Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (10): 5223-5231.DOI: 10.16085/j.issn.1000-6613.2022-2123
• Industrial catalysis • Previous Articles Next Articles
Preparation of Au-Pd/MnO2 catalyst and its catalytic performance for benzyl alcohol oxidation
GUO Xiaoyu( ), LI Dongchen, ZHAO Wei, DU Zhenyi, LI Xiaoliang(
), LI Dongchen, ZHAO Wei, DU Zhenyi, LI Xiaoliang( )
)
- State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-11-16Revised:2022-12-31Online:2023-11-11Published:2023-10-15 -
Contact:LI Xiaoliang
Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能
- 太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
-
通讯作者:李晓良 -
作者简介:郭晓宇(1996—),男,硕士研究生,研究方向为催化氧化。E-mail: guoxiaoyu0823@link.tyut.edu.cn。 -
基金资助:山西省基础研究计划(自由探索类)自然科学研究面上项目(20210302123093)
CLC Number:
Cite this article
GUO Xiaoyu, LI Dongchen, ZHAO Wei, DU Zhenyi, LI Xiaoliang. Preparation of Au-Pd/MnO2 catalyst and its catalytic performance for benzyl alcohol oxidation[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5223-5231.
郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2123
| 催化剂 | Au/% | Pd/% | Au/Pd摩尔比 | ||
|---|---|---|---|---|---|
| 理论值 | 实际值 | 理论值 | 实际值 | ||
| Au-Pd/α-MnO2 | 1.0 | 0.98 | 1.0 | 0.97 | 0.54 |
| Au-Pd/β-MnO2 | 1.0 | 0.97 | 1.0 | 0.95 | 0.55 |
| Au-Pd/γ-MnO2 | 1.0 | 0.99 | 1.0 | 0.96 | 0.55 |
| 催化剂 | Au/% | Pd/% | Au/Pd摩尔比 | ||
|---|---|---|---|---|---|
| 理论值 | 实际值 | 理论值 | 实际值 | ||
| Au-Pd/α-MnO2 | 1.0 | 0.98 | 1.0 | 0.97 | 0.54 |
| Au-Pd/β-MnO2 | 1.0 | 0.97 | 1.0 | 0.95 | 0.55 |
| Au-Pd/γ-MnO2 | 1.0 | 0.99 | 1.0 | 0.96 | 0.55 |
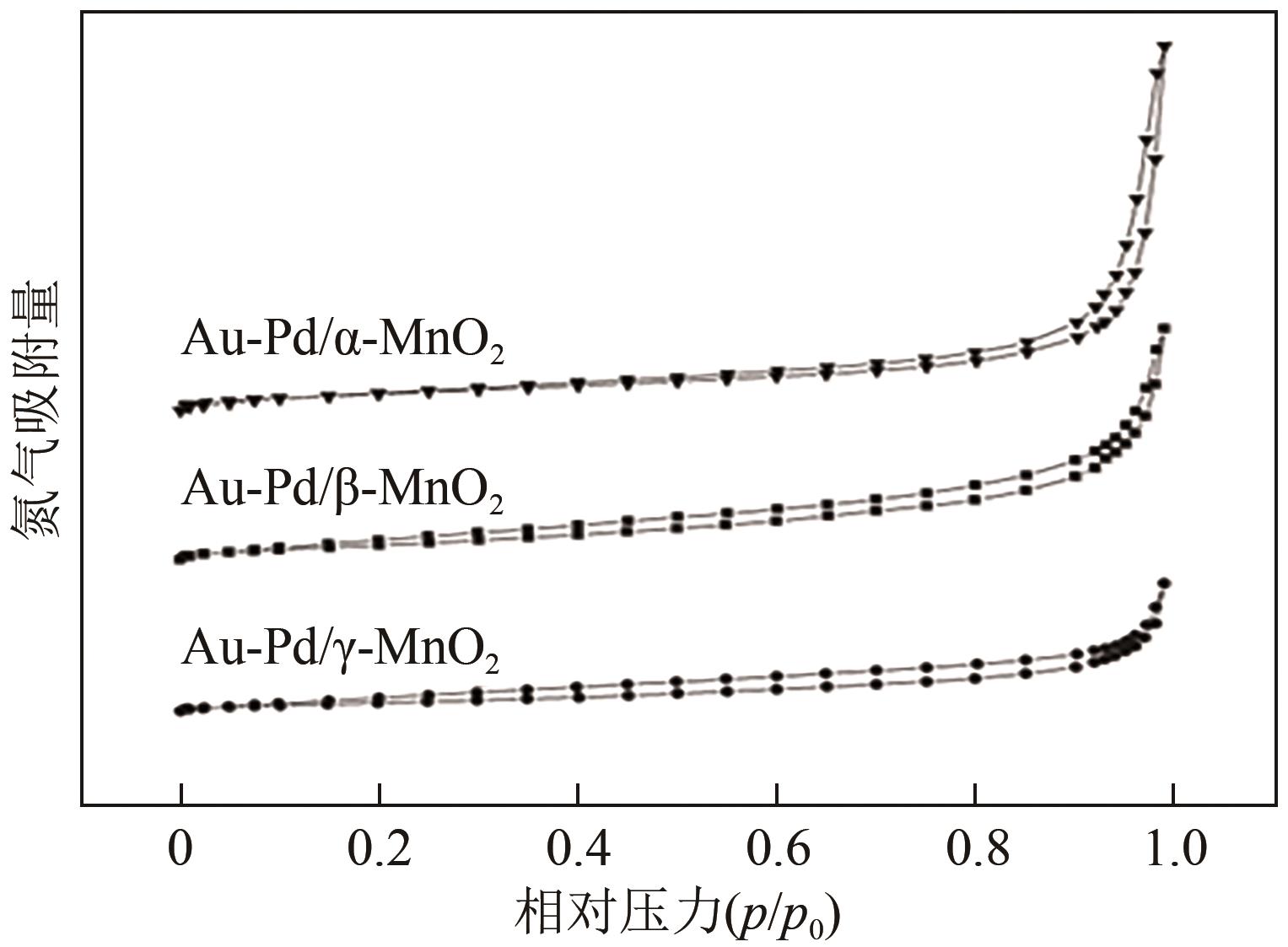
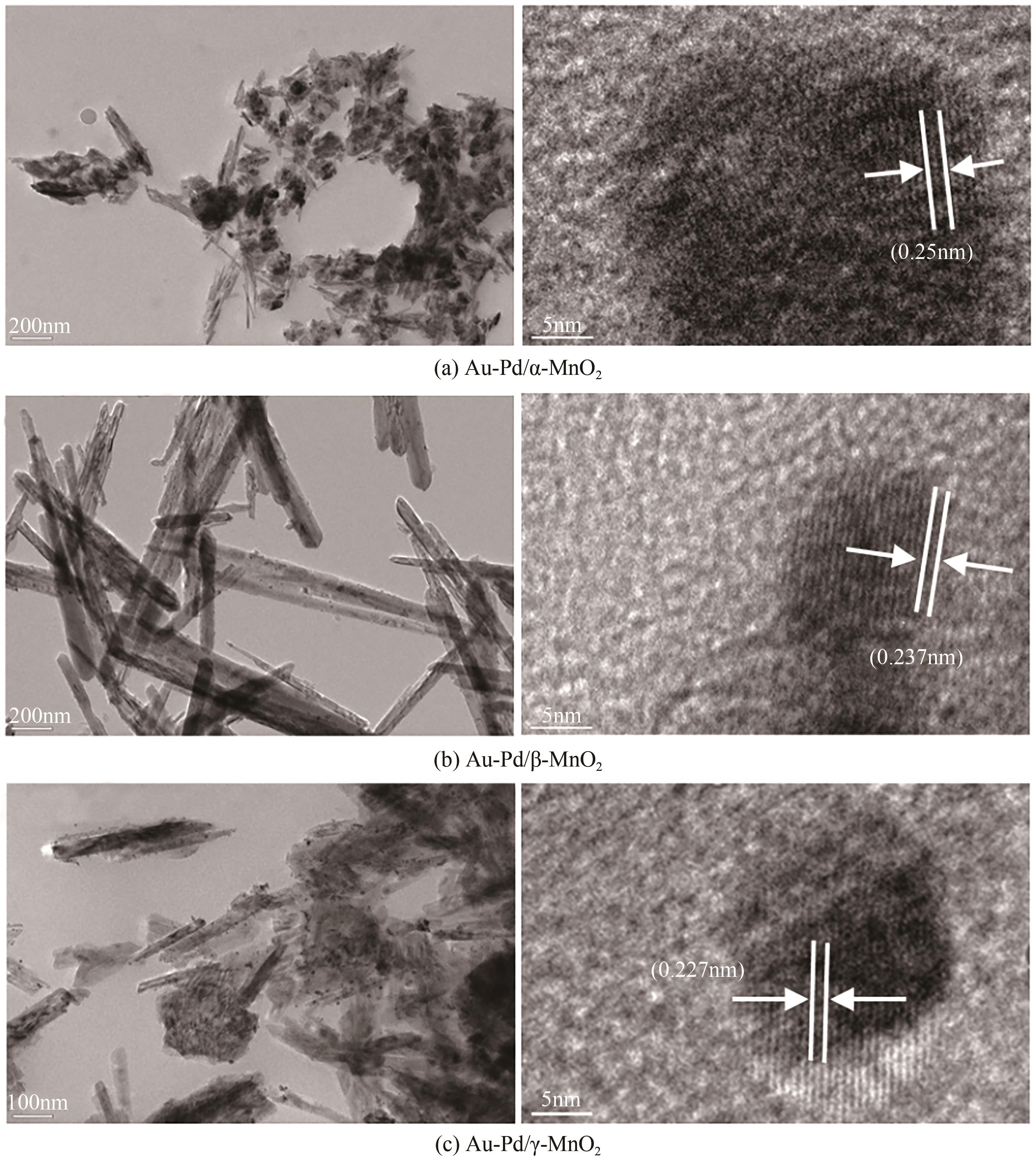
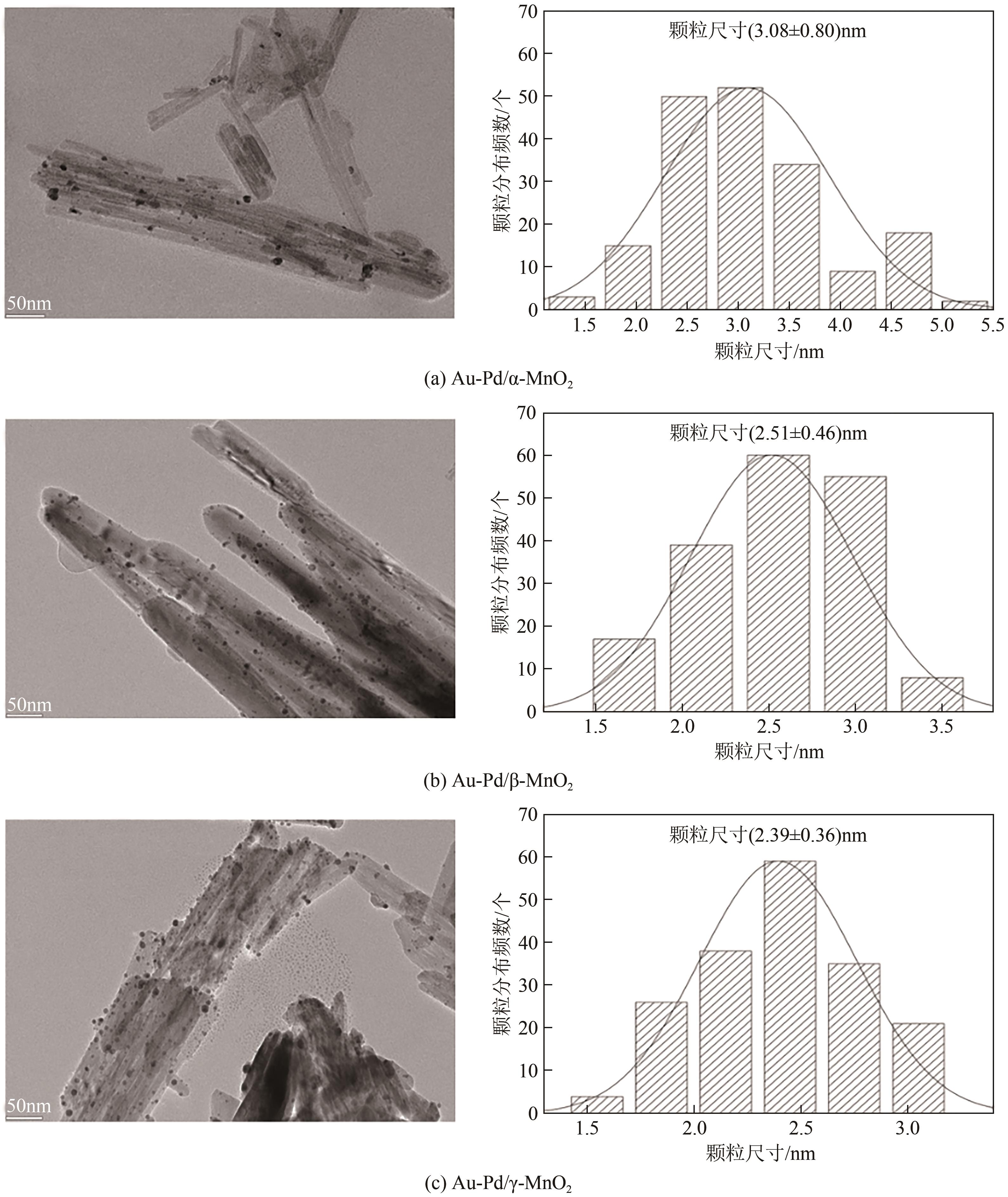
| 催化剂 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径/nm | 载体平均颗粒 尺寸①/nm |
|---|---|---|---|---|
| Au-Pd/α-MnO2 | 63.5 | 0.38 | 3.1 | 18.3 |
| Au-Pd/β-MnO2 | 27.6 | 0.13 | 3.4 | 25.5 |
| Au-Pd/γ-MnO2 | 56.9 | 0.25 | 3.1 | 14.7 |
| 催化剂 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径/nm | 载体平均颗粒 尺寸①/nm |
|---|---|---|---|---|
| Au-Pd/α-MnO2 | 63.5 | 0.38 | 3.1 | 18.3 |
| Au-Pd/β-MnO2 | 27.6 | 0.13 | 3.4 | 25.5 |
| Au-Pd/γ-MnO2 | 56.9 | 0.25 | 3.1 | 14.7 |
| 催化剂 | 结合能/eV | Pd0摩尔 分数/% | Au0摩尔 分数/% | |||
|---|---|---|---|---|---|---|
| 3d3/2 Pd0 | 3d3/2 Pd2+ | 3d5/2 Pd0 | 3d5/2 Pd2+ | |||
| Au-Pd/α-MnO2 | 340.9 | 342.8 | 335.3 | 337.3 | 27.2 | 35.69 |
| Au-Pd/β-MnO2 | 340.8 | 342.6 | 335.2 | 337.1 | 25.6 | 38.41 |
| Au-Pd/γ-MnO2 | 341.1 | 342.8 | 335.4 | 337.4 | 42.8 | 40.53 |
| 催化剂 | 结合能/eV | Pd0摩尔 分数/% | Au0摩尔 分数/% | |||
|---|---|---|---|---|---|---|
| 3d3/2 Pd0 | 3d3/2 Pd2+ | 3d5/2 Pd0 | 3d5/2 Pd2+ | |||
| Au-Pd/α-MnO2 | 340.9 | 342.8 | 335.3 | 337.3 | 27.2 | 35.69 |
| Au-Pd/β-MnO2 | 340.8 | 342.6 | 335.2 | 337.1 | 25.6 | 38.41 |
| Au-Pd/γ-MnO2 | 341.1 | 342.8 | 335.4 | 337.4 | 42.8 | 40.53 |
| 催化剂 | 结合能/eV | Oads/Olatt摩尔比 | Mn3+/(Mn3++Mn4+)摩尔比 | ||
|---|---|---|---|---|---|
| Mn3+ | Mn4+ | O1s (Olatt, Oads, OadsOH) | |||
| Au-Pd/α-MnO2 | 642.1 | 643.5 | 529.5, 531.0, 533.3 | 0.55 | 0.55 |
| Au-Pd/β-MnO2 | 641.6 | 643.9 | 529.1, 530.9, 533.0 | 0.74 | 0.48 |
| Au-Pd/γ-MnO2 | 641.9 | 643.7 | 529.5, 531.0, 533.0 | 0.76 | 0.61 |
| 催化剂 | 结合能/eV | Oads/Olatt摩尔比 | Mn3+/(Mn3++Mn4+)摩尔比 | ||
|---|---|---|---|---|---|
| Mn3+ | Mn4+ | O1s (Olatt, Oads, OadsOH) | |||
| Au-Pd/α-MnO2 | 642.1 | 643.5 | 529.5, 531.0, 533.3 | 0.55 | 0.55 |
| Au-Pd/β-MnO2 | 641.6 | 643.9 | 529.1, 530.9, 533.0 | 0.74 | 0.48 |
| Au-Pd/γ-MnO2 | 641.9 | 643.7 | 529.5, 531.0, 533.0 | 0.76 | 0.61 |
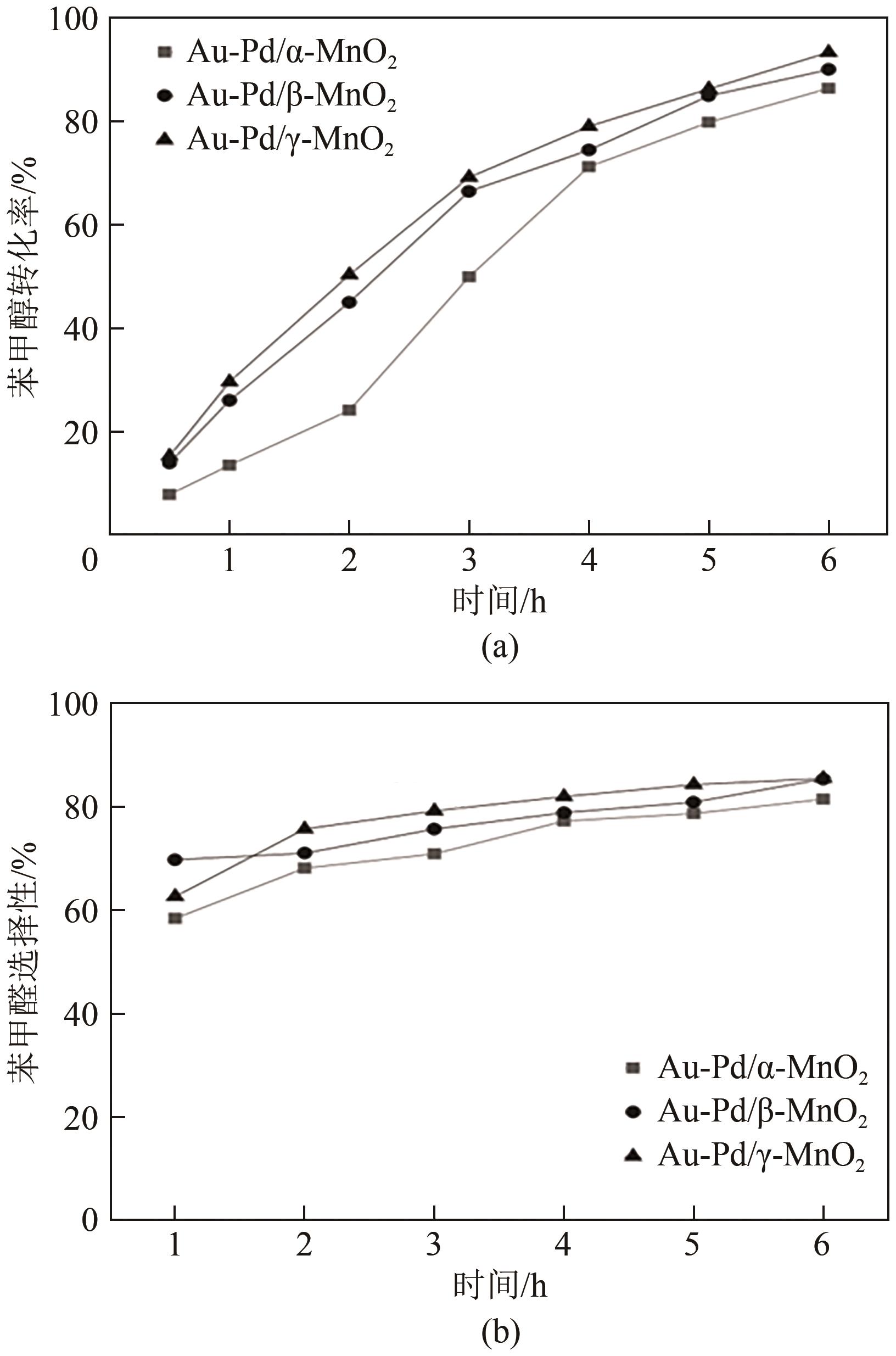
| 产物 | Au-Pd/α-MnO2选择性/% | Au-Pd/β-MnO2选择性/% | Au-Pd/γ-MnO2选择性/% |
|---|---|---|---|
| 苯 | 0.84 | 0.99 | 1.18 |
| 甲苯 | 18.08 | 10.81 | 8.76 |
| 苯甲醛 | 77.42 | 85.46 | 85.60 |
| 苯甲酸 | 0.48 | 0.79 | 2.26 |
| 苯甲酸苄酯 | 3.18 | 1.95 | 2.20 |
| 碳平衡 | 97.85 | 99.16 | 99.04 |
| 产物 | Au-Pd/α-MnO2选择性/% | Au-Pd/β-MnO2选择性/% | Au-Pd/γ-MnO2选择性/% |
|---|---|---|---|
| 苯 | 0.84 | 0.99 | 1.18 |
| 甲苯 | 18.08 | 10.81 | 8.76 |
| 苯甲醛 | 77.42 | 85.46 | 85.60 |
| 苯甲酸 | 0.48 | 0.79 | 2.26 |
| 苯甲酸苄酯 | 3.18 | 1.95 | 2.20 |
| 碳平衡 | 97.85 | 99.16 | 99.04 |
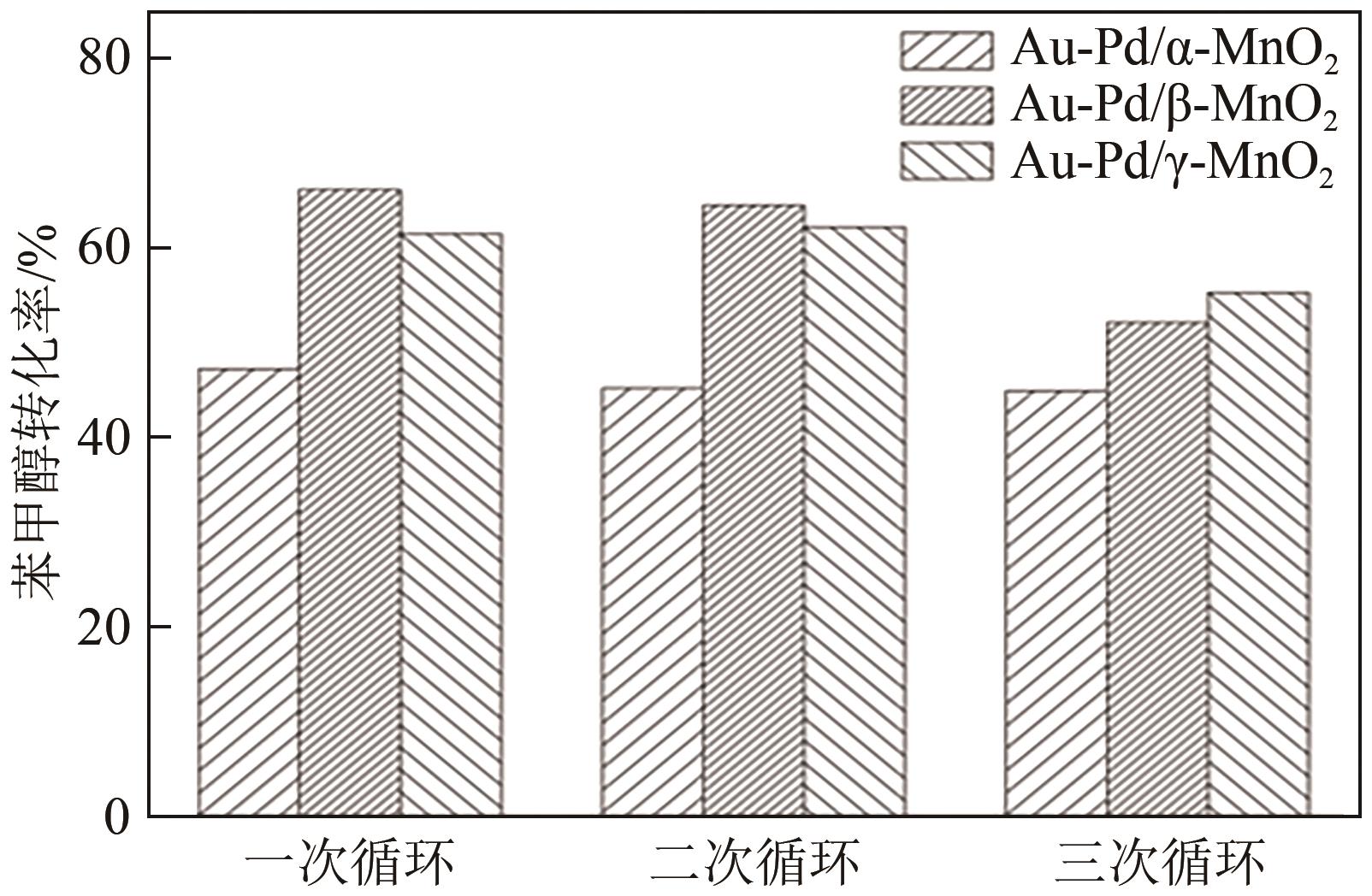
| 催化剂 | 1次循环/% | 2次循环/% | 3次循环/% |
|---|---|---|---|
| Au-Pd/α-MnO2 | 66.91 | 66.01 | 65.48 |
| Au-Pd/β-MnO2 | 74.93 | 72.55 | 70.90 |
| Au-Pd/γ-MnO2 | 77.31 | 76.23 | 72.11 |
| 催化剂 | 1次循环/% | 2次循环/% | 3次循环/% |
|---|---|---|---|
| Au-Pd/α-MnO2 | 66.91 | 66.01 | 65.48 |
| Au-Pd/β-MnO2 | 74.93 | 72.55 | 70.90 |
| Au-Pd/γ-MnO2 | 77.31 | 76.23 | 72.11 |
| 催化剂 | TOF/h-1 | 0.5h转化率 /% | 1次循环 /% | 2次循环 /% | 3次循环 /% |
|---|---|---|---|---|---|
| Au-Pd/α-MnO2 | 35280 | 7.9 | 47.2 | 45.1 | 44.9 |
| Au-Pd/β-MnO2 | 62299 | 13.9 | 66.1 | 64.5 | 52.1 |
| Au-Pd/γ-MnO2 | 68283 | 15.2 | 61.5 | 62.1 | 55.2 |
| 催化剂 | TOF/h-1 | 0.5h转化率 /% | 1次循环 /% | 2次循环 /% | 3次循环 /% |
|---|---|---|---|---|---|
| Au-Pd/α-MnO2 | 35280 | 7.9 | 47.2 | 45.1 | 44.9 |
| Au-Pd/β-MnO2 | 62299 | 13.9 | 66.1 | 64.5 | 52.1 |
| Au-Pd/γ-MnO2 | 68283 | 15.2 | 61.5 | 62.1 | 55.2 |
| 1 | 吴鑫干, 尹娟娟, 胡在君. 苯甲醛的合成进展[J]. 精细石油化工, 2002, 19(4): 57-63. |
| WU Xingan, YIN Juanjuan, HU Zaijun. Progress in benzaldehyde synthesis[J]. Speciality Petrochemicals, 2002, 19(4): 57-63. | |
| 2 | 胡乐晓, 郜胜, 吴东方, 等. 苯甲醛合成工艺研究进展[J]. 精细石油化工进展, 2011, 12(9): 42-47. |
| HU Lexiao, GAO Sheng, WU Dongfang, et al. Progress in technology for synthesizing benzaldehyde[J]. Advances in Fine Petrochemicals, 2011, 12(9): 42-47. | |
| 3 | 王海英, 王晓丹, 姜恒, 等. 杂多酸催化下苯甲醇氧化合成苯甲醛反应研究[J]. 化工科技, 2002, 10(3): 7-9. |
| WANG Haiying, WANG Xiaodan, JIANG Heng, et al. Catalytic oxidation of benzyl alcohol by heteropoly acid for the synthesis of benzaldehyde[J]. Science & Technology in Chemical Industry, 2002, 10(3): 7-9. | |
| 4 | SHELDON Roger A. Recent advances in green catalytic oxidations of alcohols in aqueous media[J]. Catalysis Today, 2015, 247: 4-13. |
| 5 | CHEN Yuanting, Huimin LIM, TANG Qinghu, et al. Solvent-free aerobic oxidation of benzyl alcohol over Pd monometallic and Au-Pd bimetallic catalysts supported on SBA-16 mesoporous molecular sieves[J]. Applied Catalysis A: General, 2010, 380(1/2): 55-65. |
| 6 | HONG Yingling, JING Xiaolian, HUANG Jiale, et al. Biosynthesized bimetallic Au-Pd nanoparticles supported on TiO2 for solvent-free oxidation of benzyl alcohol[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(7): 1752-1759. |
| 7 | VILLA Alberto, WANG Di, DIMITRATOS Nikolaos, et al. Pd on carbon nanotubes for liquid phase alcohol oxidation[J]. Catalysis Today, 2010, 150(1/2): 8-15. |
| 8 | SI Rui, Maria FLYTZANI-STEPHANOPOULOS. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction[J]. Angewandte Chemie International Edition, 2008, 120(15): 2926-2929. |
| 9 | CHE Jianwei, HAO Mengjia, YI Wuzhong, et al. Selective suppression of toluene formation in solvent-free benzyl alcohol oxidation using supported Pd-Ni bimetallic nanoparticles[J]. Chinese Journal of Catalysis, 2017, 38(11): 1870-1879. |
| 10 | 余伟雄, 程高, 崔晓娥, 等. 不同形貌ε-MnO2催化剂的制备及其催化燃烧甲苯性能[J]. 化工进展, 2021, 40(10): 5547-5553. |
| YU Weixiong, CHENG Gao, CUI Xiaoe, et al. Preparation of ɛ-MnO2 with different morphologies and their catalytic combustion of toluene[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5547-5553. | |
| 11 | HUANG Xiaoqiao, CUI Wenyao, YU Jianying, et al. Preparation of mesoporous MnO2 catalysts with different morphologies for catalytic ozonation of organic compounds[J]. Catalysis Letters, 2022, 152(5): 1441-1450. |
| 12 | GONG Jiayang, RONG Shaopeng, WANG Xiaohui, et al. Critical review of catalytic degradation of formaldehyde via MnO2: From the perspective of process intensification[J]. Journal of Cleaner Production, 2022, 377: 134242. |
| 13 | GAO Fengyu, TANG Xiaolong, YI Honghong, et al. In-situ DRIFTS for the mechanistic studies of NO oxidation over α-MnO2, β-MnO2 and γ-MnO2 catalysts[J]. Chemical Engineering Journal, 2017, 322: 525-537. |
| 14 | WANG Xun, LI Yadong. Selected-control hydrothermal synthesis of α- and β-MnO2 single crystal nanowires[J]. Journal of the American Chemical Society, 2002, 124(12): 2880-2881. |
| 15 | ZHANG Lixia, LIU Chengshuai, ZHUANG Li, et al. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells[J]. Biosensors and Bioelectronics, 2009, 24(9): 2825-2829. |
| 16 | ZHANG X G, SHEN C M, LI H L. Preparation of γ-MnO2/carbon composite material by a wet chemical method[J]. Materials Research Bulletin, 2001, 36(3/4): 541-546. |
| 17 | ROBINSON David M, GO Yong Bok, Michelle MUI, et al. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis[J]. Journal of the American Chemical Society, 2013, 135(9): 3494-3501. |
| 18 | YANG R J, FAN Y Y, YE R Q, et al. MnO2-based materials for environmental applications[J]. Advanced Materials, 2021, 33(9): 2004862. |
| 19 | WANG Fagen, LI Huaju, SHEN Wenjie. Influence of Au particle size on Au/CeO2 catalysts for CO oxidation[J]. Catalysis Today, 2011, 175(1): 541-545. |
| 20 | HARUTA M, TSUBOTA S, KOBAYASHI T, et al. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4 [J]. Journal of Catalysis, 1993, 144(1): 175-192. |
| 21 | XU Jing, WHITE Tim, LI Ping, et al. Biphasic Pd-Au alloy catalyst for low-temperature CO oxidation[J]. Journal of the American Chemical Society, 2010, 132(30): 10398-10406. |
| 22 | NASCENTE P A P, DE CASTRO S G C, LANDERS R, et al. X-ray photoemission and Auger energy shifts in some gold-palladium alloys[J]. Physical Review B, Condensed Matter, 1991, 43(6): 4659-4666. |
| 23 | CHOU T S, PERLMAN M L, WATSON R E. Electronegativity and electron binding in gold alloys[J]. Physical Review B, 1976, 14(8): 3248-3250. |
| 24 | TAN Huiteng, CHEN Yuanting, ZHOU Chunmei, et al. Palladium nanoparticles supported on Manganese oxide-CNT composites for solvent-free aerobic oxidation of alcohols: Tuning the properties of Pd active sites using MnO x [J]. Applied Catalysis B: Environmental, 2012, 119/120: 166-174. |
| 25 | WANG Zhitong, SONG Yujie, ZOU Junhua, et al. The cooperation effect in the Au-Pd/LDH for promoting photocatalytic selective oxidation of benzyl alcohol[J]. Catalysis Science & Technology, 2018, 8(1): 268-275. |
| 26 | HOU Wenbo, DEHM Nicole A, SCOTT Robert W J. Alcohol oxidations in aqueous solutions using Au, Pd, and bimetallic AuPd nanoparticle catalysts[J]. Journal of Catalysis, 2008, 253(1): 22-27. |
| 27 | TSUNOYAMA Hironori, SAKURAI Hidehiro, NEGISHI Yuichi, et al. Size-specific catalytic activity of polymer-stabilized gold nanoclusters for aerobic alcohol oxidation in water[J]. Journal of the American Chemical Society, 2005, 127(26): 9374-9375. |
| 28 | JIRÁTOVÁ K, MIKULOVÁ J, KLEMPA J, et al. Modification of Co-Mn-Al mixed oxide with potassium and its effect on deep oxidation of VOC[J]. Applied Catalysis A: General, 2009, 361(1/2): 106-116. |
| 29 | SANTOS V P, PEREIRA M F R, ÓRFÃO J J M, et al. The role of lattice oxygen on the activity of Manganese oxides towards the oxidation of volatile organic compounds[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 353-363. |
| 30 | Andrés PELUSO M, GAMBARO Luis A, PRONSATO Estela, et al. Synthesis and catalytic activity of Manganese dioxide (type OMS-2) for the abatement of oxygenated VOCs[J]. Catalysis Today, 2008, 133/134/135: 487-492. |
| 31 | LI Xiaoliang, FENG Jiangjiang, PERDJON Michal, et al. Investigations of supported Au-Pd nanoparticles on synthesized CeO2 with different morphologies and application in solvent-free benzyl alcohol oxidation[J]. Applied Surface Science, 2020, 505: 144473. |
| [1] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [2] | WANG Shuaiqi, WANG Congxin, WANG Xuelin, TIAN Zhijian. Solvent-free rapid synthesis of ZSM-12 zeolite [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3561-3571. |
| [3] | WANG Keju, ZHAO Cheng, HU Xiaomei, YUN Junge, WEI Ninghan, JIANG Xueying, ZOU Yun, CHEN Zhihang. Research progress of low temperature catalytic oxidation of VOCs by metal oxides [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2402-2412. |
| [4] | ZHANG Ming, GAO Yongkang, JI Delong, LIU Fujie, ZHU Wenshuai, LI Huaming. Research progress of polyoxometalate materials for fuel oil desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4782-4789. |
| [5] | ZHU Feifei, MA Lei, LONG Huimin. Research progresses on the preparation and application of PdxSy catalysts [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 740-749. |
| [6] | GAO Tian, ZHANG Yili, XIONG Zhuo, ZHAO Yongchun, ZHANG Junying. Research progress of modified titanium oxide photocatalytic oxidation of elemental mercury and its influencing factors [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 690-700. |
| [7] | NIE Zimeng, YANG Dian, XIONG Yulu, LI Yingjie, TIAN Senlin, NING Ping. Performance and mechanism of electrolytic manganese slag slurry for flue gas desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1063-1072. |
| [8] | WANG Jikun, LI Yang, CHEN Guifeng, LIU Min, KOU Lihong, WANG Qi, HE Yicong. Catalytic oxidation mechanism of organics degradation by ozone in high-salt wastewater of coal chemical industry [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 493-502. |
| [9] | ZHANG Xuan, SONG Xiaosan, ZHAO Po, DONG Yuanhua, LIU Yun. A critical review of advanced oxidation technology to treat 1,4-dioxane pollution [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 380-388. |
| [10] | SU Biyun, RAN Liangtao, HU Yahe, ZHANG Ao, HAN Qiaoqiao, WU Jindi, LIU Yiting, MENG Zuchao. Research progress on demulsification of petroleum Pickering emulsion by molecular oxidation, photocatalytic oxidation and electrochemical oxidation [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3995-4002. |
| [11] | SUN Hao, HE Xueying, HU Yichao, LIU Zheyi, ZHANG Yingjie. Research progress of iron-manganese oxide film simultaneous removal of iron manganese and ammonia nitrogen from micro-polluted surface water [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1634-1642. |
| [12] | HUANG Jianxiong, GUO Yingming, YANG Jing, XU Wei, WANG Xu, ZHANG Ruifeng. Removal of bisphenol A in water by iron-manganese co-oxide film and its influencing factors [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1551-1557. |
| [13] | ZHANG Wei, TANG Yunhao, YIN Yanshan, GONG Weicheng, SONG Jian, MA Ying, RUAN Min, XU Huifang, CHEN Donglin. Research progress in enhanced catalytic oxidation of VOCs by modified La-based perovskite catalyst [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1425-1437. |
| [14] | Yi ZHOU, Wenyi DENG, Yaxin SU. Research progress in catalytic oxidation of NO by carbon-based active materials at room temperature [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 859-869. |
| [15] | Guangzhu LI, Shangjing ZENG, Shuhai SUN, Kaicheng XU, Dejun BIAN. Preparation of biochar supported iron oxides composites and its application in water treatment [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 917-931. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||