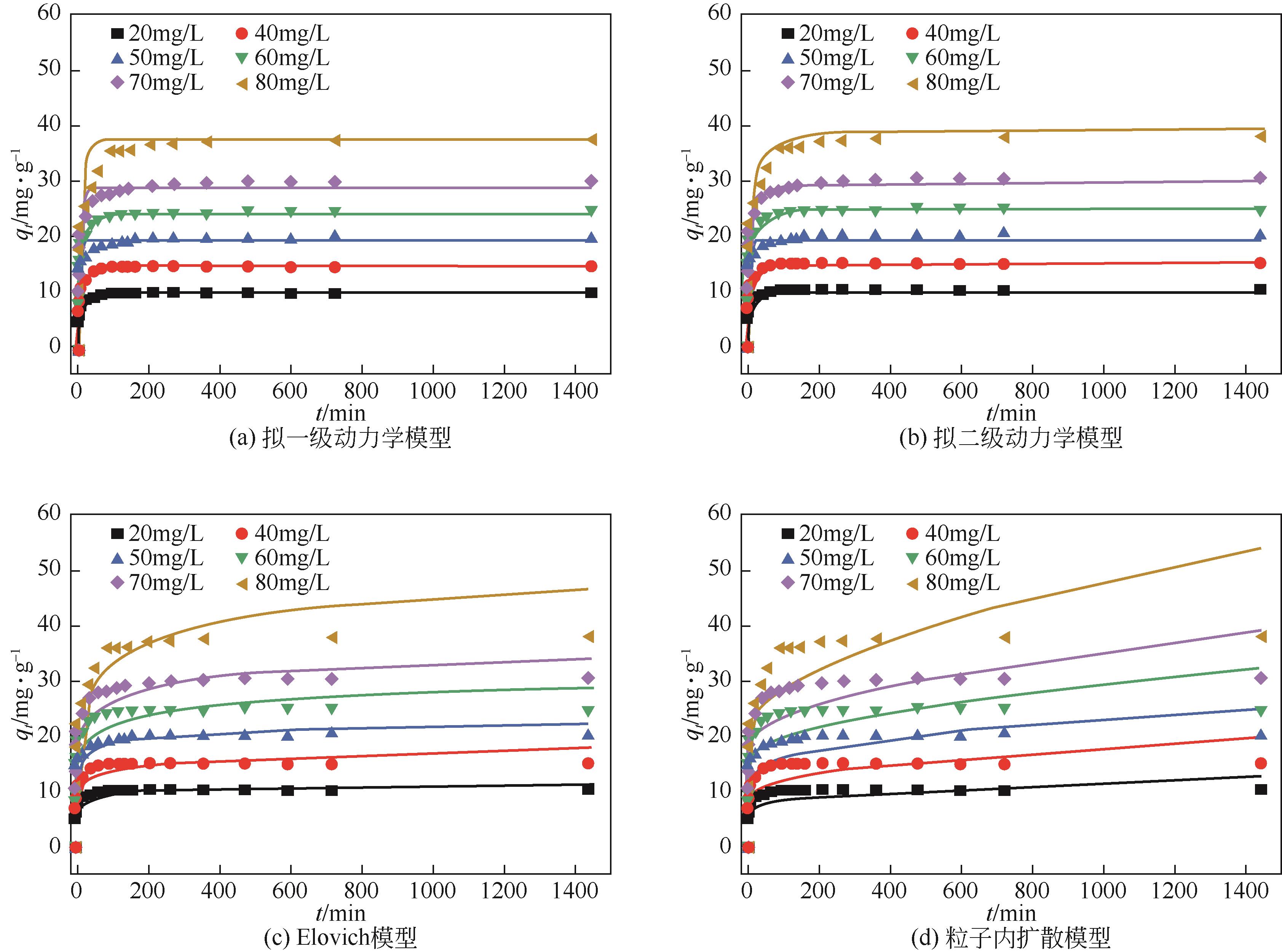
C0 /mg·L-1 | qe,exp /mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | Elovich模型 | 颗粒内扩散模型 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1 /min-1 | R2 | qe,cal /mg·g-1 | k2 /g·mg-1·min-1 | R2 | A /mg·g-1·min-1 | B /g·mg-1 | R2 | C /mg·g-1 | kid /mg·g-1·min-1/2 | R2 | |||||
| 20 | 10 | 9.8 | 0.314 | 0.943 | 10.1 | 0.0574 | 0.986 | 6.057 | 0.706 | 0.918 | 6.82 | 0.149 | 0.301 | |||
| 30 | 15 | 14.6 | 0.165 | 0.933 | 15 | 0.018 | 0.973 | 6.923 | 1.419 | 0.914 | 9.035 | 0.275 | 0.397 | |||
| 40 | 20 | 19 | 0.309 | 0.911 | 19.6 | 0.027 | 0.97 | 11.242 | 1.496 | 0.951 | 12.869 | 0.322 | 0.373 | |||
| 50 | 25 | 24.2 | 0.185 | 0.948 | 25 | 0.011 | 0.981 | 11.468 | 2.356 | 0.913 | 14.96 | 0.458 | 0.4 | |||
| 60 | 30 | 28.6 | 0.188 | 0.939 | 29.6 | 0.009 | 0.985 | 13.285 | 2.846 | 0.937 | 17.268 | 0.561 | 0.438 | |||
| 80 | 39.95 | 35.5 | 0.101 | 0.937 | 37.48 | 0.0039 | 0.982 | 10.744 | 4.608 | 0.941 | 18.327 | 0.85 | 0.484 | |||
C0 /mg·L-1 | qe,exp /mg·g-1 | 拟一级动力学模型 | 拟二级动力学模型 | Elovich模型 | 颗粒内扩散模型 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1 /min-1 | R2 | qe,cal /mg·g-1 | k2 /g·mg-1·min-1 | R2 | A /mg·g-1·min-1 | B /g·mg-1 | R2 | C /mg·g-1 | kid /mg·g-1·min-1/2 | R2 | |||||
| 20 | 10 | 9.8 | 0.314 | 0.943 | 10.1 | 0.0574 | 0.986 | 6.057 | 0.706 | 0.918 | 6.82 | 0.149 | 0.301 | |||
| 30 | 15 | 14.6 | 0.165 | 0.933 | 15 | 0.018 | 0.973 | 6.923 | 1.419 | 0.914 | 9.035 | 0.275 | 0.397 | |||
| 40 | 20 | 19 | 0.309 | 0.911 | 19.6 | 0.027 | 0.97 | 11.242 | 1.496 | 0.951 | 12.869 | 0.322 | 0.373 | |||
| 50 | 25 | 24.2 | 0.185 | 0.948 | 25 | 0.011 | 0.981 | 11.468 | 2.356 | 0.913 | 14.96 | 0.458 | 0.4 | |||
| 60 | 30 | 28.6 | 0.188 | 0.939 | 29.6 | 0.009 | 0.985 | 13.285 | 2.846 | 0.937 | 17.268 | 0.561 | 0.438 | |||
| 80 | 39.95 | 35.5 | 0.101 | 0.937 | 37.48 | 0.0039 | 0.982 | 10.744 | 4.608 | 0.941 | 18.327 | 0.85 | 0.484 | |||
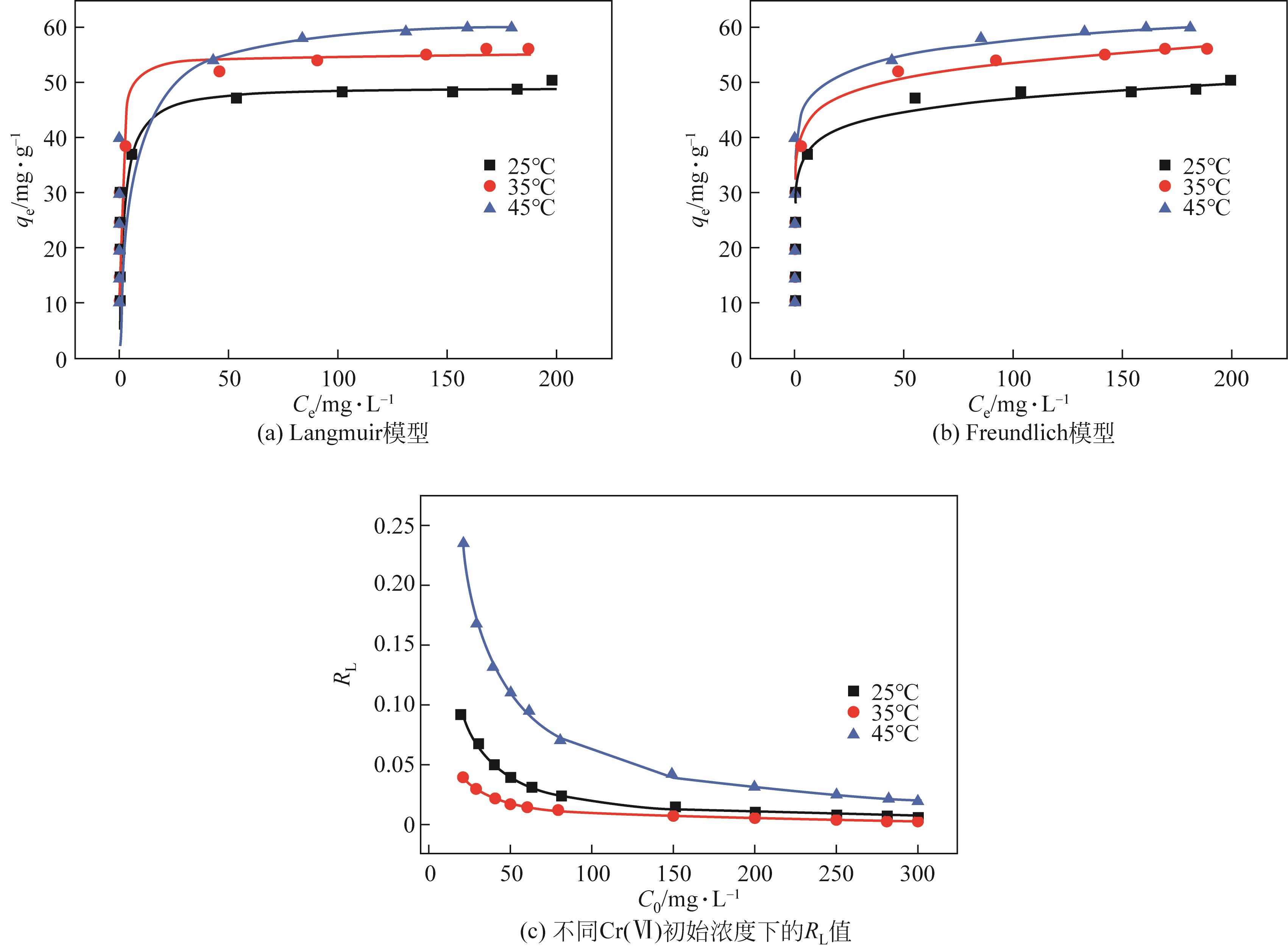
| T/℃ | qe,exp/mg·g-1 | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qmax/mg·g-1 | RL | R2 | KF/mg1-n ·L n ·g-1 | 1/n | R2 | |||
| 25 | 50.33 | 0.483 | 49.597 | 0.005~0.1 | 0.975 | 32.610 | 0.082 | 0.950 | |
| 35 | 56.2 | 1.193 | 55.133 | 0.002~0.05 | 0.953 | 37.428 | 0.079 | 0.985 | |
| 45 | 60 | 0.161 | 62.043 | 0.02~0.3 | 0.993 | 41.845 | 0.070 | 0.945 | |
| T/℃ | qe,exp/mg·g-1 | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qmax/mg·g-1 | RL | R2 | KF/mg1-n ·L n ·g-1 | 1/n | R2 | |||
| 25 | 50.33 | 0.483 | 49.597 | 0.005~0.1 | 0.975 | 32.610 | 0.082 | 0.950 | |
| 35 | 56.2 | 1.193 | 55.133 | 0.002~0.05 | 0.953 | 37.428 | 0.079 | 0.985 | |
| 45 | 60 | 0.161 | 62.043 | 0.02~0.3 | 0.993 | 41.845 | 0.070 | 0.945 | |
| 吸附剂 | 温度/℃ | pH | 吸附剂投入量/g·L-1 | 初始浓度C0/mg·L-1 | 吸附量qmax/mg·g-1 |
|---|---|---|---|---|---|
| 聚硫橡胶改性活性炭[ | 22 | 4 | 3 | 20 | 8.9 |
| 改性污泥[ | 30 | 3 | 10 | 50 | 26.3 |
| 球磨炭[ | 22 | 7 | 50 | 1000 | 28.9 |
| TA改性炭[ | 22 | 5 | 0.8 | 20 | 31.0 |
| 铁负载炭[ | 25 | 3.3 | 1 | 50 | 53.4 |
| 小麦秸秆生物炭[ | 25 | 2 | — | 600 | 24.6 |
| 核桃壳基活性炭(本文) | 45 | 2 | 2 | 300 | 62.0 |
| 吸附剂 | 温度/℃ | pH | 吸附剂投入量/g·L-1 | 初始浓度C0/mg·L-1 | 吸附量qmax/mg·g-1 |
|---|---|---|---|---|---|
| 聚硫橡胶改性活性炭[ | 22 | 4 | 3 | 20 | 8.9 |
| 改性污泥[ | 30 | 3 | 10 | 50 | 26.3 |
| 球磨炭[ | 22 | 7 | 50 | 1000 | 28.9 |
| TA改性炭[ | 22 | 5 | 0.8 | 20 | 31.0 |
| 铁负载炭[ | 25 | 3.3 | 1 | 50 | 53.4 |
| 小麦秸秆生物炭[ | 25 | 2 | — | 600 | 24.6 |
| 核桃壳基活性炭(本文) | 45 | 2 | 2 | 300 | 62.0 |
| 活性炭 | ∆H⊖/kJ·mol-1 | ∆S⊖/J·mol-1·K-1 | ∆G⊖/kJ·mol-1 | ||
|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | |||
| PBC1-400 | 68.52 | 33.84 | -15.38 | -18.14 | -13.44 |
| 活性炭 | ∆H⊖/kJ·mol-1 | ∆S⊖/J·mol-1·K-1 | ∆G⊖/kJ·mol-1 | ||
|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | |||
| PBC1-400 | 68.52 | 33.84 | -15.38 | -18.14 | -13.44 |
| 1 | BUAISHA M, BALKU S, YAMAN Ş Ö. Heavy metal removal investigation in conventional activated sludge systems[J]. Civil Engineering Journal, 2020, 6(3): 470-477. |
| 2 | KANG Li, YANG Hanpei, WANG Lina, et al. Facile integration of FeS and titanate nanotubes for efficient removal of total Cr from aqueous solution: Synergy in simultaneous reduction of Cr(VI) and adsorption of Cr(III)[J]. Journal of Hazardous Materials, 2020, 398: 122834. |
| 3 | ALVARADO L, TORRES I R, CHEN A C. Integration of ion exchange and electrodeionization as a new approach for the continuous treatment of hexavalent chromium wastewater[J]. Separation and Purification Technology, 2013, 105: 55-62. |
| 4 | ZHANG Jishi, ZHENG Pengwei. A preliminary investigation of the mechanism of hexavalent chromium removal by corn-bran residue and derived chars[J]. RSC Advances, 2015, 5(23): 17768-17774. |
| 5 | LIU Wujun, ZENG Fanxin, JIANG Hong, et al. Preparation of high adsorption capacity bio-chars from waste biomass[J]. Bioresource Technology, 2011, 102(17): 8247-8252. |
| 6 | XIAO Xin, CHEN Baoliang, CHEN Zaiming, et al. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review[J]. Environmental Science & Technology, 2018, 52(9): 5027-5047. |
| 7 | LUO Mingke, LIN Hai, LI Bing, et al. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water[J]. Bioresource Technology, 2018, 259: 312-318. |
| 8 | XU Shuang, YU Weiguang, LIU Sen, et al. Adsorption of hexavalent chromium using banana pseudostem biochar and its mechanism[J]. Sustainability, 2018, 10(11): 4250. |
| 9 | ENNIYA I, RGHIOUI L, JOURANI A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels[J]. Sustainable Chemistry and Pharmacy, 2018, 7: 9-16. |
| 10 | NJOKU V O, HAMEED B H. Preparation and characterization of activated carbon from corncob by chemical activation with H3PO4 for 2,4-dichlorophenoxyacetic acid adsorption[J]. Chemical Engineering Journal, 2011, 173(2): 391-399. |
| 11 | RIVERA-UTRILLA J, SÁNCHEZ-POLO M, GÓMEZ-SERRANO V, et al. Activated carbon modifications to enhance its water treatment applications. an overview[J]. Journal of Hazardous Materials, 2011, 187(1/2/3): 1-23. |
| 12 | GUPTA G K, RAM M, BALA R, et al. Pyrolysis of chemically treated corncob for biochar production and its application in Cr(VI) removal[J]. Environmental Progress & Sustainable Energy, 2018, 37(5): 1606-1617. |
| 13 | LYUBCHIK S B, BENOIT R, BÉGUIN F. Influence of chemical modification of anthracite on the porosity of the resulting activated carbons[J]. Carbon, 2002, 40(8): 1287-1294. |
| 14 | HAN Qiaoning, WANG Jing, GOODMAN Bernard A, et al. High adsorption of methylene blue by activated carbon prepared from phosphoric acid treated eucalyptus residue[J]. Powder Technology, 2020, 366: 239-248. |
| 15 | ZHANG Huiyan, YUE Xiupeng, LI Fei, et al. Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups[J]. Science of the Total Environment, 2018, 631/632: 795-802. |
| 16 | CAO Jiashun, LIN Junxiong, FANG Fang, et al. A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies[J]. Bioresource Technology, 2014, 163: 199-205. |
| 17 | DOVI E, ARYEE A A, KANI A N, et al. Functionalization of walnut shell by grafting amine groups to enhance the adsorption of Congo red from water in batch and fixed-bed column modes[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106301. |
| 18 | ZHAO Nan, ZHAO Chuanfang, Yizhong LYU, et al. Adsorption and coadsorption mechanisms of Cr(VI) and organic contaminants on H3PO4 treated biochar[J]. Chemosphere, 2017, 186: 422-429. |
| 19 | GEORGIEVA V G, GONSALVESH L, TAVLIEVA M P. Thermodynamics and kinetics of the removal of nickel(II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells[J]. Journal of Molecular Liquids, 2020, 312: 112788. |
| 20 | FANG Yi, YANG Ke, ZHANG Yipeng, et al. Highly surface activated carbon to remove Cr(VI) from aqueous solution with adsorbent recycling[J]. Environmental Research, 2021, 197: 111151. |
| 21 | HAYASHI J, KAZEHAYA A, MUROYAMA K, et al. Preparation of activated carbon from lignin by chemical activation[J]. Carbon, 2000, 38(13): 1873-1878. |
| 22 | BANERJEE M, BASU R K, DAS S K. Cr(VI) adsorption by a green adsorbent walnut shell: adsorption studies, regeneration studies, scale-up design and economic feasibility[J]. Process Safety and Environmental Protection, 2018, 116: 693-702. |
| 23 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 24 | THOMMES M, KANEKO K, NEIMARK A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 25 | MYGLOVETS M, PODDUBNAYA O I, SEVASTYANOVA O, et al. Preparation of carbon adsorbents from lignosulfonate by phosphoric acid activation for the adsorption of metal ions[J]. Carbon, 2014, 80: 771-783. |
| 26 | 王鲁元, 金春江, 陈惠敏, 等. 一步热解活化法制备纳米木质素基多孔炭材料[J]. 化工进展, 2022, 41(5): 2582-2592. |
| WANG Luyuan, JIN Chunjiang, CHEN Huimin, et al. Preparation of nano-lignin-based porous carbon materials by one-step pyrolysis activation method[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2582-2592. | |
| 27 | SOCRATES G. Infrared characteristic group frequencies[M]. 2nd ed. New York: Wiley, 1994. |
| 28 | CORBRJDGE D E C. Infra-red analysis of phosphorus compounds[J]. Journal of Applied Chemistry, 2007, 6(10): 456-465. |
| 29 | ZHOU Xiang, LIU Xiaohao, QI Fenglei, et al. Efficient preparation of P-doped carbon with ultra-high mesoporous ratio from furfural residue for dye removal[J]. Separation and Purification Technology, 2022, 292: 120954. |
| 30 | SUN Xiaoming, LI Yadong. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles[J]. Angewandte Chemie International Edition, 2004, 43(5): 597-601. |
| 31 | MENG Huan, FAN Ke, Jingxiang LOW, et al. Electrochemically reduced graphene oxide on silicon nanowire arrays for enhanced photoelectrochemical hydrogen evolution[J]. Dalton Transactions, 2016, 45(35): 13717-13725. |
| 32 | SHEN Xuqian, XIAO Fan, ZHAO Hongying, et al. In situ formed PdFe nanoalloy and carbon defects in cathode for synergic reduction-oxidation of chlorinated pollutants in electro-Fenton process[J]. Environmental Science & Technology, 2020, 54(7): 4564-4572. |
| 33 | SADEZKY A, MUCKENHUBER H, GROTHE H, et al. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information[J]. Carbon, 2005, 43(8): 1731-1742. |
| 34 | Honghong LYU, TANG Jingchun, HUANG Yao, et al. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite[J]. Chemical Engineering Journal, 2017, 322: 516-524. |
| 35 | GENOVESE M, JIANG J H, LIAN K, et al. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob[J]. Journal of Materials Chemistry A, 2015, 3(6): 2903-2913. |
| 36 | ZHONG Mengqi, CHEN Si, WANG Teng, et al. Co-pyrolysis of polyester and cotton via thermogravimetric analysis and adsorption mechanism of Cr(VI) removal by carbon in aqueous solution[J]. Journal of Molecular Liquids, 2022, 354: 118902. |
| 37 | JIA Xiuxiu, ZHANG Yunqiu, HE Zhuang, et al. Mesopore-rich badam-shell biochar for efficient adsorption of Cr(VI) from aqueous solution[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105634. |
| 38 | SHI Shunquan, YANG Jiakuan, LIANG Sha, et al. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles[J]. Science of the Total Environment, 2018, 628/629: 499-508. |
| 39 | 周春地, 阳婷, 闵熙泽, 等. 零价铁、铜改性生物炭及其对Cr(Ⅵ)吸附性能的影响[J]. 化工进展, 2020, 39(10): 4275-4282. |
| ZHOU Chundi, YANG Ting, MIN Xize, et al. Influence of zero valent iron and copper modified biochar on Cr(Ⅵ) adsorption[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4275-4282. | |
| 40 | SONG Li, JING Shichao, QIU Yixing, et al. Efficient removal of Cr(Ⅲ)-carboxyl complex from neutral and high-salinity wastewater by nitrogen doped biomass-based composites[J]. Chinese Chemical Letters. 2023, 34: 107180. |
| 41 | DOVI E, ARYEE A A, KANI A N, et al. High-capacity amino-functionalized walnut shell for efficient removal of toxic hexavalent chromium ions in batch and column mode[J]. Journal of Environmental Chemical Engineering, 2023, 10(2): 107180. |
| 42 | GUPTA G K, MONDAL M K. Mechanism of Cr(VI) uptake onto sagwan sawdust derived biochar and statistical optimization via response surface methodology[J]. Biomass Conversion and Biorefinery, 2020: 1-17. |
| 43 | LIU Na, ZHANG Yuting, XU Chao, et al. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study[J]. Journal of Hazardous Materials, 2020, 384: 121371. |
| 44 | WANG Fayuan, WANG Hui, MA Jianwei. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent — Bamboo charcoal[J]. Journal of Hazardous Materials, 2010, 177(1/2/3): 300-306. |
| 45 | DENG Hui, YANG Le, TAO Guanghui, et al. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation — Application in methylene blue adsorption from aqueous solution[J]. Journal of Hazardous Materials, 2009, 166(2/3): 1514-1521. |
| 46 | MORTAZAVIAN S, SABER A, HONG J, et al. Synthesis, characterization, and kinetic study of activated carbon modified by polysulfide rubber coating for aqueous hexavalent chromium removal[J]. Journal of Industrial and Engineering Chemistry, 2019, 69: 196-210. |
| 47 | KYZAS G Z, KOSTOGLOU M. Green adsorbents for wastewaters: A critical review[J]. Materials, 2014, 7(1): 333-364. |
| 48 | LIU Hai, LIANG Shuang, GAO Jinhong, et al. Enhancement of Cr(VI) removal by modifying activated carbon developed from Zizania caduciflora with tartaric acid during phosphoric acid activation[J]. Chemical Engineering Journal, 2014, 246: 168-174. |
| 49 | LI Z Z, KATSUMI T, INUI T, et al. Woods charred at low temperatures and their modification for the adsorption of Cr(VI) ions from aqueous solution[J]. Adsorption Science & Technology, 2010, 28(5): 419-435. |
| 50 | TYTŁAK A, OLESZCZUK P, DOBROWOLSKI R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions[J]. Environmental Science and Pollution Research, 2015, 22(8): 5985-5994. |
| 51 | ZENG Huiting, ZENG Honghu, ZHANG Hua, et al. Efficient adsorption of Cr(VI) from aqueous environments by phosphoric acid activated eucalyptus biochar[J]. Journal of Cleaner Production, 2021, 286: 124964. |
| [1] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [2] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [3] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [6] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [7] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [8] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [9] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [10] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [11] | WANG Shuaiqing, YANG Siwen, LI Na, SUN Zhanying, AN Haoran. Research progress on element doped biomass carbon materials for electrochemical energy storage [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4296-4306. |
| [12] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [13] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [14] | WU Ya, ZHAO Dan, FANG Rongmiao, LI Jingyao, CHANG Nana, DU Chunbao, WANG Wenzhen, SHI Jun. Research progress on highly efficient demulsifiers for complex crude oil emulsions and their applications [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4398-4413. |
| [15] | ZHENG Mengqi, WANG Chengye, WANG Yan, WANG Wei, YUAN Shoujun, HU Zhenhu, HE Chunhua, WANG Jie, MEI Hong. Application and prospect of algal-bacterial symbiosis technology in zero liquid discharge of industrial wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4424-4431. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||