| 1 |
ZHANG Xiaolong, LIU Yanfeng, LIU Long, et al. Microbial production of sialic acid and sialylated human milk oligosaccharides: Advances and perspectives[J]. Biotechnology Advances, 2019,37(5): 787-800.
|
| 2 |
YANG Haiquan, LU Liping, CHEN Xianzhong. An overview and future prospects of sialic acids[J]. Biotechnology Advances, 2021, 46: 107678.
|
| 3 |
LING Alvin Jin Wei, ChANG Lee Sin, BABJI Abdul Salam, et al. Review of sialic acid’s biochemistry, sources, extraction and functions with special reference to edible bird’s nest[J]. Food Chemistry, 2022, 367: 130755.
|
| 4 |
SHEN Gwo Jenn, DATTA Arun K, IZUMI M, et al. Expression of α2,8/2,9-polysialyltransferase fromescherichia coli k92: Characterization of the enzyme and its reaction products[J]. Journal of Biological Chemistry, 1999, 274(49): 35139-35146.
|
| 5 |
JAHAN M, WYNN P, WANG B. Concentration and distribution of sialic acid in sow milk during lactation[J]. Journal of Nutrition & Intermediary Metabolism, 2016, 4: 36.
|
| 6 |
VARKI Ajit. Sialic acids in human health and disease[J]. Trends in Molecular Medicine, 2008,14(8): 351-360.
|
| 7 |
SPICHTIG Véronique, MICHAUD Julien, AUSTIN Sean. Determination of sialic acids in milks and milk-based products[J]. Analytical Biochemistry, 2010, 405(1): 28-40.
|
| 8 |
CHEESEMAN Jack, KUHNLE Gunter, SPENCER Daniel I R, et al. Assays for the identification and quantification of sialic acids: Challenges, opportunities and future perspectives[J]. Bioorganic & Medicinal Chemistry, 2021, 30: 115882.
|
| 9 |
CHAN Tak-Hang, LEE Ming-Chao. Indium-mediated coupling of α- (bromomethyl)acrylic acid with carbonyl compounds in aqueous media. concise syntheses of (+)-3-deoxy-D-glycero-D-galacto-nonulosonic acid and N-acetylneuraminic acid[J]. The Journal of Organic Chemistry, 1995, 60(13): 4228-4232.
|
| 10 |
Claudine AUGÉ, SERJE D, CHRISTINE G. Synthesis with immobilized enzyme of the most important sialic acid[J]. Tetrahedron Letters, 1984, 25(41): 4663-4664.
|
| 11 |
SIMON Ethan S, BEDNARSKI Mark D, WHITESIDES George M. Synthesis of CMP-NeuAc from N-acetylglucosamine: Generation of CTP from CMP using adenylate kinase[J]. Journal of the American Chemical Society, 1988, 110(21): 7159-7163.
|
| 12 |
JUNEJA Lekh Raj, KOKETSU M, KIM M. Large-scale preparation of sialic acid from chalaza and egg-yolk membrane[J]. Carbohydrate Research, 1991, 214(1): 179-186.
|
| 13 |
WHITEHOUSE M W, ZILLIKEN F. Isolation and determination of neuraminic (sialic) acids[J]. Methods of Biochemical Analysis, 1960, 8: 199-220.
|
| 14 |
SCHAUER R, Kelm S, Reuter G, et al. In biology of the sialic acids[M]. New York: Plenum,1995.
|
| 15 |
ZHANG Yinan, TAO Fei, DU Miaofen, et al. An efficient method for N-acetyl-d-neuraminic acid production using coupled bacterial cells with a safe temperature-induced system[J]. Applied Microbiology and Biotechnology, 2010, 86(2): 481-489.
|
| 16 |
邱从平, 刘明霞.一种唾液酸的生产方法:CN1523031A[P]. 2004-08-25.
|
|
QIU Congping, LIU Mingxia. Method for producing sialic acid: CN1523031A[P]. 2004-08-25.
|
| 17 |
NAKAMURA T, KAWASE H, KIMURA K, et al. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation[J]. Journal of Dairy Science, 2003, 86(4): 1315-1320.
|
| 18 |
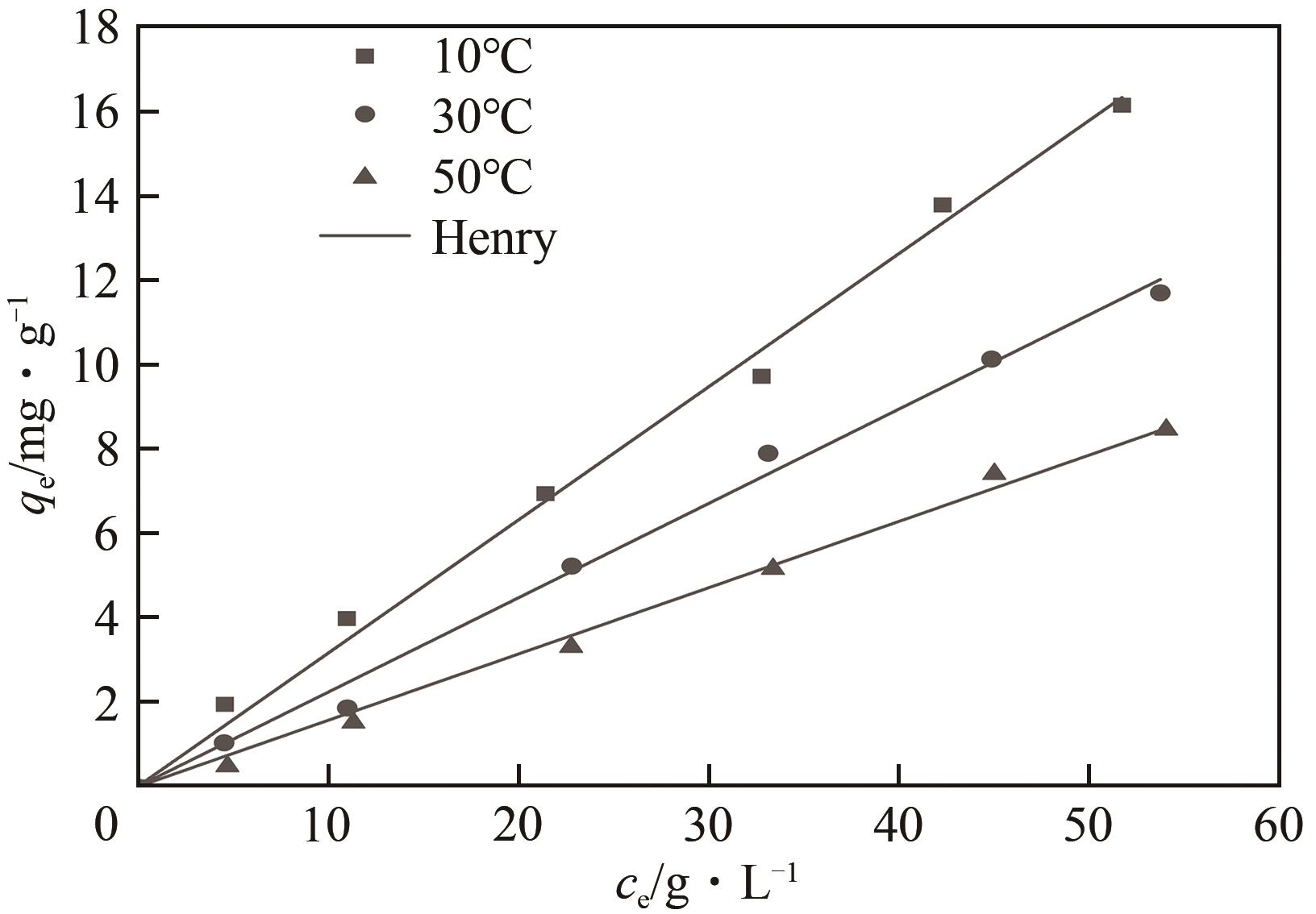
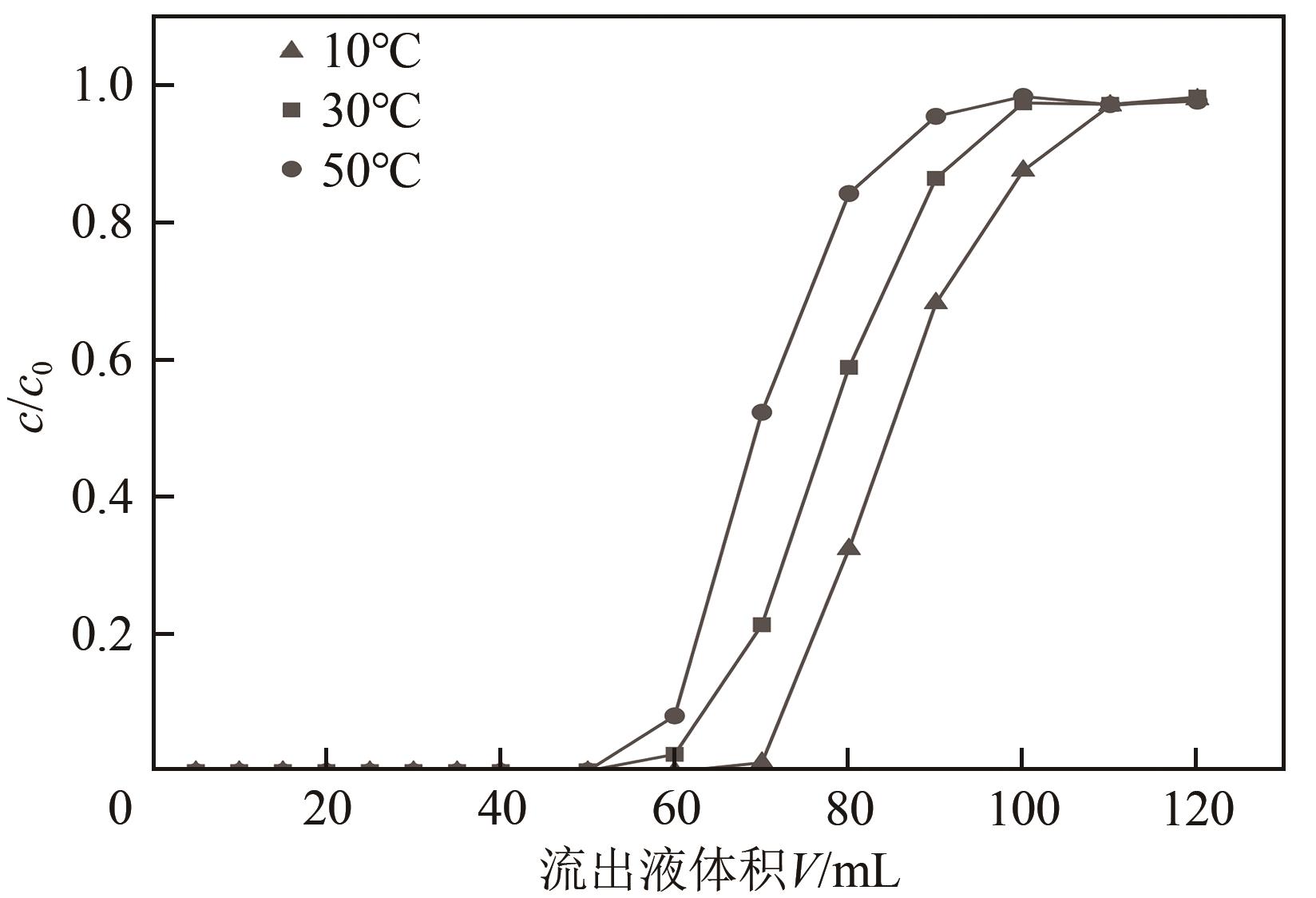
FOO K Y. Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal, 2010, 156(1): 2-10.
|
| 19 |
NEBAGHE K C, BOUNDATI Y EL, ZIAT K, et al. Comparison of linear and non-linear method for determination of optimum equilibrium isotherm for adsorption of copper (Ⅱ) onto treated Martil sand[J]. Fluid Phase Equilibria, 2016, 430: 188-194.
|
| 20 |
LIU Yu, LIU Yajuan. Biosorption isotherms, kinetics and thermodynamics[J]. Separation and Purification Technology, 2008, 61(3): 229-242.
|
| 21 |
LIU Zhengang, ZHANG Fushen. Removal of copper (Ⅱ) and phenol from aqueous solution using porous carbons derived from hydrothermal chars[J]. Desalination, 2011, 267(1):101-106.
|
| 22 |
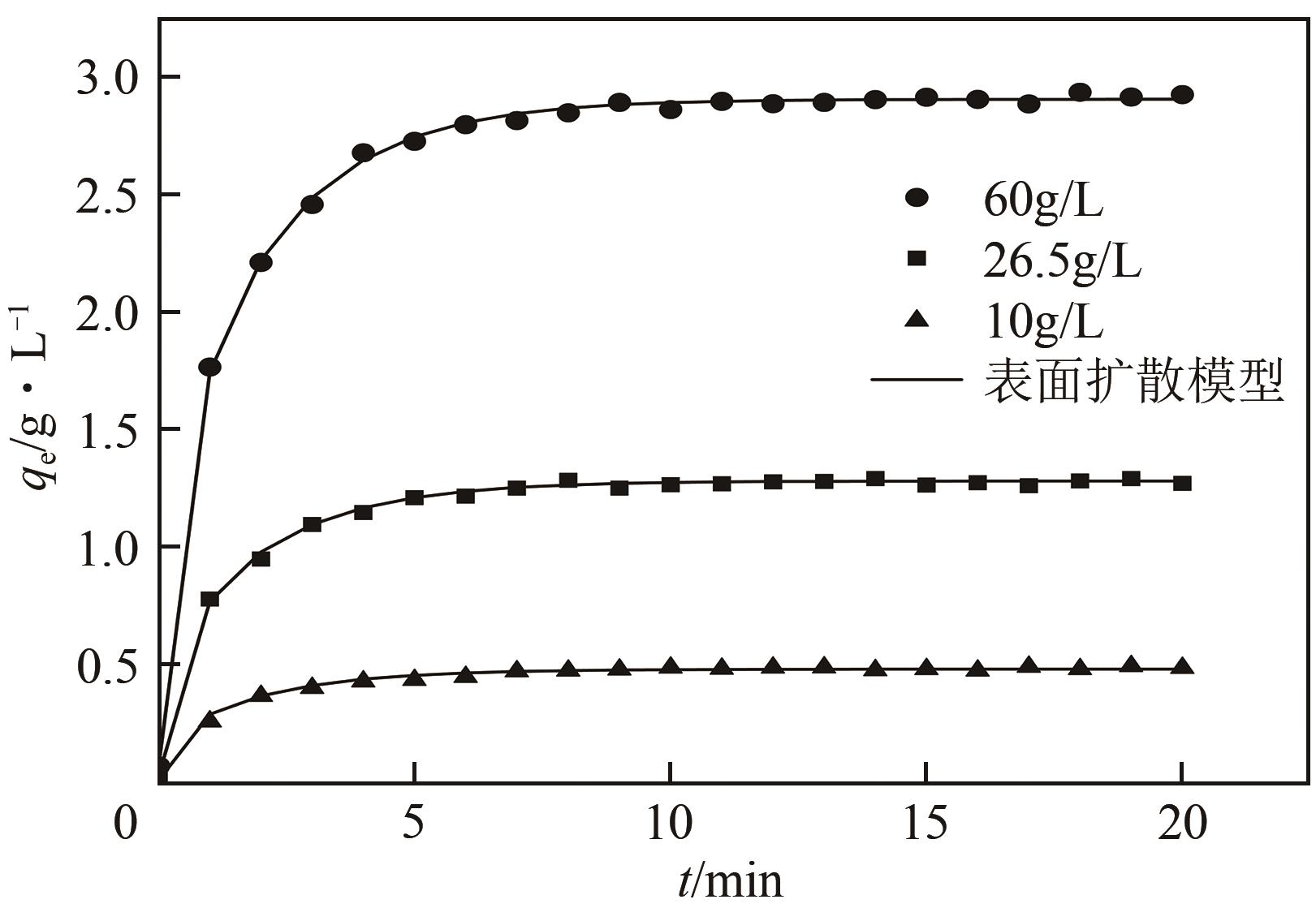
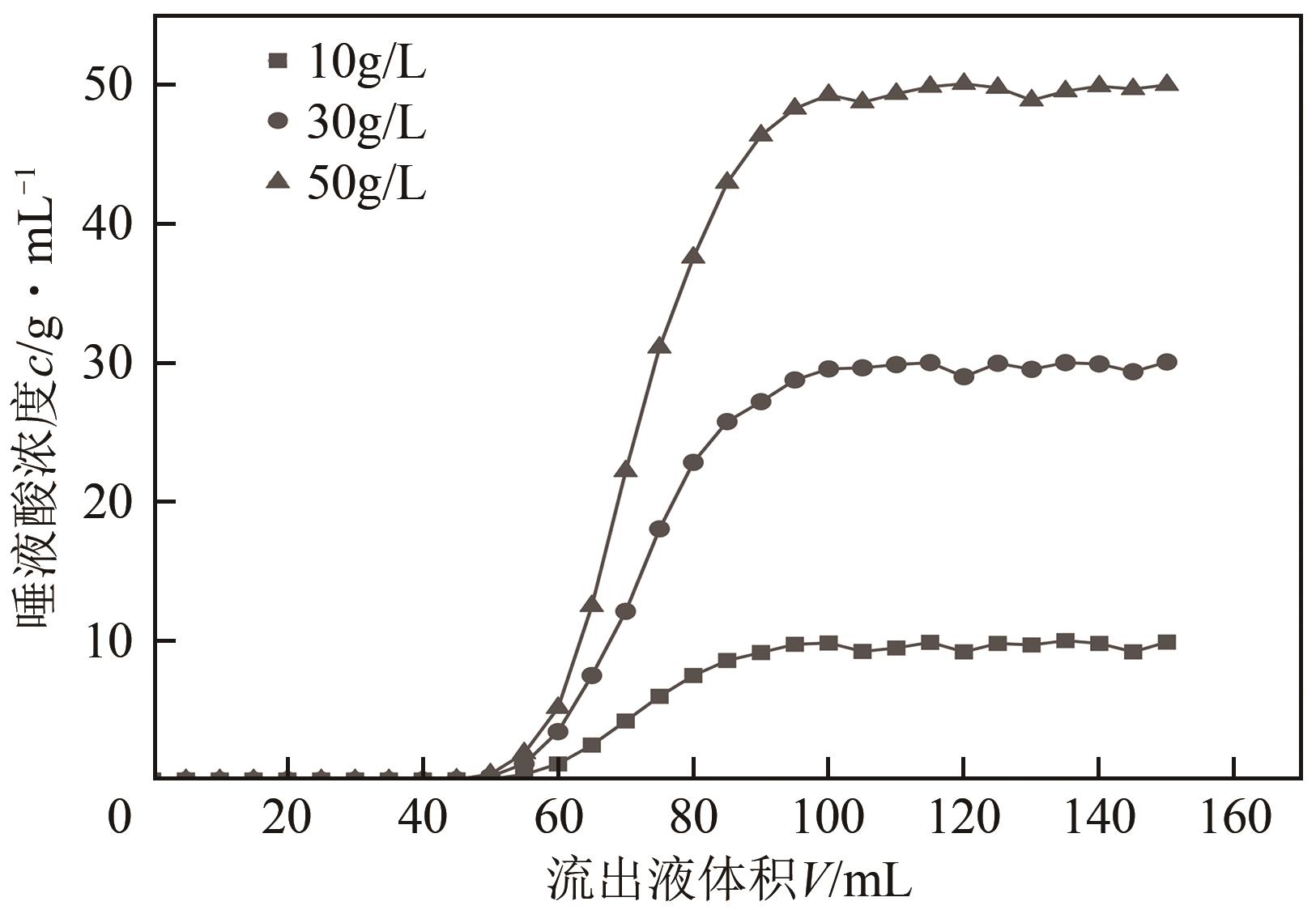
LI Xin, ZHANG Lei, CHANG Yonghui, et al. Kinetics of adsorption of thymopentin on a gel-type strong cation-exchange resin[J]. Chromatographia, 2007, 66(3/4): 231-235.
|
| 23 |
WU Jinglan, WANG Lili, ZHOU Jingwei, et al. Recovery of acetoin from the aqueous solution by means of a novel hyper-cross-linked resin: Equilibrium and kinetics[J]. Journal of Food Engineering, 2013, 119(4): 714-723.
|
| 24 |
MOTA Maria Inês F, PINTO Paula C Rodrigues, LOUREIRO José Miguel, et al. Adsorption of vanillin and syringaldehyde onto a macroporous polymeric resin[J]. Chemical Engineering Journal, 2016, 288: 869-879.
|
 ), LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao(
), LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao( )
)