Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (1): 407-413.DOI: 10.16085/j.issn.1000-6613.2023-1318
• Industrial catalysis • Previous Articles
Deactivation mechanism of sodium poisoning hydrodesulfurization catalyst
SUN Jin( ), CHEN Xiaozhen, LIU Mingrui, LIU Li, NIU Shikun, GUO Rong(
), CHEN Xiaozhen, LIU Mingrui, LIU Li, NIU Shikun, GUO Rong( )
)
- SINOPEC Dalian Research Institute of Petroleum and Petrochemicals Co. , Ltd. , Dalian 116045, Liaoning, China
-
Received:2023-08-01Revised:2023-10-08Online:2024-02-05Published:2024-01-20 -
Contact:GUO Rong
加氢脱硫催化剂钠中毒失活机理
- 中石化(大连)石油化工研究院有限公司,辽宁 大连 116045
-
通讯作者:郭蓉 -
作者简介:孙进(1982—),男,硕士研究生,研究方向为工业催化。E-mail:sunjin.fshy@sinopec.com。 -
基金资助:辽宁省“兴辽人才计划”(XLYC2002102)
CLC Number:
Cite this article
SUN Jin, CHEN Xiaozhen, LIU Mingrui, LIU Li, NIU Shikun, GUO Rong. Deactivation mechanism of sodium poisoning hydrodesulfurization catalyst[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 407-413.
孙进, 陈晓贞, 刘名瑞, 刘丽, 牛世坤, 郭蓉. 加氢脱硫催化剂钠中毒失活机理[J]. 化工进展, 2024, 43(1): 407-413.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1318
| 样品 | C质量分数/% | Na2O质量分数/% |
|---|---|---|
| A | 5.35 | — |
| B | 5.67 | — |
| C | 10.54 | — |
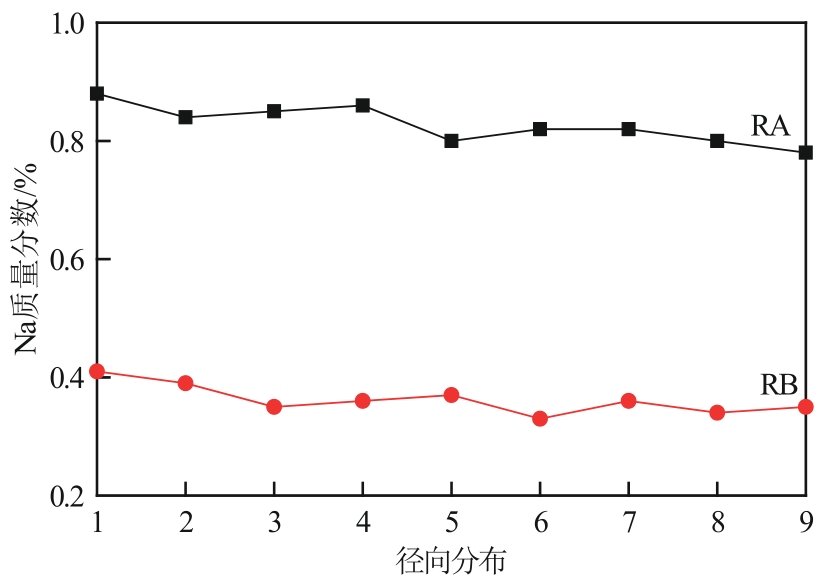
| RA | — | 1.11 |
| RB | — | 0.51 |
| RC | — | 0.02 |
| 样品 | C质量分数/% | Na2O质量分数/% |
|---|---|---|
| A | 5.35 | — |
| B | 5.67 | — |
| C | 10.54 | — |
| RA | — | 1.11 |
| RB | — | 0.51 |
| RC | — | 0.02 |
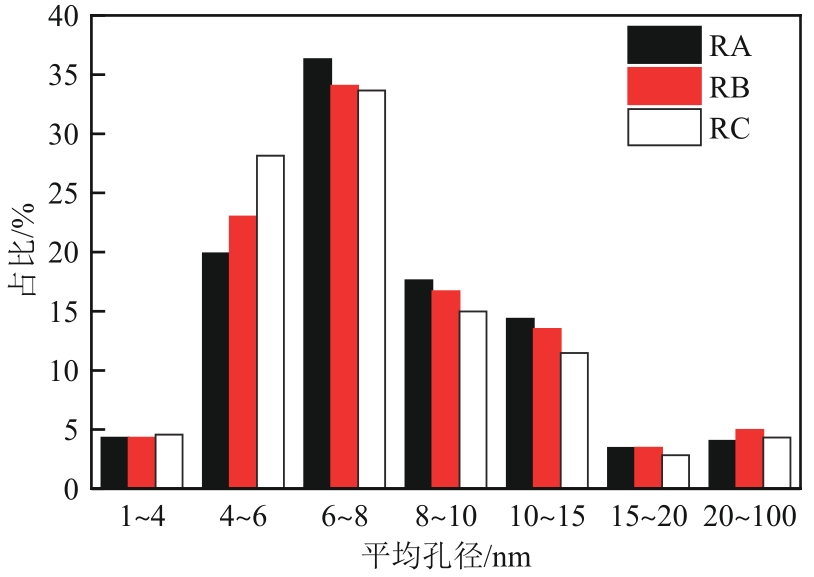
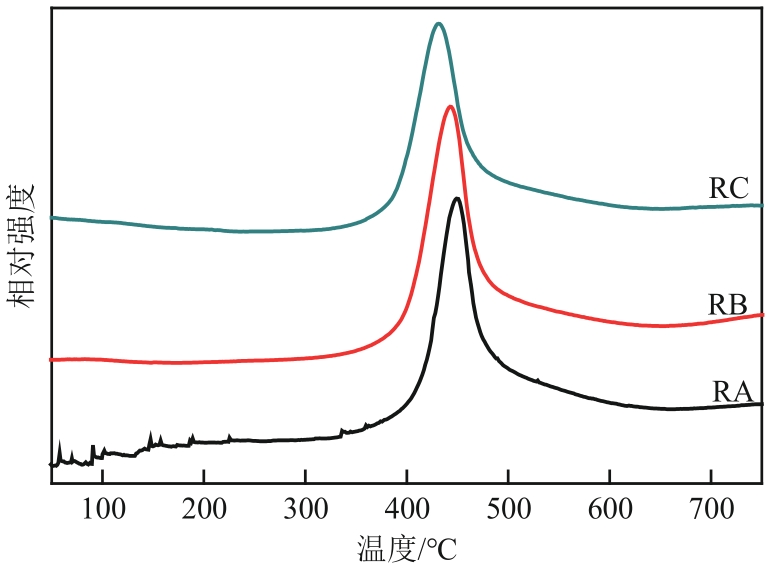
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 总酸/mmol·g-1 | B酸/mmol·g-1 | L酸/mmol·g-1 | B酸/L酸 |
|---|---|---|---|---|---|---|---|
| 新鲜催化剂 | 149.3 | 0.35 | 6.51 | — | — | — | — |
| RA | 138.1 | 0.32 | 6.88 | 0.326 | 0.289 | 0.037 | 0.13 |
| RB | 143.8 | 0.33 | 6.82 | 0.424 | 0.368 | 0.056 | 0.15 |
| RC | 145.0 | 0.34 | 6.55 | 0.545 | 0.462 | 0.083 | 0.18 |
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 总酸/mmol·g-1 | B酸/mmol·g-1 | L酸/mmol·g-1 | B酸/L酸 |
|---|---|---|---|---|---|---|---|
| 新鲜催化剂 | 149.3 | 0.35 | 6.51 | — | — | — | — |
| RA | 138.1 | 0.32 | 6.88 | 0.326 | 0.289 | 0.037 | 0.13 |
| RB | 143.8 | 0.33 | 6.82 | 0.424 | 0.368 | 0.056 | 0.15 |
| RC | 145.0 | 0.34 | 6.55 | 0.545 | 0.462 | 0.083 | 0.18 |
| 参数 | D1 | D2 |
|---|---|---|
| 密度(20℃)/cm3·g-1 | 0.8525 | 0.8657 |
| 总硫含量/μg·g-1 | 11900 | 19400 |
| 馏程/℃ | ||
| 初馏点(IBP) | 208.5 | 210.4 |
| 10%~30% | 273.6~292.1 | 283.2~203.8 |
| 50%~70% | 303.4~313.4 | 315.5~329.1 |
| 90%~95% | 329.5~336.9 | 348.9~360.9 |
| 终馏点(FBP) | 341.8 | 371.4 |
| 参数 | D1 | D2 |
|---|---|---|
| 密度(20℃)/cm3·g-1 | 0.8525 | 0.8657 |
| 总硫含量/μg·g-1 | 11900 | 19400 |
| 馏程/℃ | ||
| 初馏点(IBP) | 208.5 | 210.4 |
| 10%~30% | 273.6~292.1 | 283.2~203.8 |
| 50%~70% | 303.4~313.4 | 315.5~329.1 |
| 90%~95% | 329.5~336.9 | 348.9~360.9 |
| 终馏点(FBP) | 341.8 | 371.4 |
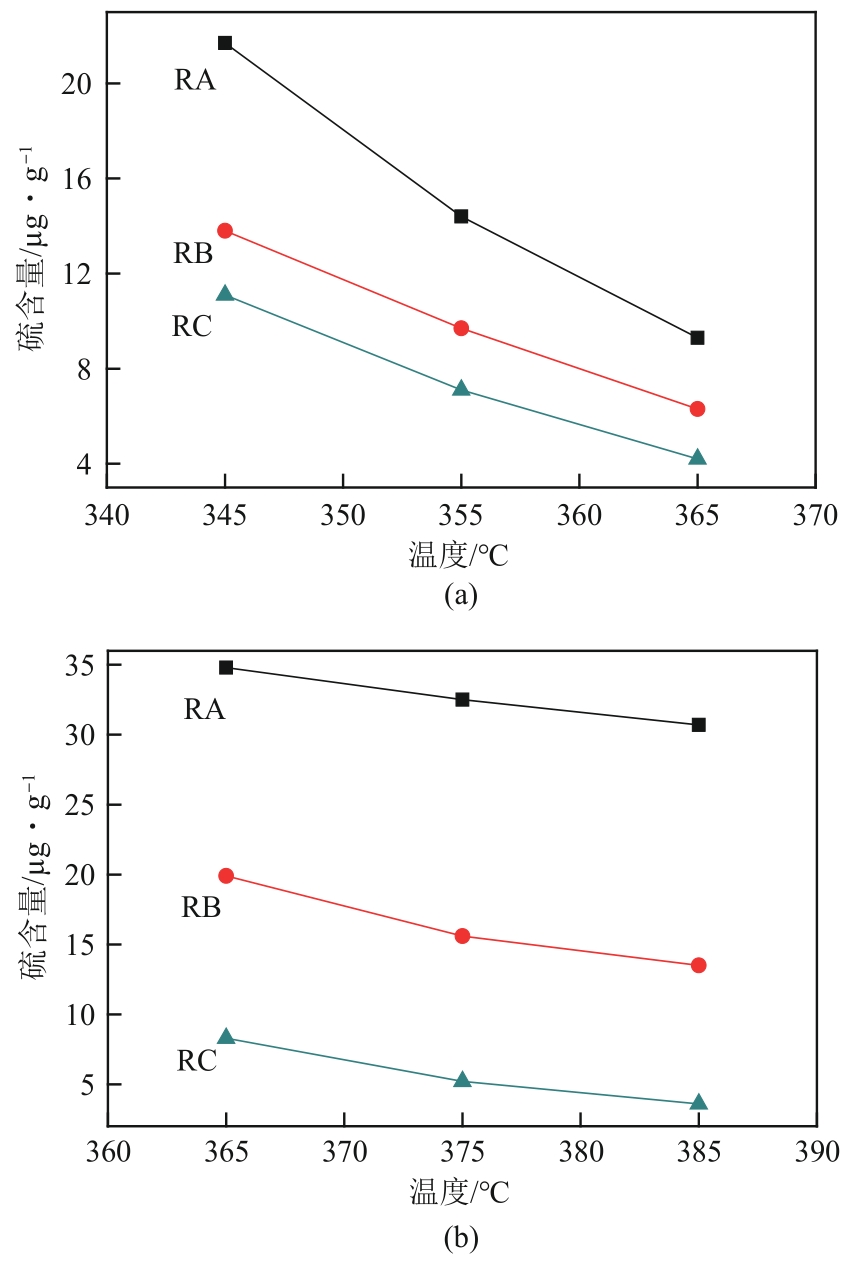
| 硫化物 | 相对脱硫速率 | 沸点/℃ |
|---|---|---|
| 噻吩 | 100 | 84.2 |
| 苯并噻吩 | 30 | 221 |
| 二苯并噻吩 | 30 | 333 |
| 甲基二苯并噻吩 | 5 | 339~351 |
| 二甲基二苯并噻吩 | 1 | 357~369 |
| 三甲基二苯并噻吩 | 1 | 370~387 |
| 硫化物 | 相对脱硫速率 | 沸点/℃ |
|---|---|---|
| 噻吩 | 100 | 84.2 |
| 苯并噻吩 | 30 | 221 |
| 二苯并噻吩 | 30 | 333 |
| 甲基二苯并噻吩 | 5 | 339~351 |
| 二甲基二苯并噻吩 | 1 | 357~369 |
| 三甲基二苯并噻吩 | 1 | 370~387 |
| 1 | ZHOU Jibin, ZHAO Jianping, ZHANG Jinling, et al. Regeneration of catalysts deactivated by coke deposition: A review[J]. Chinese Journal of Catalysis, 2020, 41(7): 1048-1061. |
| 2 | 孙进, 郭蓉, 杨成敏, 等. 加氢催化剂失活因素与再生活性研究[J]. 石油炼制与化工, 2017, 48(5): 43-47. |
| SUN Jin, GUO Rong, YANG Chengmin, et al. Effects of carbon and silicon deposition on activity of hydrogenation catalyst and regeneration performance[J]. Petroleum Processing and Petrochemicals, 2017, 48(5): 43-47. | |
| 3 | ELIZALDE Ignacio, ANCHEYTA Jorge. Modeling the deactivation by metal deposition of heavy oil hydrotreating catalyst[J]. Catalysis Today, 2014, 220/221/222: 221-227. |
| 4 | KOHLI K, PRAJAPATI R, MAITY S K, et al. Deactivation of a hydrotreating catalyst during hydroprocessing of synthetic crude by metal bearing compounds[J]. Fuel, 2019, 243: 579-589. |
| 5 | 左艳梅. 超声波-电脱盐技术的工业应用[J]. 石油炼制与化工, 2018, 49(2): 100-103. |
| ZUO Yanmei. Industrial application of ultrasonic-electric desalination technology[J]. Petroleum Processing and Petrochemicals, 2018, 49(2): 100-103. | |
| 6 | 王振宇, 张峰, 沈明欢, 等. 高钠原油的脱钠研究[J]. 石油炼制与化工, 2020, 51(7): 1-5. |
| WANG Zhenyu, ZHANG Feng, SHEN Minghuan, et al. Study of sodium removal of high-sodium crude oil[J]. Petroleum Processing and Petrochemicals, 2020, 51(7): 1-5. | |
| 7 | 李大东. 加氢处理工艺与工程[M]. 北京: 中国石化出版社, 2004: 511-512. |
| LI Dadong. Hydrogenation process and engineering[M]. Beijing: China Petrochemical Press, 2004: 511-512. | |
| 8 | 陈禹霏, 辛靖, 韩龙年, 等. 液相加氢失活催化剂的物理性质分析及其再生性能[J]. 炼油技术与工程, 2022, 52(9): 45-48. |
| CHEN Yufei, XIN Jing, HAN Longnian, et al. Analysis of physical properties and regeneration performance of liquid-phase hydrogenation deactivated catalyst[J]. Petroleum Refinery Engineering, 2022, 52(9): 45-48. | |
| 9 | 张玉婷, 张国辉, 朱金剑, 等. 加氢精制催化剂载体脱钠工艺研究[J]. 无机盐工业, 2019, 51(11): 88-90. |
| ZHANG Yuting, ZHANG Guohui, ZHU Jinjian, et al. Study on process of removing sodium from hydrogenation refining agent carrier[J]. Inorganic Chemicals Industry, 2019, 51(11): 88-90. | |
| 10 | 张杰潇, 刘子阳, 周灵萍, 等. 催化裂化催化剂脱钠工艺及微反活性研究[J]. 石油炼制与化工, 2015, 46(2): 43-48. |
| ZHANG Jiexiao, LIU Ziyang, ZHOU Lingping, et al. Study on sodium removal process and microactivity of catalytic cracking catalyst[J]. Petroleum Processing and Petrochemicals, 2015, 46(2): 43-48. | |
| 11 | 刘子林, 林德海, 何发泉, 等. 钠化焙烧法回收废SCR催化剂中钒和钨的浸出机理及浸出动力学研究[J]. 材料导报, 2021, 35(S1): 429-433. |
| LIU Zilin, LIN Dehai, HE Faquan, et al. Study of leaching mechanism and kinetics of vanadium and tungsten on the process of recovery spent SCR catalyst by sodium roasted[J]. Materials Reports, 2021, 35(S1): 429-433. | |
| 12 | 李明明. 废催化剂钠化焙烧—水浸过程钒、钼、铝的转化行为研究[D]. 昆明: 昆明理工大学, 2014. |
| LI Mingming. Study on transformation behavior of vanadium, molybdenum and aluminum in sodium roasting-water immersion process of spent catalyst[D].Kunming: Kunming University of Science and Technology, 2014. | |
| 13 | WANG Jun, LI Quanzhi, YAO Jiandong. The effect of metal-acid balance in Pt-loading dealuminated Y zeolite catalysts on the hydrogenation of benzene[J]. Applied Catalysis A: General, 1999, 184(2): 181-188. |
| 14 | 姜凤华, 王安杰, 胡永康, 等. 载体的酸性和钠含量对Ni-Mo催化剂加氢脱硫性能的影响[J]. 石油炼制与化工, 2011, 42(1): 20-27. |
| JIANG Fenghua, WANG Anjie, HU Yongkang, et al. The effect of acidity and sodium content of support on the HDS performace of Ni-Mo catalysts[J]. Petroleum Processing and Petrochemicals, 2011, 42(1): 20-27. | |
| 15 | FRITZ Paul O, LUNSFORD Jack H. The effect of sodium poisoning on dealuminated Y-type zeolites[J]. Journal of Catalysis, 1989, 118(1): 85-98. |
| 16 | PHUNG Thanh Khoa, HERRERA Concepción, LARRUBIA María Ángeles, et al. Surface and catalytic properties of some γ-Al2O3 powders[J]. Applied Catalysis A: General, 2014, 483: 41-51. |
| 17 | LA PAROLA V, DEGANELLO G, VENEZIA A M. CoMo catalysts supported on aluminosilicates: Synergy between support and sodium effects[J]. Applied Catalysis A: General, 2004, 260(2): 237-247. |
| 18 | VENEZIA Anna Maria, LA PAROLA Valeria, LIOTTA Leonarda Francesca. Structural and surface properties of heterogeneous catalysts: Nature of the oxide carrier and supported particle size effects[J]. Catalysis Today, 2017, 285: 114-124. |
| 19 | MUNDOTIYA Sharda, SINGH Rupesh, SAHA Sulay, et al. Effect of sodium on Ni-promoted MoS2 catalyst for hydrodesulfurization reaction: Combined experimental and simulation study[J]. Energy & Fuels, 2021, 35(3): 2368-2378. |
| 20 | LI Xiang, WANG Anjie, WANG Yao, et al. Hydrodesulfurization of dibenzothiophene over Ni-Mo sulfides supported by proton-exchanged siliceous MCM-41[J]. Catalysis Letters, 2002, 84: 107-113. |
| 21 | ORTEGA-DOMÍNGUEZ Rodrigo A, Arturo MENDOZA-NIETO J, Patricia HERNÁNDEZ-HIPÓLITO, et al. Influence of Na content on behavior of NiMo catalysts supported on titania nanotubes in hydrodesulfurization[J]. Journal of Catalysis, 2015, 329: 457-470. |
| 22 | 郭蓉, 周勇, 高娜, 等. 原料油性质对柴油加氢装置长周期稳定运行的影响[J]. 炼油技术与工程, 2021, 51(3): 1-4. |
| GUO Rong, ZHOU Yong, GAO Na, et al. Effect of feedstock properties on long-term stable operation of diesel hydrogenation unit[J]. Petroleum Refinery Engineering, 2021, 51(3): 1-4. |
| [1] | WANG Darui, SUN Hongmin, WANG Yiyan, TANG Zhimou, LI Rui, FAN Xueyan, YANG Weimin. Recent progress in zeolite for efficient catalytic reaction process [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 1-18. |
| [2] | LUO Fen, YANG Xiaoqi, DUAN Fanglin, LI Xiaojiang, WU Liang, XU Tongwen. Recent advances in the bipolar membrane and its applications [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 145-163. |
| [3] | GAI Hongwei, ZHANG Chenjun, QU Jingying, SUN Huailu, TUO Yongxiao, WANG Bin, JIN Xu, ZHANG Xi, FENG Xiang, CHEN De. Research progress on catalytic dehydrogenation process intensification for liquid organic hydride carrier hydrogen storage [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 164-185. |
| [4] | ZHANG Jiahao, LI Yingying, XU Yanlin, YIN Jiabin, ZHANG Jisong. Research advancement of continuous reductive amination in microreactors [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 186-197. |
| [5] | HENG Linyu, DENG Zhuoran, CHENG Daojian, WEI Bin, ZHAO Liqiang. Progress of high-throughput synthesis device for process reinforcement of metal catalyst preparation [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 246-259. |
| [6] | WANG Yiyan, WANG Darui, SHEN Zhenhao, HE Junlin, SUN Hongmin, YANG Weimin. Preparation and catalytic performance of fully crystalline MCM-22 zeolite catalyst [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 285-291. |
| [7] | YU Xiaoxiao, CHAO Yanhong, LIU Haiyan, ZHU Wenshuai, LIU Zhichang. Enhanced photoelectric properties and photocatalytic CO2 conversion by D-A conjugated polymerization [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 292-301. |
| [8] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [9] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [10] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [11] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [12] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [13] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [14] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [15] | WANG Jingang, ZHANG Jianbo, TANG Xuejiao, LIU Jinpeng, JU Meiting. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4636-4648. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||