Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (12): 6996-7018.DOI: 10.16085/j.issn.1000-6613.2024-1929
• Materials science and technology • Previous Articles
Research progress on hydrogen absorption and desorption performance of metal oxide catalyzed solid-state hydrogen storage materials
WU Guojie1,2( ), LIU Quanyu1, PENG Cheng1, XIA Siqi1, HUANG Dongfang1, ZHOU Quanbao3, LYU Peng1,2(
), LIU Quanyu1, PENG Cheng1, XIA Siqi1, HUANG Dongfang1, ZHOU Quanbao3, LYU Peng1,2( ), WANG Xuegang1,2(
), WANG Xuegang1,2( )
)
- 1.School of Water Resources and Environmental Engineering, East China University of Technology, Nanchang 330013, Jiangxi, China
2.Jiangxi Provincial Key Laboratory of Genesis and Remediation of Groundwater Pollution (East China University of Technology), Nanchang 330013, Jiangxi, China
3.School of Chemistry and Materials Science, East China University of Technology, Nanchang 330013, Jiangxi, China
-
Received:2024-11-22Revised:2025-01-31Online:2026-01-06Published:2025-12-25 -
Contact:LYU Peng, WANG Xuegang
金属氧化物催化固态储氢材料吸放氢性能的研究进展
吴国界1,2( ), 刘泉宇1, 彭程1, 夏思奇1, 黄东方1, 周权宝3, 吕朋1,2(
), 刘泉宇1, 彭程1, 夏思奇1, 黄东方1, 周权宝3, 吕朋1,2( ), 王学刚1,2(
), 王学刚1,2( )
)
- 1.东华理工大学水资源与环境工程学院,江西 南昌 330013
2.地下水污染成因与修复江西省重点实验室 (东华理工大学),江西 南昌 330013
3.东华理工大学化学与材料科学学院,江西 南昌 330013
-
通讯作者:吕朋,王学刚 -
作者简介:吴国界(2000—),男,硕士研究生,研究方向为环境友好功能材料。E-mail:2251697307@qq.com。 -
基金资助:国家自然科学基金(12205042)
CLC Number:
Cite this article
WU Guojie, LIU Quanyu, PENG Cheng, XIA Siqi, HUANG Dongfang, ZHOU Quanbao, LYU Peng, WANG Xuegang. Research progress on hydrogen absorption and desorption performance of metal oxide catalyzed solid-state hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2025, 44(12): 6996-7018.
吴国界, 刘泉宇, 彭程, 夏思奇, 黄东方, 周权宝, 吕朋, 王学刚. 金属氧化物催化固态储氢材料吸放氢性能的研究进展[J]. 化工进展, 2025, 44(12): 6996-7018.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1929
| 复合体系 | 储氢容量/% | 材料开始吸氢至最大氢容量90%所需时间/s | 扩散系数/m2·s-1 |
|---|---|---|---|
| Nd2Mg17-50% Ni | 3.192 | 2.90 | 7.160×10–6 |
| Nd2Mg17-50% Ni-0.5% CeO2 | 3.329 | 0.55 | 7.237×10–6 |
| Nd2Mg17-50% Ni-1.0% CeO2 | 3.376 | 0.65 | 9.302×10–6 |
| Nd2Mg17-50% Ni-1.5% CeO2 | 3.320 | 0.62 | 9.306×10–6 |
| Nd2Mg17-50% Ni-2.0% CeO2 | 3.199 | 0.67 | 10.265×10–6 |
| 复合体系 | 储氢容量/% | 材料开始吸氢至最大氢容量90%所需时间/s | 扩散系数/m2·s-1 |
|---|---|---|---|
| Nd2Mg17-50% Ni | 3.192 | 2.90 | 7.160×10–6 |
| Nd2Mg17-50% Ni-0.5% CeO2 | 3.329 | 0.55 | 7.237×10–6 |
| Nd2Mg17-50% Ni-1.0% CeO2 | 3.376 | 0.65 | 9.302×10–6 |
| Nd2Mg17-50% Ni-1.5% CeO2 | 3.320 | 0.62 | 9.306×10–6 |
| Nd2Mg17-50% Ni-2.0% CeO2 | 3.199 | 0.67 | 10.265×10–6 |
| 复合体系 | 起始脱氢温度/℃ | 峰值脱氢温度/℃ | 脱氢量(质量分数)/% | 理论吸氢量(质量分数)/% |
|---|---|---|---|---|
| As-milled MgH2 | 361.1 | 422.7 | 7.47 | 7.60 |
| MgH2+10% ZrO2 | 297.0 | 410.2 | 5.54 | 6.84 |
| MgH2+10% Ni | 282.2 | 385.5 | 5.95 | 6.84 |
| MgH2+5% Ni+5% ZrO2 | 255.1 | 365.5 | 6.40 | 6.84 |
| 复合体系 | 起始脱氢温度/℃ | 峰值脱氢温度/℃ | 脱氢量(质量分数)/% | 理论吸氢量(质量分数)/% |
|---|---|---|---|---|
| As-milled MgH2 | 361.1 | 422.7 | 7.47 | 7.60 |
| MgH2+10% ZrO2 | 297.0 | 410.2 | 5.54 | 6.84 |
| MgH2+10% Ni | 282.2 | 385.5 | 5.95 | 6.84 |
| MgH2+5% Ni+5% ZrO2 | 255.1 | 365.5 | 6.40 | 6.84 |
| 种类 | 催化机理及效果 | 应用体系 | 环境影响 | 文献 |
|---|---|---|---|---|
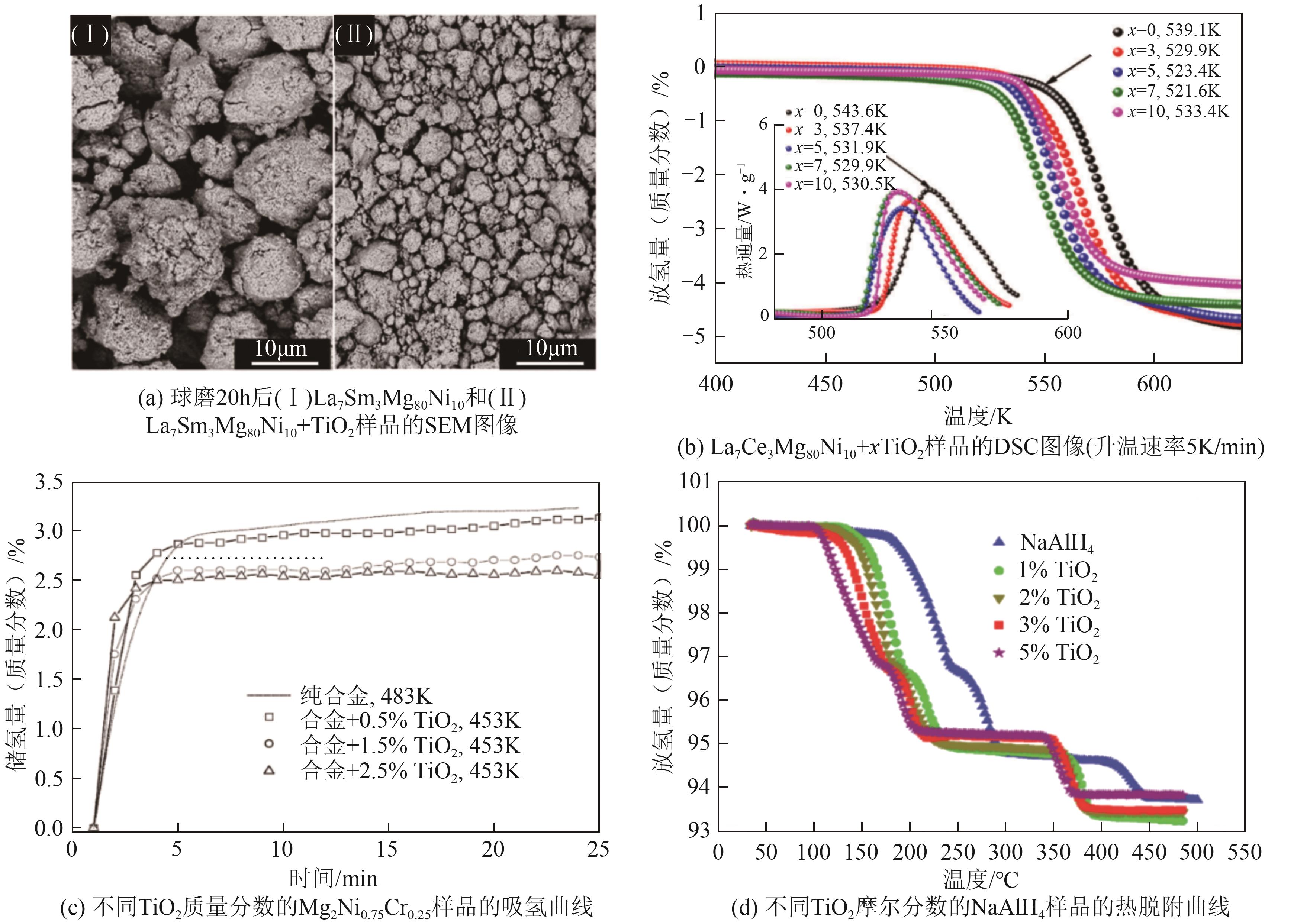
| TiO2 | 改善固态储氢材料的微观结构,减小材料的颗粒尺寸、减少材料颗粒团聚,增强固态储氢材料的吸放氢性能 | Mg2Ni、NaAlH4、La7Ce3Mg80Ni1、Mg2Ni0.75Cr0.25和La7Sm3Mg80Ni10等 | TiO2是相对无害的,并且其不易在环境中降解或迁移 | [ |
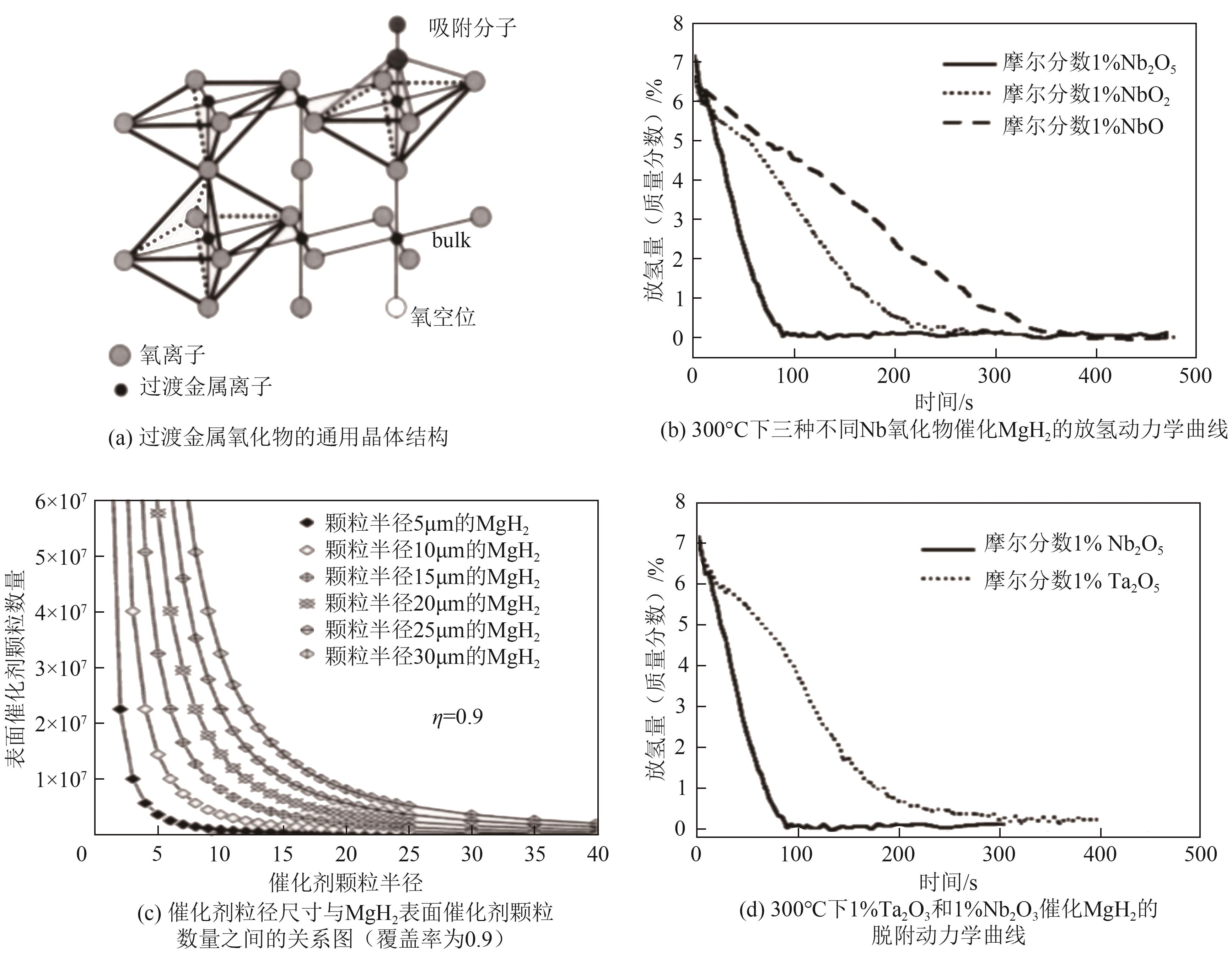
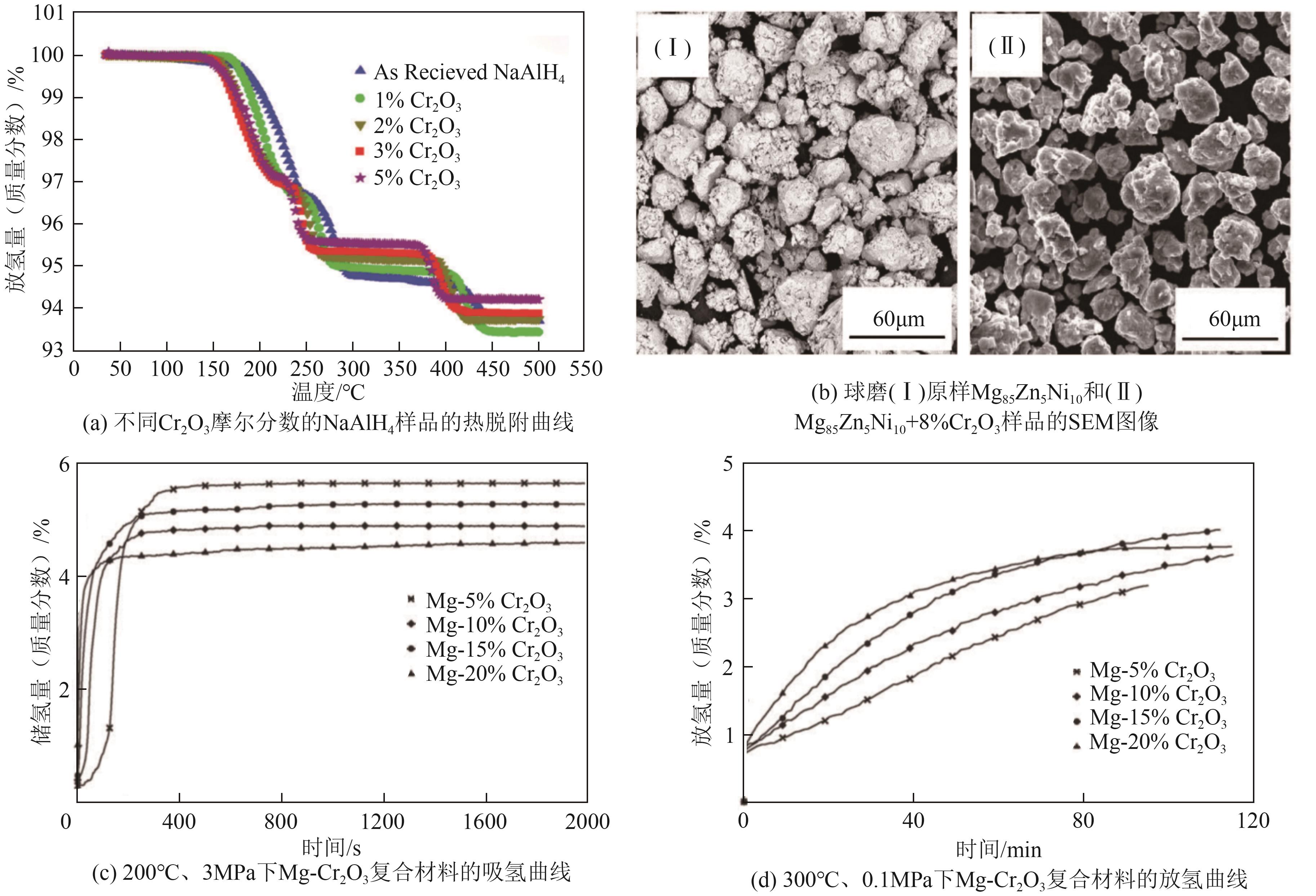
| Cr2O3 | 提供表面活性位点,促进氢分子吸附和解离,减小颗粒尺寸,促进相界面、氢扩散通道及成核位点生成,进而改善固态储氢材料的吸放氢动力学性能,增强材料的热稳定性和循环稳定性 | MgH2、LiAlH4、Mg85Zn5Ni10和Mg等 | Cr2O3是相对稳定且低毒的氧化物,同时还不易溶于水,对环境的直接危害也相对较低 | [ |
| Nb2O5 | “氢泵”效应,促进氢分子吸附和解离,显著降低固态储氢材料的起始脱氢温度,改善脱氢动力学 | NaAlH4、MgH2、Mg-23.5-Ni和LiAlH4等 | Nb2O5是一种稳定的化合物,在环境中不会轻易降解或释放铌元素,对环境的直接影响较小 | [ |
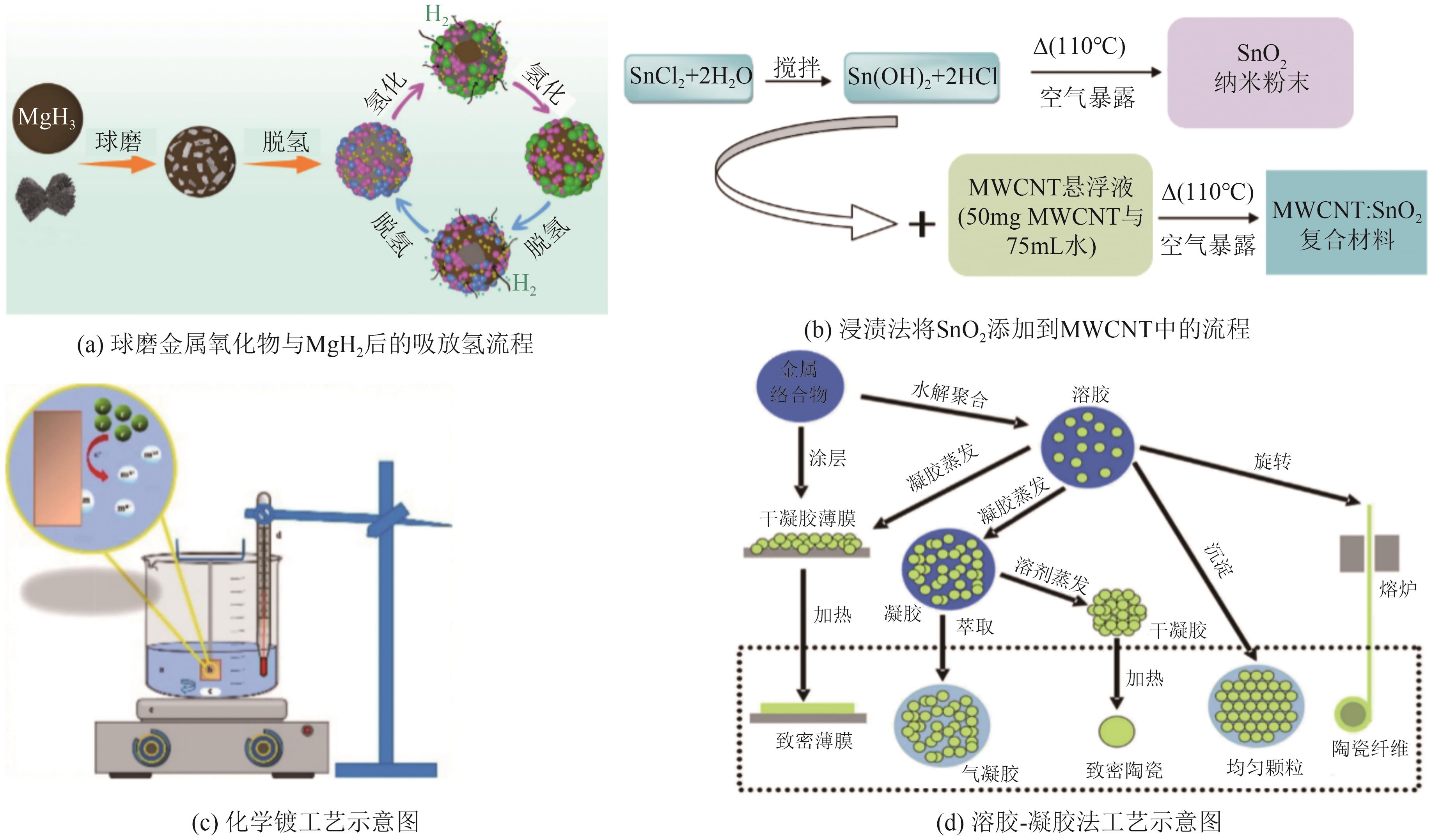
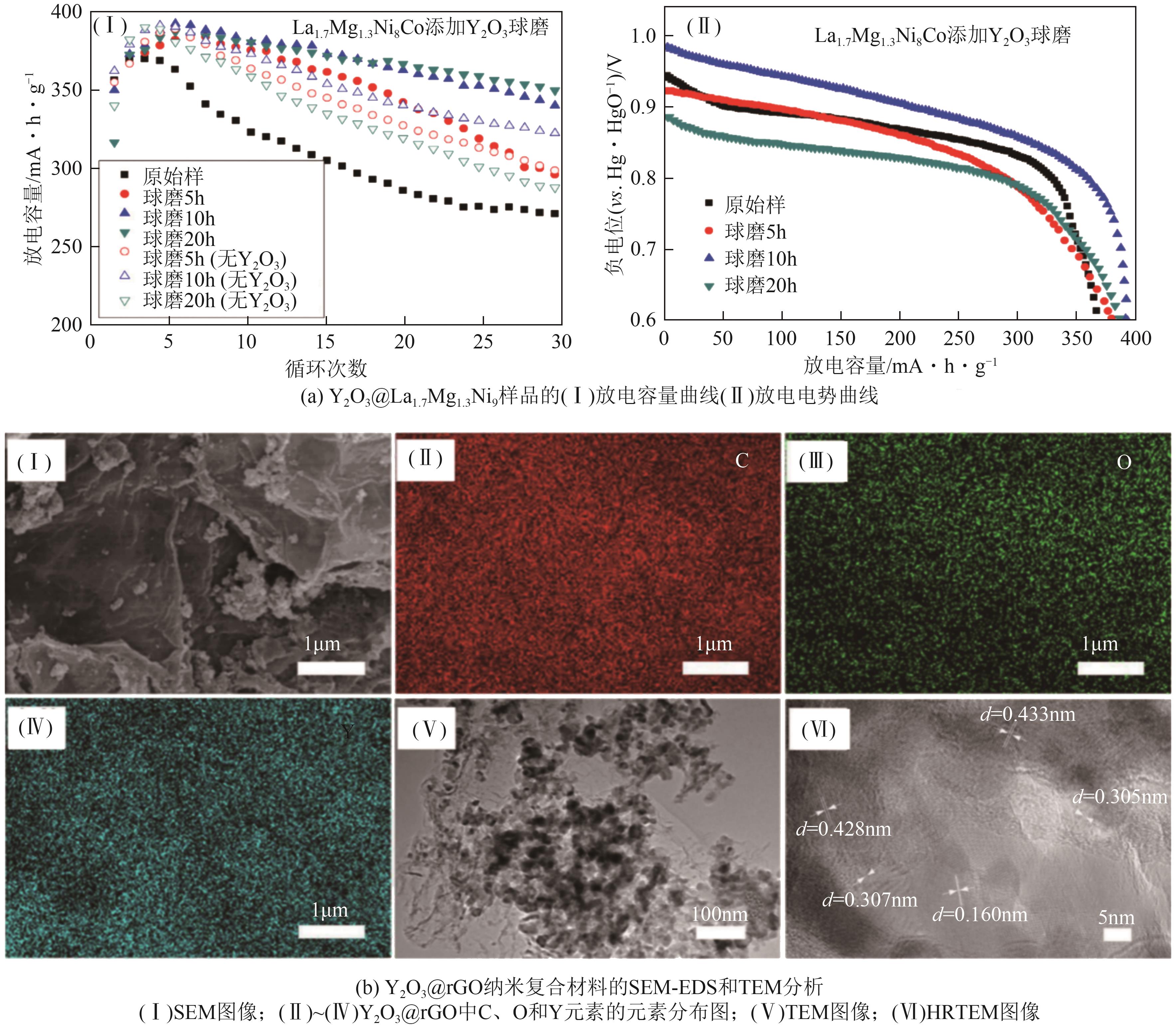
| Y2O3 | 有助于形成更多的氢扩散通道和活性成核位点,提高固态储氢材料的脱氢速率 | Mg-Al、Mg0.97Zn0.03、Na2LiAlH6、La1.7Mg1.3Ni9和MgH2等 | Y2O3在环境中较为稳定,通常不会对环境造成显著影响 | [ |
| La2O3 | 催化作用相对较弱,可以改善固态储氢材料的脱氢动力学性能,但是可能会降低储氢容量 | Mg2Ni、MgH2和NaAlH4等 | La2O3的环境稳定性高 | [ |
| CeO2 | 细化固态储氢材料的颗粒尺寸,增加比表面积,改善储氢热力学参数及降低吸放氢反应活化能等 | Nd2Mg17-50% Ni复合体系、Mg96La3Ni、YMg11Ni、Mg85Cu5Ni10、MgH2和NaAlH4等 | CeO2在环境中相对稳定,对环境影响有限 | [ |
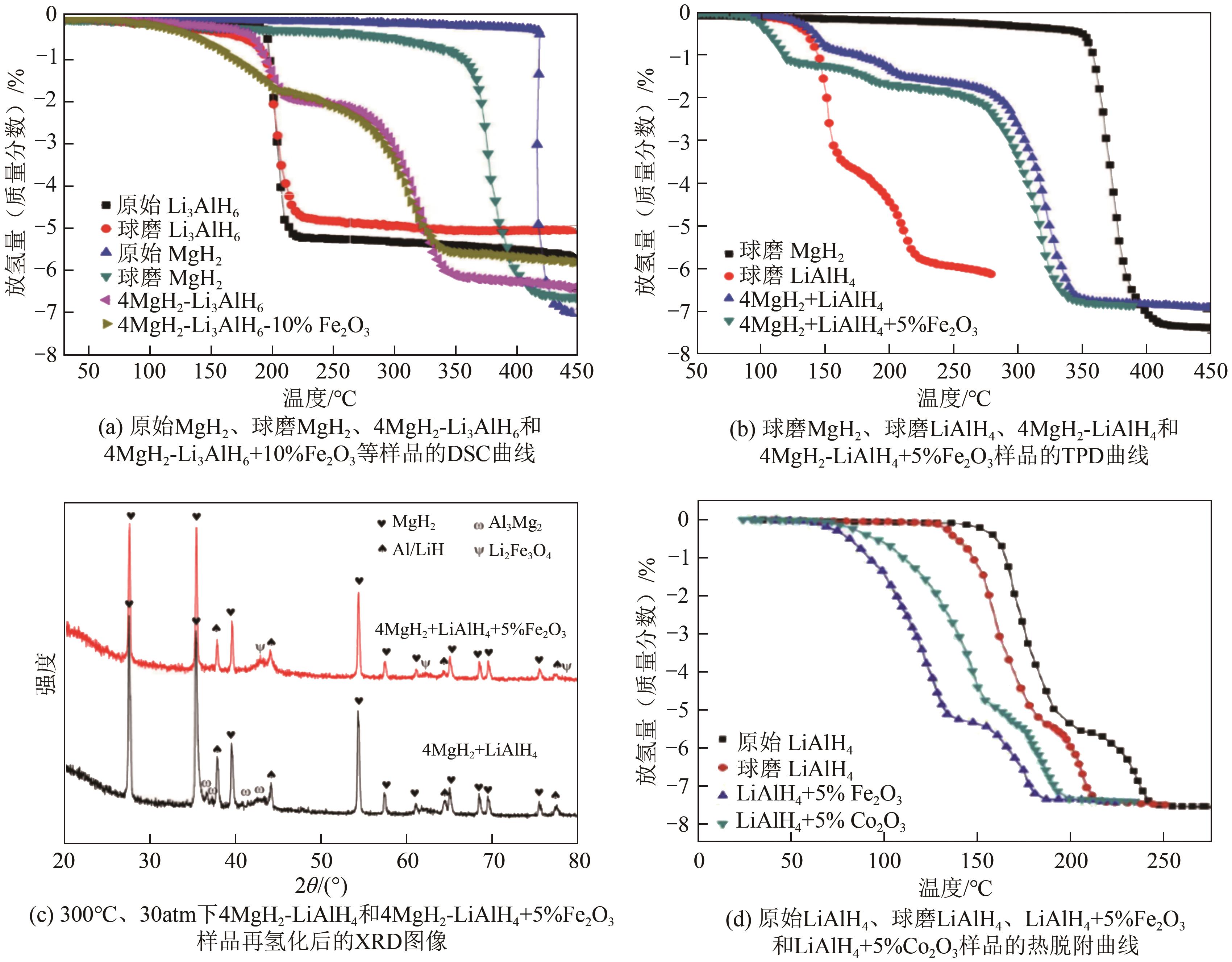
| Fe2O3 | 表面键态和电子态与颗粒内部不同,导致其表面的活性反应位点数量多,降低活化能,改善固态储氢材料的脱氢动力学性能 | 4MgH2-Li3AlH6复合体系和LiAlH4等 | Fe2O3对环境的影响通常较小 | [ |
| ZrO2 | 可以作为催化剂,也可以作用作为催化剂载体,减少固态储氢材料的晶粒尺寸,改善固态储氢材料的吸放氢动力学性能 | MgH2等 | ZrO2是一种无毒的化合物,对环境影响较小 | [ |
| CuO | 可以抑制某些固态储氢材料在吸放氢过程中结构的变化和活性的损失,提高储氢容量和循环稳定性 | MgNi、MgH2和多壁碳纳米管等 | CuO是一种稳定的铜化合物,但在水环境中可能导致水体污染 | [ |
| Co2O3 | 促进氢扩散通道的形成,降低固态储氢材料的脱氢反应活化能及增加活性反应位点等,改善固态储氢材料的脱氢性能 | LiAlH4等 | Co2O3是一种相对稳定的氧化物,但钴化合物对环境和健康的潜在风险需引起重视 | [ |
| SnO2 | 减少颗粒的团聚,提高比表面积,降低起始脱氢温度 | MgH2等 | SnO2是一种稳定的化合物,通常认为对环境无害 | [ |
| Sm2O3 | 显著改善固态储氢材料的脱氢动力学,增强其循环稳定性 | NaAlH4等 | Sm2O3的环境稳定性高 | [ |
| 种类 | 催化机理及效果 | 应用体系 | 环境影响 | 文献 |
|---|---|---|---|---|
| TiO2 | 改善固态储氢材料的微观结构,减小材料的颗粒尺寸、减少材料颗粒团聚,增强固态储氢材料的吸放氢性能 | Mg2Ni、NaAlH4、La7Ce3Mg80Ni1、Mg2Ni0.75Cr0.25和La7Sm3Mg80Ni10等 | TiO2是相对无害的,并且其不易在环境中降解或迁移 | [ |
| Cr2O3 | 提供表面活性位点,促进氢分子吸附和解离,减小颗粒尺寸,促进相界面、氢扩散通道及成核位点生成,进而改善固态储氢材料的吸放氢动力学性能,增强材料的热稳定性和循环稳定性 | MgH2、LiAlH4、Mg85Zn5Ni10和Mg等 | Cr2O3是相对稳定且低毒的氧化物,同时还不易溶于水,对环境的直接危害也相对较低 | [ |
| Nb2O5 | “氢泵”效应,促进氢分子吸附和解离,显著降低固态储氢材料的起始脱氢温度,改善脱氢动力学 | NaAlH4、MgH2、Mg-23.5-Ni和LiAlH4等 | Nb2O5是一种稳定的化合物,在环境中不会轻易降解或释放铌元素,对环境的直接影响较小 | [ |
| Y2O3 | 有助于形成更多的氢扩散通道和活性成核位点,提高固态储氢材料的脱氢速率 | Mg-Al、Mg0.97Zn0.03、Na2LiAlH6、La1.7Mg1.3Ni9和MgH2等 | Y2O3在环境中较为稳定,通常不会对环境造成显著影响 | [ |
| La2O3 | 催化作用相对较弱,可以改善固态储氢材料的脱氢动力学性能,但是可能会降低储氢容量 | Mg2Ni、MgH2和NaAlH4等 | La2O3的环境稳定性高 | [ |
| CeO2 | 细化固态储氢材料的颗粒尺寸,增加比表面积,改善储氢热力学参数及降低吸放氢反应活化能等 | Nd2Mg17-50% Ni复合体系、Mg96La3Ni、YMg11Ni、Mg85Cu5Ni10、MgH2和NaAlH4等 | CeO2在环境中相对稳定,对环境影响有限 | [ |
| Fe2O3 | 表面键态和电子态与颗粒内部不同,导致其表面的活性反应位点数量多,降低活化能,改善固态储氢材料的脱氢动力学性能 | 4MgH2-Li3AlH6复合体系和LiAlH4等 | Fe2O3对环境的影响通常较小 | [ |
| ZrO2 | 可以作为催化剂,也可以作用作为催化剂载体,减少固态储氢材料的晶粒尺寸,改善固态储氢材料的吸放氢动力学性能 | MgH2等 | ZrO2是一种无毒的化合物,对环境影响较小 | [ |
| CuO | 可以抑制某些固态储氢材料在吸放氢过程中结构的变化和活性的损失,提高储氢容量和循环稳定性 | MgNi、MgH2和多壁碳纳米管等 | CuO是一种稳定的铜化合物,但在水环境中可能导致水体污染 | [ |
| Co2O3 | 促进氢扩散通道的形成,降低固态储氢材料的脱氢反应活化能及增加活性反应位点等,改善固态储氢材料的脱氢性能 | LiAlH4等 | Co2O3是一种相对稳定的氧化物,但钴化合物对环境和健康的潜在风险需引起重视 | [ |
| SnO2 | 减少颗粒的团聚,提高比表面积,降低起始脱氢温度 | MgH2等 | SnO2是一种稳定的化合物,通常认为对环境无害 | [ |
| Sm2O3 | 显著改善固态储氢材料的脱氢动力学,增强其循环稳定性 | NaAlH4等 | Sm2O3的环境稳定性高 | [ |
| 种类 | 催化机理及效果 | 应用体系 | 环境影响 | 文献 |
|---|---|---|---|---|
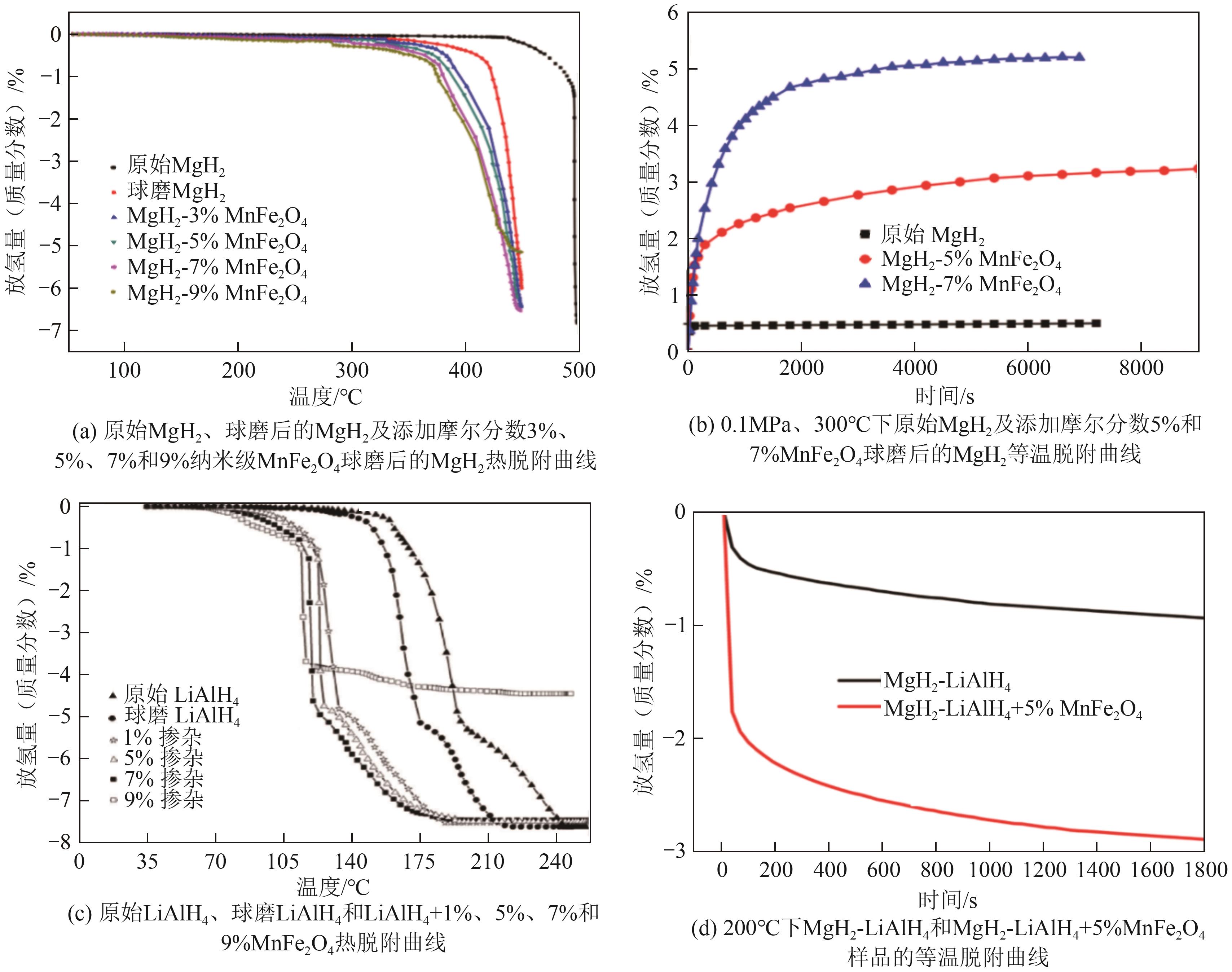
| MnFe2O4 | 提供活性位点,促进氢分子解离和扩散,降低脱氢反应活化能,改善脱氢性能 | NaAlH4、LiAlH4和MgH2等 | 高浓度MnFe2O4可能对环境有一定的风险 | [ |
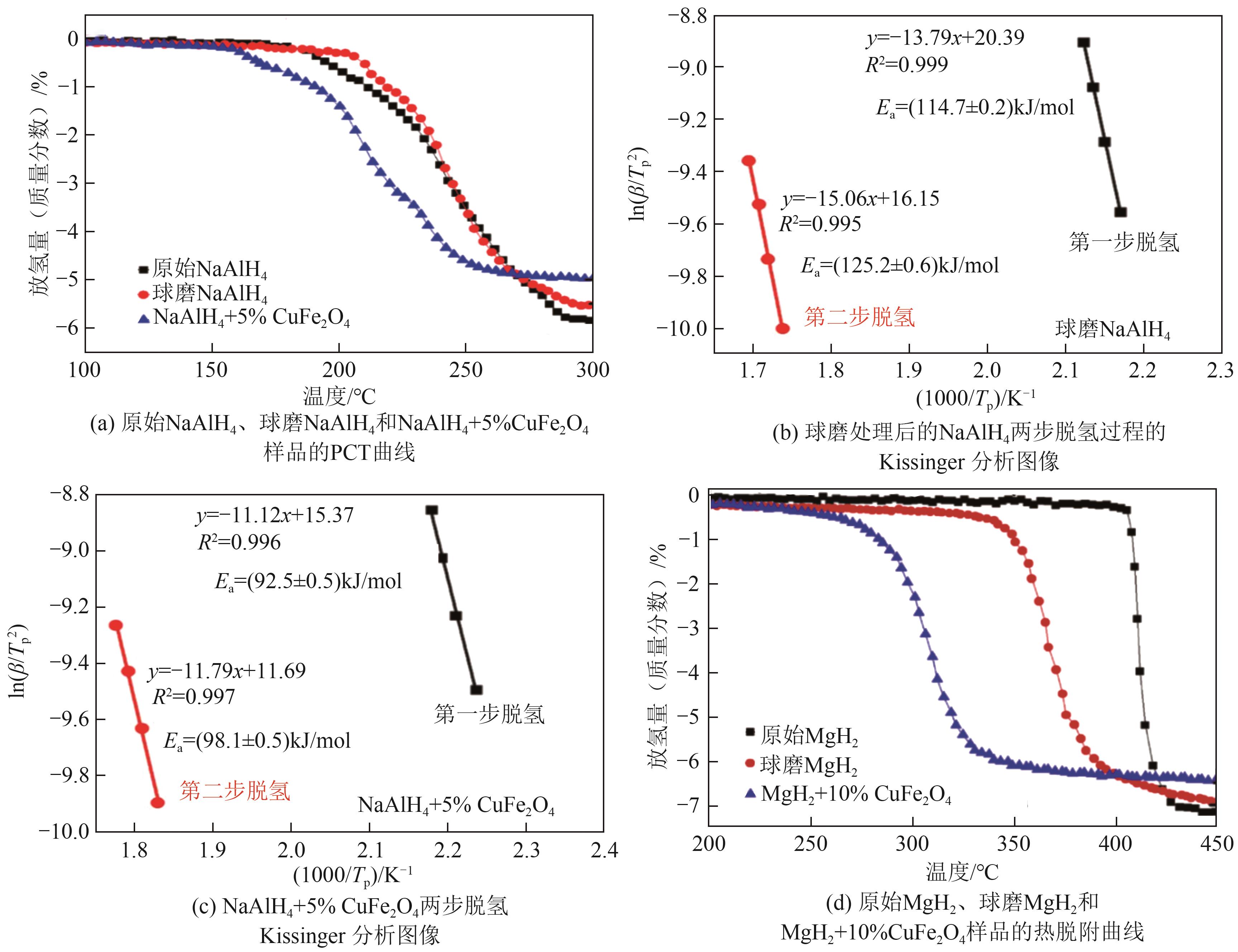
| CuFe2O4 | 减少颗粒尺寸,缓解团聚现象,增加晶界和比表面积,形成具有催化活性的产物,促进氢原子的扩散。降低活化能,可提高脱氢性能 | NaAlH4和MgH2等 | CuFe2O4在水环境中可能导致水体污染 | [ |
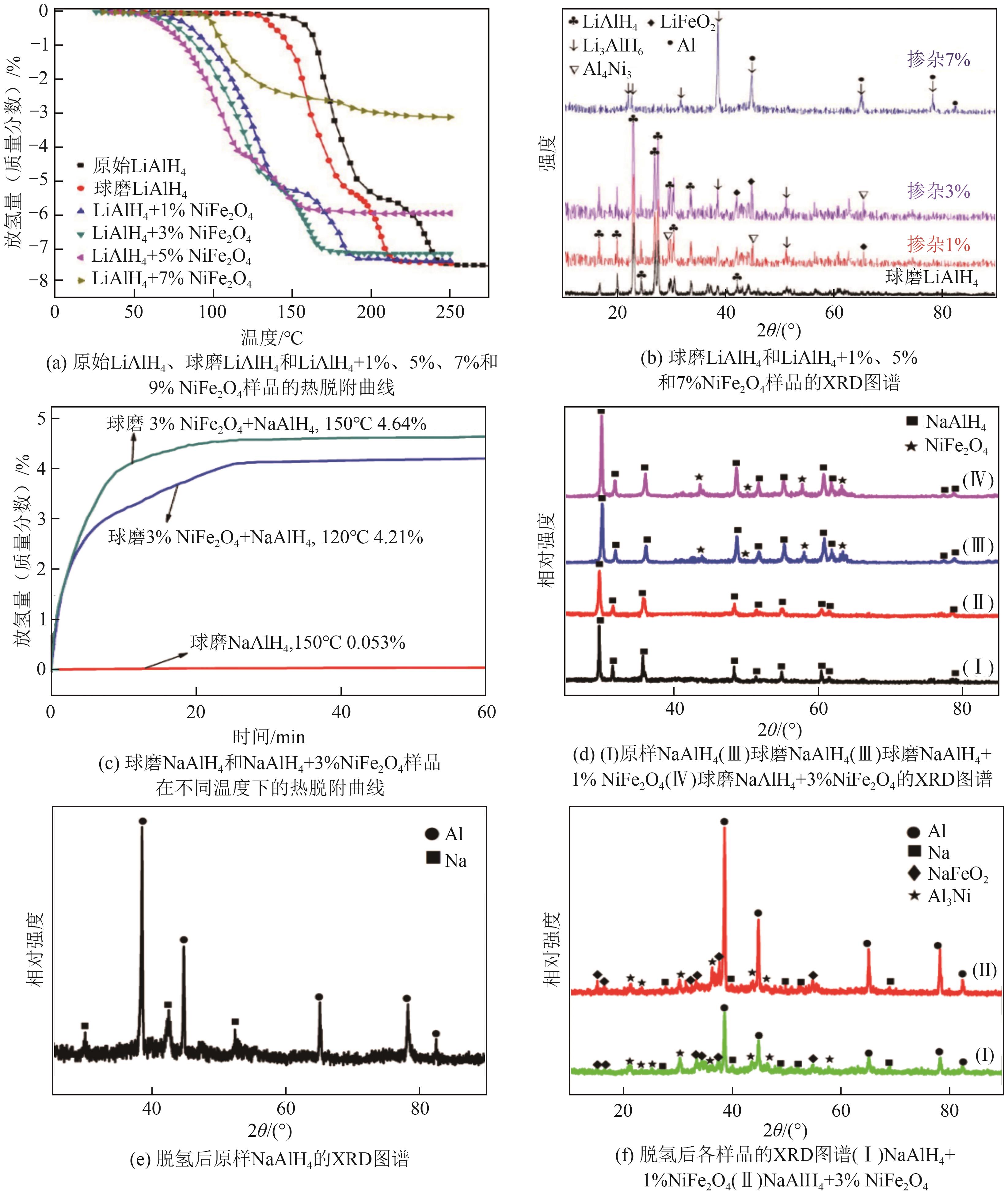
| NiFe2O4 | 在球磨过程中,可以与部分固态储氢材料形成多价态且能够提供活性位点的中间产物,降低脱氢的起始脱氢温度,提高脱氢速率 | LiAlH4和NaAlH4等 | NiFe2O4对环境影响主要由镍浓度主导,高浓度的NiFe2O4可能对水体产生一定的影响 | [ |
| MgFe2O4 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | Na3AlH6-4LiBH4、MgH2、NaBH4和LiAlH4等 | MgFe2O4对环境影响总体较低 | [ |
| LaFeO3 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4和MgH2等 | 作为一种无机氧化物材料,其在使用过程中不会释放有害的副产物 | [ |
| CoTiO3 | 具有高氧化还原活性和稳定性,降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4和MgH2等 | CoTiO3对环境影响主要与钴的含量相关,钴化合物在高浓度下对水生生物有毒,可能导致水体污染 | [ |
| MnTiO3 | 在吸放氢过程中,可以与部分固态储氢材料反应,可以形成多价态及多元素的氧化物,提供催化环境,降低起始脱氢温度并提高脱氢速率 | MgH2等 | 作为一种无机氧化物材料,具有相对良好的环境兼容性和较低的环境负担 | [ |
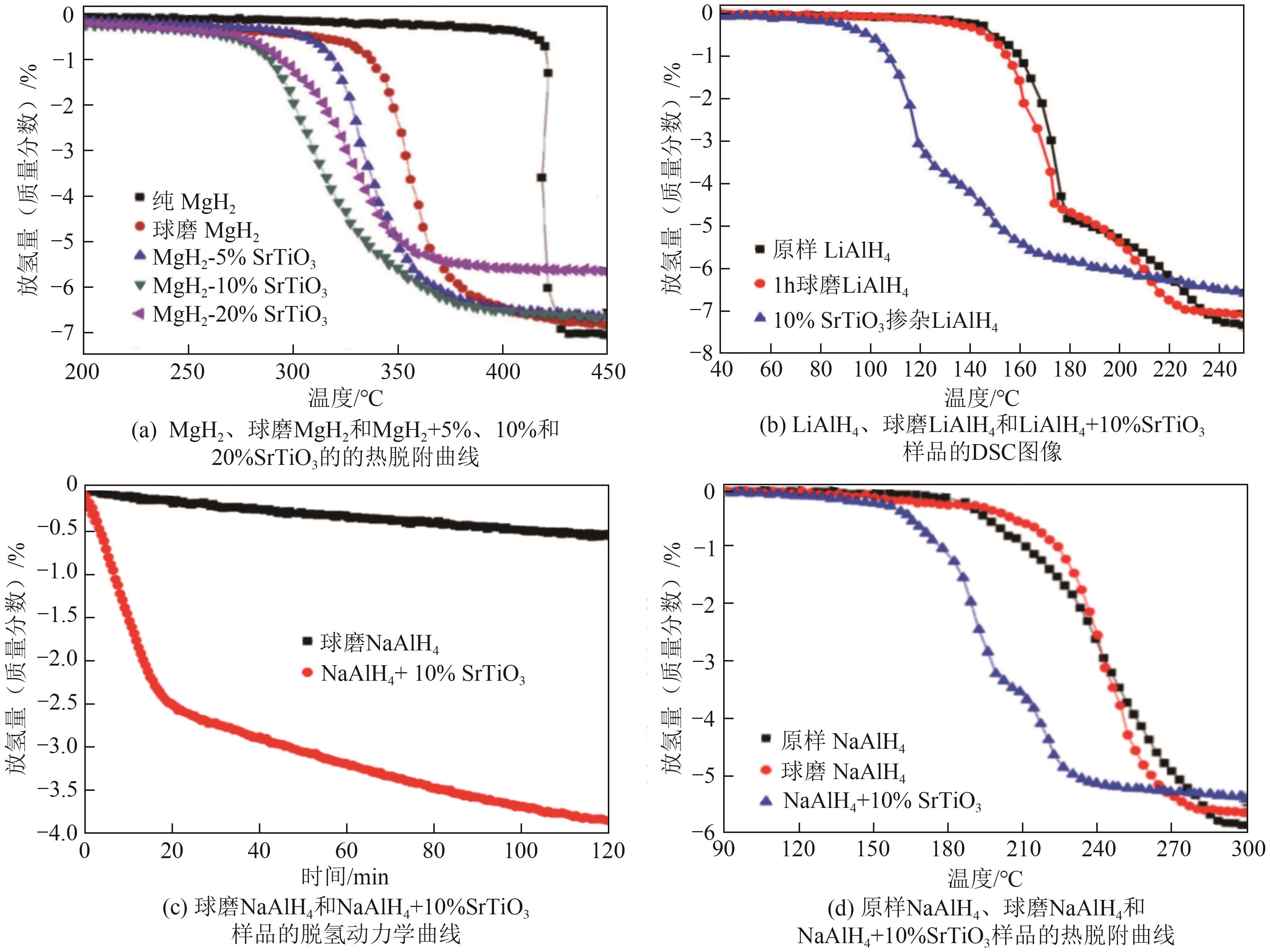
| SrTiO3 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4、NaAlH4和MgH2等 | 作为一种无机材料,其通常具有较好的化学稳定性和环境兼容性 | [ |
| MnCoO4.5 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | MgH2等 | 高浓度MnCoO4.5可能对水体和生态系统造成负面影响 | [ |
| TiNb2O7 | 球磨过程中生成了多种具有催化活性的产物(如Ti、TiO、NbO2和Nb等),上述产物对脱氢反应具有促进作用,降低脱氢反应活化能,降低起始脱氢温度 | LiAlH4和NaAlH4等 | TiNb2O7对环境影响较低,不易释放有害物质 | [ |
| 种类 | 催化机理及效果 | 应用体系 | 环境影响 | 文献 |
|---|---|---|---|---|
| MnFe2O4 | 提供活性位点,促进氢分子解离和扩散,降低脱氢反应活化能,改善脱氢性能 | NaAlH4、LiAlH4和MgH2等 | 高浓度MnFe2O4可能对环境有一定的风险 | [ |
| CuFe2O4 | 减少颗粒尺寸,缓解团聚现象,增加晶界和比表面积,形成具有催化活性的产物,促进氢原子的扩散。降低活化能,可提高脱氢性能 | NaAlH4和MgH2等 | CuFe2O4在水环境中可能导致水体污染 | [ |
| NiFe2O4 | 在球磨过程中,可以与部分固态储氢材料形成多价态且能够提供活性位点的中间产物,降低脱氢的起始脱氢温度,提高脱氢速率 | LiAlH4和NaAlH4等 | NiFe2O4对环境影响主要由镍浓度主导,高浓度的NiFe2O4可能对水体产生一定的影响 | [ |
| MgFe2O4 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | Na3AlH6-4LiBH4、MgH2、NaBH4和LiAlH4等 | MgFe2O4对环境影响总体较低 | [ |
| LaFeO3 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4和MgH2等 | 作为一种无机氧化物材料,其在使用过程中不会释放有害的副产物 | [ |
| CoTiO3 | 具有高氧化还原活性和稳定性,降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4和MgH2等 | CoTiO3对环境影响主要与钴的含量相关,钴化合物在高浓度下对水生生物有毒,可能导致水体污染 | [ |
| MnTiO3 | 在吸放氢过程中,可以与部分固态储氢材料反应,可以形成多价态及多元素的氧化物,提供催化环境,降低起始脱氢温度并提高脱氢速率 | MgH2等 | 作为一种无机氧化物材料,具有相对良好的环境兼容性和较低的环境负担 | [ |
| SrTiO3 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | LiAlH4、NaAlH4和MgH2等 | 作为一种无机材料,其通常具有较好的化学稳定性和环境兼容性 | [ |
| MnCoO4.5 | 降低脱氢反应活化能,降低起始脱氢温度,提高脱氢速率 | MgH2等 | 高浓度MnCoO4.5可能对水体和生态系统造成负面影响 | [ |
| TiNb2O7 | 球磨过程中生成了多种具有催化活性的产物(如Ti、TiO、NbO2和Nb等),上述产物对脱氢反应具有促进作用,降低脱氢反应活化能,降低起始脱氢温度 | LiAlH4和NaAlH4等 | TiNb2O7对环境影响较低,不易释放有害物质 | [ |
| [82] | LEE Gil-Jae, SHIM Jae-Hyeok, CHO Young Whan, et al. Reversible hydrogen storage in NaAlH4 catalyzed with lanthanide oxides[J]. International Journal of Hydrogen Energy, 2007, 32(12): 1911-1915. |
| [83] | LIU Jinming, YONG Hui, ZHAO Yang, et al. Synergistic enhancement of hydrogen storage performance of Mg-La-Ni alloy by CeO2@C composite catalyst[J]. International Journal of Hydrogen Energy, 2024, 55: 141-152. |
| [84] | LI Xia, ZHANG Yanghuan, YANG Tai, et al. Hydriding/dehydriding properties of NdMgNi alloy with catalyst CeO2 [J]. Journal of Rare Earths, 2016, 34(4): 407-412. |
| [85] | YUAN Zeming, SUI Yongqi, ZHAI Tingting, et al. Influence of CeO2 nanoparticles on microstructure and hydrogen storage performance of Mg-Ni-Zn alloy[J]. Materials Characterization, 2021, 178: 111248. |
| [86] | ZHANG Yanghuan, LI Yaqin, SHANG Hongwei, et al. Hydrogen storage performance of the as-milled YMgNi alloy catalyzed by CeO2 [J]. International Journal of Hydrogen Energy, 2018, 43(3): 1643-1650. |
| [87] | YIN Yi, LI Bo, YUAN Zeming, et al. Enhanced hydrogen storage performance of Mg-Cu-Ni system catalyzed by CeO2 additive[J]. Journal of Rare Earths, 2020, 38(9): 983-993. |
| [88] | ISMAIL M, HALIM YAP F A, YAHYA M S. Improved hydrogen storage properties of Mg-Li-Al-H composite system by milling with Fe2O3 powder[J]. Advanced Powder Technology, 2017, 28(9): 2151-2158. |
| [89] | MUSTAFA N S, ISMAIL M. Enhanced hydrogen storage properties of 4MgH2+LiAlH4 composite system by doping with Fe2O3 nanopowder[J]. International Journal of Hydrogen Energy, 2014, 39(15): 7834-7841. |
| [90] | LI Ziliang, LI Ping, WAN Qi, et al. Dehydrogenation improvement of LiAlH4 catalyzed by Fe2O3 and Co2O3 nanoparticles[J]. The Journal of Physical Chemistry C, 2013, 117(36): 18343-18352. |
| [91] | TOME Kudzaishe Caren, XI Senliang, FU Yuanyi, et al. Remarkable catalytic effect of Ni and ZrO2nanoparticles on the hydrogen sorption properties of MgH2 [J]. International Journal of Hydrogen Energy, 2022, 47(7): 4716-4724. |
| [92] | GUEMOU Samuel, GAO Dongqiang, WU Fuying, et al. Enhanced hydrogen storage kinetics of MgH2 by the synergistic effect of Mn3O4/ZrO2 nanoparticles[J]. Dalton Transactions, 2023, 52(3): 609-620. |
| [93] | SHUKLA Vivek, YADAV Thakur Prasad. Notable catalytic activity of CuO nanoparticles derived from metal-organic frameworks for improving the hydrogen sorption properties of MgH2 [J]. International Journal of Energy Research, 2022, 46(9): 12804-12819. |
| [94] | RATHER Sami ullah. Hydrogen uptake of cobalt and copper oxide-multiwalled carbon nanotube composites [J]. International Journal of Hydrogen Energy, 2017, 42(16): 11553-11559. |
| [95] | ZHANG Yanhui, JIAO Lifang, YUAN Huatang, et al. Study on the electrochemical properties of MgNi-CuO hydrogen storage composite materials[J]. Journal of Alloys and Compounds, 2009, 481(1/2): 639-643. |
| [96] | JUBAIR Ahammed, RAHMAN Md Akhlakur, KHAN Md Maksudur Rahman, et al. Catalytic role of binary oxides (CuO and Al2O3) on hydrogen storage in MgH2 [J]. Environmental Progress & Sustainable Energy, 2023, 42(1): e13940. |
| [97] | ABDELLATIEF M, CAMPOSTRINI R, LEONI M, et al. Effects of SnO2 on hydrogen desorption of MgH2 [J]. International Journal of Hydrogen Energy, 2013, 38(11): 4664-4669. |
| [98] | LI Ping, WAN Qi, LI Ziliang, et al. MgH2 dehydrogenation properties improved by MnFe2O4 nanoparticles[J]. Journal of Power Sources, 2013, 239: 201-206. |
| [99] | ZHAI Fuqiang, LI Ping, SUN Aizhi, et al. Significantly improved dehydrogenation of LiAlH4 destabilized by MnFe2O4 nanoparticles[J]. The Journal of Physical Chemistry C, 2012, 116(22): 11939-11945. |
| [100] | WAN Qi, LI Ping, LI Ziliang, et al. Improved hydrogen storage performance of MgH2-LiAlH4 composite by addition of MnFe2O4 [J]. The Journal of Physical Chemistry C, 2013, 117(51): 26940-26947. |
| [101] | IDRIS N H, MUSTAFA N S, ISMAIL M. MnFe2O4 nanopowder synthesised via a simple hydrothermal method for promoting hydrogen sorption from MgH2 [J]. International Journal of Hydrogen Energy, 2017, 42(33): 21114-21120. |
| [102] | WAN Qi, LI Ping, LI Ziliang, et al. NaAlH4 dehydrogenation properties enhanced by MnFe2O4 nanoparticles[J]. Journal of Power Sources, 2014, 248: 388-395. |
| [103] | MUSTAFA N S, SAZELEE N A, ALI N A, et al. Enhancement of hydrogen storage properties of NaAlH4 catalyzed by CuFe2O4 [J]. International Journal of Hydrogen Energy, 2023, 48(90): 35197-35205. |
| [104] | ISMAIL M, MUSTAFA N S, ALI N A, et al. The hydrogen storage properties and catalytic mechanism of the CuFe2O4-doped MgH2 composite system[J]. International Journal of Hydrogen Energy, 2019, 44(1): 318-324. |
| [105] | LI Ping, LI Ziliang, ZHAI Fuqiang, et al. NiFe2O4 nanoparticles catalytic effects of improving LiAlH4 dehydrogenation properties[J]. The Journal of Physical Chemistry C, 2013, 117(49): 25917-25925. |
| [106] | HUANG Yihui, LI Ping, WAN Qi, et al. Improved dehydrogenation performance of NaAlH4 using NiFe2O4 nanoparticles[J]. Journal of Alloys and Compounds, 2017, 709: 850-856. |
| [107] | HALIM YAP F A, ALI N A, IDRIS N H, et al. Catalytic effect of MgFe2O4 on the hydrogen storage properties of Na3AlH6-LiBH4 composite system[J]. International Journal of Hydrogen Energy, 2018, 43(45): 20882-20891. |
| [108] | ALI N A, IDRIS N H, SAZELEE N A, et al. Catalytic effects of MgFe2O4 addition on the dehydrogenation properties of LiAlH4 [J]. International Journal of Hydrogen Energy, 2019, 44(52): 28227-28234. |
| [109] | ALI N A, IDRIS Nurul Hayati, DIN M F MD, et al. Nanolayer-like-shaped MgFe2O4 synthesised via a simple hydrothermal method and its catalytic effect on the hydrogen storage properties of MgH2 [J]. RSC Advances, 2018, 8(28): 15667-15674. |
| [110] | SAZELEE N A, YAHYA M S, IDRIS N H, et al. Desorption properties of LiAlH4 doped with LaFeO3 catalyst[J]. International Journal of Hydrogen Energy, 2019, 44(23): 11953-11960. |
| [111] | Nurul Amirah ALI, AHMAD Muhammad Amirul Nawi, YAHYA Muhammad Syarifuddin, et al. Improved dehydrogenation properties of LiAlH4 by addition of nanosized CoTiO3 [J]. Nanomaterials, 2022, 12(21): 3921. |
| [112] | Nurul Amirah ALI, YAHYA Muhammad Syarifuddin, SAZELEE Noratiqah, et al. Influence of nanosized CoTiO3 synthesized via a solid-state method on the hydrogen storage behavior of MgH2 [J]. Nanomaterials, 2022, 12(17): 3043. |
| [113] | ALI N A, YUSNIZAM N Y, SAZELEE N A, et al. Inclusion of CoTiO3 to ameliorate the re/dehydrogenation properties of the Mg-Na-Al system[J]. Journal of Magnesium and Alloys, 2024, 12(3): 1215-1226. |
| [114] | MA Chao, ZHAO Baozhou, YUAN Jianguang, et al. Superior synergistic effect derived from MnTiO3 nanodiscs for the reversible hydrogen storage properties of MgH2 [J]. Journal of Alloys and Compounds, 2023, 968: 171774. |
| [115] | YAHYA M S, ISMAIL M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2 [J]. Journal of Energy Chemistry, 2019, 28: 46-53. |
| [116] | ISMAIL M, SAZELEE N A, ALI N A, et al. Catalytic effect of SrTiO3 on the dehydrogenation properties of LiAlH4 [J]. Journal of Alloys and Compounds, 2021, 855: 157475. |
| [117] | SAZELEE Noratiqah, MUSTAFA Nurul Shafikah, YAHYA Muhammad Syarifuddin, et al. Enhanced dehydrogenation performance of NaAlH4 by the addition of spherical SrTiO3 [J]. International Journal of Energy Research, 2021, 45(6): 8648-8658. |
| [1] | OUYANG Liuzhang, LIU Fen, WANG Hui, et al. Magnesium-based hydrogen storage compounds: A review[J]. Journal of Alloys and Compounds, 2020, 832: 154865. |
| [2] | ZHANG Yanghuan, JIA Zhichao, YUAN Zeming, et al. Development and application of hydrogen storage[J]. Journal of Iron and Steel Research, International, 2015, 22(9): 757-770. |
| [3] | SCHLAPBACH L, ZÜTTEL A. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414(6861): 353-358. |
| [4] | 崔祥勇, 王阳峰. “双碳”背景下石化企业氢气系统协同优化技术研究及应用[J]. 广东化工, 2023, 50(12): 85-88. |
| CUI Xiangyong, WANG Yangfeng. Research and application of collaborative optimization technology for hydrogen system in refinery under the background of “Double Carbon” [J]. Guangdong Chemical Industry, 2023, 50(12): 85-88. | |
| [5] | 俞和胜, 祁海鹰, 谭忠超. “双碳”背景下传统化石能源脱碳制氢增值化利用技术[J]. 清华大学学报(自然科学版), 2023, 63(8): 1226-1235. |
| YU Hesheng, QI Haiying, TAN Zhongchao. Decarbonization, hydrogen production, and value-added utilization of conventional fossil fuels under the background of “double-carbon” [J]. Journal of Tsinghua University (Science and Technology), 2023, 63(8): 1226-1235. | |
| [6] | RASUL M G, HAZRAT M A, SATTAR M A, et al. The future of hydrogen: Challenges on production, storage and applications[J]. Energy Conversion and Management, 2022, 272: 116326. |
| [7] | 廖红波, 张雪霞, 岳嘉玲, 等. 氢气储运技术发展及展望[J]. 太阳能学报, 2024, 45(11): 691-699. |
| LIAO Hongbo, ZHANG Xuexia, YUE Jialing, et al. Development and prospect of hydrogen storage and transportation technology[J]. Acta Energiae Solaris Sinica, 2024, 45(11): 691-699. | |
| [8] | 韩利, 李琦, 冷国云, 等. 氢能储存技术最新进展[J]. 化工进展, 2022, 41(S1): 108-117. |
| HAN Li, LI Qi, LENG Guoyun, et al. Latest research progress of hydrogen energy storage technology[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 108-117. | |
| [9] | PETITPAS Guillaume. Simulation of boil-off losses during transfer at a LH2 based hydrogen refueling station [J]. International Journal of Hydrogen Energy, 2018, 43(46): 21451-21463. |
| [10] | ALLENDORF Mark D, STAVILA Vitalie, SNIDER J L, et al. Challenges to developing materials for the transport and storage of hydrogen[J]. Nature Chemistry, 2022, 14(11): 1214-1223. |
| [11] | 刘木子, 史柯柯, 赵强, 等. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769. |
| LIU Muzi, SHI Keke, ZHAO Qiang, et al. Research progress of solid hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4746-4769. | |
| [12] | 刘泉宇, 彭程, 黄东方, 等. 表面处理技术在储氢材料中的应用研究进展[J]. 材料导报, 2024, 38(20): 25-36. |
| LIU Quanyu, PENG Cheng, HUANG Dongfang, et al. Research progress on the application of surface treatment technology in hydrogen storage materials[J]. Materials Review, 2024, 38(20): 25-36. | |
| [13] | 高佳佳, 米媛媛, 周洋, 等. 新型储氢材料研究进展[J]. 化工进展, 2021, 40(6): 2962-2971. |
| GAO Jiajia, MI Yuanyuan, ZHOU Yang, et al. Recent developments in new hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2962-2971. | |
| [14] | SUN Ze, LU Xiong, NYAHUMA Farai Michael, et al. Enhancing hydrogen storage properties of MgH2 by transition metals and carbon materials: A brief review[J]. Frontiers in Chemistry, 2020, 8: 552. |
| [15] | 邓文琪. 金属氧化物对镁基材料储氢性能影响及机理研究[D]. 沈阳: 沈阳工业大学, 2024. |
| Deng Wenqi. Influence and mechanism of metal oxides on the hydrogen storageperformance of magnesium-based[D]. Shenyang: Shenyang University of Technology, 2024. | |
| [16] | ALI N A, ISMAIL M. Modification of NaAlH4 properties using catalysts for solid-state hydrogen storage: A review[J]. International Journal of Hydrogen Energy, 2021, 46(1): 766-782. |
| [17] | 顾婷婷, 顾坚, 张喻, 等. 金属硼氢化物基固态储氢体系[J]. 化学进展, 2020, 32(5): 665-686. |
| [118] | ZHANG Lingchao, WANG Ke, LIU Yongfeng, et al. Highly active multivalent multielement catalysts derived from hierarchical porous TiNb2O7 nanospheres for the reversible hydrogen storage of MgH2 [J]. Nano Research, 2021, 14(1): 148-156. |
| [119] | HU Song, ZHANG Huanhuan, YUAN Zhenluo, et al. Ultrathin K2Ti8O17 nanobelts for improving the hydrogen storage kinetics of MgH2 [J]. Journal of Alloys and Compounds, 2021, 881: 160571. |
| [120] | LONG Shiteng, QIN Yang, FU Huafeng, et al. Hydrogen storage properties of MgH2 modified by efficient Co3V2O8 catalyst[J]. Separation and Purification Technology, 2024, 341: 126901. |
| [121] | SAZELEE N A, RATHER Sami-ullah, SININ A M, et al. Improved dehydrogenation performance of LiAlH4 doped with BaMnO3 [J]. Heliyon, 2024, 10(10): e31190. |
| [122] | LIU Heng, ZHAI Xiaojie, LI Zhe, et al. Improved electrochemical hydrogen storage performance of Ti49Zr26Ni25 quasicrystal alloy by doping with mesoporous α-Fe2O3 particles[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7447-7455. |
| [17] | GU Tingting, GU Jian, ZHANG Yu, et al. Metal borohydride-based system for solid-state hydrogen storage[J]. Progress in Chemistry, 2020, 32(5): 665-686. |
| [18] | ZHANG Yanghuan, LI Chen, YUAN Zeming, et al. Research progress of TiFe-based hydrogen storage alloys[J]. Journal of Iron and Steel Research International, 2022, 29(4): 537-551. |
| [19] | 吕朋, 周兴盛, 刘芝辰, 等. 基于机械形变方法的TiFe基储氢合金的研究进展[J]. 东华理工大学学报(自然科学版), 2021, 44(1): 90-95. |
| Peng LYU, ZHOU Xingsheng, LIU Zhichen, et al. Research progress of TiFe-based hydrogen storage alloys processed by mechanical deformation method[J]. Journal of East China University of Technology (Natural Science), 2021, 44(1): 90-95. | |
| [20] | RUI Zebao, TANG Minni, JI Weikang, et al. Insight into the enhanced performance of TiO2 nanotube supported Pt catalyst for toluene oxidation[J]. Catalysis Today, 2017, 297: 159-166. |
| [21] | LIU Guozhu, TIAN Yajie, ZHANG Bofeng, et al. Catalytic combustion of VOC on sandwich-structured Pt@ZSM-5 nanosheets prepared by controllable intercalation[J]. Journal of Hazardous Materials, 2019, 367: 568-576. |
| [22] | DU Jinpeng, QU Zhenping, DONG Cui, et al. Low-temperature abatement of toluene over Mn-Ce oxides catalysts synthesized by a modified hydrothermal approach[J]. Applied Surface Science, 2018, 433: 1025-1035. |
| [23] | FENG Xinyi, GUO Jiaxiu, WEN Xinru, et al. Enhancing performance of Co/CeO2 catalyst by Sr doping for catalytic combustion of toluene[J]. Applied Surface Science, 2018, 445: 145-153. |
| [24] | 王旭凤, 刘建. 掺杂金属氧化物对Mg基储氢合金性能的影响[J]. 稀土, 2018, 39(5): 122-134. |
| WANG Xufeng, LIU Jian. Effect of doping of metal oxides on performance of Mg-based hydrogen storage alloy[J].Chinese Rare Earths, 2018, 39(5): 122-134. | |
| [25] | 蔡浩, 顾昊, 朱云峰, 等. 催化剂对镁基储氢材料储氢性能影响的研究进展[J]. 材料导报, 2008, 22(11): 115-119. |
| CAI Hao, GU Hao, ZHU Yunfeng, et al. Research progress in the influence of catalysts on hydrogen storage property of magnesium-based hydrogen storage materials[J]. Materials Review, 2008, 22(11): 115-119. | |
| [26] | 王诗雯, 鲁杨帆, 丁朝, 等. Ti基催化剂改性Mg基储氢材料的研究进展[J]. 稀有金属, 2023, 47(12): 1642-1656. |
| WANG Shiwen, LU Yangfan, DING Zhao, et al. Research progress on Ti-based catalysts modified Mg-based hydrogen storage materials[J].Chinese Journal of Rare Metals, 2023, 47(12): 1642-1656. | |
| [27] | 任壮禾, 张欣, 高明霞, 等. Ti基催化剂改性的NaAlH4储氢材料研究进展[J]. 稀有金属, 2021, 45(5): 569-582. |
| REN Zhuanghe, ZHANG Xin, GAO Mingxia, et al. Research progress in Ti-based catalysts-modified NaAlH4 hydrogen storage materials[J]. Chinese Journal of Rare Metals, 2021, 45(5): 569-582. | |
| [28] | 徐晓红. 复合金属氧化物多孔材料限域催化协同改善LiBH4储氢性能研究[D]. 天津: 南开大学, 2017. |
| XU Xiaohong. An investigation on the hydrogen storage properties of LiBH4 catalyzed and confined by the porous mixed metal oxide materials[D]. Tianjin: Nankai University, 2017. | |
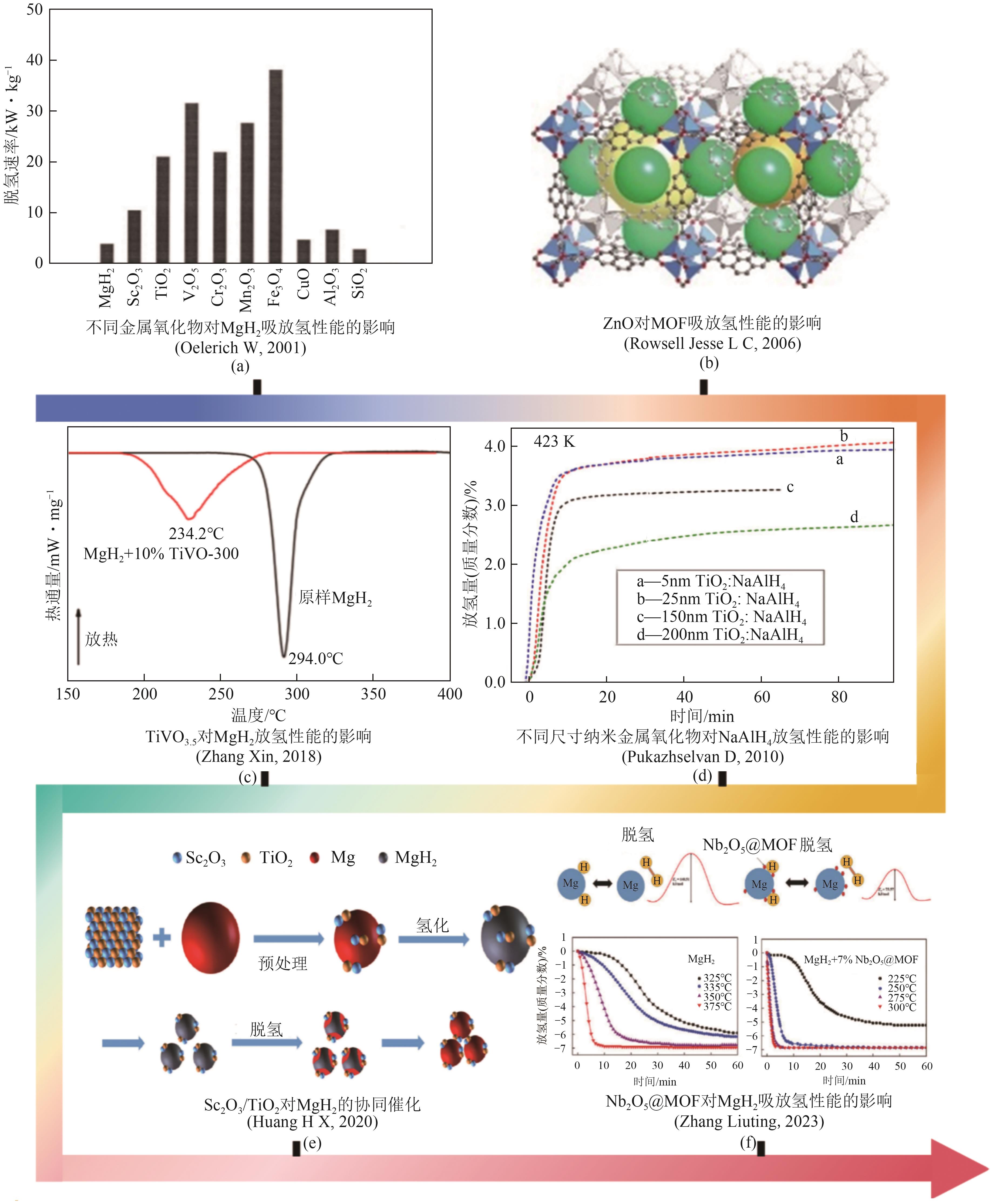
| [29] | OELERICH W, KLASSEN T, BORMANN R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials[J]. Journal of Alloys and Compounds, 2001, 315(1/2): 237-242. |
| [30] | ROWSELL Jesse L C, YAGHI Omar M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| [31] | PUKAZHSELVAN D, M Sterlin Leo HUDSON, SINHA A S K, et al. Studies on metal oxide nanoparticles catalyzed sodium aluminum hydride[J]. Energy, 2010, 35(12): 5037-5042. |
| [32] | ZHANG Xin, SHEN Zhengyang, JIAN Ni, et al. A novel complex oxide TiVO3.5 as a highly active catalytic precursor for improving the hydrogen storage properties of MgH2 [J]. International Journal of Hydrogen Energy, 2018, 43(52): 23327-23335. |
| [33] | HUANG H X, YUAN J G, ZHANG B, et al. A noteworthy synergistic catalysis on hydrogen sorption kinetics of MgH2 with bimetallic oxide Sc2O3/TiO2 [J]. Journal of Alloys and Compounds, 2020, 839: 155387. |
| [34] | ZHANG Liuting, NYAHUMA Farai Michael, ZHANG Haoyu, et al. Metal organic framework supported niobium pentoxide nanoparticles with exceptional catalytic effect on hydrogen storage behavior of MgH2 [J]. Green Energy & Environment, 2023, 8(2): 589-600. |
| [35] | JAIN Ankur, AGARWAL Shivani, ICHIKAWA Takayuki. Catalytic tuning of sorption kinetics of lightweight hydrides: A review of the materials and mechanism[J]. Catalysts, 2018, 8(12): 651. |
| [36] | SAZELEE N A, IDRIS N H, DIN M F MD, et al. LaFeO3 synthesised by solid-state method for enhanced sorption properties of MgH2 [J]. Results in Physics, 2020, 16: 102844. |
| [37] | ZHUANG Guoxin, CHEN Yawen, ZHUANG Zanyong, et al. Oxygen vacancies in metal oxides: Recent progress towards advanced catalyst design[J]. Science China Materials, 2020, 63(11): 2089-2118. |
| [38] | BORGSCHULTE A, RECTOR J H, DAM B, et al. The role of niobium oxide as a surface catalyst for hydrogen absorption[J]. Journal of Catalysis, 2005, 235(2): 353-358. |
| [39] | BARKHORDARIAN Gagik, KLASSEN Thomas, Rüdiger BORMANN. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction[J]. The Journal of Physical Chemistry B, 2006, 110(22): 11020-11024. |
| [40] | POLANSKI M, BYSTRZYCKI J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride[J]. Journal of Alloys and Compounds, 2009, 486(1/2): 697-701. |
| [41] | BARTOLETTI Andrea, GONDOLINI Angela, SANGIORGI Nicola, et al. Identification of structural changes in CaCu3Ti4O12 on high energy ball milling and their effect on photocatalytic performance[J]. Catalysis Science & Technology, 2023, 13(4): 1041-1058. |
| [42] | CUI Jie, WANG Hui, LIU Jiangwen, et al. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts[J]. Journal of Materials Chemistry A, 2013, 1(18): 5603-5611. |
| [43] | HUANG Z G, GUO Z P, CALKA A, et al. Effects of iron oxide (Fe2O3, Fe3O4) on hydrogen storage properties of Mg-based composites[J]. Journal of Alloys and Compounds, 2006, 422(1/2): 299-304. |
| [44] | WANG Shuai, YONG Hui, YAO Jiwei, et al. Influence of the phase evolution and hydrogen storage behaviors of Mg-RE alloy by a multi-valence Mo-based catalyst[J]. Journal of Energy Storage, 2023, 58: 106397. |
| [45] | OELERICH W, KLASSEN T, BORMANN R. Comparison of the catalytic effects of V, V2O5, VN, and VC on the hydrogen sorption of nanocrystalline Mg[J]. Journal of Alloys and Compounds, 2001, 322(1/2): L5-L9. |
| [46] | ZHANG Meng, XIAO Xuezhang, LUO Bosang, et al. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures[J]. Journal of Energy Chemistry, 2020, 46: 191-198. |
| [47] | SCHWAB George-Maria. Alloy catalysts in dehydrogenation[J]. Discussions of the Faraday Society, 1950, 8: 166. |
| [48] | RATHI Bhawna, AGARWAL Shivani, SHRIVASTAVA Kriti, et al. Recent advances in designing metal oxide-based catalysts to enhance the sorption kinetics of magnesium hydride[J]. International Journal of Hydrogen Energy, 2024, 53: 131-162. |
| [49] | ZHANG Yiwen, XIAO Xuezhang, LUO Bosang, et al. Synergistic effect of LiBH4 and LiAlH4 additives on improved hydrogen storage properties of unexpected high capacity magnesium hydride[J]. The Journal of Physical Chemistry C, 2018, 122(5): 2528-2538. |
| [50] | RAFI-UD-DIN, QU Xuanhui, LI Ping, et al. Hydrogen sorption improvement of LiAlH4 catalyzed by Nb2O5 and Cr2O3 nanoparticles[J]. The Journal of Physical Chemistry C, 2011, 115(26): 13088-13099. |
| [51] | WEI Xin, LI Chen, GE Qilu, et al. Improved hydrogen storage performances of the as-milled Mg-Al-Y alloy by co-doping of Tm@C (Tm=Fe, Co, Cu)[J]. Journal of Alloys and Compounds, 2022, 929: 167317. |
| [52] | MATANOVIC Ivana, ARTYUSHKOVA Kateryna, ATANASSOV Plamen. Understanding PGM-free catalysts by linking density functional theory calculations and structural analysis: Perspectives and challenges[J]. Current Opinion in Electrochemistry, 2018, 9: 137-144. |
| [53] | JACOBSEN Claus J H, Søren DAHL, BOISEN Astrid, et al. Optimal catalyst curves: Connecting density functional theory calculations with industrial reactor design and catalyst selection[J]. Journal of Catalysis, 2002, 205(2): 382-387. |
| [54] | 路站宽. 基于密度泛函理论的高效ORR双原子催化剂的性能筛选和机理研究[D]. 北京: 北京化工大学, 2024. |
| LU Zhankuan. Performance screening and mechanism study of efficient ORR diatomic catalysts based on DFT[D]. Beijing: Beijing University of Chemical Technology, 2024. | |
| [55] | Jorge BENAVIDES-HERNÁNDEZ, DUMEIGNIL Franck. From characterization to discovery: Artificial intelligence, machine learning and high-throughput experiments for heterogeneous catalyst design[J]. ACS Catalysis, 2024, 14(15): 11749-11779. |
| [56] | LAI Nung Siong, Yi Shen TEW, ZHONG Xialin, et al. Artificial intelligence (AI) workflow for catalyst design and optimization[J]. Industrial & Engineering Chemistry Research, 2023, 62(43): 17835-17848. |
| [57] | 董毅. 基于机器学习的多元金属氧化物基催化剂的高效开发与性能研究[D]. 杭州: 浙江大学, 2024. |
| DONG Yi. Machine learning-based efficient development and performance study of multimetal oxide-based catalysts[D]. Hangzhou: Zhejiang University, 2024. | |
| [58] | ZHANG Yanghuan, WEI Xin, ZHANG Wei, et al. Catalytic effect comparison of TiO2 and La2O3 on hydrogen storage thermodynamics and kinetics of the as-milled La-Sm-Mg-Ni-based alloy[J]. Journal of Magnesium and Alloys, 2021, 9(6): 2063-2077. |
| [59] | MA X Z, MARTINEZ-FRANCO E, DORNHEIM M, et al. Catalyzed Na2LiAlH6 for hydrogen storage[J]. Journal of Alloys and Compounds, 2005, 404: 771-774. |
| [60] | SONG Jianzheng, HAN Shumin, FU Ruidong. Effect of La2O3-CaO composite additive on the hydrogen storage properties of Mg2Ni alloy[J]. Materials Science and Engineering: B, 2014, 188: 114-118. |
| [61] | SONG Myoungyoup, KWON Sungnam, Jong-Soo BAE, et al. Hydrogen-storage properties of melt spun Mg-23.5wt%Ni milled with nano Nb2O5 [J]. Journal of Alloys and Compounds, 2009, 478(1/2): 501-506. |
| [62] | ZHOU Shiming, WEI Dan, WAN Haiyi, et al. Efficient catalytic effect of the page-like MnCo2O4.5 catalyst on the hydrogen storage performance of MgH2 [J]. Inorganic Chemistry Frontiers, 2022, 9(21): 5495-5506. |
| [63] | VELLINGIRI Lathapriya, ANNAMALAI Karthigeyan, KANDASAMY Ramamurthi, et al. Synthesis and characterization of MWCNT impregnated with different loadings of SnO2 nanoparticles for hydrogen storage applications[J]. International Journal of Hydrogen Energy, 2018, 43(2): 848-860. |
| [64] | MOLAEI Maryam, ATAPOUR Masoud. Nickel-based coatings as highly active electrocatalysts for hydrogen evolution reaction: A review on electroless plating cost-effective technique[J]. Sustainable Materials and Technologies, 2024, 40: e00991. |
| [65] | LIU Xianzhe, ZHANG Xu, TAO Hong, et al. Research progress of tin oxide-based thin films and thin-film transistors prepared by sol-gel method[J]. Acta Physica Sinica, 2020, 69(22): 228102. |
| [66] | SHAHCHERAGHI A, DEHGHANI F, RAEISSI K, et al. Effects of TiO2 additive on electrochemical hydrogen storage properties of nanocrystalline/amorphous Mg2Ni intermetallic alloy[J]. Iranian Journal of Materials Science and Engineering, 2013, 10: 1-9. |
| [67] | ZHANG Yanghuan, ZHANG Wei, WEI Xin, et al. Catalytic effects of TiO2 on hydrogen storage thermodynamics and kinetics of the as-milled Mg-based alloy [J]. Materials Characterization, 2021, 176: 111118. |
| [68] | GAO R G, TU J P, WANG X L, et al. The absorption and desorption properties of nanocrystalline Mg2Ni0.75Cr0.25 alloy containing TiO2 nanoparticles[J]. Journal of Alloys and Compounds, 2003, 356: 649-653. |
| [69] | RAFI-UD-DIN, QU Xuanhui, LI Ping, et al. Superior catalytic effects of Nb2O5, TiO2, and Cr2O3 nanoparticles in improving the hydrogen sorption properties of NaAlH4 [J]. The Journal of Physical Chemistry C, 2012, 116(22): 11924-11938. |
| [70] | SONG Wenjie, MA Wenhao, HE Shuai, et al. TiO2@C catalyzed hydrogen storage performance of Mg-Ni-Y alloy with LPSO and ternary eutectic structure[J]. Journal of Magnesium and Alloys, 2024, 12(2): 767-778. |
| [71] | XIAN Kaicheng, NIE Bo, LI Zigen, et al. TiO2decorated porous carbonaceous network structures offer confinement, catalysis and thermal conductivity for effective hydrogen storage of LiBH4 [J]. Chemical Engineering Journal, 2021, 407: 127156. |
| [72] | YIN Yi, LI Bo, YUAN Zeming, et al. Microstructure and improved hydrogen storage properties of Mg85Zn5Ni10 alloy catalyzed by Cr2O3 nanoparticles[J]. Journal of Physics and Chemistry of Solids, 2019, 134: 295-306. |
| [73] | VIJAY R, SUNDARESAN R, MAIYA M P, et al. Hydrogen storage properties of Mg-Cr2O3 nanocomposites: The role of catalyst distribution and grain size[J]. Journal of Alloys and Compounds, 2006, 424(1/2): 289-293. |
| [74] | POLANSKI Marek, BYSTRZYCKI Jerzy, PLOCINSKI Tomasz. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles[J]. International Journal of Hydrogen Energy, 2008, 33(7): 1859-1867. |
| [75] | HANADA Nobuko, ICHIKAWA Takayuki, HINO Satoshi, et al. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5 [J]. Journal of Alloys and Compounds, 2006, 420(1/2): 46-49. |
| [76] | BARKHORDARIAN Gagik, KLASSEN Thomas, Rüdiger BORMANN. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg[J]. Journal of Alloys and Compounds, 2004, 364(1/2): 242-246. |
| [77] | OSORIO-GARCÍA M, SUÁREZ-ALCÁNTARA K, TODAKA Y, et al. Low-temperature hydrogenation of Mg-Ni-Nb2O5 alloy processed by high-pressure torsion[J]. Journal of Alloys and Compounds, 2021, 878: 160309. |
| [78] | ZHANG Huaiwei, FU Li, XUAN Weidong, et al. Surface modification of the La1.7Mg1.3Ni9 alloy with trace Y2O3 related to the electrochemical hydrogen storage properties[J]. Renewable Energy, 2020, 145: 1572-1577. |
| [79] | ZHONG H C, HUANG Y S, DU Z Y, et al. Enhanced hydrogen ab/de-sorption of Mg(Zn) solid solution alloy catalyzed by YH2/Y2O3 nanocomposite[J]. International Journal of Hydrogen Energy, 2020, 45(51): 27404-27412. |
| [80] | WANG Hui, HU Jie, HAN Fuguo, et al. Enhanced joint catalysis of YH2/Y2O3 on dehydrogenation of MgH2 [J]. Journal of Alloys and Compounds, 2015, 645: S209-S212. |
| [81] | LAN Zhiqiang, SUN Zhenzhen, DING Yuchuan, et al. Catalytic action of Y2O3@graphene nanocomposites on the hydrogen-storage properties of Mg-Al alloys[J]. Journal of Materials Chemistry A, 2017, 5(29): 15200-15207. |
| [1] | LIU Zhe, ZHOU Shunli, LI Yongxiang, ZHANG Chengxi, LIU Yipeng. Research progress on alkyl naphthalene synthesis catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 144-158. |
| [2] | LIN Yijie, QIAO Peng, LI Xinrui, ZHANG Hongbin, WANG Xueqin. Construction and application of heterostructures of photocatalyst TiO2 nanomaterials [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 159-177. |
| [3] | WANG Tao, ZHANG Xuebing, ZHANG Qi, CHEN Qiang, ZHANG Kui, MEN Zhuowu. Effects of reduction-carburization temperature and inlet CO concentration on industrial precipitated iron-based catalyst for Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 178-184. |
| [4] | BAO Xinde, LIU Biye, HUANG Renwei, HONG Yuhao, GUAN Xin, LIN Jinguo. Preparation of biomass-based@CuNiOS composite catalysts for the reduction of organic dye [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 185-196. |
| [5] | ZHAO Siyang, LI Chenran, LIU Yang. Process optimization for regulating diene selectivity of MTO regenerated catalyst through pre-carbon deposition using C4 by-product [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 205-212. |
| [6] | ZHAO Yulong, CAI Kai, YU Shanqing. Influence of pore structure of alumina on the adsorption, diffusion and reactivity of hydrocarbon molecules in catalytic cracking [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 213-221. |
| [7] | LI Junliang, LI Yue, SUN Daolai. Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 222-231. |
| [8] | LIU Chao, DING Chengao, WU Baoshun, LEI Xinyu, WANG Guangying, YU Zhengwei. Effect of TiO2 support particle size on the denitrification and water/sulfur poisoning resistance of RuO x -V2O5-WO3/TiO2 catalyst [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 232-242. |
| [9] | ZHANG Hanlin, YUE Xuehai, LIU Junxi, YIN Fengjun. Fabrication of high stability electrocatalyst for oxygen evolution reaction by Ru-Sr-Ir electrodeposition [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 243-251. |
| [10] | CHEN Zizhao, HE Fangshu, HU Qiang, YANG Yang, CHEN Hanping, YANG Haiping. Research progress on anti-carbon deposition Ni-based catalysts for dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4968-4978. |
| [11] | WANG Zhen, ZHANG Yaoyuan, WU Qin, SHI Daxin, CHEN Kangcheng, LI Hansheng. Development of Ni/Al2O3-based catalysts for the dry reforming of methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4979-4998. |
| [12] | ZHANG Haipeng, QIN Shanshan, WANG Yuxuan, YU Haibiao. Preparation of 3.0F-Ag x Co catalysts for N2O decomposition [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 4999-5005. |
| [13] | SUN Mengyuan, LU Shijian, LIU Ling, XUE Yanyang, ZHANG Yunrong, DONG Qi, KANG Guojun. Research progress of MOF and their derivatives in carbon capture [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5339-5350. |
| [14] | WANG Wenjun, LIU Ruixin, WANG Jun, ZHANG Qinglei, HOU Li’an. Research progress of visible light degradation of indoor VOCs by titanium dioxide materials [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5351-5362. |
| [15] | ZENG Jin, GAO Yan, WANG Zhaopeng, XIE Yuyun, LIU Jun, LIANG Qi, WANG Chunying. Degradation mechanism of 2,4-dichlorophenoxyacetic acid by NaYF4:Yb,Tm composite TiO2/Bi2WO6 photocatalyst and evaluation of products toxicity [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5416-5431. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||