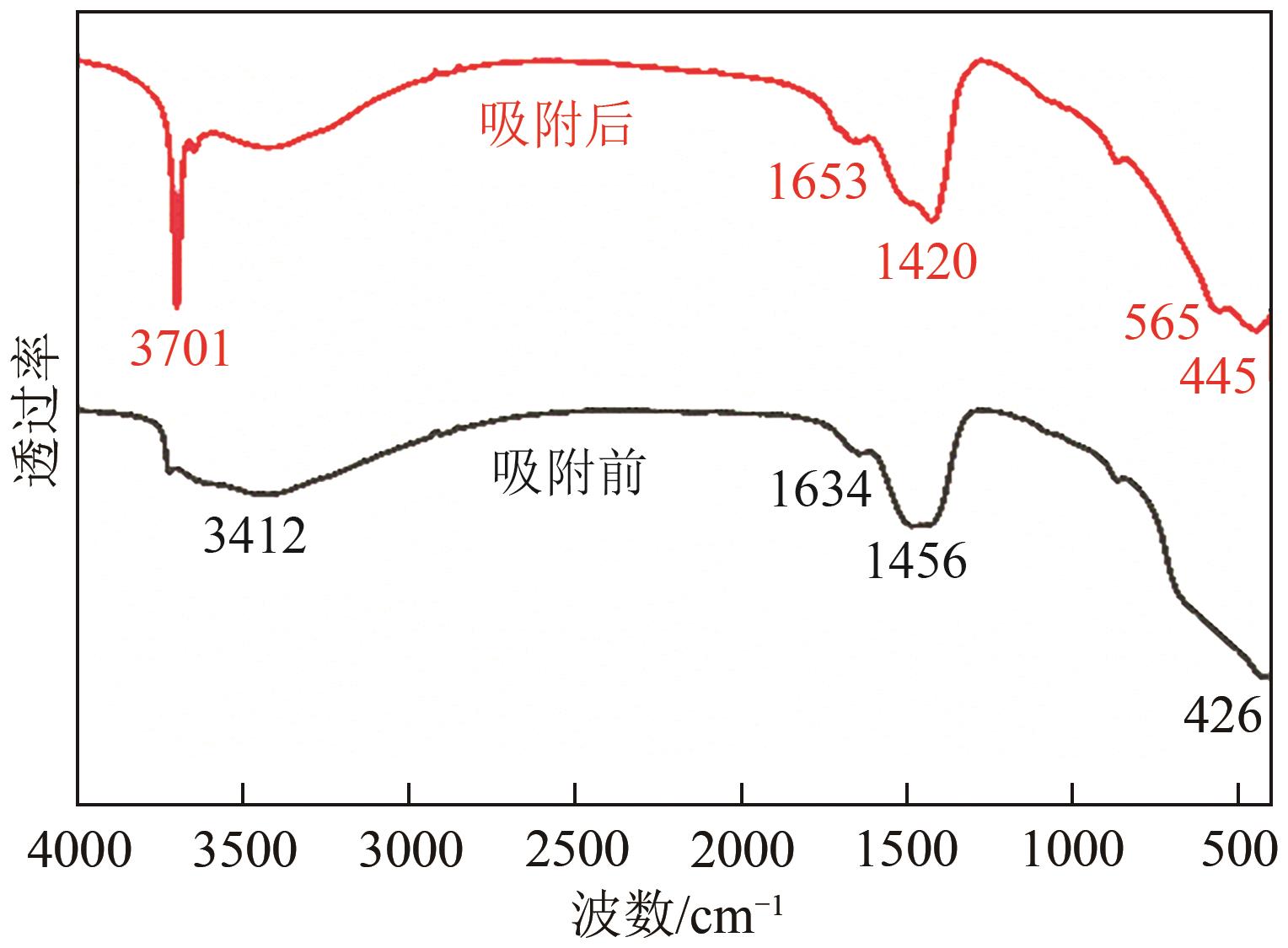
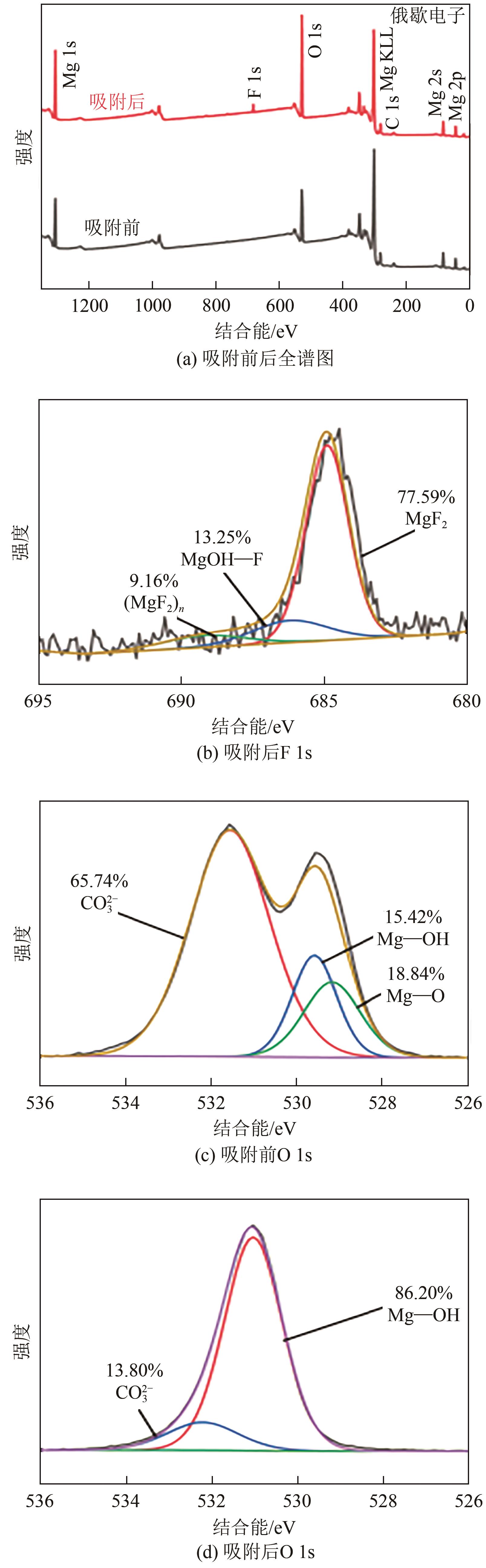
| 1 |
GHOSH Aniruddha, MUKHERJEE Kakali, GHOSH Sumanta K, et al. Sources and toxicity of fluoride in the environment[J]. Research on Chemical Intermediates, 2013, 39(7): 2881-2915.
|
| 2 |
KANNO Cynthia M, SANDERS Rebecca L, FLYNN Steven M, et al. Novel apatite-based sorbent for defluoridation: Synthesis and sorption characteristics of nano-micro-crystalline hydroxyapatite-coated-limestone[J]. Environmental Science & Technology, 2014, 48(10): 5798-5807.
|
| 3 |
KIM Eun-Ah, PARK Ji Hye, HAN Sung-Hee, et al. Exploratory factor analysis of fluoride removal efficiency associated with the chemical properties of geomaterials[J]. Journal of Hazardous Materials, 2017, 334: 178-184.
|
| 4 |
GAN Yonghai, WANG Xiaomeng, ZHANG Li, et al. Coagulation removal of fluoride by zirconium tetrachloride: Performance evaluation and mechanism analysis[J]. Chemosphere, 2019, 218: 860-868.
|
| 5 |
YU Chenglong, LIU Lin, WANG Xiaodong, et al. Fluoride removal performance of highly porous activated alumina[J]. Journal of Sol-Gel Science and Technology, 2023, 106: 471-479.
|
| 6 |
EKKA Basanti, DHAKA Rajendra S, PATEL Raj Kishore, et al. Fluoride removal in waters using ionic liquid-functionalized alumina as a novel adsorbent[J]. Journal of Cleaner Production, 2017, 151: 303-318.
|
| 7 |
HE Junyong, CAI Xingguo, CHEN Kai, et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal[J]. Journal of Colloid and Interface Science, 2016, 484: 162-172.
|
| 8 |
JADHAV Sachin V, BRINGAS Eugenio, YADAV Ganapati D, et al. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal[J]. Journal of Environmental Management, 2015, 162: 306-325.
|
| 9 |
田追, 张震, 卢嫚, 等. 新型除氟吸附材料的研究进展[J]. 化工进展, 2022, 41(6): 3051-3062.
|
|
TIAN Zhui, ZHANG Zhen, LU Man, et al. New adsorption materials for removing fluoride from wastewater: A review[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3051-3062.
|
| 10 |
KUMAR Eva, BHATNAGAR Amit, KUMAR Umesh, et al. Defluoridation from aqueous solutions by nano-alumina: Characterization and sorption studies[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1042-1049.
|
| 11 |
MALIYEKKAL Shihabudheen M, SHUKLA Sanjay, PHILIP Ligy, et al. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules[J]. Chemical Engineering Journal, 2008, 140(1/2/3): 183-192.
|
| 12 |
HE Yuxuan, ZHANG Liming, AN Xiao, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: Adsorption isotherms, kinetics, thermodynamics and mechanism[J]. Science of the Total Environment, 2019, 688: 184-198.
|
| 13 |
CHANG Qing, ZHU Lihua, LUO Zhihong, et al. Sono-assisted preparation of magnetic magnesium-aluminum layered double hydroxides and their application for removing fluoride[J]. Ultrasonics Sonochemistry, 2011, 18(2): 553-561.
|
| 14 |
KAMEDA Tomohito, Jumpei OBA, YOSHIOKA Toshiaki. Recyclable Mg-Al layered double hydroxides for fluoride removal: Kinetic and equilibrium studies[J]. Journal of Hazardous Materials, 2015, 300: 475-482.
|
| 15 |
ARAGA Ramya, KALI Suresh, SHARMA Chandra S. Coconut‐shell‐derived carbon/carbon nanotube composite for fluoride adsorption from aqueous solution[J]. CLEAN-Soil Air Water, 2019, 47(5): 1800286.
|
| 16 |
MEDELLÍN-CASTILLO Nahum Andres, CRUZ-BRIANO Sergio Armando, Roberto LEYVA-RAMOS, et al. Use of bone char prepared from an invasive species, pleco fish (Pterygoplichthys spp.), to remove fluoride and Cadmium(Ⅱ) in water[J]. Journal of Environmental Management, 2020, 256: 109956.
|
| 17 |
AFFONSO Lutiane N, MARQUES Jorge L, LIMA Valéria V C, et al. Removal of fluoride from fertilizer industry effluent using carbon nanotubes stabilized in chitosan sponge[J]. Journal of Hazardous Materials, 2020, 388: 122042.
|
| 18 |
SONG Jiangyan, YU Yongyi, HAN Xiaoshuai, et al. Novel MOF(Zr)-on-MOF(Ce) adsorbent for elimination of excess fluoride from aqueous solution[J]. Journal of Hazardous Materials, 2024, 463: 132843.
|
| 19 |
JEYASEELAN Antonysamy, ASWIN KUMAR Ilango, VISWANATHAN Natrayasamy, et al. Development and characterization of hydroxyapatite layered lanthanum organic frameworks by template method for defluoridation of water[J]. Journal of Colloid and Interface Science, 2022, 622: 228-238.
|
| 20 |
冯江涛, 王睎, 赵旭阳, 等. 改性聚吡咯材料去除水中氟离子的性能[J]. 化工进展, 2021, 40(7): 4036-4046.
|
|
FENG Jiangtao, WANG Xi, ZHAO Xuyang, et al. Removal of fluoride from water by modified polypyrrole[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4036-4046.
|
| 21 |
ZHANG Yangzhong, HUANG Kai. Grape pomace as a biosorbent for fluoride removal from groundwater[J]. RSC Advances, 2019, 9(14): 7767-7776.
|
| 22 |
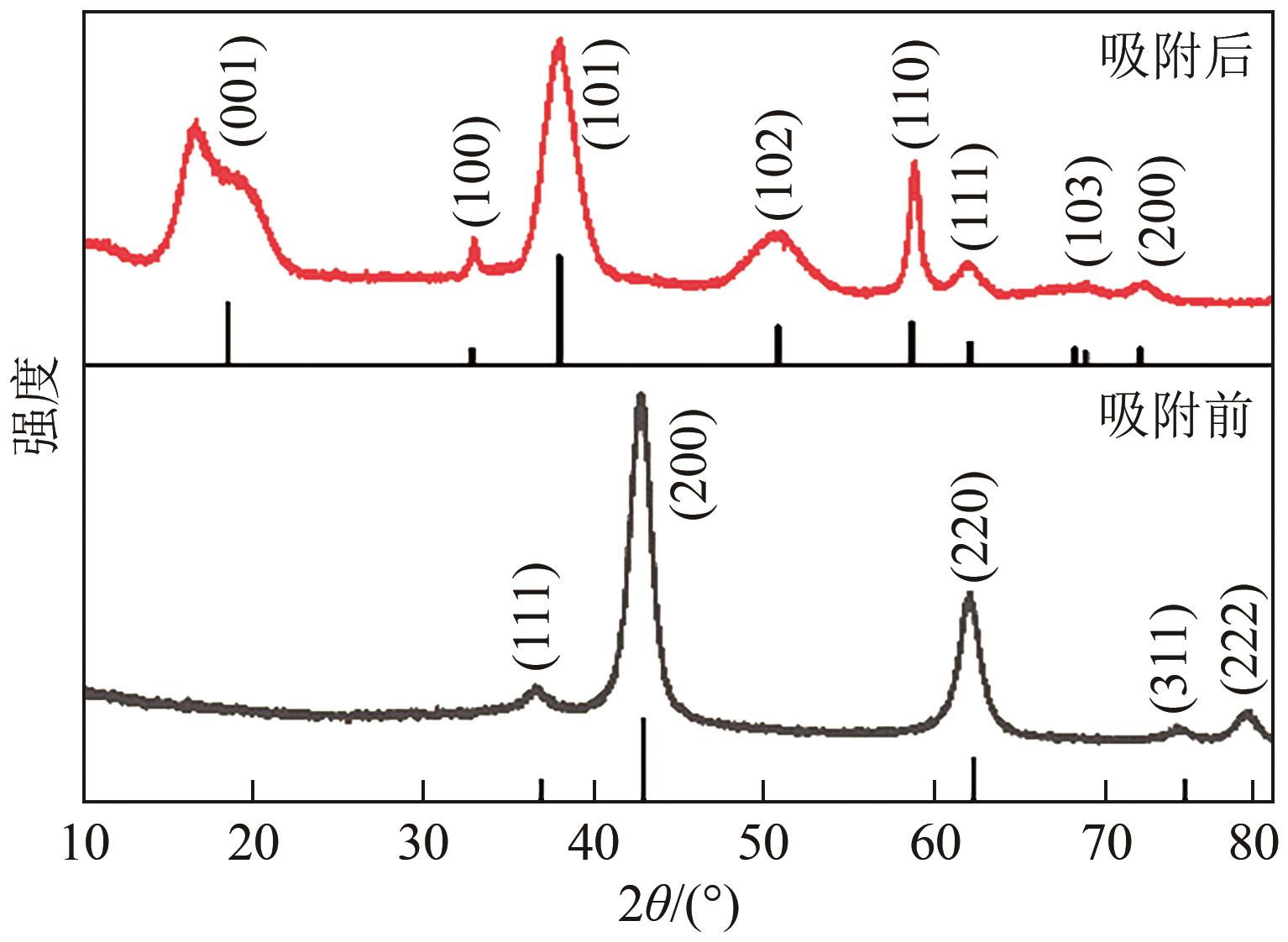
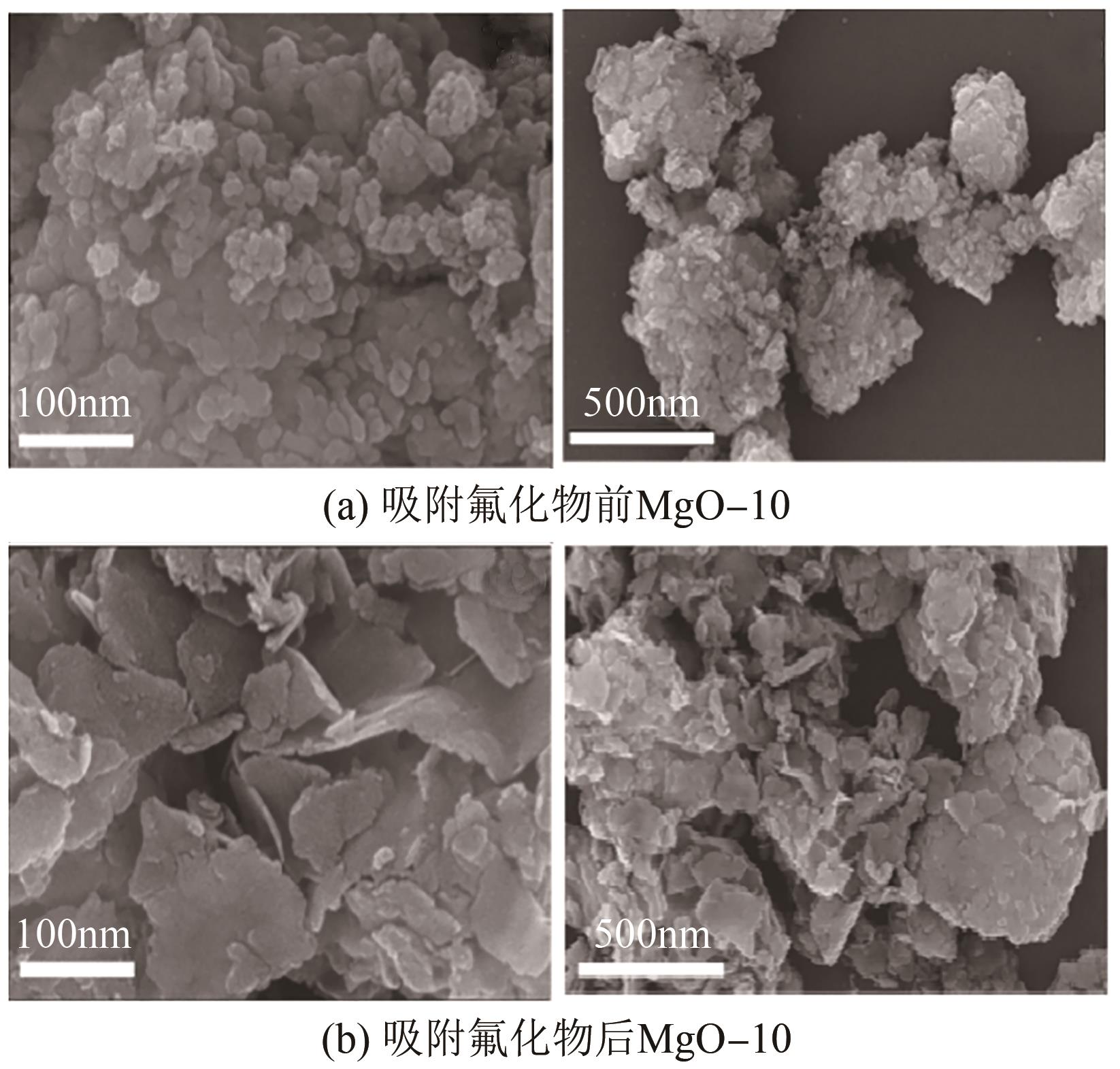
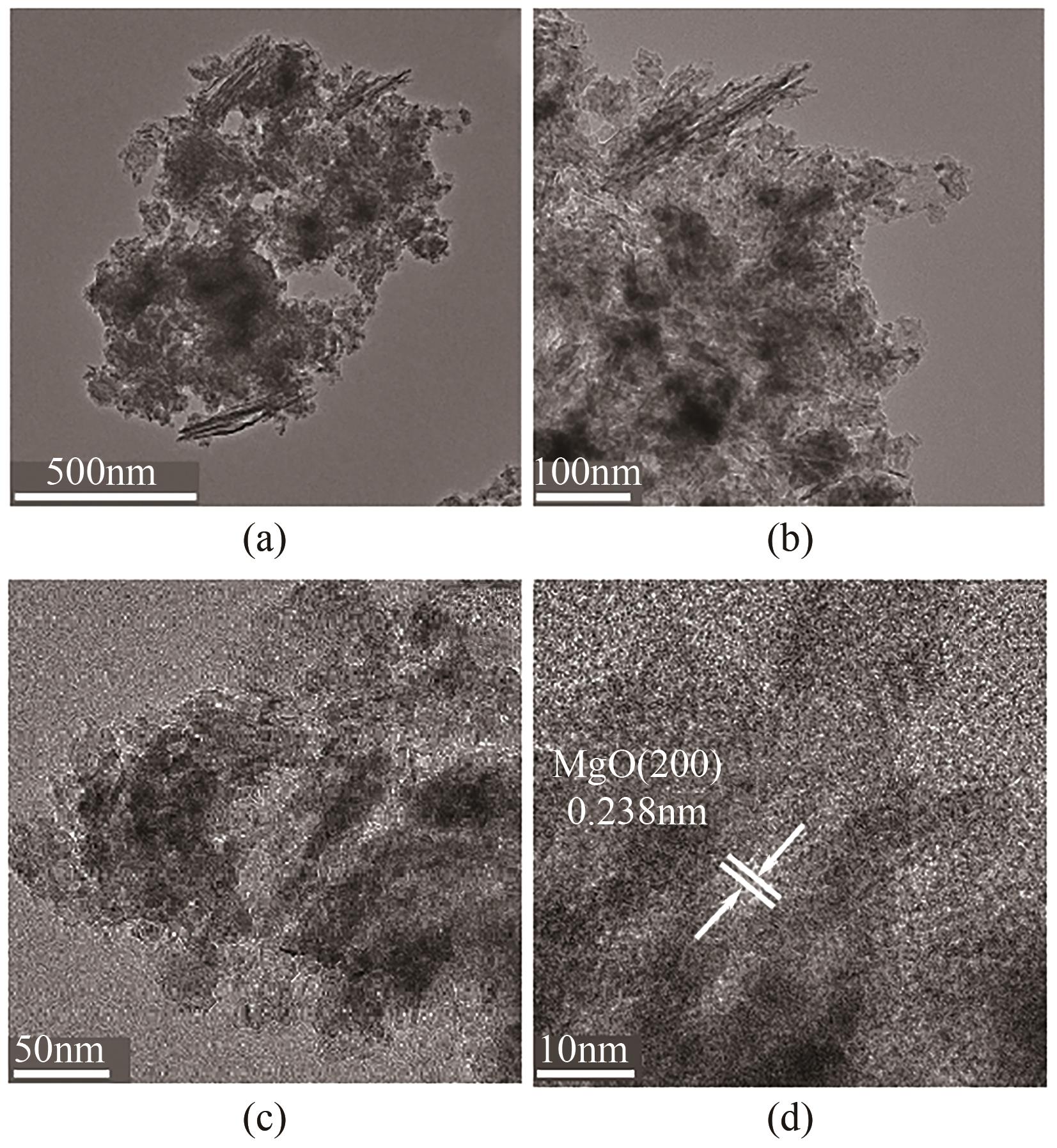
JIN Zhen, JIA Yong, ZHANG Kaisheng, et al. Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism[J]. Journal of Alloys and Compounds, 2016, 675: 292-300.
|
| 23 |
康宁, 由昆, 徐丽, 等. 多孔球状活性氧化镁的制备及除氟效能研究[J]. 工业水处理, 2022, 42(11): 65-75.
|
|
KANG Ning, YOU Kun, XU Li, et al. Research on porous spherical activated MgO preparation and its defluorination efficacy[J]. Industrial Water Treatment, 2022, 42(11): 65-75.
|
| 24 |
LI Lianxiang, XU Di, LI Xiaoqin, et al. Excellent fluoride removal properties of porous hollow MgO microspheres[J]. New Journal of Chemistry, 2014, 38(11): 5445-5452.
|
| 25 |
YU Zhichao, XU Chonghe, YUAN Kangkang, et al. Template-free synthesis of MgO mesoporous nanofibers with superior adsorption for fluoride and Congo red[J]. Ceramics International, 2018, 44(8): 9454-9462.
|
| 26 |
GUO Wei, LIN Hongfei, ZHU Hongxiang, et al. Preparation and application of magnesium oxide nanoparticles for superiorly fluoride removal[J]. Journal of Alloys and Compounds, 2023, 960: 170935.
|
| 27 |
TOLKOU Athanasia K, MANOUSI Natalia, ZACHARIADIS George A, et al. Recently developed adsorbing materials for fluoride removal from water and fluoride analytical determination techniques: A review[J]. Sustainability, 2021, 13(13): 7061-7083.
|
| 28 |
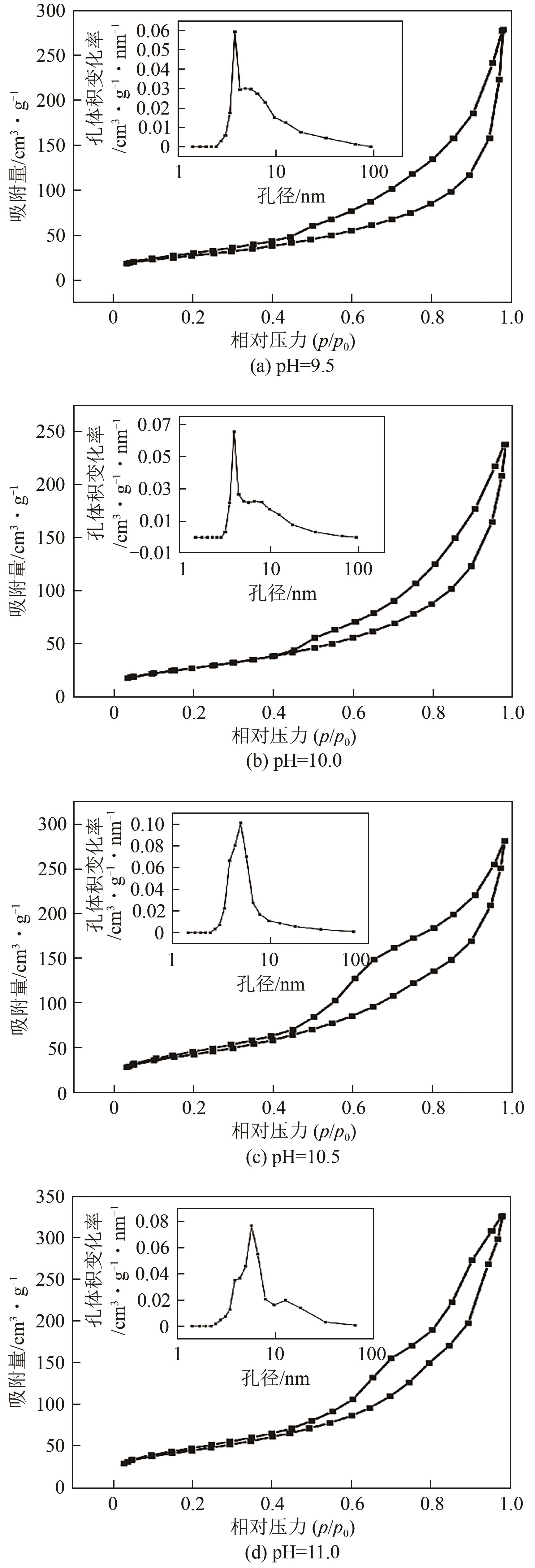
SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619.
|
| 29 |
TANG Dandan, ZHANG Gaoke. Efficient removal of fluoride by hierarchical Ce-Fe bimetal oxides adsorbent: Thermodynamics, kinetics and mechanism[J]. Chemical Engineering Journal, 2016, 283: 721-729.
|
| 30 |
PARASHAR Kamya, BALLAV Niladri, DEBNATH Sushanta, et al. Rapid and efficient removal of fluoride ions from aqueous solution using a polypyrrole coated hydrous tin oxide nanocomposite[J]. Journal of Colloid and Interface Science, 2016, 476: 103-118.
|
| 31 |
WANG Kaixiang, WEI Tingting, LI Yinuo, et al. Flocculation-to-adsorption transition of novel salt-responsive polyelectrolyte for recycling of highly polluted saline textile effluents[J]. Chemical Engineering Journal, 2021, 413: 127410.
|
| 32 |
ZHU Kecheng, DUAN Yanyan, WANG Fu, et al. Silane-modified halloysite/Fe3O4 nanocomposites: Simultaneous removal of Cr(Ⅵ) and Sb(Ⅴ) and positive effects of Cr(Ⅵ) on Sb(Ⅴ) adsorption[J]. Chemical Engineering Journal, 2017, 311: 236-246.
|
| 33 |
ZHANG Mengxue, XU Liheng, QI Changli, et al. Highly effective removal of Tetracycline from water by hierarchical porous carbon: Batch and column adsorption[J]. Industrial & Engineering Chemistry Research, 2019, 58(43): 20036-20046.
|
| 34 |
TANG Yulin, GUAN Xiaohong, WANG Jianmin, et al. Fluoride adsorption onto granular ferric hydroxide: Effects of ionic strength, pH, surface loading, and major co-existing anions[J]. Journal of Hazardous Materials, 2009, 171(1/2/3): 774-779.
|
| 35 |
MOHAPATRA M, ROUT K, SINGH P, et al. Fluoride adsorption studies on mixed-phase nano iron oxides prepared by surfactant mediation-precipitation technique[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1751-1757.
|
| 36 |
LANGMUIR Irving. The constitution and fundamental properties of solids and liquids. Part Ⅰ. Solids[J]. Journal of the American Chemical Society, 1916, 38(11): 2221-2295.
|
| 37 |
YIN Chun, HUANG Qilan, ZHU Guiping, et al. High-performance lanthanum-based metal-organic framework with ligand tuning of the microstructures for removal of fluoride from water[J]. Journal of Colloid and Interface Science, 2022, 607: 1762-1775.
|
| 38 |
GOGOI Sweety, DUTTA Robin K. Mechanism of fluoride removal by phosphoric acid-enhanced limestone: Equilibrium and kinetics of fluoride sorption[J]. Desalination and Water Treatment, 2016, 57(15): 6838-6851.
|
| 39 |
SAHU Sumanta, MALLIK Laxmi, PAHI Souman, et al. Facile synthesis of poly o-toluidine modified lanthanum phosphate nanocomposite as a superior adsorbent for selective fluoride removal: A mechanistic and kinetic study[J]. Chemosphere, 2020, 252: 126551.
|
| 40 |
GAO Panpan, TIAN Xike, YANG Chao, et al. Fabrication, performance and mechanism of MgO meso-/macroporous nanostructures for simultaneous removal of As(Ⅲ) and F in a groundwater system[J]. Environmental Science: Nano, 2016, 3(6): 1416-1424.
|
| 41 |
ZHANG Kaisheng, WU Shibiao, WANG Xuelong, et al. Wide pH range for fluoride removal from water by MHS-MgO/MgCO3 adsorbent: Kinetic, thermodynamic and mechanism studies[J]. Journal of Colloid and Interface Science, 2015, 446: 194-202.
|
| 42 |
NIU Haixia, YANG Qing, TANG Kaibin, et al. Large-scale synthesis of single-crystalline MgO with bone-like nanostructures[J]. Journal of Nanoparticle Research, 2006, 8(6): 881-888.
|
| 43 |
JIN Zhen, JIA Yong, LUO Tao, et al. Efficient removal of fluoride by hierarchical MgO microspheres: Performance and mechanism study[J]. Applied Surface Science, 2015, 357: 1080-1088.
|
| 44 |
XU Rong. ZENG Huachun. Dimensional control of cobalt-hydroxide-carbonate nanorods and their thermal conversion to one-dimensional arrays of Co3O4 nanoparticles[J]. Journal of Physical Chemistry B, 2003, 107(46): 12643-12649.
|
| 45 |
SUGAMA T, SABATINI R, PETRAKIS L. Decomposition of chrysotile asbestos by fluorosulfonic acid[J]. Industrial & Engineering Chemistry Research, 1998, 37(1): 79-88.
|
| 46 |
WANG Lanting, XIE Yanhua, YANG Jinglong, et al. Insight into mechanisms of fluoride removal from contaminated groundwater using lanthanum-modified bone waste[J]. RSC Advances, 2017, 7(85): 54291-54305.
|
| 47 |
WUTTKE Stefan, COMAN Simona M, SCHOLZ Gudrun, et al. Novel Sol-gel synthesis of acidic MgF2- x (OH) x materials[J]. Chemistry:A European Journal, 2008, 14(36): 11488-11499.
|
| 48 |
MAO Chunfeng, YIN Kai, YANG Chenghan, et al. Fe-based MOFs@Pd@COFs with spatial confinement effect and electron transfer synergy of highly dispersed Pd nanoparticles for Suzuki-Miyaura coupling reaction[J]. Journal of Colloid and Interface Science, 2022, 608: 809-819.
|
 ), SHI Ling, ZHANG Dongqiang, LI Ning
), SHI Ling, ZHANG Dongqiang, LI Ning