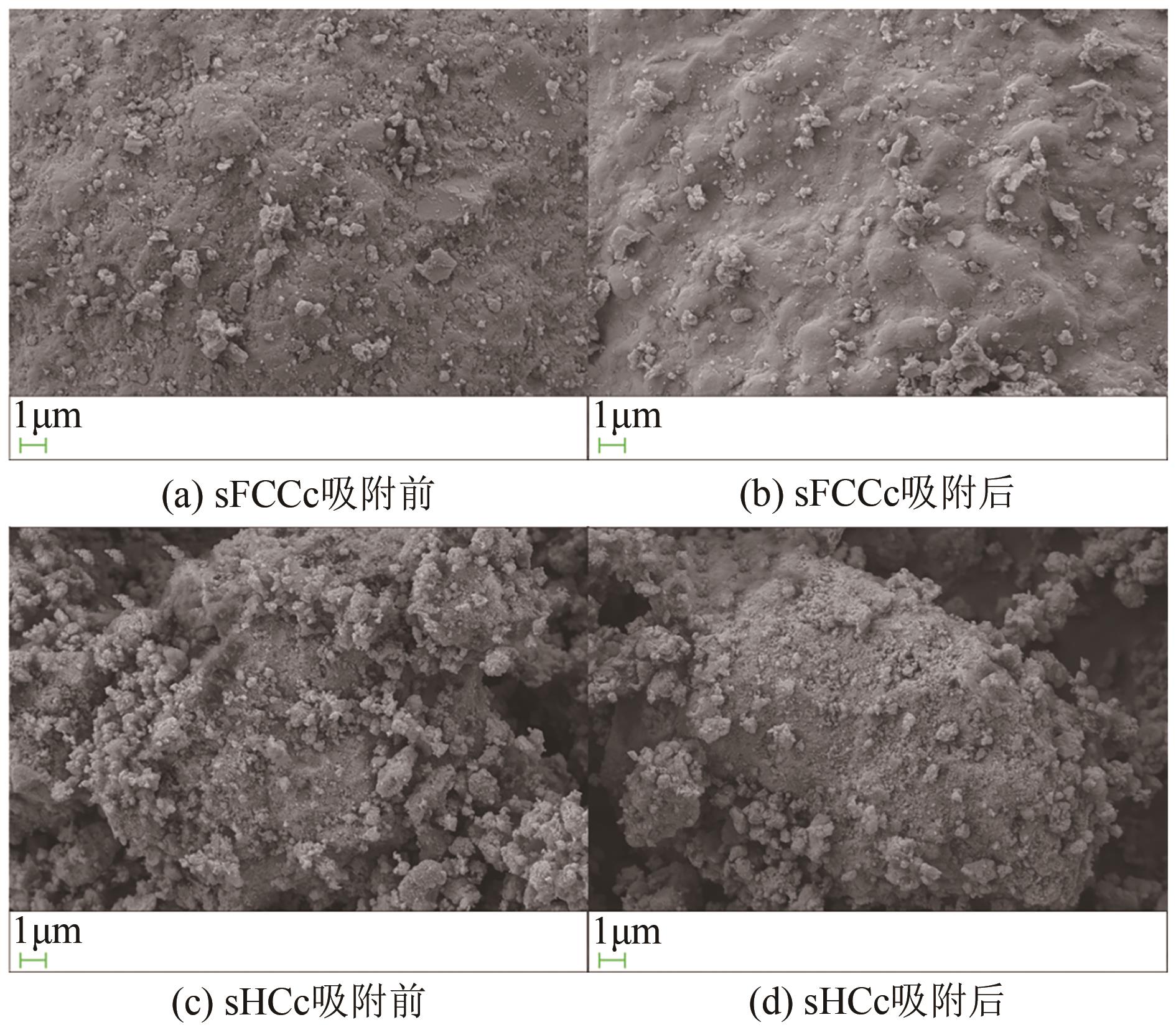
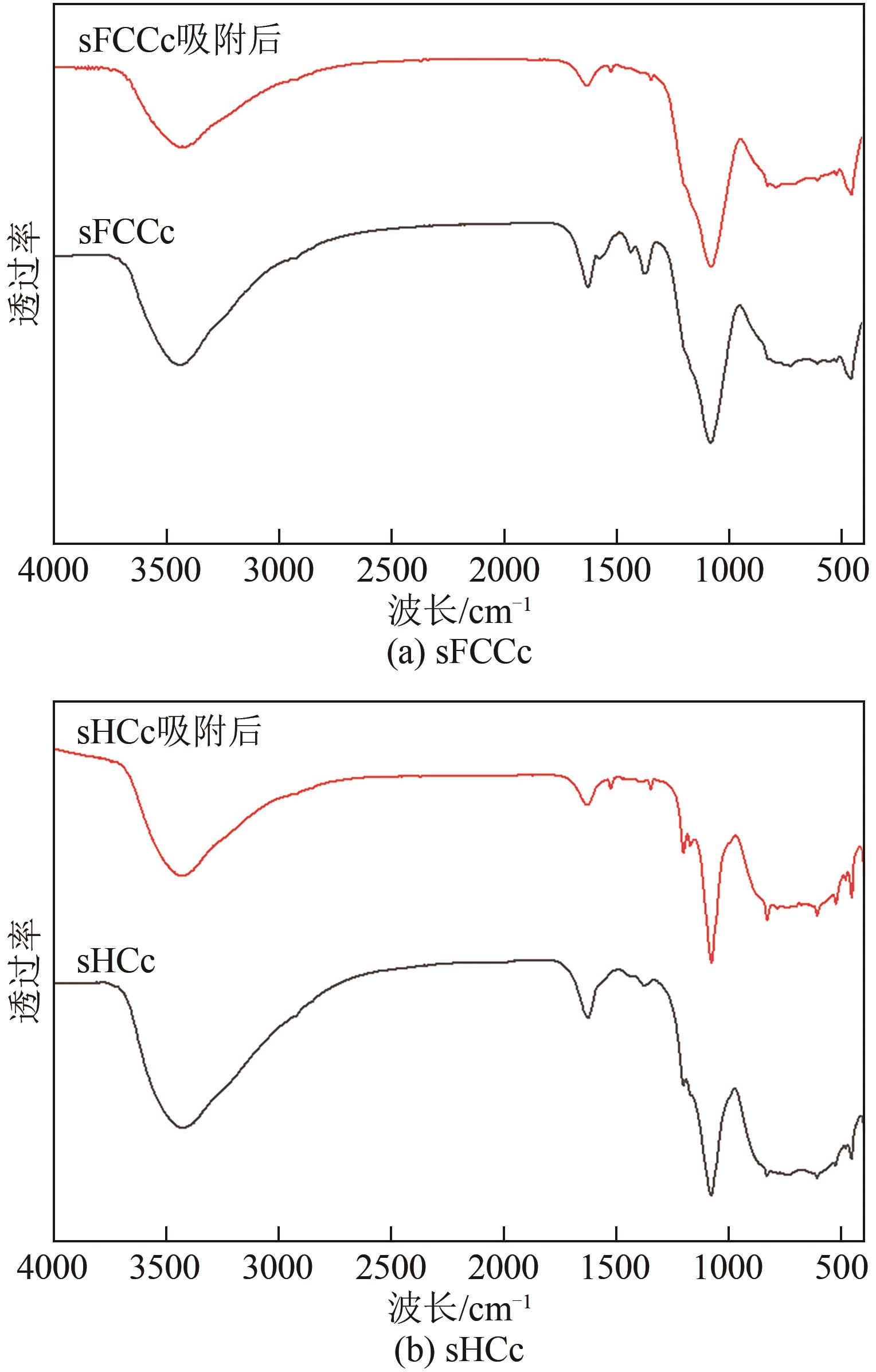
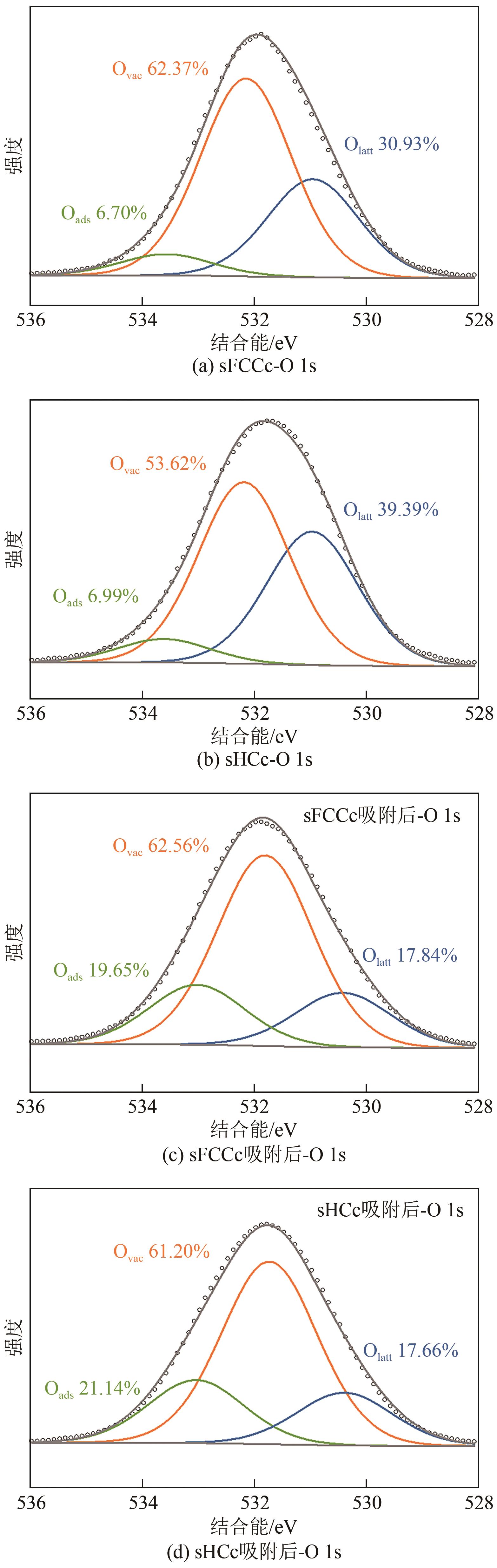
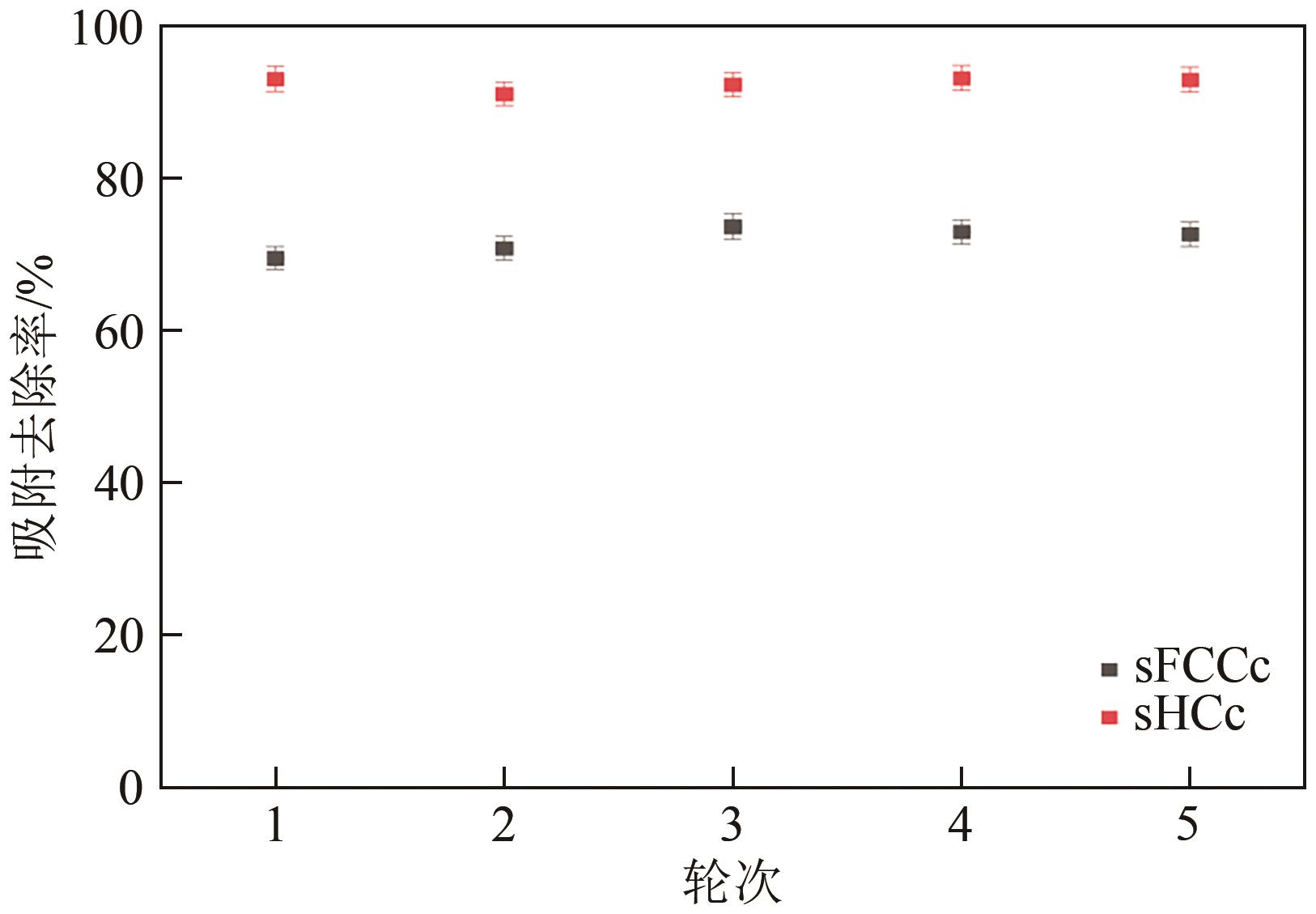
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (2): 1076-1087.DOI: 10.16085/j.issn.1000-6613.2024-0127
• Resources and environmental engineering • Previous Articles
Effects and mechanism on the removal of nitrobenzene from water by adsorption of refining waste catalysts
LI Zhuoyu1( ), YU Meiqi1, CHEN Xiaoyan2, HU Ruohui1, WANG Qinghong1, CHEN Chunmao1(
), YU Meiqi1, CHEN Xiaoyan2, HU Ruohui1, WANG Qinghong1, CHEN Chunmao1( ), ZHAN Yali1
), ZHAN Yali1
- 1.College of Chemical Engineering and Environment, State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Beijing), Beijing 102249, China
2.HSE Department, SINOPEC Group, Beijing 100728, China
-
Received:2024-01-16Revised:2024-05-10Online:2025-03-10Published:2025-02-25 -
Contact:CHEN Chunmao
炼油废催化剂吸附去除水中硝基苯的特性与机制
李琢宇1( ), 余美琪1, 陈孝彦2, 胡若晖1, 王庆宏1, 陈春茂1(
), 余美琪1, 陈孝彦2, 胡若晖1, 王庆宏1, 陈春茂1( ), 詹亚力1
), 詹亚力1
- 1.中国石油大学(北京)化学工程与环境学院,重质油全国重点实验室,北京 102249
2.中国石油化工集团 有限公司健康安全环保管理部,北京 100728
-
通讯作者:陈春茂 -
作者简介:李琢宇(1992—),女,博士,讲师,研究方向为石油石化污染处理与控制。E-mail:Lizyhit@163.com。 -
基金资助:国家自然科学基金青年基金(22308382);国家自然科学基金面上项目(22278436)
CLC Number:
Cite this article
LI Zhuoyu, YU Meiqi, CHEN Xiaoyan, HU Ruohui, WANG Qinghong, CHEN Chunmao, ZHAN Yali. Effects and mechanism on the removal of nitrobenzene from water by adsorption of refining waste catalysts[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1076-1087.
李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0127
| 催化剂 | O | Al | Si | La | Ce | Fe | Ni | W | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | |
| FCCc | 37.70 | 51.62 | 36.39 | 29.86 | 22.10 | 17.42 | 1.41 | 0.22 | 0.89 | 0.14 | 0.78 | 0.31 | 1.14 | 0.43 | — | — |
| sFCCc | 26.61 | 41.91 | 32.64 | 30.48 | 24.39 | 21.88 | 2.65 | 0.48 | 2.92 | 0.43 | 4.50 | 2.03 | 6.28 | 2.69 | — | — |
| HCc | 40.95 | 65.00 | 28.41 | 26.74 | 3.05 | 2.76 | — | — | — | — | 0.48 | 0.22 | 5.20 | 2.25 | 21.91 | 3.03 |
| sHCc | 34.70 | 53.72 | 41.15 | 37.78 | 5.10 | 4.50 | — | — | — | — | 0.59 | 0.26 | 4.37 | 1.85 | 14.08 | 1.90 |
| 催化剂 | O | Al | Si | La | Ce | Fe | Ni | W | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | 质量 分数/% | 原子 分数/% | |
| FCCc | 37.70 | 51.62 | 36.39 | 29.86 | 22.10 | 17.42 | 1.41 | 0.22 | 0.89 | 0.14 | 0.78 | 0.31 | 1.14 | 0.43 | — | — |
| sFCCc | 26.61 | 41.91 | 32.64 | 30.48 | 24.39 | 21.88 | 2.65 | 0.48 | 2.92 | 0.43 | 4.50 | 2.03 | 6.28 | 2.69 | — | — |
| HCc | 40.95 | 65.00 | 28.41 | 26.74 | 3.05 | 2.76 | — | — | — | — | 0.48 | 0.22 | 5.20 | 2.25 | 21.91 | 3.03 |
| sHCc | 34.70 | 53.72 | 41.15 | 37.78 | 5.10 | 4.50 | — | — | — | — | 0.59 | 0.26 | 4.37 | 1.85 | 14.08 | 1.90 |
| 催化剂 | Olatt相对质量分数/% | Ovac相对质量分数/% | Oads相对质量分数/% |
|---|---|---|---|
| FCCc | 16.91 | 80.51 | 2.58 |
| HCc | 19.40 | 64.37 | 16.24 |
| sFCCc | 30.93 | 62.37 | 6.70 |
| sHCc | 39.39 | 53.62 | 6.99 |
| 催化剂 | Olatt相对质量分数/% | Ovac相对质量分数/% | Oads相对质量分数/% |
|---|---|---|---|
| FCCc | 16.91 | 80.51 | 2.58 |
| HCc | 19.40 | 64.37 | 16.24 |
| sFCCc | 30.93 | 62.37 | 6.70 |
| sHCc | 39.39 | 53.62 | 6.99 |
| 催化剂 | Si与Al质量比/% | 总酸量 /mmol∙g-1 | B酸量 /mmol∙g-1 | L酸量 /mmol∙g-1 |
|---|---|---|---|---|
| FCCc | 60.73 | 19.00 | 5.59 | 13.42 |
| sFCCc | 74.72 | 8.54 | 1.28 | 7.26 |
| HCc | 10.74 | 32.19 | 2.77 | 29.42 |
| sHCc | 11.91 | 18.20 | 1.75 | 16.46 |
| 催化剂 | Si与Al质量比/% | 总酸量 /mmol∙g-1 | B酸量 /mmol∙g-1 | L酸量 /mmol∙g-1 |
|---|---|---|---|---|
| FCCc | 60.73 | 19.00 | 5.59 | 13.42 |
| sFCCc | 74.72 | 8.54 | 1.28 | 7.26 |
| HCc | 10.74 | 32.19 | 2.77 | 29.42 |
| sHCc | 11.91 | 18.20 | 1.75 | 16.46 |
| 催化剂 | 总比表面积/m2∙g-1 | 微孔比表面积/m2∙g-1 | 外比表面积/m2∙g-1 | 总孔体积/cm³∙g-1 | 微孔体积/cm³∙g-1 | 平均孔径/nm |
|---|---|---|---|---|---|---|
| FCCc | 219.44 | 134.91 | 84.53 | 0.18 | 0.066 | 3.36 |
| sFCCc | 116.91 | 72.00 | 44.91 | 0.16 | 0.035 | 5.40 |
| HCc | 272.14 | 127.89 | 144.25 | 0.35 | 0.062 | 5.17 |
| sHCc | 236.56 | 106.86 | 129.69 | 0.47 | 0.051 | 7.96 |
| 催化剂 | 总比表面积/m2∙g-1 | 微孔比表面积/m2∙g-1 | 外比表面积/m2∙g-1 | 总孔体积/cm³∙g-1 | 微孔体积/cm³∙g-1 | 平均孔径/nm |
|---|---|---|---|---|---|---|
| FCCc | 219.44 | 134.91 | 84.53 | 0.18 | 0.066 | 3.36 |
| sFCCc | 116.91 | 72.00 | 44.91 | 0.16 | 0.035 | 5.40 |
| HCc | 272.14 | 127.89 | 144.25 | 0.35 | 0.062 | 5.17 |
| sHCc | 236.56 | 106.86 | 129.69 | 0.47 | 0.051 | 7.96 |
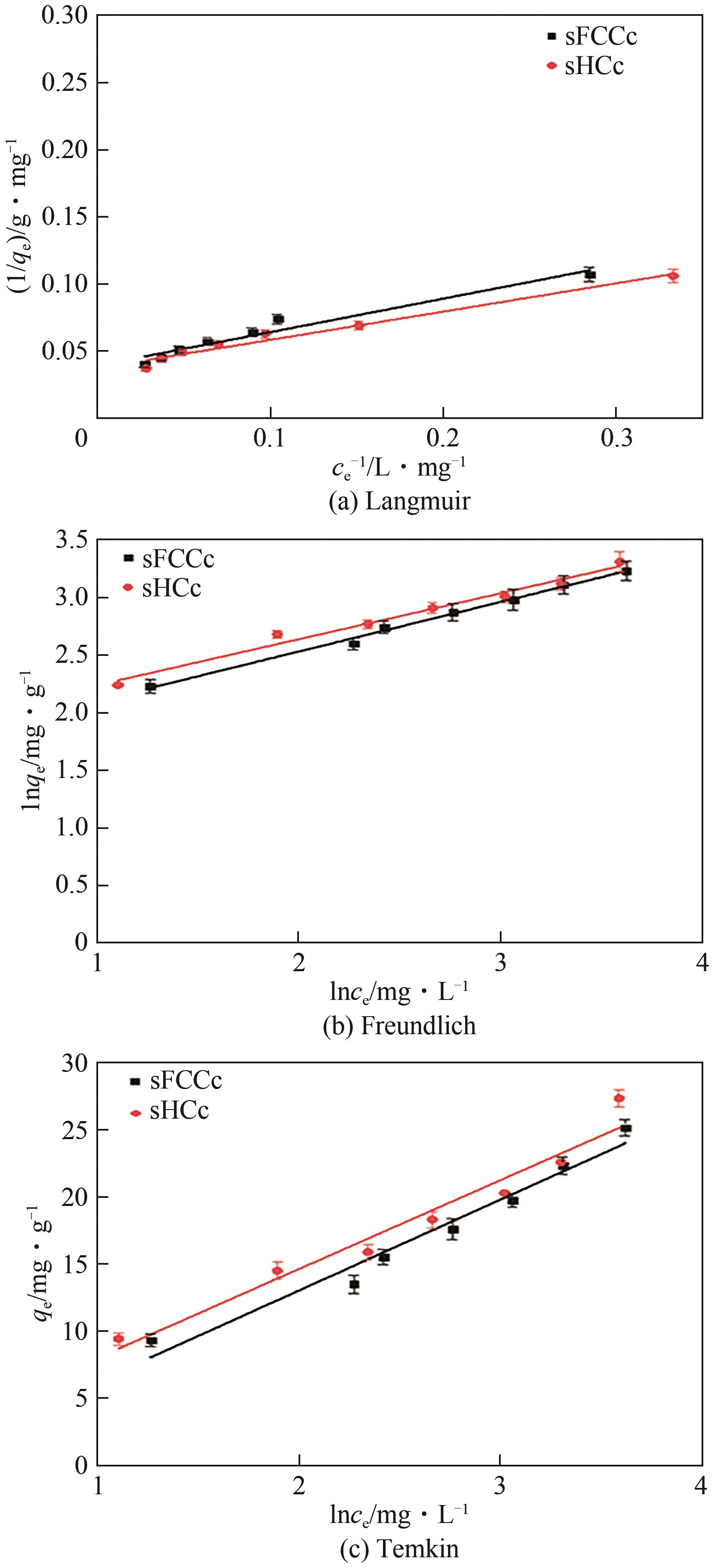
| 催化剂 | Langmuir模型 | Freundlich模型 | Temkin模型 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL | qm/mg∙g-1 | RL | R2 | KF | 1/n | R2 | KT | R2 | |
| sFCCc | 0.1580 | 25.38 | 0.11 | 0.9527 | 5.3411 | 0.430 | 0.9946 | 0.9416 | 0.9666 |
| sHCc | 0.1780 | 26.68 | 0.10 | 0.9745 | 6.3256 | 0.398 | 0.9921 | 1.2475 | 0.9560 |
| 催化剂 | Langmuir模型 | Freundlich模型 | Temkin模型 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KL | qm/mg∙g-1 | RL | R2 | KF | 1/n | R2 | KT | R2 | |
| sFCCc | 0.1580 | 25.38 | 0.11 | 0.9527 | 5.3411 | 0.430 | 0.9946 | 0.9416 | 0.9666 |
| sHCc | 0.1780 | 26.68 | 0.10 | 0.9745 | 6.3256 | 0.398 | 0.9921 | 1.2475 | 0.9560 |
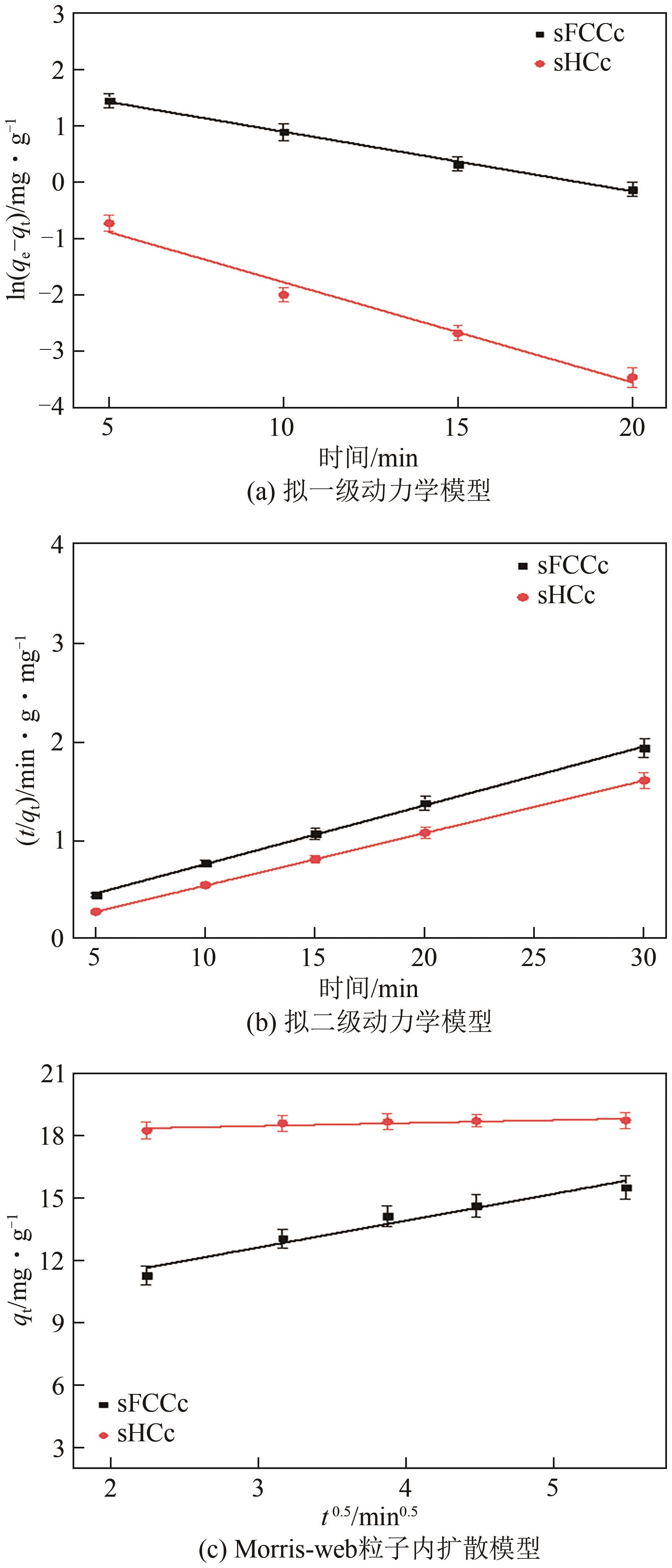
| 催化剂 | 拟一级动力学模型 | 拟二级动力学模型 | Morris-web 粒子内扩散模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe/mg∙g-1 | qcal/mg∙g-1 | k1/min-1 | R2 | qcal/mg∙g-1 | H/mg∙g-1∙min-1 | k2/mg∙g-1∙min-1 | R2 | k3/mg∙g-1∙ | R2 | |
| sFCCc | 15.54 | 7.06 | 0.408 | 0.9975 | 16.31 | 0.43 | 0.206 | 0.9999 | 0.9395 | 0.9669 |
| sHCc | 18.76 | 1.01 | 0.408 | 0.9790 | 18.87 | 6.54 | 0.347 | 0.9999 | 1.2493 | 0.9566 |
| 催化剂 | 拟一级动力学模型 | 拟二级动力学模型 | Morris-web 粒子内扩散模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe/mg∙g-1 | qcal/mg∙g-1 | k1/min-1 | R2 | qcal/mg∙g-1 | H/mg∙g-1∙min-1 | k2/mg∙g-1∙min-1 | R2 | k3/mg∙g-1∙ | R2 | |
| sFCCc | 15.54 | 7.06 | 0.408 | 0.9975 | 16.31 | 0.43 | 0.206 | 0.9999 | 0.9395 | 0.9669 |
| sHCc | 18.76 | 1.01 | 0.408 | 0.9790 | 18.87 | 6.54 | 0.347 | 0.9999 | 1.2493 | 0.9566 |
| 催化剂 | 温度/K | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J·mol-1∙K-1 |
|---|---|---|---|---|
| sFCCc | 298 | 0.36 | 5.19 | 16.20 |
| 308 | 0.20 | |||
| 318 | 0.04 | |||
| 328 | 0.03 | |||
| sHCc | 298 | -3.87 | -20.97 | -57.39 |
| 308 | -3.29 | |||
| 318 | -2.72 | |||
| 328 | -2.15 |
| 催化剂 | 温度/K | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J·mol-1∙K-1 |
|---|---|---|---|---|
| sFCCc | 298 | 0.36 | 5.19 | 16.20 |
| 308 | 0.20 | |||
| 318 | 0.04 | |||
| 328 | 0.03 | |||
| sHCc | 298 | -3.87 | -20.97 | -57.39 |
| 308 | -3.29 | |||
| 318 | -2.72 | |||
| 328 | -2.15 |
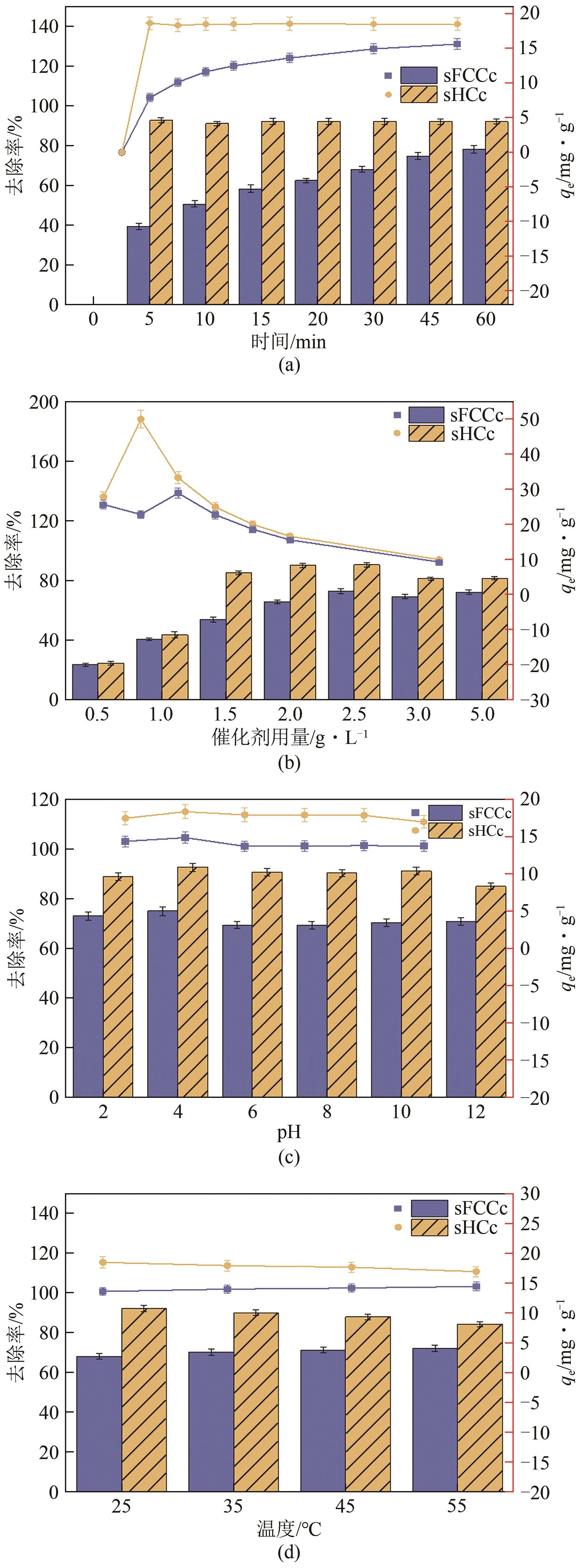
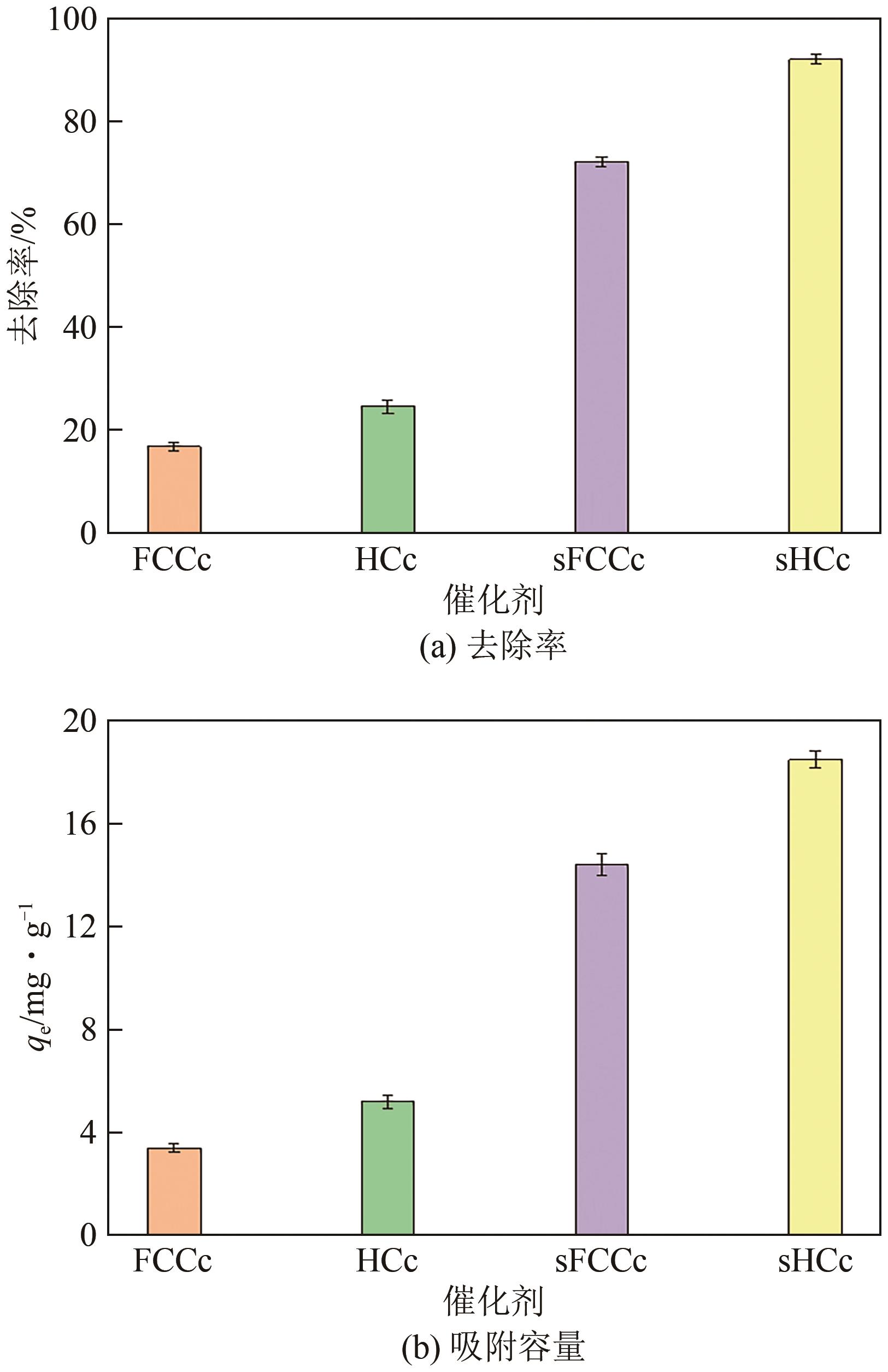
| 吸附剂种类 | 最优条件 | 去除率/% | 参考文献 |
|---|---|---|---|
| 炼油废催化剂 | 投加量2.5g/L,温度55℃(sFCCc)、25℃(sHCc),吸附平衡时间30min(sFCCc)、5min(sHCc) | 72.25(sFCCc),92.19(sHCc) | 本研究 |
| 大孔树脂 | pH=1,投加量70g/L,吸附平衡时间3.5h | 98.8 | [ |
| 活性炭纤维毡 | 投加量22.9g/L,吸附平衡时间110~120min | >46 | [ |
| 粉状活性炭 | 投加量14.2g/L,吸附平衡时间60~70min | >80 | [ |
| 颗粒活性炭 | 投加量3.14g/L,吸附平衡时间40~50min | >94 | [ |
| 碳纳米管/聚氨酯复合材料 | pH=5.4,投加量2mg/L,吸附平衡时间24h | >60 | [ |
| 膨胀石墨 | 投加量0.4g/L,温度70℃,吸附平衡时间120min | >50 | [ |
| 改性硅藻土 | pH=5,温度50℃,投加量4g/L,吸附平衡时间2h | 99.5 | [ |
| 有机改性膨润土 | 投加量20g/L,吸附平衡时间80min | 62.4 | [ |
| SiO2气凝胶 | pH=8.35,温度25℃,投加量3.33g/L,吸附平衡时间30min | 62.4 | [ |
| 吸附剂种类 | 最优条件 | 去除率/% | 参考文献 |
|---|---|---|---|
| 炼油废催化剂 | 投加量2.5g/L,温度55℃(sFCCc)、25℃(sHCc),吸附平衡时间30min(sFCCc)、5min(sHCc) | 72.25(sFCCc),92.19(sHCc) | 本研究 |
| 大孔树脂 | pH=1,投加量70g/L,吸附平衡时间3.5h | 98.8 | [ |
| 活性炭纤维毡 | 投加量22.9g/L,吸附平衡时间110~120min | >46 | [ |
| 粉状活性炭 | 投加量14.2g/L,吸附平衡时间60~70min | >80 | [ |
| 颗粒活性炭 | 投加量3.14g/L,吸附平衡时间40~50min | >94 | [ |
| 碳纳米管/聚氨酯复合材料 | pH=5.4,投加量2mg/L,吸附平衡时间24h | >60 | [ |
| 膨胀石墨 | 投加量0.4g/L,温度70℃,吸附平衡时间120min | >50 | [ |
| 改性硅藻土 | pH=5,温度50℃,投加量4g/L,吸附平衡时间2h | 99.5 | [ |
| 有机改性膨润土 | 投加量20g/L,吸附平衡时间80min | 62.4 | [ |
| SiO2气凝胶 | pH=8.35,温度25℃,投加量3.33g/L,吸附平衡时间30min | 62.4 | [ |
| 1 | 朱庆云, 曾令志, 鲜楠莹, 等. 全球主要炼油催化剂发展现状及趋势[J]. 石化技术与应用, 2019, 37(3): 153-157. |
| ZHU Qingyun, ZENG Lingzhi, XIAN Nanying, et al. Current situation and development trend of global main refining catalysts[J]. Petrochemical Technology & Application, 2019, 37(3): 153-157. | |
| 2 | ZHAO Y X, WOJCIECHOWSKI B W. The consequences of steam dilution in catalytic cracking[J]. Journal of Catalysis, 1996, 163(2): 365-373. |
| 3 | AL-KHATTAF S. The influence of Y-zeolite unit cell size on the performance of FCC catalysts during gas oil catalytic cracking[J]. Applied Catalysis A: General, 2002, 231(1/2): 293-306. |
| 4 | M-F REYNIERS, BEIRNAERT H, MARIN G B. Influence of coke formation on the conversion of hydrocarbons[J]. Applied Catalysis A: General, 2000, 202(1): 49-63. |
| 5 | 许本静, 刘振, 邢伟, 等. 与SiCl反应铵交换催化裂化废催化剂的复活方法: CN201510176129.2[P]. 2017-03-15. |
| 6 | RAMEZANI A, EMAMI S M, NEMAT S. Reuse of spent FCC catalyst, waste serpentine and kiln rollers waste for synthesis of cordierite and cordierite-mullite ceramics[J]. Journal of Hazardous Materials, 2017, 338: 177-185. |
| 7 | VELÁZQUEZ S, MONZÓ J, BORRACHERO M V, et al. Evaluation of the pozzolanic activity of spent FCC catalyst/fly ash mixtures in Portland cement pastes[J]. Thermochimica Acta, 2016, 632: 29-36. |
| 8 | 何捍卫, 孟佳, 解东梅, 等. 废FCC催化剂吸附苯酚废水[J]. 粉末冶金材料科学与工程, 2012, 17(1): 69-75. |
| HE Hanwei, MENG Jia, XIE Dongmei, et al. Adsorption of phenol by spent FCC catalyst residue from wastewater[J]. Materials Science and Engineering of Powder Metallurgy, 2012, 17(1): 69-75. | |
| 9 | 戚霖, 宫红, 王锐, 等. 废FCC催化剂吸附双酚A水溶液的研究[J]. 应用化工, 2018, 47(9): 1892-1895, 1899. |
| QI Lin, GONG Hong, WANG Rui, et al. Study on the adsorption of bisphenol A aqueous solution by spent FCC catalyst[J]. Applied Chemical Industry, 2018, 47(9): 1892-1895, 1899. | |
| 10 | HUSSAIN Zakir, KUMAR Rakesh, MEGHAVATHU Deepa. Kinetics and thermodynamics of adsorption process using A spent-FCC catalyst[J]. International Journal of Engineering & Technology, 2018, 7(4.5): 284. |
| 11 | WEBER Thomas W, CHAKRAVORTI Ranjit K. Pore and solid diffusion models for fixed-bed adsorbers[J]. AIChE Journal, 1974, 20(2): 228-238. |
| 12 | LU Hainan, XU Jiacheng, FENG Zhengjun, et al. Effects of different modifiers on the sorption and structural properties of biochar derived from wheat stalk[J]. Environmental Science and Pollution Research, 2022, 29(36): 54988-55002. |
| 13 | ABDEDAYEM Asma, GUIZA Monia, OUEDERNI Abdelmottaleb. Adsorption/regeneration coupling process using ozone on cobalt supported on activated carbon for nitrobenzene degradation[J]. Ozone: Science & Engineering, 2021, 43(1): 32-47. |
| 14 | NEMATOLLAHZADEH Ali, BABAPOOR Aziz, MOUSAVI Seyyed Mojtaba, et al. Nitrobenzene adsorption from aqueous solution onto polythiophene-modified magnetite nanoparticles[J]. Materials Chemistry and Physics, 2021, 262: 124266. |
| 15 | LI Xi, ZHANG Xiao, XU Yanhua, et al. Removal of nitrobenzene from aqueous solution by using modified magnetic diatomite[J]. Separation and Purification Technology, 2020, 242: 116792. |
| 16 | YAĞMUR Hatice Karaer, İsmet KAYA. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue[J]. Journal of Molecular Structure, 2021, 1232: 130071. |
| 17 | HADERLEIN Stefan B, WEISSMAHR Kenneth W, SCHWARZENBACH René P. Specific adsorption of nitroaromatic explosives and pesticides to clay minerals[J]. Environmental Science and Technology, 1996, 30(2): 612-622. |
| 18 | QIN Qingdong, XU Yan. Enhanced nitrobenzene adsorption in aqueous solution by surface silylated MCM-41[J]. Microporous and Mesoporous Materials, 2016, 232: 143-150. |
| 19 | YOSEFI Leila, HAGHIGHI Mohammad. Fabrication of nanostructured flowerlike p-BiOI/p-NiO heterostructure and its efficient photocatalytic performance in water treatment under visible-light irradiation[J]. Applied Catalysis B: Environmental, 2018, 220: 367-378. |
| 20 | HU Peiwei, YANG Huaming, OUYANG Jing. Synthesis and characterization of Sb-SnO2/kaolinites nanoparticles [J]. Applied Clay Science, 2012, 55: 151-157. |
| 21 | KRÓL M, ROŻEK P, CHLEBDA D, et al. Influence of alkali metal cations/type of activator on the structure of alkali-activated fly ash-ATR-FTIR studies[J]. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy, 2018, 198: 33-37. |
| 22 | NEZAMPOUR Fahime, GHIACI Mehran, MASOOMI Kianoosh. Activated carbon and graphitic carbon nitride immobilized on mesoporous silica for adsorption of nitrobenzene[J]. Journal of Chemical & Engineering Data, 2018, 63(6): 1977-1986. |
| 23 | EL-MAGHRABI Heba H, HOSNY R, RAMZI M, et al. Novel mesoporous silica (MCM-41) and its characterization for oil adsorption from produced water injected in water injection projects using fixed bed column processes[J]. Desalination and Water Treatment, 2017, 60: 70-77. |
| 24 | FELLENZ Nicolás, PEREZ-ALONSO Francisco J, MARTIN Pedro P, et al. Chromium (Ⅵ) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents[J]. Microporous and Mesoporous Materials, 2017, 239: 138-146. |
| 25 | KECIRA Zoubida, BENTURKI Oumessaâd, BENTURKI Asma, et al. High adsorption capacity of nitrobenzene from aqueous solution using activated carbons prepared from vegetable waste[J]. Environmental Progress & Sustainable Energy, 2020, 39(6): e13463. |
| 26 | TARIQ Muqaddas, DURRANI Arjumand Iqbal, FAROOQ Umar, et al. Efficacy of spent black tea for the removal of nitrobenzene from aqueous media[J]. Journal of Environmental Management, 2018, 223: 771-778. |
| 27 | SAHA Papita, CHOWDHURY Shamik. Insight into adsorption thermodynamics[M]//InTech, 2011: 1111-1125. |
| 28 | 温凯云, 李红艳, 崔建国, 等. 核桃壳活性炭的制备及对水中硝基苯的吸附[J]. 工业水处理, 2021, 41(2): 62-66. |
| WEN Kaiyun, LI Hongyan, CUI Jianguo, et al. Preparation of activated carbon from walnut shell and its adsorption of nitrobenzene in water[J]. Industrial Water Treatment, 2021, 41(2): 62-66. | |
| 29 | 李树鹏, 方虎, 李雪松, 等. 大孔树脂吸附法处理含硝基苯类工业废水试验研究[J]. 工业用水与废水, 2011, 42(5): 32-37. |
| LI Shupeng, FANG Hu, LI Xuesong, et al. Treatment of industrial wastewater containing nitrobenzene compounds by macroporous resin adsorption[J]. Industrial Water & Wastewater, 2011, 42(5): 32-37. | |
| 30 | 周欣梅, 宋雷蕾, 刘建华. 活性炭纤维毡、粉末活性炭、颗粒活性炭对水中硝基苯的静态吸附研究[J]. 中国科技信息, 2015(7): 54, 56. |
| ZHOU Xinmei, SONG Leilei, LIU Jianhua. Study on static adsorption of nitrobenzene in water by activated carbon fiber felt, powdered activated carbon and granular activated carbon[J]. China Science and Technology Information, 2015(7): 54, 56. | |
| 31 | 谢刚, 毛宁, 周林成, 等. 改进碳纳米管/聚氨酯复合材料吸附硝基苯[J]. 环境工程学报, 2015, 9(3): 1117-1123. |
| XIE Gang, MAO Ning, ZHOU Lincheng, et al. Adsorption of nitrobenzene by improving carbon nanotubes and polyurethane composite[J]. Chinese Journal of Environmental Engineering, 2015, 9(3): 1117-1123. | |
| 32 | 郑思宁, 谢茹胜, 连锦明, 等. 膨胀石墨对硝基苯的吸附性能研究[J]. 福建师范大学学报(自然科学版), 2010, 26(5): 50-52. |
| ZHENG Sining, XIE Rusheng, LIAN Jinming, et al. Study on adsorption of nitrobenzene onto expanded graphite[J]. Journal of Fujian Normal University (Natural Science Edition), 2010, 26(5): 50-52. | |
| 33 | 陈碧霄, 于鹏, 陈宋辉, 等. 改性硅藻土的制备及其对硝基苯的吸附性能研究[J]. 环境污染与防治, 2014, 36(8): 111. |
| CHEN Bixiao, YU Peng, CHEN Songhui, et al. Preparation of modified diatomite and its adsorption performance for nitrobenzene[J]. Environmental Pollution & Control, 2014, 36(8): 111. | |
| 34 | 金伟, 任建敏. 有机改性膨润土对硝基苯的吸附研究[J]. 重庆工商大学学报(自然科学版), 2012, 29(6): 85-87, 95. |
| JIN Wen, REN Jianmin. Research on adsorption of nitrobenzene by modified oganic bentonite[J]. Journal of Chongqing Technology and Business University (Natural Science Edition), 2012, 29(6): 85-87, 95. | |
| 35 | 崔升, 刘学涌, 刘渝, 等. SiO2气凝胶对废水中硝基苯的吸附性能研究[J]. 中国科学: 技术科学, 2011, 41(2): 229-233. |
| CUI Sheng, LIU Xueyong, LIU Yu, et al. Study on adsorption performance of SiO2 aerogel for nitrobenzene in wastewater[J]. Scientia Sinica (Technologica), 2011, 41(2): 229-233. |
| [1] | ZHANG Qiang, SUN Nan, ZHENG Junjie, WU Qiang, LIU Chuanhai, LI Yuanji. Effect of mixed thermodynamic promoters on kinetic and recovery study of hydration separation coal mine gas [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 192-201. |
| [2] | LIU Xinwei, GAO Shan, WANG Hongtao, WANG Jiancheng. Activation of gasification fine slag and aluminum ash and their adsorption properties [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 558-571. |
| [3] | ZHAO Liyang, LI Qian, HE Peixi, PAN Honghui, LIU Yan, LIU Xixiang. Tetracycline adsorption properties of sludge-based biochar ball-milled co-modified by phosphomolybdic acid-Fe3O4 [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 583-595. |
| [4] | ZHANG Wei, HUANG Jiu, ZHU Xiaofang, LI Peng. Performance and mechanism of lead adsorption using attapulgite-based cobalt-tungsten hydrotalcite adsorbent [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 596-606. |
| [5] | SHI Lei, WANG Qian, ZHAO Xiaosheng, LIU Hongchen, CHE Yuanjun, DUAN Yu, LI Qing. Synthesis and methyl blue adsorption performance of oil shale ash-based zeolites [J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 650-661. |
| [6] | LIU Li, FENG Bo, WEN Yang, GU Qixiong. Research progress in synthesis, functionalization and metal adsorption of silica-based mesoporous materials [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5063-5078. |
| [7] | CAO Shuyang, SHI Jingbo, DONG Youming, LYU Jianxiong. Water adsorption and desorption isotherms and thermodynamic properties of Eucalyptus obliqua woods at different temperatures [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5095-5105. |
| [8] | WU Yuqi, LI Jiangtao, DING Jianzhi, SONG Xiulan, SU Bingqin. Calcined Mg/Al hydrotalcites for CO2 removal in anaerobic digestion biogas: Performances and mechanisms [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5250-5261. |
| [9] | YANG Xinheng, JI Zhiyong, GUO Zhiyuan, LIU Qi, ZHANG Panpan, WANG Jing, LIU Jie, BI Jingtao, ZHAO Yingying, YUAN Junsheng. Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance [J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5262-5274. |
| [10] | BIAN Weibai, ZHANG Ruixuan, PAN Jianming. Research progress on preparation methods of inorganic metal lithium ion sieve materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4173-4186. |
| [11] | HE Haixia, WAN Yameng, LI Fanfan, NIU Xinyu, ZHANG Jingwen, LI Tao, REN Baozeng. Kinetics and crystallization process of naphazoline hydrochloride in methanol-ethyl acetate system [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4230-4245. |
| [12] | YIN Chenyang, LIU Yongfeng, CHEN Ruizhe, ZHANG Lu, SONG Jin’ou, LIU Haifeng. Kinetic simulation of n-hexane pyrolysis reaction based on quantitative calculations [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4273-4282. |
| [13] | WANG Jia, LI Wencui, WU Fan, GAO Xinqian, LU Anhui. Regulation active components distribution of NiMo/Al2O3 catalysts for hydrodesulfurization [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4393-4402. |
| [14] | WANG Yufei, JIA Yu, ZHANG Yisheng, XUE Wei, LI Fang, WANG Yanji. Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4421-4431. |
| [15] | ZHENG Yunxiang, GAO Yilun, LI Yanru, LIU Qinglin, ZHANG Haoteng, WANG Xiangpeng. Preparation and adsorption properties of porous double-network hydrogels modified by nitrilotriacetic acid anhydride [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4542-4549. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||