Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (S1): 411-419.DOI: 10.16085/j.issn.1000-6613.2023-0305
• Resources and environmental engineering • Previous Articles Next Articles
Research progresses on zeolite-based CO2 adsorbents
CHEN Chongming1( ), CHEN Qiu1, GONG Yunqian1, CHE Kai1, YU Jinxing1(
), CHEN Qiu1, GONG Yunqian1, CHE Kai1, YU Jinxing1( ), SUN Nannan2
), SUN Nannan2
- 1.State Grid Hebei Electric Power Research Institute, Shijiazhuang 050021, Hebei, China
2.Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China
-
Received:2023-03-01Revised:2023-04-18Online:2023-11-30Published:2023-10-25 -
Contact:YU Jinxing
分子筛基CO2吸附剂研究进展
陈崇明1( ), 陈秋1, 宫云茜1, 车凯1, 郁金星1(
), 陈秋1, 宫云茜1, 车凯1, 郁金星1( ), 孙楠楠2
), 孙楠楠2
- 1.国网河北省电力有限公司电力科学研究院,河北 石家庄 050021
2.中国科学院上海高等研究院,上海 201210
-
通讯作者:郁金星 -
作者简介:陈崇明(1983-),男,正高级工程师,研究方向为火电机组烟气污染物治理技术、二氧化碳捕集技术。E-mail: dyy_chencm@163.com。 -
基金资助:国网河北省电力有限公司电力科学研究院自主课题(TSS 2021-06)
CLC Number:
Cite this article
CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419.
陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0305
| 改性方法 | 优势 | 劣势 |
|---|---|---|
| 离子交换改性 | 改性理论、技术成熟;底物适用性广;可选阳离子种类多;过程简易,易放大;对分子筛材料的晶体结构和多孔性质影响小 | 含水工况下CO2吸附性能的提升相对有限;可能造成吸附选择性和循环稳定性的下降 |
| 胺基化改性 | 吸附量和选择性同时提升;含水工况影响小 | 制备成本增加;循环稳定性易受操作条件和杂质影响解吸难度高 |
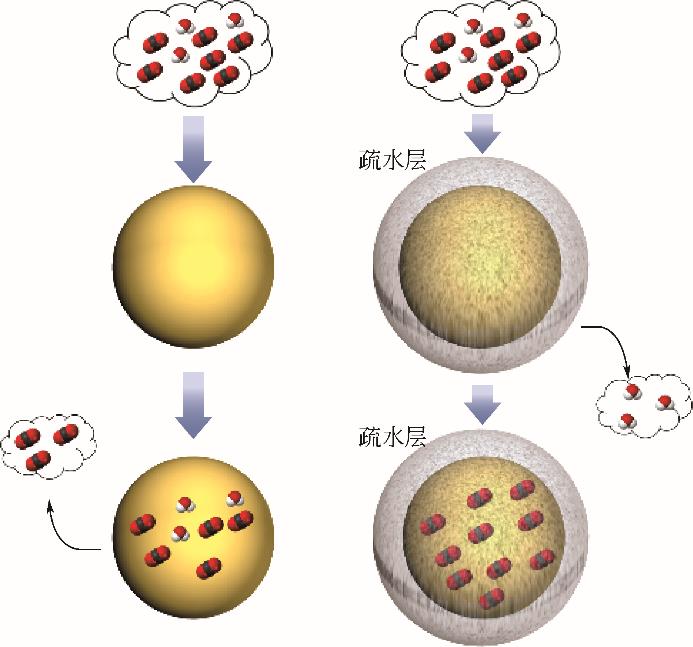
| 疏水化改性 | 抑制分子筛骨架中Si—O键水解断裂;缓解水的竞争吸附,改善含水工况条件下CO2的吸附选择性和循环稳定性 | 制备成本增加;适用范围受限;影响分子筛的孔道结构;可能造成CO2吸附量显著下降 |
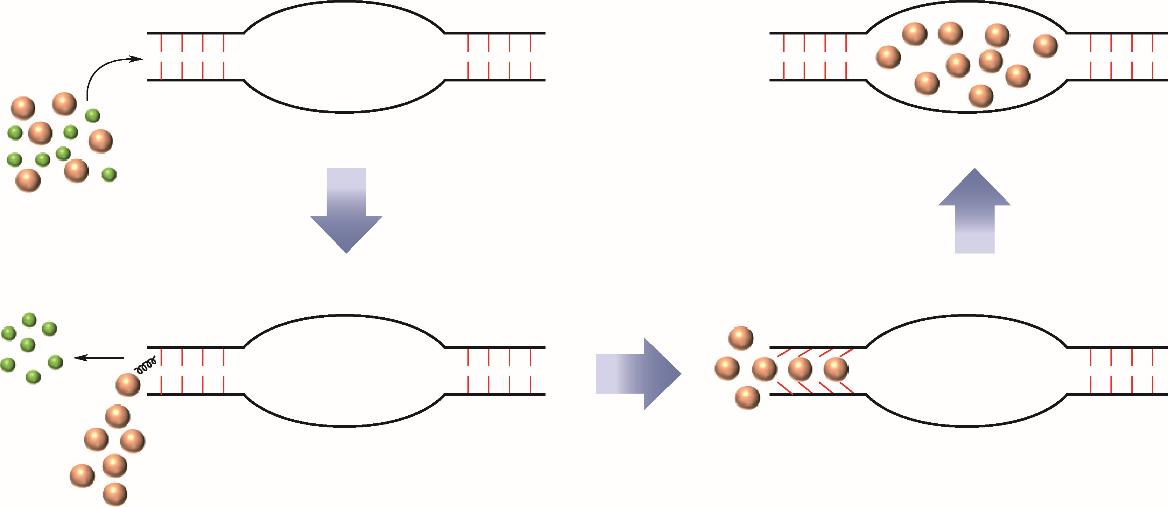
| “Trapdoor”新策略 | 颠覆性创新策略 | 成熟度低,关键科学问题缺乏认识;适用范围可能有限 |
| 改性方法 | 优势 | 劣势 |
|---|---|---|
| 离子交换改性 | 改性理论、技术成熟;底物适用性广;可选阳离子种类多;过程简易,易放大;对分子筛材料的晶体结构和多孔性质影响小 | 含水工况下CO2吸附性能的提升相对有限;可能造成吸附选择性和循环稳定性的下降 |
| 胺基化改性 | 吸附量和选择性同时提升;含水工况影响小 | 制备成本增加;循环稳定性易受操作条件和杂质影响解吸难度高 |
| 疏水化改性 | 抑制分子筛骨架中Si—O键水解断裂;缓解水的竞争吸附,改善含水工况条件下CO2的吸附选择性和循环稳定性 | 制备成本增加;适用范围受限;影响分子筛的孔道结构;可能造成CO2吸附量显著下降 |
| “Trapdoor”新策略 | 颠覆性创新策略 | 成熟度低,关键科学问题缺乏认识;适用范围可能有限 |
| 1 | SOLOMON S, PLATTNER G K, KNUTTI R, et al. Irreversible climate change due to carbon dioxide emissions[J]. Proceedings of the National Academy of Sciences, 2009, 106(6): 1704-1709. |
| 2 | 辛保安, 陈梅, 赵鹏, 等. 碳中和目标下考虑供电安全约束的我国煤电退减路径研究[J]. 中国电机工程学报, 2022, 42(19): 6919-6930. |
| XIN Baoan, CHEN Mei, ZHAO Peng, et al. Research on coal power generation reduction path considering power supply adequacy constraints under carbon neutrality target in China[J]. Proceedings of the CSEE, 2022, 42(19): 6919-6930. | |
| 3 | 张贤, 李阳, 马乔, 等. 我国碳捕集利用与封存技术发展研究[J]. 中国工程科学, 2021, 23(6): 70-80. |
| ZHANG Xian, LI Yang, MA Qiao, et al. Development of carbon capture, utilization and storage technology in China[J]. Strategic Study of CAE, 2021, 23(6): 70-80. | |
| 4 | Change Intergovernmental Panel on Climate Change. Ipcc[J]. Climate Change, 2014. |
| 5 | 国家科学技术部社会发展科技司, 中国21世纪议程管理中心. 中国碳捕集利用与封存技术发展路线图(2019)[R]. 2019. |
| Department of Social Development Science and Technology, Ministry of Science and Technology and Management Centre of Agendum in the 21st Century. China's carbon capture, utilization and storage technology development roadmap (2019)[R]. 2019. | |
| 6 | MONDAL M K, BALSORA H K, VARSHNEY P. Progress and trends in CO2 capture/separation technologies: A review[J]. Energy, 2012, 46(1): 431-441. |
| 7 | 吴彬, 黄坤荣, 刘子健. 化学吸收法捕集二氧化碳研究进展[J]. 广州化工, 2017, 45(11): 11-14. |
| WU Bin, HUANG Kunrong, LIU Zijian. Research progress on carbon dioxide capture by chemical absorption[J]. Guangzhou Chemical Industry, 2017, 45(11): 11-14. | |
| 8 | 王继锋. 变温吸附在焦炉煤气净化中的应用[J]. 煤炭与化工, 2018, 41(12): 130-132. |
| WANG Jifeng. Application of TSA in purge of coke oven gas[J]. Coal and Chemical Industry, 2018, 41(12): 130-132. | |
| 9 | 高腾飞, 常超, 杨阳, 等. 碳捕集变压吸附技术工艺及吸附材料研究进展[J]. 辽宁化工, 2020, 49(11): 1389-1394. |
| GAO Tengfei, CHANG Chao, YANG Yang, et al. Research progress of pressure swing adsorption technology and adsorption materials for carbon capture[J]. Liaoning Chemical Industry, 2020, 49(11): 1389-1394. | |
| 10 | MOHSEN K, MOHAMMAD S, JOSÉ A, et al. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions[J]. Journal of CO2 Utilization, 2022, 57: 101890. |
| 11 | KUMAR S, SRIVASTAVA R, KOH J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives[J]. Journal of CO2 Utilization, 2021, 41: 101251. |
| 12 | 耿一琪, 郭彦霞, 樊飙, 等. CaO基吸附剂捕集CO2及其抗烧结改性研究进展[J]. 燃料化学学报, 2021, 49(7): 998-1013. |
| GENG Yiqi, GUO Yanxia, FAN Biao, et al. Research progress of calcium-based adsorbents for CO2 capture and anti-sintering modification[J]. Journal of Fuel Chemistry and Technology, 2021, 49(7): 998-1013. | |
| 13 | 何凯武, 唐思扬, 刘长军, 等. 有机胺功能化介孔固体吸附剂吸附分离CO2性能研究[J]. 化工学报, 2018, 69(9): 3887-3895. |
| HE Kaiwu, TANG Siyang, LIU Changjun, et al. Performance of amine functionalized mesoporous adsorbents for CO2 adsorption[J]. CIESC Journal, 2018, 69(9): 3887-3895. | |
| 14 | 鲁雪婷, 蒲彦锋, 李磊, 等. 氨基修饰的金属有机框架Cu3(BTC)2的制备及其CO2吸附性能研究[J]. 燃料化学学报, 2019, 47(3): 338-343. |
| LU Xueting, PU Yanfeng, LI Lei, et al. Preparation of metal-organic frameworks Cu3(BTC)2 with amino-functionalization for CO2 adsorption[J]. Journal of Fuel Chemistry and Technology, 2019, 47(3): 338-343. | |
| 15 | OZDEMIR J, MOSLEH I, ABOLHASSANI M, et al. Covalent organic frameworks for the capture, fixation, or reduction of CO2 [J]. Frontiers in Energy Research, 2019, 7: 77. |
| 16 | 刘鑫博, 唐建峰, 胡苏阳, 等. 5种沸石分子筛的吸附脱碳对比实验[J]. 煤气与热力, 2021, 41(9): 19-24, 29, 45-46. |
| LIU Xinbo, TANG Jianfeng, HU Suyang, et al. Comparative experiment on adsorption and decarbonization of five kinds of zeolite molecular sieves[J]. Gas & Heat, 2021, 41(9): 19-24, 29, 45-46. | |
| 17 | 王烁天, 李冠泓, 杨月峰, 等. 分子筛对二氧化碳的穿透吸附性能测定[J]. 精细石油化工, 2022, 39(3): 58-62. |
| WANG Shuotian, LI Guanhong, YANG Yuefeng, et al. Penetrating adsorption property of carbon dioxide for zeolites[J]. Speciality Petrochemicals, 2022, 39(3): 58-62. | |
| 18 | CHUE K T, KIM J N, YOO Y J, et al. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption[J]. Industrial & Engineering Chemistry Research, 1995, 34(2): 591-598. |
| 19 | MULGUNDMATH V P, TEZEL F H, SAATCIOGLU T, et al. Adsorption and separation of CO2/N2 and CO2/CH4 by 13X zeolite[J]. The Canadian Journal of Chemical Engineering, 2012, 90(3): 730-738. |
| 20 | 胡苏阳, 刘鑫博, 唐建峰, 等. 13X沸石分子筛对低浓度CO2动态吸附[J]. 化工进展, 2022, 41(1): 153-160. |
| HU Suyang, LIU Xinbo, TANG Jianfeng, et al. Dynamic adsorption of low concentration CO2 over 13X zeolite[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 153-160. | |
| 21 | PRODINGER S, VEMURI R S, VARGA T, et al. Impact of chabazite SSZ-13 textural properties and chemical composition on CO2 adsorption applications[J]. New Journal of Chemistry, 2016, 40(5): 4375-4385. |
| 22 | 王煜瑶, 张强, 于吉红. 多级孔NaX分子筛的合成及CO2吸附性能[J]. 高等学校化学学报, 2020, 41(4): 616-622. |
| WANG Yuyao, ZHANG Qiang, YU Jihong. Synthesis of Hierarchical NaX zeolite and Its CO2 adsorption performance[J]. Chemical Journal of Chinese Universities, 2020, 41(4): 616-622. | |
| 23 | YANG S T, KIM J, AHN W S. CO2 adsorption over ion-exchanged zeolite beta with alkali and alkaline earth metal ions[J]. Microporous and Mesoporous Materials, 2010, 135(1-3): 90-94. |
| 24 | BOWER J K, BARPAGA D, PRODINGER S, et al. Dynamic adsorption of CO2/N2 on cation-exchanged chabazite SSZ-13: a breakthrough analysis[J]. ACS Applied Materials & Interfaces, 2018, 10(17): 14287-14291. |
| 25 | WALTON K S, ABNEY M B, DOUGLAS LEVAN M. CO2 adsorption in Y and X zeolites modified by alkali metal cation exchange[J]. Microporous and Mesoporous Materials, 2006, 91(1-3): 78-84. |
| 26 | SUN M, GU Q, HANIF A, et al. Transition metal cation-exchanged SSZ-13 zeolites for CO2 capture and separation from N2 [J]. Chemical Engineering Journal, 2019, 370: 1450-1458. |
| 27 | REMY T, GOBECHIYA E, DANACI D, et al. Biogas upgrading through kinetic separation of carbon dioxide and methane over Rb- and Cs-ZK-5 zeolites[J]. RSC Advances, 2014, 4(107): 62511-62524. |
| 28 | HUDSON M R, QUEEN W L, MASON J A, et al. Unconventional, highly selective CO2 adsorption in zeolite SSZ-13[J]. Journal of the American Chemical Society, 2012, 134(4): 1970-1973. |
| 29 | LEE C H, HYEON D H, JUNG H, et al. Effects of pore structure and PEI impregnation on carbon dioxide adsorption by ZSM-5 zeolites[J]. Journal of Industrial and Engineering Chemistry, 2015, 23: 251-256. |
| 30 | LIANG W, HUANG J, XIAO P, et al. Amine-immobilized HY zeolite for CO2 capture from hot flue gas[J]. Chinese Journal of Chemical Engineering, 2022, 43: 335-342. |
| 31 | MADDEN D, CURTIN T. Carbon dioxide capture with amino-functionalised zeolite-β: A temperature programmed desorption study under dry and humid conditions[J]. Microporous and Mesoporous Materials, 2016, 228: 310-317. |
| 32 | TIEN H N, SUNGJUNE K, MINYOUNG Y, et al. Hierarchical zeolites with amine-functionalized mesoporous domains for carbon dioxide capture[J]. ChemSusChem, 2016, 9: 455-461. |
| 33 | HYUNCHUL J, SUNBIN J, DONG HYUN J, et al. Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents[J]. Chemical Engineering Journal, 2017, 307: 836-844. |
| 34 | KIM C, CHO H S, CHANG S, et al. An ethylenediamine-grafted Y zeolite: A highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation [J]. Energy & Environmental Science, 2016, 9(5): 1803-1811. |
| 35 | MINJAE K, JAE W L, SEONGGON K, et al. CO2 adsorption on zeolite 13X modified with hydrophobic octadecyltrimethoxysilane for indoor application[J]. Journal of Cleaner Production, 2022, 337: 130597. |
| 36 | DONG-IL K, JEONG-CHUL K, HAESOL L, et al. Engineering micropore walls of beta zeolites by post-functionalization for CO2 adsorption performance screening under humid conditions[J]. Chemical Engineering Journal, 2022, 427: 131461. |
| 37 | MANABU M, SHUMPEI O, KODIA K, et al. High water tolerance of a core-shell-structured zeolite for CO2 adsorptive separation under wet conditions[J]. ChemSusChem, 2018, 11: 1756-1760. |
| 38 | GAO F, LI Y, BIAN Z, et al. Dynamic hydrophobic hindrance effect of zeolite@zeolitic imidazolate framework composites for CO2 capture in the presence of water[J]. Journal of Material Chemistry A, 2015, 3: 8091-8097. |
| 39 | SHUVO J D, CHUTHARAT K, ZHEN H L, et al. CO2 capture from humid flue gases and humid atmosphere using a microporous coppersilicate[J]. Science, 2015, 350: 302-306. |
| 40 | SHANG J, LI G, SINGH R, et al. Discriminative separation of gases by a “molecular trapdoor” mechanism in chabazite zeolites [J]. Journal of the American Chemical Society, 2012, 134(46): 19246-19253. |
| 41 | SHANG J, LI G, RANJEET S, et al. Determination of composition range for “molecular trapdoor” effect in chabazite zeolite[J]. Journal of Physical Chemistry C, 2013, 117: 12841-12847. |
| 42 | DU T, FANG X, LIU L, et al. An optimal trapdoor zeolite for exclusive admission of CO2 at industrial carbon capture operating temperatures[J]. Chemical Communications, 2018, 54: 3134-3137. |
| 43 | LI G, SHANG J, GU Q, et al. Temperature-regulated guest admission and release in microporous materials[J]. Nature Communications, 2017, 8: 15777. |
| 44 | LOZINSKA M M, MOWAT J P S, WEIGHT P A, et al. Cation gating and relocation during the highly selective “trapdoor” adsorption of CO2 on univalent cation forms of zeolite Rho[J]. Chemistry of Materials, 2014, 26: 2052-2061. |
| 45 | COUDERT F, KOHEN D. Molecular insight into CO2 “trapdoor” adsorption in zeolite Na-RHO[J]. Chemistry of Materials, 2017, 29: 2724-2730. |
| [1] | WANG Jiaqing, SONG Guangwei, LI Qiang, GUO Shuaicheng, DAI Qingli. Rubber-concrete interface modification method and performance enhancement path [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 328-343. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [5] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [6] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [7] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [8] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [9] | WANG Jingang, ZHANG Jianbo, TANG Xuejiao, LIU Jinpeng, JU Meiting. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4636-4648. |
| [10] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [11] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [12] | PAN Yichang, ZHOU Rongfei, XING Weihong. Advanced microporous membranes for efficient separation of same-carbon-number hydrocarbon mixtures: State-of-the-art and challenges [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3926-3942. |
| [13] | LI Xuejia, LI Peng, LI Zhixia, JIN Dunshang, GUO Qiang, SONG Xufeng, SONG Peng, PENG Yuelian. Experimental comparation on anti-scaling and anti-wetting ability of hydrophilic and hydrophobic modified membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4458-4464. |
| [14] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [15] | CHEN Junjun, FEI Chang’en, DUAN Jintang, GU Xueping, FENG Lianfang, ZHANG Cailiang. Research progress on chemical modification of polyether ether ketone for the high bioactivity [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4015-4028. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||