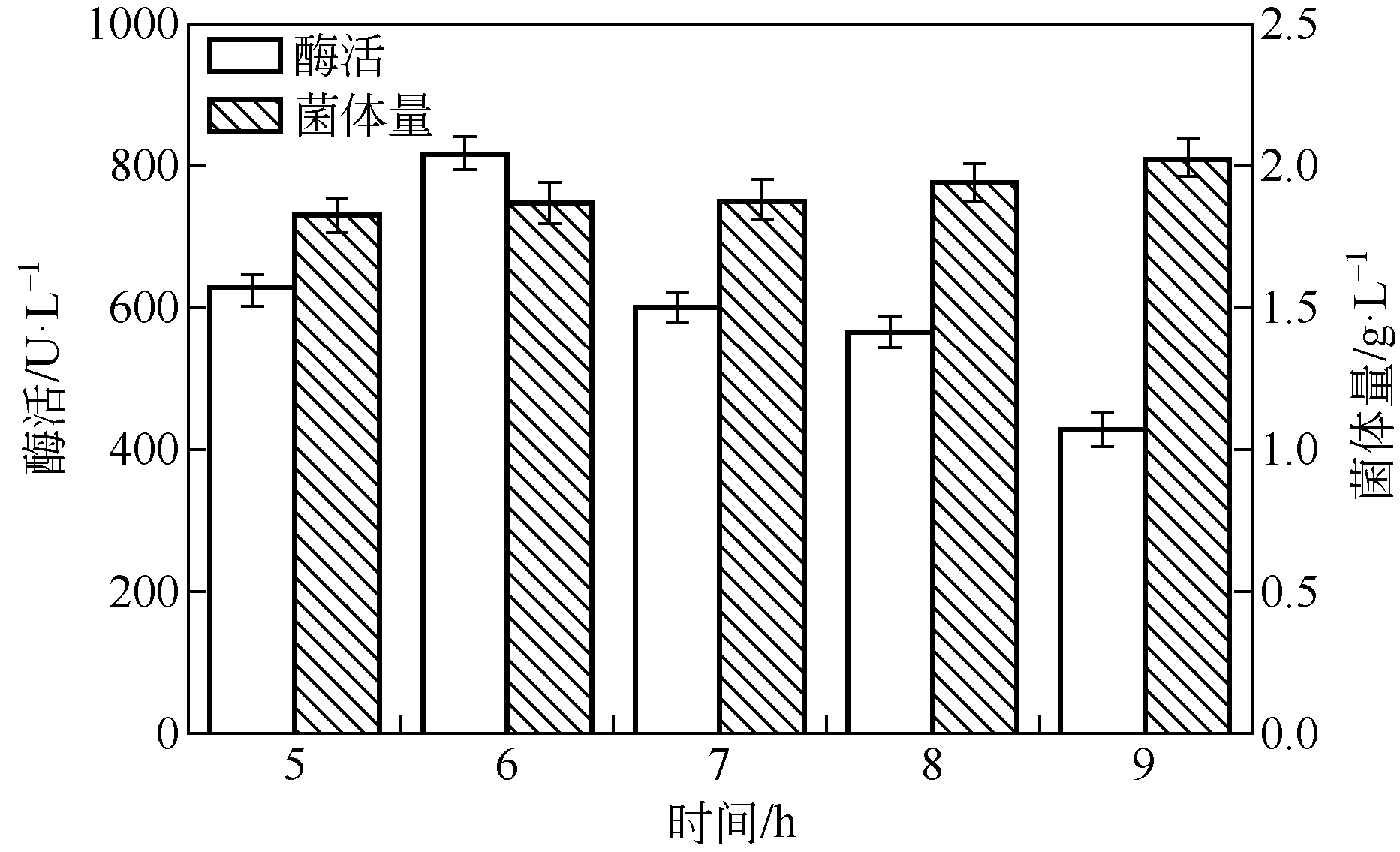
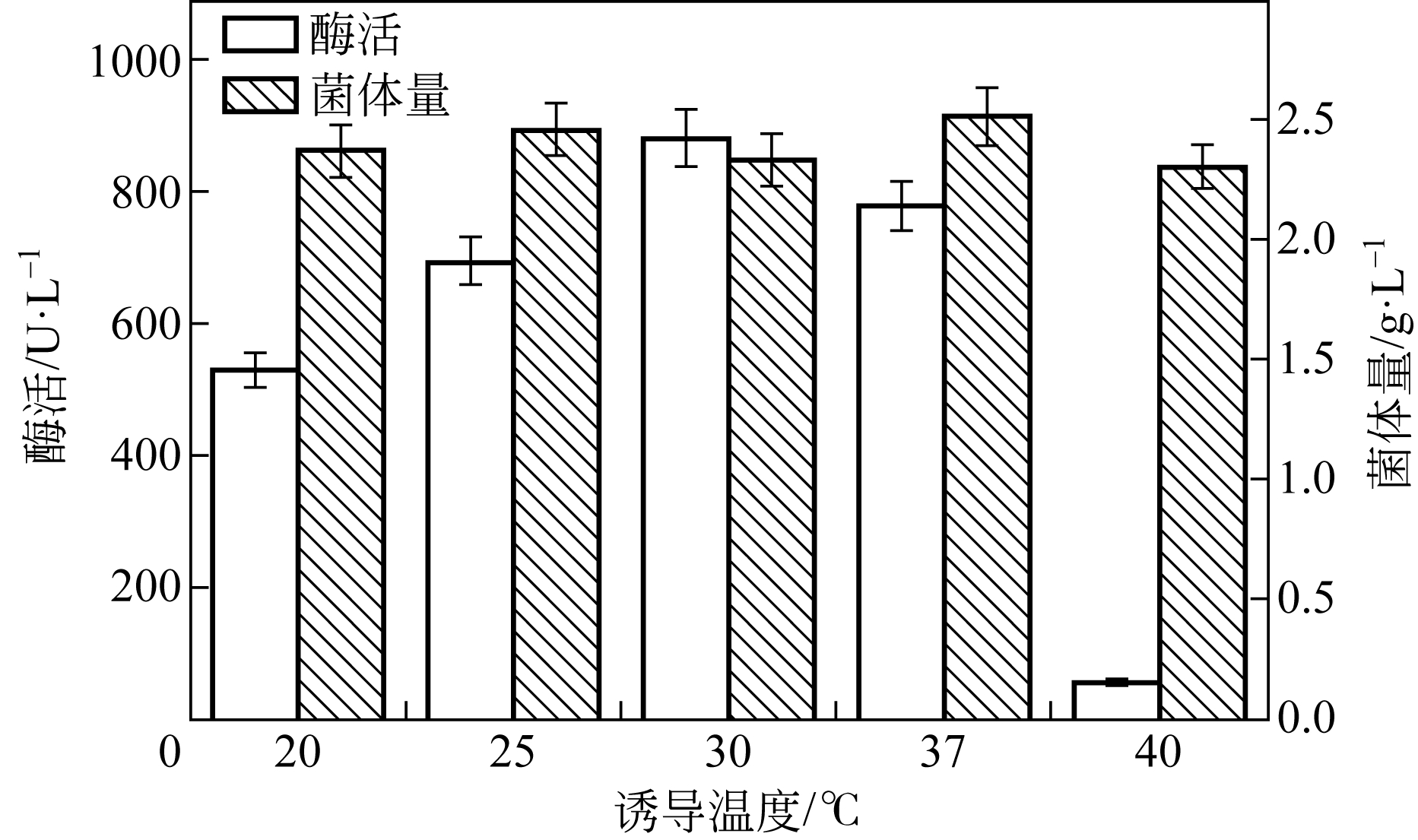
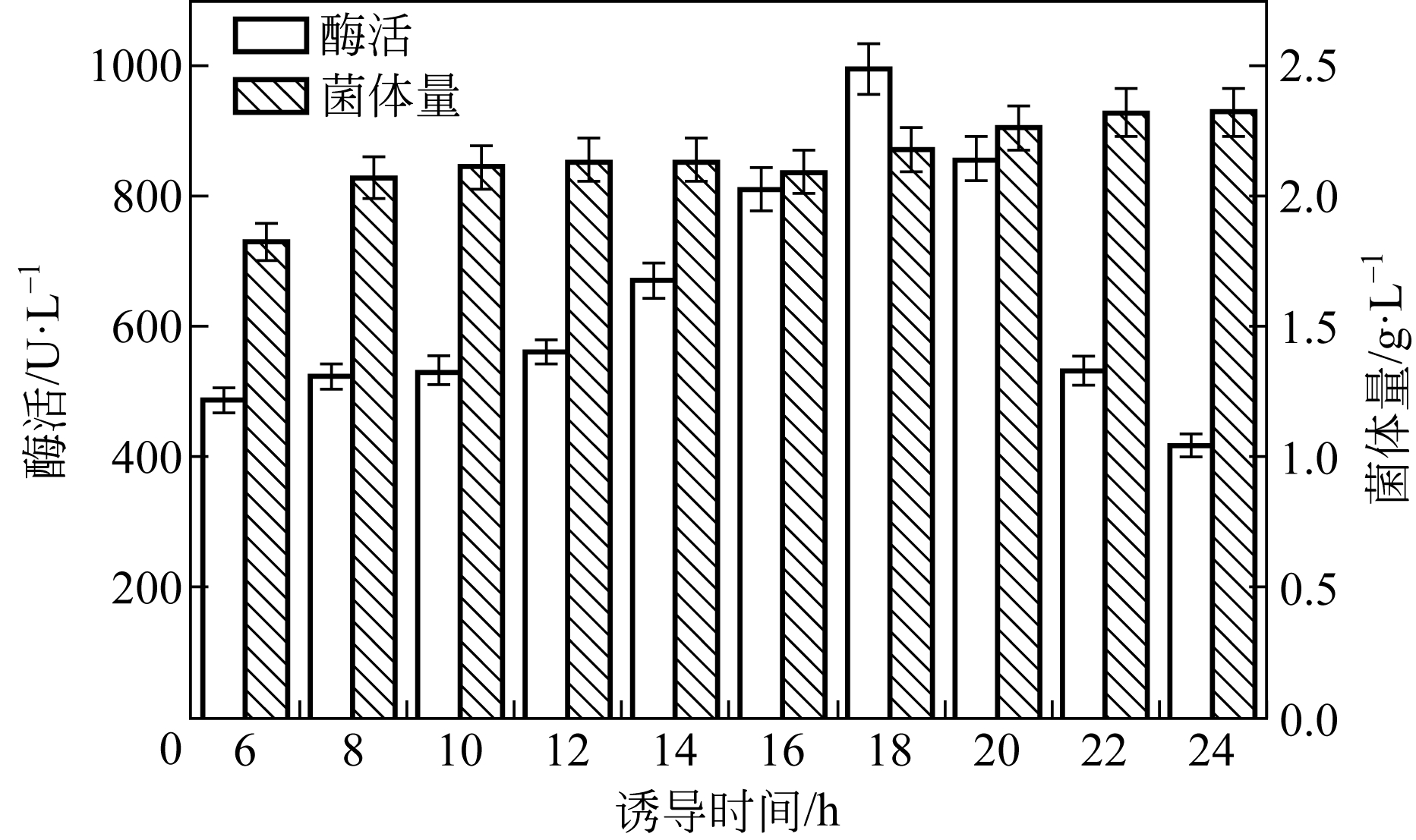
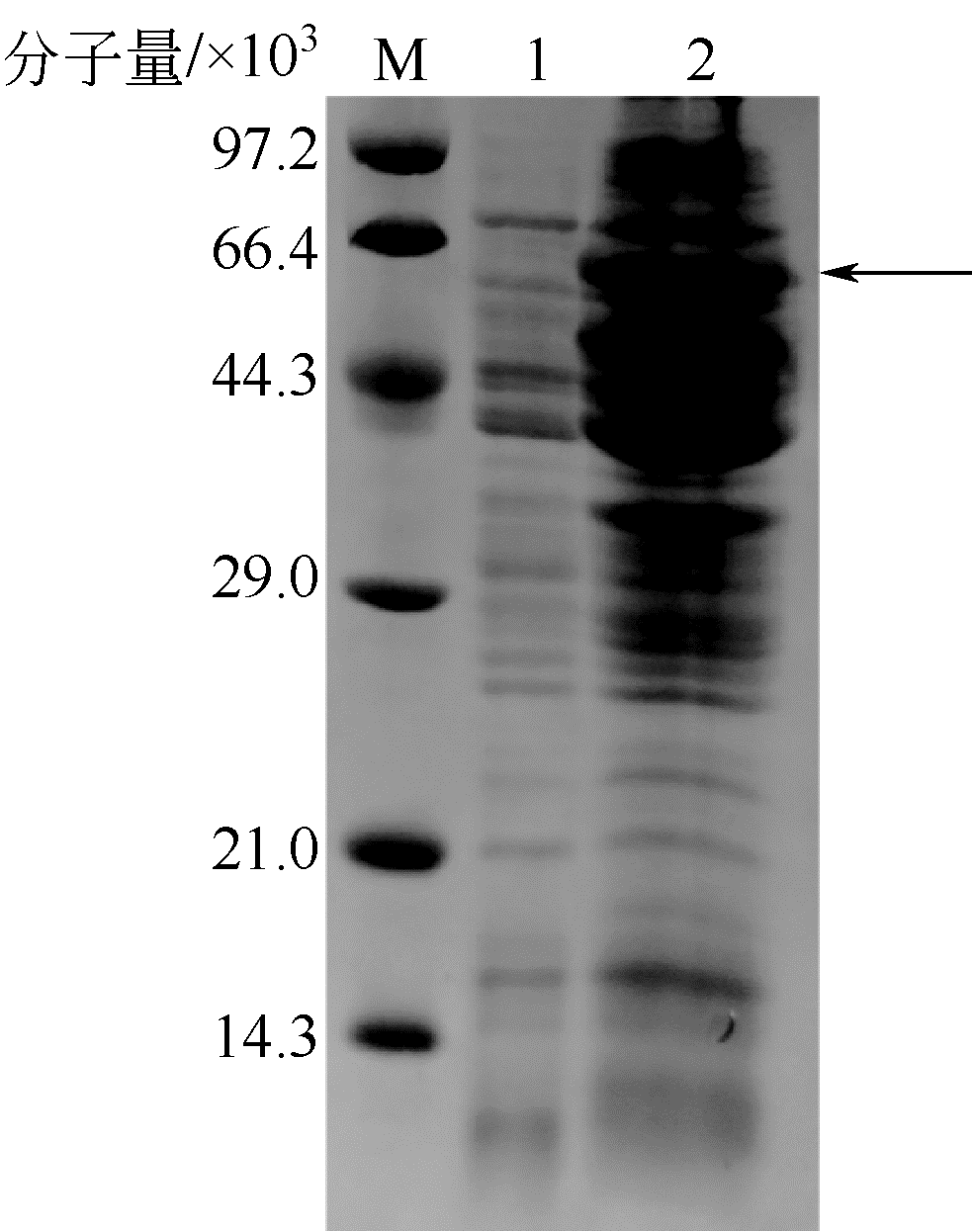
| 1 |
WENNEKES L M , GOOSEN T , VANDEN P J , et al . Purification and characterization of glucose-6-phosphate dehydrogenase from Aspergillus niger and Aspergillus nidulans [J]. Journal General Microbiology, 1993, 139(11): 2793-2800.
|
| 2 |
徐娴, 谢承佳, 何冰芳 . 枯草芽孢杆菌葡萄糖脱氢酶基因的克隆及高效表达 [J]. 食品与生物技术学报, 2007, 26(5): 75-78.
|
|
XU X , XIE C J , HE B F . Cloning and high-level expression of glucose dehydrogenase gene from Bacillus subtilis [J]. Journal of Foods and Biotechnology, 2007, 26(5): 75-78.
|
| 3 |
IGARASHI S , OKUDA J , IKEBUKURO K , et al . Molecular engineering of PQQGDH and its applications[J]. Archives of Biochemistry and Biophysics, 2004, 428(1): 52-63.
|
| 4 |
MORI K , NAKAJIMA M , KOJIMA K , et al . Screening of Aspergillus-derived FAD-glucose dehydrogenases from fungal genome database [J]. Biotechnology Letters, 2011, 33(11): 2255-2263.
|
| 5 |
YANG Y F , HUANG L , WANG J F , et al . Efficient expression, purification, and characterization of a novel FAD-dependent glucose dehydrogenase from Aspergillus terreus in Pichia pastoris [J]. Journal of Microbiology and Biotechnology, 2014, 24(11): 1516-1524.
|
| 6 |
CHRISTOPH S , PETRA S , MIRIAM K , et al . Heterologous overexpression of Glomerella cingulata FAD-dependent glucose dehydrogenase in Escherichia coli and Pichia pastoris [J]. Microbial Cell Factories, 2011, 10: 106.
|
| 7 |
MURATA K , AKATSUKA W , SADAKANE T , et al . Glucose oxidation catalyzed by FAD-dependent glucose dehydrogenase within Os complex-tethered redox polymer hydrogel[J]. Electrochimica Acta, 2014, 136: 537-541.
|
| 8 |
SU L Q , HONG R Y , WU J . Enhanced extracellular expression of gene-optimized Thermobifida fusca cutinase in Escherichia coli by optimization of induction strategy[J]. Process Biochemistry, 2015, 50(7): 1039-1046.
|
| 9 |
陈 亮, 任随周, 许玫英 . 乳糖替代IPTG诱导脱色酶TpmD基因在大肠杆菌中的高效表达[J]. 微生物学通报, 2009, 36(4): 551-556.
|
|
CHEN L , REN S Z , XU M Y . Expression of TpmD gene in lactase induced by lactose instead of IPTG in E.coli [J]. Microbiology, 2009, 36(4): 551-556.
|
| 10 |
杨海麟, 王长城, 张玲 . 高密度培养重组E.coli产胆固醇氧化酶的乳糖诱导策略 [J]. 食品工业科技, 2011, 32(2): 162-165.
|
|
YANG H L , WANG C C , ZHANG L . High-density culture of lactose induced cholesterol oxidase in recombinant E.coli [J]. Food Industry Science and Technology, 2011, 32(2): 162-165.
|
| 11 |
张翔, 张彦昊, 刘孝永 . 产壳聚糖酶菌株ncps116发酵条件优化及其酶学性质 [J]. 化工进展, 2018, 37(6): 2354-2363.
|
|
ZHANG X , ZHANG Y H , LIU X Y . Optimization of fermentation conditions and enzymatic properties of the chitosan producing strain ncps116[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2354-2363.
|
| 12 |
孙艳, 王长城, 张玲 . 重组大肠杆菌产胆固醇氧化酶的指数流加策略 [J]. 化工进展, 2010, 29(1): 130-133.
|
|
SUN Y , WANG C C , ZHANG L . Indexed federation strategies for cholesterol oxidase production from recombinant E.coli [J]. Chemical Industry and Engineering Progress, 2010, 29(1): 130-133.
|
| 13 |
SU L Q , HUANG Y , WU J . Enhanced production of recombinant Escherichia coli glutamate decarboxylase through optimization of induction strategy and addition of pyridoxine[J]. Bioresource Technology, 2015, 198: 63-69.
|
| 14 |
CHEN X , ZHOU L , TIAN K , et al . Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production[J]. Biotechnology Advances, 2013, 31(8): 1200-1223.
|
| 15 |
FRUCHTL M , SAKON J , BEITLE R . Alternate carbohydrate and nontraditional inducer leads to increased productivity of a collagen binding domain fusion protein via fed-batch fermentation[J]. Journal of Biotechnology, 2016, 226: 65-73.
|
| 16 |
相场洋志 . 新型葡萄糖脱氢酶: CN1962859[P]. 2006-03-13.
|
|
YOSHIHIKO F . New glucose dehydrogenase: CN1962859[P]. 2006-03-13.
|
| 17 |
田中宪彰, 结城健介 .黄素腺嘌呤双核苷酸结合型葡萄糖脱氢酶: CN103981157A[P]. 2014-08-13.
|
|
TANAKA M , YUKI K . Flavin adenine dinucleotide binding glucose dehydrogenase: CN103981157A[P].. 2014-08-13.
|
| 18 |
田岛辽子, 一柳敦, 市川惠一, 等 . 黄素结合型葡萄糖脱氢酶:CN102292439A[P].. 2010-04-19.
|
|
TIDAO L , YILIU D , ICHIKAWA K , et al . Flavin-binding glucose dehydrogenase: CN102292439[P]. 2010-04-19.
|
| 19 |
YANG Y , HUANG L , WANG J , et al . Expression, characterization and mutagenesis of an FAD-dependent glucose dehydrogenase from Aspergillus terreus [J]. Enzyme and Microbial Technology, 2015, 68: 43-49.
|
| 20 |
SYGMUND C , STAUDIGL P , KLAUSBERGER M . Heterologous overexpression of Glomerella cingulata FAD-dependent glucose dehydrogenase in Escherichia coli and Pichia pastoris [J]. Microbial Cell Factories, 2011, 10: 106-115.
|
 ),Zukun SONG,Rong LIN,Nan WANG,Hailin YANG(
),Zukun SONG,Rong LIN,Nan WANG,Hailin YANG( )
)