Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (S1): 469-478.DOI: 10.16085/j.issn.1000-6613.2024-0635
• Fine chemicals • Previous Articles Next Articles
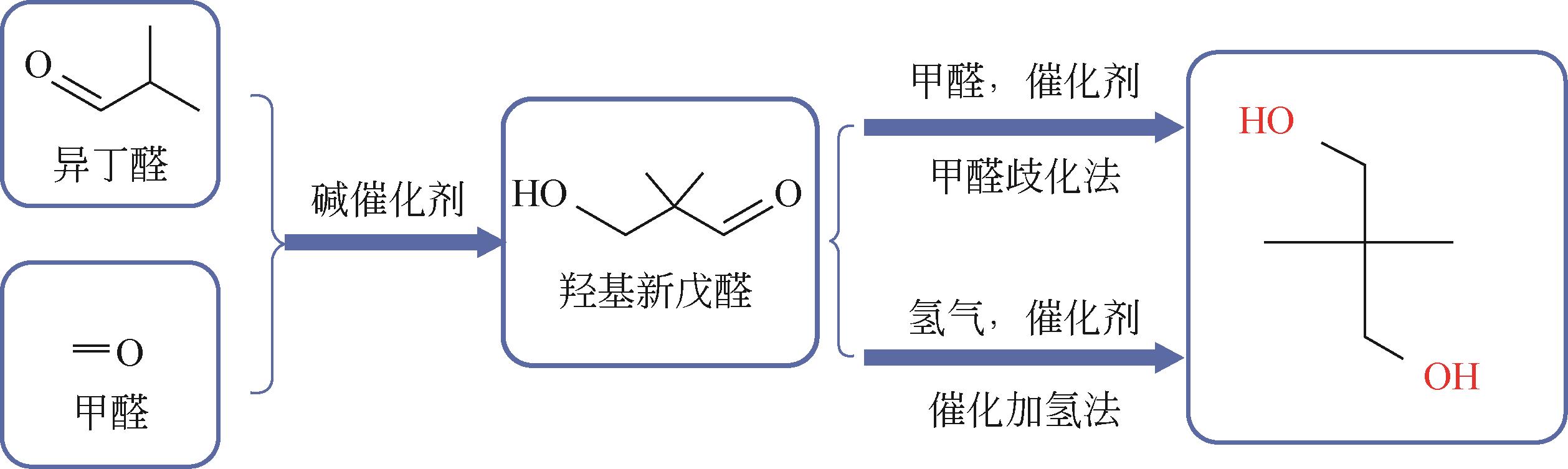
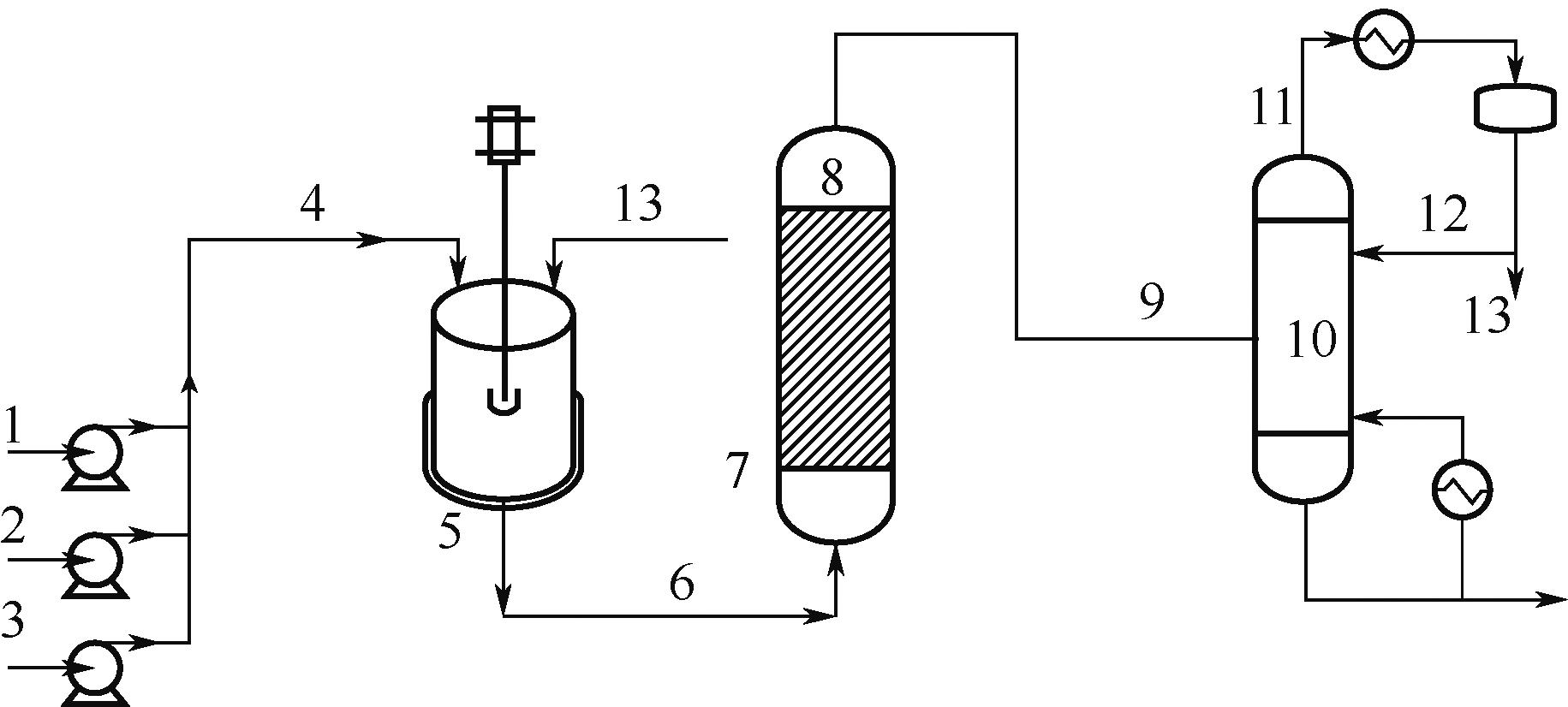
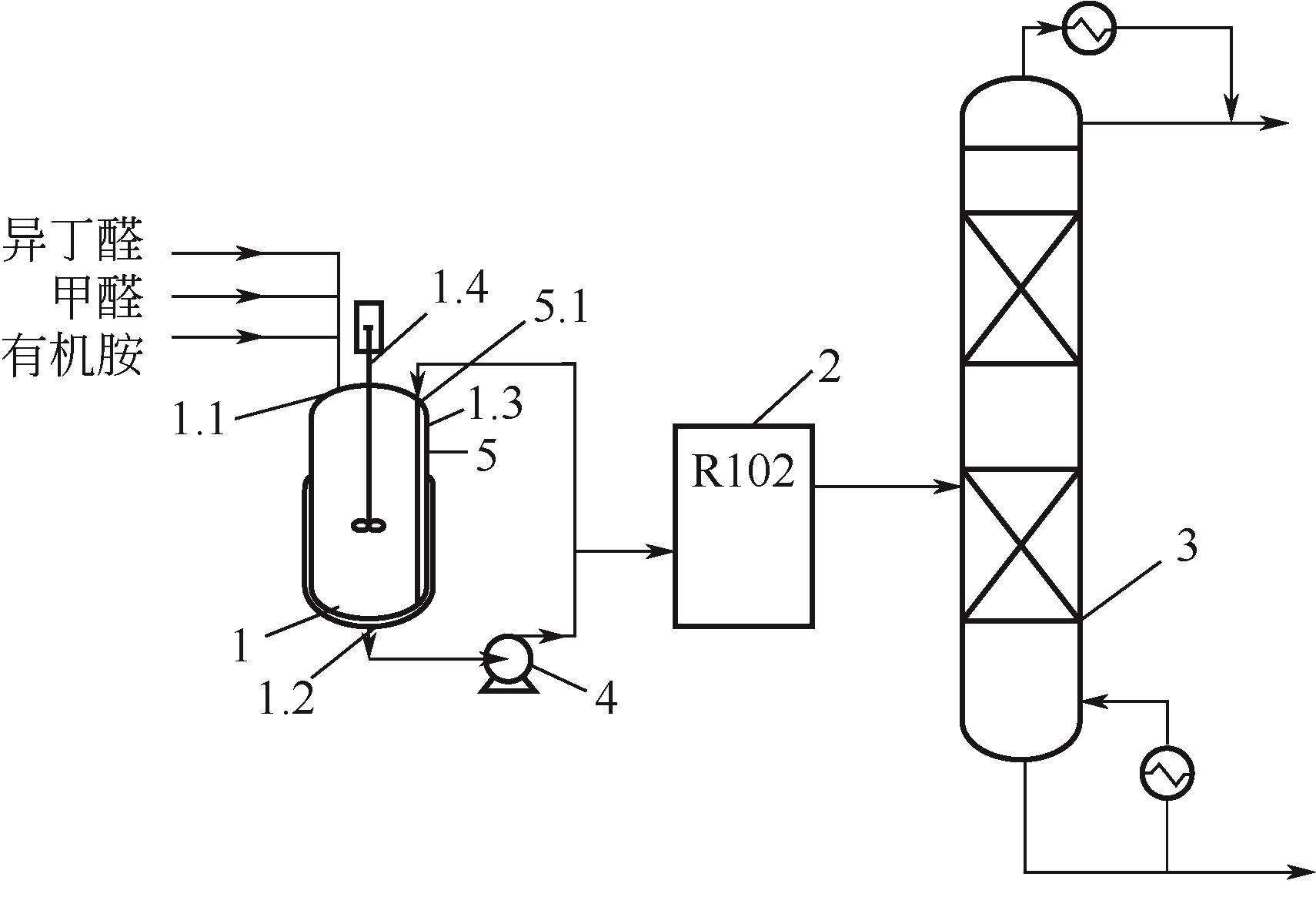
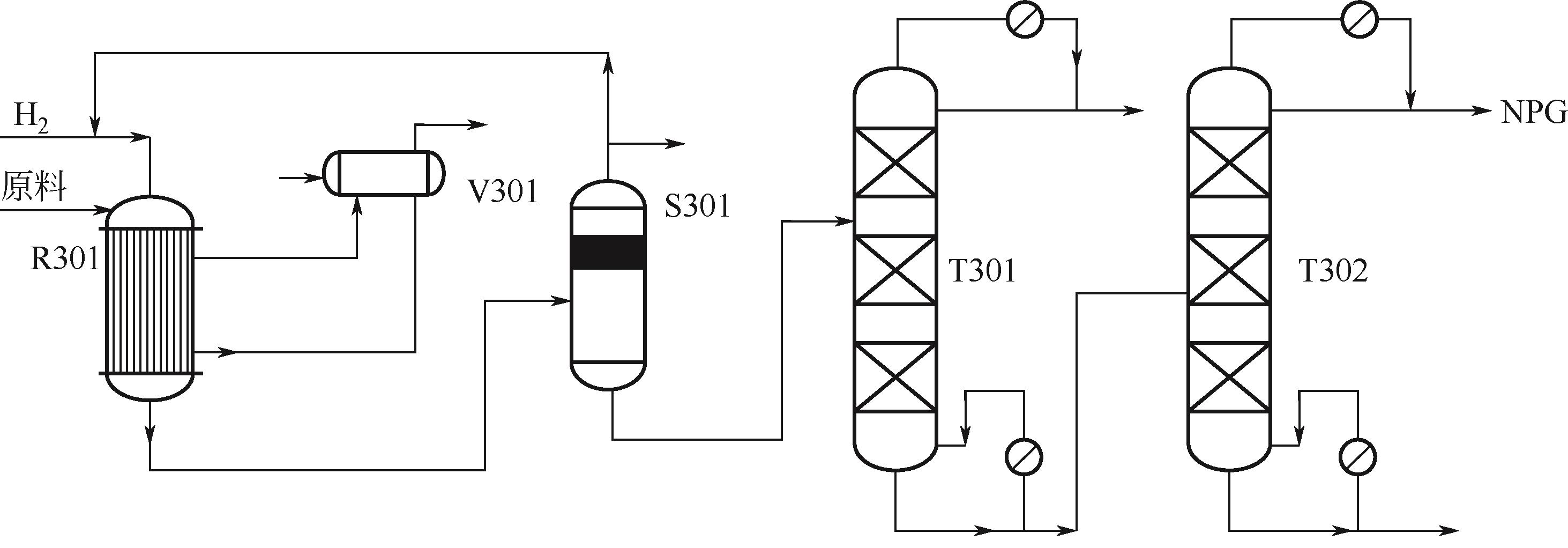
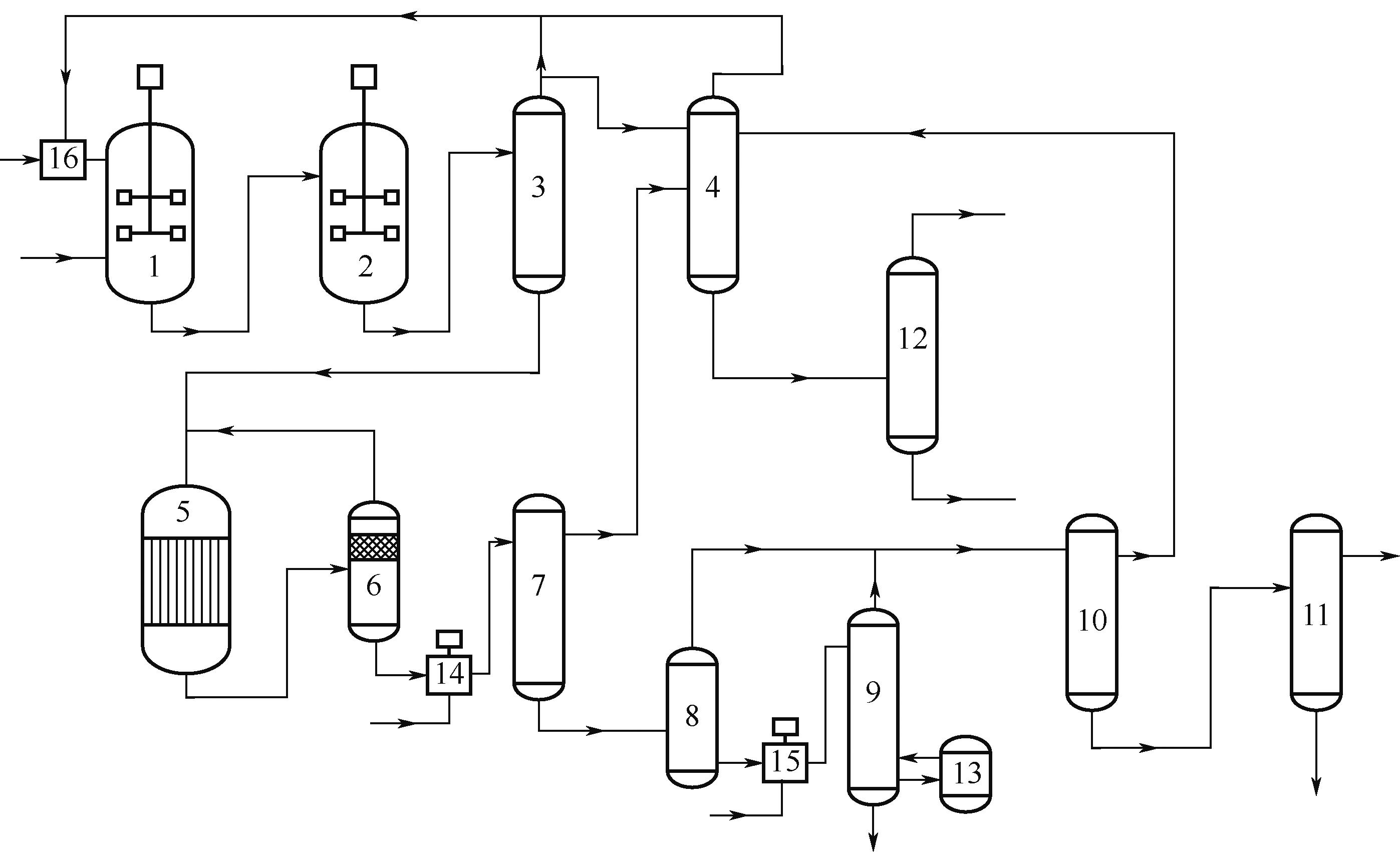
Synthesis process of neopentyl glycol
GAO Congzhi( ), ZHANG Yaxuan, LIN Lu, DENG Xiaoting, YIN Xia, DING Yigang, XIAO Yanhua, DU Zhiping(
), ZHANG Yaxuan, LIN Lu, DENG Xiaoting, YIN Xia, DING Yigang, XIAO Yanhua, DU Zhiping( )
)
- School of Chemical Engineering and Pharmacy, Hubei Key Laboratory of Novel Reactor & Green Chemical Technology, Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan University of Engineering, Wuhan 430000, Hubei, China
-
Received:2024-04-15Revised:2024-06-16Online:2024-12-06Published:2024-11-20 -
Contact:DU Zhiping
新戊二醇的合成工艺
高聪志( ), 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平(
), 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平( )
)
- 武汉工程大学化工与制药学院,绿色化工过程教育部重点实验室,湖北省新型反应器与绿色化学工艺 重点实验室,湖北 武汉 430000
-
通讯作者:杜治平 -
作者简介:高聪志(2000—),女,硕士研究生,研究方向为绿色化学。E-mail:2486333072@qq.com。 -
基金资助:湖北省自然科学基金(21S067)
CLC Number:
Cite this article
GAO Congzhi, ZHANG Yaxuan, LIN Lu, DENG Xiaoting, YIN Xia, DING Yigang, XIAO Yanhua, DU Zhiping. Synthesis process of neopentyl glycol[J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 469-478.
高聪志, 张雅萱, 林璐, 邓晓婷, 殷霞, 丁一刚, 肖艳华, 杜治平. 新戊二醇的合成工艺[J]. 化工进展, 2024, 43(S1): 469-478.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0635
| 反应工艺 | 反应器 | 工艺产率 | 优点 | 缺点 |
|---|---|---|---|---|
| 缩合工艺 | 射流式环流缩合反应器 | 甲醛的转化率达92%,异丁醛转化率为98% | 传热传质效率高 | 能耗高,运行和维护成本大 |
| 微通道反应器 | 羟基新戊醛收率为94% | 传质传热效率高,控温精准 | 易堵塞,生产能力受限 | |
| 加氢工艺 | 釜式反应器 | 新戊二醇的收率和选择性都为95% | 设备简单方便,制造维护成本低,搅拌效率高 | 换热面积小,控温难,停留时间不一致 |
| 固定床反应器 | 羟基新戊醛转化率95%以上,新戊二醇收率为96.5% | 返混小,产物选择性大,催化剂机械损耗小,维护成本低 | 传热差,易飞温,催化剂更换困难 | |
| 列管式反应器 | 羟基新戊醛转化率为99% | 高效的热交换能力,返混小 | 制造成本高,催化剂更换不便,不易清洗 | |
| 滴流床反应器 | 新戊二醇总收率为97% | 转化率高,副反应少,传热效率高 | 易形成局部热点,加速催化剂失活 |
| 反应工艺 | 反应器 | 工艺产率 | 优点 | 缺点 |
|---|---|---|---|---|
| 缩合工艺 | 射流式环流缩合反应器 | 甲醛的转化率达92%,异丁醛转化率为98% | 传热传质效率高 | 能耗高,运行和维护成本大 |
| 微通道反应器 | 羟基新戊醛收率为94% | 传质传热效率高,控温精准 | 易堵塞,生产能力受限 | |
| 加氢工艺 | 釜式反应器 | 新戊二醇的收率和选择性都为95% | 设备简单方便,制造维护成本低,搅拌效率高 | 换热面积小,控温难,停留时间不一致 |
| 固定床反应器 | 羟基新戊醛转化率95%以上,新戊二醇收率为96.5% | 返混小,产物选择性大,催化剂机械损耗小,维护成本低 | 传热差,易飞温,催化剂更换困难 | |
| 列管式反应器 | 羟基新戊醛转化率为99% | 高效的热交换能力,返混小 | 制造成本高,催化剂更换不便,不易清洗 | |
| 滴流床反应器 | 新戊二醇总收率为97% | 转化率高,副反应少,传热效率高 | 易形成局部热点,加速催化剂失活 |
| 分离方法 | 工作原理 | 优点 | 缺点 |
|---|---|---|---|
| 蒸馏法 | 板式精馏塔:由塔体和一定间距的若干塔板组成,气液逆流接触,轻组分升至高层塔板,重组分向下流动实现分离 | 传质效率高,有较高的生产能力 | 压降较大,增加成本,有雾沫夹带现象,影响板效率,易堵塞 |
| 降膜蒸发器:顶部有液体分布器和垂直排列的加热管,料液沿管内壁形成薄膜流动,被壳程的加热介质蒸发 | 传热系数较高,停留时间较短,蒸发速度快,能耗低 | 制造精度要求高,结垢后维护成本高 | |
| 结晶法 | 羟基新戊醛在水中溶解度小,新戊二醇在有机溶剂(苯、甲苯)中溶解度小 | 产品纯度高,操作简单,成本低 | 生产不连续,影响生产效率 |
| 萃取法 | 利用辛醇萃取羟基新戊醛,利用水逆流萃取新戊二醇 | 多级提取能提高分离效率,设备简单,成本低 | 操作时间长,影响生产效率 |
| 分离方法 | 工作原理 | 优点 | 缺点 |
|---|---|---|---|
| 蒸馏法 | 板式精馏塔:由塔体和一定间距的若干塔板组成,气液逆流接触,轻组分升至高层塔板,重组分向下流动实现分离 | 传质效率高,有较高的生产能力 | 压降较大,增加成本,有雾沫夹带现象,影响板效率,易堵塞 |
| 降膜蒸发器:顶部有液体分布器和垂直排列的加热管,料液沿管内壁形成薄膜流动,被壳程的加热介质蒸发 | 传热系数较高,停留时间较短,蒸发速度快,能耗低 | 制造精度要求高,结垢后维护成本高 | |
| 结晶法 | 羟基新戊醛在水中溶解度小,新戊二醇在有机溶剂(苯、甲苯)中溶解度小 | 产品纯度高,操作简单,成本低 | 生产不连续,影响生产效率 |
| 萃取法 | 利用辛醇萃取羟基新戊醛,利用水逆流萃取新戊二醇 | 多级提取能提高分离效率,设备简单,成本低 | 操作时间长,影响生产效率 |
| 1 | 刘丽秀, 孟宪兴, 鲁琳琳, 等. 新戊二醇合成工艺研究[J]. 山东化工, 2008, 37(8): 1-3, 6. |
| LIU Lixiu, MENG Xianxing, LU Linlin, et al. Research on synthesis of neopentyl glycol[J]. Shandong Chemical Industry, 2008, 37(8): 1-3, 6. | |
| 2 | 蒋贵仲, 张华西. 新戊二醇生产工艺研究进展[J]. 四川化工, 2014, 17(1): 23-25. |
| JIANG Guizhong, ZHANG Huaxi. Progress in production of neopentyl glycol[J]. Sichuan Chemical Industry, 2014, 17(1): 23-25. | |
| 3 | 张照明, 袁利明, 赵艳丰, 等. 国内新戊二醇研究与产业化进展[J]. 当代化工, 2011, 40(10): 1058-1061. |
| ZHANG Zhaoming, YUAN Liming, ZHAO Yanfeng, et al. Research and industrialization of neopentyl glycol in China[J]. Contemporary Chemical Industry, 2011, 40(10): 1058-1061. | |
| 4 | 卢俊典, 刘晓杰, 燕晓宇. 新戊二醇市场分析[J]. 化学工业, 2021, 39(2): 52-56. |
| LU Jundian, LIU Xiaojie, YAN Xiaoyu. The analysis of neopentyl glycol market[J]. Chemical Industry, 2021, 39(2): 52-56. | |
| 5 | 王剑, 李雪梅, 罗鸽, 等. 新戊二醇的合成[J]. 上海化工, 2012, 37(8): 9-12. |
| WANG Jian, LI Xuemei, LUO Ge, et al. Synthesis of neopentyl glycol[J]. Shanghai Chemical Industry, 2012, 37(8): 9-12. | |
| 6 | 张倩. 新戊二醇在聚酯合成中的应用[J]. 精细与专用化学品, 2013, 21(7): 46-47. |
| ZHANG Qian. Application of neopentyl glycol in polyester synthesis[J]. Fine and Specialty Chemicals, 2013, 21(7): 46-47. | |
| 7 | WEI Xinlai, WANG Yaoming, YAN Haiyang, et al. A sustainable valorization of neopentyl glycol salt waste containing sodium formate via bipolar membrane electrodialysis[J]. Separation and Purification Technology, 2021, 254: 117563. |
| 8 | MONASTERSKA Edyta, CHROBOK Anna, PANKALLA Ewa, et al. Development of methods for the synthesis of neopentyl glycol by hydrogenation of hydroxypivaldehyde[J]. Molecules, 2021, 26(19): 5822. |
| 9 | 刘岩. 新戊二醇的发展前景[J]. 科技展望, 2016, 26(32): 69. |
| LIU Yan. Development prospect of neopentyl glycol[J]. Science and Technology, 2016, 26(32): 69. | |
| 10 | 关鹏. 浅述新戊二醇的发展[J]. 天津化工, 2022, 36(2): 5-8. |
| GUAN Peng. Discussion on the development of neopentyl glycol[J]. Tianjin Chemical Industry, 2022, 36(2): 5-8. | |
| 11 | SERRANO Angel, DAUVERGNE Jean-Luc, DOPPIU Stefania, et al. Neopentyl glycol as active supporting media in shape-stabilized PCMs[J]. Materials, 2019, 12(19): 3169. |
| 12 | 王钧. 新戊二醇的生产技术和市场前景[J]. 中国石油和化工标准与质量, 2014, 34(9): 20-21. |
| WANG Jun. Production technology and market prospect of neopentyl glycol[J]. China Petroleum and Chemical Standard and Quality, 2014, 34(9): 20-21. | |
| 13 | 孙宗连. 国内外新戊二醇生产现状及市场分析[J]. 精细与专用化学品, 2005, 13(23): 31-33. |
| SUN Zonglian. Production status and market analysis of dimethyltrimethylene glycol at home and abroad[J]. Fine and Specialty Chemicals, 2005, 13(23): 31-33. | |
| 14 | DAI Zhaofeng, SHE Xiaohui, WANG Chen, et al. Thermodynamic analysis on the performance of barocaloric refrigeration systems using neopentyl glycol as the refrigerant[J]. Journal of Thermal Science. 2023, 32(3): 1063-1073. |
| 15 | DAI Zhaofeng, SHE Xiaohui, SHAO Bohan, et al. Plastic crystal neopentyl glycol/multiwall carbon nanotubes composites for highly efficient barocaloric refrigeration system[J]. Journal of Thermal Science, 2024, 33(1): 383-393. |
| 16 | DAI Zhaofeng, SHE Xiaohui, WANG Chen, et al. Dynamic simulation and performance analysis of a solid-state barocaloric refrigeration system[J]. Energy, 2024, 294: 130800. |
| 17 | 吕志果, 郭振美, 刘义勇. 一种制备新戊二醇方法: CN102249853A[P]. 2011-11-23. |
| Zhiguo LYU, GUO Zhenmei, LIU Yiyong. A method for preparing neopentyl glycol: CN102249853A[P]. 2011-11-23. | |
| 18 | 宫一鸣, 张德鲁, 张超, 等. 阳离子交换树脂催化合成新戊二醇过程研究[J]. 青岛科技大学学报(自然科学版), 2023, 44(5): 26-34. |
| GONG Yiming, ZHANG Delu, ZHANG Chao, et al. Research on catalytic synthesis of neopentyl glycol via cation exchange resins[J]. Journal of Qingdao University of Science and Technology (Natural Science Edition), 2023, 44(5): 26-34. | |
| 19 | 刘齐琼, 于鹏浩, 程双, 等. 羟基新戊醛合成工艺及其动力学[J]. 化学反应工程与工艺, 2016, 32(1): 66-72. |
| LIU Qiqiong, YU Penghao, CHENG Shuang, et al. Study on synthetic process and kinetics of hydroxypivalaldehyde[J]. Chemical Reaction Engineering and Technology, 2016, 32(1): 66-72. | |
| 20 | 郝庆亮. 新戊二醇行业发展分析[J]. 精细与专用化学品, 2017, 25(12): 5-8. |
| HAO Qingliang. Development analysis of neopentyl glycol industry[J]. Fine and Specialty Chemicals, 2017, 25(12): 5-8. | |
| 21 | 赵宁博. 固体碱催化甲醛与异丁醛液相缩合反应的研究[D]. 上海: 华东理工大学, 2012. |
| ZHAO Ningbo. Liquid phase aldol condensation of formaldehyde and isobutyraldehyde over solid base catalysts[D]. Shanghai: East China University of Science and Technology, 2012. | |
| 22 | 章意坚. 新戊二醇合成的研究[D]. 杭州: 浙江大学, 2006. |
| ZHANG Yijian. Synthesis of neopentyl glycol[D]. Hangzhou: Zhejiang University, 2006. | |
| 23 | 叶庆国, 孙培生, 梁荣宁. 羟戊醛的合成工艺研究[J]. 化学工业与工程技术, 2008, 29(2): 3-6. |
| YE Qingguo, SUN Peisheng, LIANG Rongning. Research on synthesis process of hydroxypivalaldehyde[J]. Journal of Chemical Industry & Engineering, 2008, 29(2): 3-6. | |
| 24 | 原宇航, 王建, 张春雷, 等. 一种碱性离子液体催化合成羟基新戊醛的方法: CN101219939B[P]. 2011-03-23. |
| YUAN Yuhang, WANG Jian, ZHANG Chunlei, et al. A method for catalytic synthesis of hydroxynevalaldehyde using alkaline ionic liquids: CN101219939B[P]. 2011-03-23. | |
| 25 | HASHMI Azhar. Cross-Aldol condensation of isobutyraldehyde and formaldehyde using phase transfer catalyst[J]. Journal of Saudi Chemical Society, 2016, 20: S382-S386. |
| 26 | 刘晓晖, 赵宁博, 王艳芹, 等. 一种磷灰石类固体碱催化合成羟基新戊醛的方法: CN102417443A[P]. 2012-04-18. |
| LIU Xiaohui, ZHAO Ningbo, WANG Yanqin, et al. A method for synthesizing hydroxynevalaldehyde using a solid alkali catalyst based on apatite: CN102417443A[P]. 2012-04-18. | |
| 27 | 伍艳辉, 吴高胜, 余强, 等. 用于合成羟基新戊醛的复合镁基类氧化物催化剂及方法: CN104923200A[P]. 2015-09-23. |
| WU Yanhui, WU Gaosheng, YU Qiang, et al. Composite magnesium based oxide catalyst and method for synthesizing hydroxynevalaldehyde: CN104923200A[P]. 2015-09-23. | |
| 28 | 胡波, 李长胜, 崔龙, 等. 一种制备羟基新戊醛的催化剂及其使用方法: CN105771998B[P]. 2018-08-21. |
| HU Bo, LI Changsheng, CUI Long, et al. A catalyst for preparing hydroxynevalaldehyde and its usage method: CN105771998B[P]. 2018-08-21. | |
| 29 | 马江权, 周佳, 高晓新, 等. 一种高效制备羟基新戊醛的方法: CN109836317B[P]. 2022-03-01. |
| MA Jiangquan, ZHOU Jia, GAO Xiaoxin, et al. A method for efficient preparation of hydroxyneoganaldehyde: CN109836317B[P]. 2022-03-01. | |
| 30 | 邴威瀚, 刘喆, 梁秀霞, 等. 一种氢氧根插层钙镁铝水滑石固体碱催化剂制备及使用方法: CN110624529A[P]. 2019-12-31. |
| BING Weihan, LIU Zhe, LIANG Xiuxia, et al. Preparation and usage method of a hydroxide intercalated calcium magnesium aluminum hydrotalcite solid base catalyst: CN110624529A[P]. 2019-12-31. | |
| 31 | WANG Huimin, BING Weihan, CHEN Chunyuan, et al. Geometric effect promoted hydrotalcites catalysts towards aldol condensation reaction[J]. Chinese Journal of Catalysis, 2020, 41(8): 1279-1287. |
| 32 | BING Weihan, WANG Huimin, ZHENG Lei, et al. A CaMnAl-hydrotalcite solid basic catalyst toward the aldol condensation reaction with a comparable level to liquid alkali catalysts[J]. Green Chemistry, 2018, 20(13): 3071-3080. |
| 33 | BING Weihan, ZHENG Lei, HE Shan, et al. Insights on active sites of CaAl-hydrotalcite as a high-performance solid base catalyst toward aldol condensation[J]. ACS Catalysis, 2018, 8(1): 656-664. |
| 34 | 安华良, 吕建华, 刘继东. 一种基于催化反应精馏缩合制备羟基新戊醛的方法: CN112174794B[P]. 2021-08-03. |
| AN Hualiang, Jianhua LYU, LIU Jidong. A method for preparation of hydroxyneoganaldehyde based on condensation of catalytic reaction: CN112174794B[P]. 2021-08-03. | |
| 35 | 程双, 张新平, 张金忠, 等. 制备羟基新戊醛的方法: CN105061170A[P]. 2015-11-18. |
| CHENG Shuang, ZHANG Xinping, ZHANG Jinzhong, et al. Method for preparation of hydroxyneoganaldehyde: CN105061170[P]. 2015-11-18. | |
| 36 | 卢文新, 张大洲, 刘佳, 等. 连续合成羟基新戊醛的系统: CN112755932A[P]. 2021-05-07. |
| LU Wenxin, ZHANG Dazhou, LIU Jia, et al. System for continuous synthesis of hydroxyneoganaldehyde: CN112755932B[P]. 2021-05-07. | |
| 37 | 廖本仁, 龚磊, 袁振文, 等. 采用微通道反应器制备羟基新戊醛的方法: CN105693491A[P]. 2016-06-22. |
| LIAO Benren, GONG Lei, YUAN Zhenwen, et al. Method of preparing hydroxyneoganaldehyde using microchannel reactors: CN105693491A[P]. 2016-06-22. | |
| 38 | 龚磊, 袁振文, 代立, 等. 微通道反应器内合成羟基新戊醛[J]. 上海化工, 2016, 41(6): 19-22. |
| GONG Lei, YUAN Zhenwen, DAI Li, et al. Synthesis of hydroxypivalaldehyde in micro-channel reactor[J]. Shanghai Chemical Industry, 2016, 41(6): 19-22. | |
| 39 | 王钰, 李博, 杨赛, 等. 一种超临界条件下制备羟基新戊醛的方法: CN109232213B[P]. 2021-08-17. |
| WANG Yu, LI Bo, YANG Sai, et al. A method for preparing hydroxyneoganaldehyde under supercritical conditions: CN109232213B[P]. 2021-08-17. | |
| 40 | ZHU Jinghu, LIU Hui, ZHOU Xueyan, et al. Insights into the modifying effect of Ga on Cu-based catalysts for hydrogenation of hydroxypivalaldehyde to neopentyl glycol[J]. Catalysts, 2023, 13(4): 673. |
| 41 | TIKE M A, GHARDE A M, MAHAJANI V V. Studies to aid process development for the manufacture of neopentyl glycol from isobutyraldehyde: Aldol condensation followed by hydrogenation[J]. Asia-Pacific Journal of Chemical Engineering, 2008, 3(3): 333-346. |
| 42 | 戚明珠, 冯广军, 赵鹏, 等. 一种新戊二醇的生产工艺: CN101993351A[P]. 2011-03-30. |
| QI Mingzhu, FENG Guangjun, ZHAO Peng, et al. A production process of neopentyl glycol: CN101993351A[P]. 2011-03-30. | |
| 43 | 孙中华, 孙远龙, 袁浩然, 等. 镍系羟基新戊醛加氢制备新戊二醇的催化剂及制法: CN107970940A[P]. 2018-05-01. |
| SUN Zhonghua, SUN Yuanlong, YUAN Haoran, et al. Catalyst and preparation method for nickel based hydroxyneoganaldehyde hydrogenation to prepare neopentyl glycol: CN107970940A[P]. 2018-05-01. | |
| 44 | 刘平, 彭枝忠. 一种催化加氢制备新戊二醇的方法: CN109232177B[P]. 2021-05-07. |
| LIU Ping, PENG Zhizhong. A method for catalytic hydrogenation to prepare neopentyl glycol: CN109232177 B[P]. 2021-05-07. | |
| 45 | 王海峰, 王延坤, 闫志广, 等. 一种连续生产新戊二醇的方法: CN108623437B[P]. 2021-07-27. |
| WANG Haifeng, WANG Yankun, YAN Zhiguang, et al. A method for the continuous production of neopentyl glycol: CN108623437B[P]. 2021-07-27. | |
| 46 | 王剑, 宁春利, 罗鸽, 等. 一种制备新戊二醇的方法: CN101735015A[P]. 2010-06-16. |
| WANG Jian, NING Chunli, LUO Ge, et al. A method for the preparation of neopentyl glycol: CN101735015A[P]. 2010-06-16. | |
| 47 | 马提亚·艾泽纳赫, 库·查拉斯基, 彼得·海曼斯, 等. 新戊二醇的连续生产方法: CN104684878A[P]. 2015-06-03. |
| MATTHIAS Ezenah, KU Charashi, PETER Hainans, et al. A method for the continuous production of neopentyl glycol: CN104684878B[P]. 2016-12-07. | |
| 48 | 魏传明, 江津河, 许凤杰. 加氢法制备新戊二醇用铜基催化剂及其制备方法: CN102513107A[P]. 2012-06-27. |
| WEI Chuanming, JIANG Jinhe, XU Fengjie. Copper based catalysts and their preparation methods for the preparation of neopentyl glycol by hydrogenation method: CN102513107B[P]. 2013-09-18. | |
| 49 | 王广建, 王建爽, 王芳, 等. B助剂对Cu基催化剂羟基新戊醛加氢反应性能的影响[J]. 当代化工, 2021, 50(1): 55-59. |
| WANG Guangjian, WANG Jianshuang, WANG Fang, et al. Effect of B additive on the performance of Cu based catalyst for hydrogenation of hydroxypivalaldehyde[J]. Contemporary Chemical Industry, 2021, 50(1): 55-59. | |
| 50 | 孙远龙, 袁浩然, 孙中华, 等. 羟基新戊醛加氢制新戊二醇的催化剂及其制备方法: CN105727958A[P]. 2016-07-06. |
| SUN Yuanlong, YUAN Haoran, SUN Zhonghua, et al. Catalysts and preparation methods for hydroxyneoganaldehyde hydrogenation to produce neopentyl glycol: CN105727958A[P]. 2016-07-06. | |
| 51 | 何光文, 王中华, 李浩, 等. 一种加氢制备新戊二醇的催化剂及其制备方法: CN102728370A[P]. 2012-10-17. |
| HE Guangwen, WANG Zhonghua, LI Hao, et al. A catalyst for hydrogenation to prepare neopentyl glycol and its preparation method: CN102728370B[P]. 2014-06-11. | |
| 52 | 李作金, 初乃波, 詹吉山, 等. 一种羟基特戊醛液加氢制备新戊二醇的催化剂的制备方法: CN104258869A[P]. 2016-05-18 |
| LI Zuojin, CHU Naibo, ZHAN Jishan, et al. Preparation method of a catalyst for liquid hydroxyneoganaldehyde hydrogenation to prepare neopentanediol: CN104258869B[P]. 2016-05-18. | |
| 53 | 王荷芳, 孙洋洋, 吕建华, 等. 一种碳包覆纳米铜锌铝催化剂的制备方法: CN109046362A[P]. 2018-12-21. |
| WANG Hefang, SUN Yangyang, Jianhua LYU, et al. A preparation method for a carbon-coated copper-aluminum catalyst: CN109046362A[P]. 2018-12-21. | |
| 54 | 刘佳, 卢文新, 张大洲, 等. 用于羟基新戊醛加氢制备新戊二醇的催化剂的制备方法: CN112427038A[P]. 2021-03-02. |
| LIU Jia, LU Wenxin, ZHANG Dazhou, et al. Preparation method of catalyst for hydroxyneoganaldehyde hydrogenation to prepare neopentyl glycol: CN112427038A[P]. 2021-03-02. | |
| 55 | 曹善健, 姜庆梅, 杨在刚, 等. 一种新戊二醇的制备方法: CN103351277A[P]. 2013-10-16. |
| CAO Shanjian, JIANG Qingmei, YANG Zaigang, et al. A method for the preparation of neopentyl glycol: ZN103351277B[P]. 2014-12-31. | |
| 56 | 张大洲, 卢文新, 刘佳, 等. 连续加氢生产新戊二醇的工艺: CN112537998A[P]. 2021-03-23. |
| ZHANG Dazhou, LU Wenxin, LIU Jia, et al. A method for the continuous production of Neopentyl Glycol: CN112537998A[P]. 2021-3-23. | |
| 57 | 常怀春, 侯志扬, 任格勇, 等. 新戊二醇缩合加氢生产工艺及其装置: CN103130611A[P]. 2016-04-20. |
| CHANG Huaichun, HOU Zhiyang, REN Geyong, et al. Production process and equipment for condensation hydrogenation of neopentyl glycol: CN103130611B[P]. 2016-04-20. | |
| 58 | 杨文龙, 程鹏, 卢雁飞, 等. 镍/氧化铝加氢催化剂的制备及性能研究[J]. 无机盐工业, 2022, 54(7): 141-148. |
| YANG Wenlong, CHENG Peng, LU Yanfei, et al. Study on preparation and performance of Ni/Al2O3 hydrogenation catalyst[J]. Inorganic Chemicals Industry, 2022, 54(7): 141-148. | |
| 59 | 程双. 羟基新戊醛连续合成反应器模型化[J]. 上海化工, 2018, 43(7): 24-29. |
| CHENG Shuang. Modeling of continuous reactors for hydroxypivalaldehyde synthesis[J]. Shanghai Chemical Industry, 2018, 43(7): 24-29. | |
| 60 | 张冬梅, 马江权, 朱亚娟. 羟基新戊醛加氢制新戊二醇催化剂及工艺[J]. 精细化工, 2015, 32(7): 784-788. |
| ZHANG Dongmei, MA Jiangquan, ZHU Yajuan. A study on the catalyst and process of catalytic hydrogenation of 3-hydroxy-2,2-dimethylpropanal to neopentyl glycol[J]. Fine Chemicals, 2015, 32(7): 784-788. | |
| 61 | 杨霞, 赵彦恒, 秦利涛, 等. 一种连续分离新戊二醇和甲酸钠的方法: CN113307722A[P]. 2021-08-27. |
| YANG Xia, ZHAO Yanheng, QIN Litao, et al. A method for continuous separation of neopentyl glycol and sodium formate: CN113307722A[P]. 2021-08-27. | |
| 62 | 田文广, 李雁, 陶红秀, 等. 新戊二醇间歇精馏过程的模拟、优化与应用[J]. 计算机与应用化学, 2012, 29(11): 1355-1358. |
| TIAN Wenguang, LI Yan, TAO Hongxiu, et al. Simulation, optimization and application of batch distillation of neopentyl glycol[J]. Computers and Applied Chemistry, 2012, 29(11): 1355-1358. | |
| 63 | 周彩荣, 石晓华, 冯伟, 等. 新戊二醇在溶剂中溶解度的测定及关联[J]. 高校化学工程学报, 2010, 24(3): 365-369. |
| ZHOU Cairong, SHI Xiaohua, FENG Wei, et al. Measurement and correlation of solubilities of neopentyl glycol in solvents[J]. Journal of Chemical Engineering of Chinese Universities, 2010, 24(3): 365-369. |
| [1] | LI Lin, HUANG Guoyong, XU Shengming, YU Fengshan, WENG Yaqing, CAO Caifang, WEN Jiawei, WANG Chunxia, WANG Junlian, GU Bintao, ZHANG Yuanhua, LIU Bin, WANG Caiping, PAN Jianming, XU Zeliang, WANG Chong, WANG Ke. Recovery and regeneration preparation of aluminum-based spent catalyst support [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 640-649. |
| [2] | LI Xinyue, LI Zhenjing, HAN Yihang, GUO Yongqiang, YAN Yu, KAREMULATI Halimire, ZHAO Huiji, CHAI Yongming, LIU Dong, YIN Changlong. Research progress on catalysts for the production of green diesel by hydrodeoxidation of lipid [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 351-364. |
| [3] | WANG Bo, WANG Bin, GONG Xiang, YANG Fusheng, FANG Tao. Enhancing dehydrogenation performance of liquid organic hydrogen carriers based on reactor design: Research progress [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 189-208. |
| [4] | LI Shuaizhe, NIE Yichen, PHIDJAVARD Keomeesay, GU Wen, ZHANG Wei, LIU Na, XU Gaoxiang, LIU Ying, LI Xingyong, CHEN Yubao. Research progress on non-precious metal-catalyzed hydrogenation and deoxygenation of biomass to produce hydrocarbon-based biofuels [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 225-242. |
| [5] | XIONG Lei, DING Feiyan, LI Cong, WANG Qunle, LYU Qi, ZHAI Xiaona, LIU Feng. Recent advances in metal Pt supported heterogeneous catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 295-304. |
| [6] | SONG Caicheng, CHEN Xiaozhen, LIU Li, YANG Chengmin, ZHENG Bumei, YIN Xiaoying, SUN Jin, YAO Yunhai, DUAN Weiyu. Research progress of carbon-based carrier supported hydrodesulfurization catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 305-314. |
| [7] | HAN Hongjing, CHE Yu, TIAN Yuxuan, WANG Haiying, ZHANG Yanan, CHEN Yanguang. Advances on catalysts and solvents for catalytic hydrogenolysis of lignin [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 315-324. |
| [8] | HU Xing, LIU Yi, DU Zexue. Research progress of catalyst for direct synthesis of epichlorohydrin from 3-chloropropylene [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 325-334. |
| [9] | YU Mengjie, WU Yutong, LUO Faxiang, DOU Yibo. Research progress on structural design of photocatalysts for diluted carbon dioxide reduction [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 335-350. |
| [10] | HE Shikun, ZHANG Ronghua, LI Haoyang, PAN Hui, FENG Junfeng. Preparation of 5-hydroxymethylfurfural from glucose catalyzed by dealuminized molecular sieve solid acids [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 374-381. |
| [11] | ZHANG Ridong, LYU Jianhua, LIU Jidong, GUO Bao, LI Wensong. Ru-K-NaY catalyzed decarbonylation of dimethyl oxalate to dimethyl carbonate [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 382-390. |
| [12] | LIAO Xu, ZHOU Jun, LUO Jie, ZENG Ruilin, WANG Zeyu, LI Zunhua, LIN Jinqing. Research progress on CO2 cycloaddition reaction catalyzed by porous ionic polymers [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4925-4940. |
| [13] | XIU Haoran, WANG Yungang, BAI Yanyuan, LIU Tao, ZHANG Xingbang, ZHANG Yijia. Pilot test of H2O2 low temperature catalytic oxidation for desulfurization and denitrification [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4941-4950. |
| [14] | FU Wei, NING Shuying, CAI Chen, CHEN Jiayin, ZHOU Hao, SU Yaxin. SCR-C3H6 denitrification performance of Cu-modified MIL-100(Fe) catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4951-4960. |
| [15] | MA Hongzhou, DANG Yubo, WANG Yaoning, ZENG Jinyang, ZHAO Xiaojun, SHI Jianwei. Zinc-based desulfurizer scrap and copper-zinc-based catalyst synergistic vacuum carbothermal extraction of zinc [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5275-5281. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||